Abstract
Background
Many HIV-infected pregnant women identified during antenatal care do not enroll in long-term HIV care, resulting in deterioration of maternal health and continued risk of HIV transmission to infants.
Methods
We performed a cluster-randomized trial to evaluate the effect of integrating HIV care into ANC clinics in rural Kenya. Twelve facilities were randomized to provide either integrated services (ANC, PMTCT, and HIV care delivered in the ANC clinic; n=6 intervention facilities), or standard ANC services (including PMTCT and referral to a separate clinic for HIV care; n=6 control facilities).
Results
There were high patient attrition rates over the course of this study. Among study participants who enrolled in HIV care, there was twelve month follow up data for 256/611 (41.8%) women, and postpartum data for only 325/1172 (28%) women. By 9 months of age, 382/568 (67.3%) infants at intervention sites and 338/594 (57.0%) at control sites had tested for HIV (OR 1.45, 95% CI 0.71-2.82); 7.3% of infants tested HIV-positive at intervention sites compared to 8.0% of infants at control sites (OR 0.89, 95% CI 0.56-1.43). The composite clinical/immunologic progression into AIDS was similar in both arms (4.9% vs. 5.1 %, OR 0.83, 95% CI 0.41 - 1.68).
Conclusions
Despite the provision of integrated services, patient attrition was substantial in both arms, suggesting barriers beyond lack of service integration. Integration of HIV services into the ANC clinic was not associated with a reduced risk HIV transmission to infants and did not appear to affect short-term maternal health outcomes.
Keywords: HIV/AIDS, prevention of mother-to-child transmission, service integration, cluster randomized controlled trial, Africa
Introduction
The elimination of mother-to-child transmission of HIV (eMTCT) is a top public health priority in sub-Saharan Africa.1-4 In order to successfully eliminate vertical transmission and to improve maternal health outcomes, all HIV-infected women of child bearing age need to be identified through routine HIV testing. However, once identified, HIV-infected pregnant women need to navigate a cascade of services5,6 including continued attendance at antenatal care (ANC) clinics, enrollment in HIV care, laboratory testing for CD4 count, antiretroviral therapy (ART) for the prevention of MTCT throughout pregnancy, labor and delivery, and breastfeeding, life-long highly active ART (HAART) for those meeting criteria, and HIV testing for HIV exposed infants. Although MTCT has been reduced to < 2% in high-income countries7, MTCT rates remain high in most sub-Saharan African countries8, partially because women and infants fall out of the cascade at different steps, reducing the effectiveness of the interventions5,6,9,10.
In Kenya the majority of pregnant women undergo routine HIV testing as part of antenatal care, HIV seropositive women are given PMTCT interventions during antenatal care and are then referred to a separate clinic for enrollment in long-term HIV care and treatment.11,12 In some settings a pregnant woman diagnosed with HIV will receive care in two clinic locations, she will require twice the visits, wait in twice the queues, and have twice the number of visits to laboratory or pharmacy. Conversely, women who are established patients in HIV clinics may not be routinely evaluated for pregnancy. If a pregnancy is incidentally identified, patients are referred to ANC clinics for the duration of their pregnancy, and will continue care in two venues.
Family Aids Care and Education Services (FACES) is a U.S. Centers for Disease Control (CDC) funded collaboration between the Kenya Medical Research Institute (KEMRI) and the University of California San Francisco (UCSF), which supports the Kenyan Ministry of Health to provide HIV care, treatment, and prevention services in Nyanza Province, Kenya.13 Over time FACES program managers and national policy makers in Kenya began to recognize the missed opportunities and inefficiencies in the vertical systems of care that separate ANC, PMTCT, and HIV treatment services. In 2005, FACES program managers hypothesized that integrating ANC and HIV treatment services for pregnant women in a single clinic with a single provider would result in faster initiation of HAART for eligible women and infants, improved maternal health outcomes, and reduced mother-to-child transmission of HIV.
Methods
Study Design
This study is a cluster-randomized trial to evaluate the maternal and infant effects of integrating ANC, PMTCT, and HIV care and treatment services. Twelve clinics in Nyanza Province Kenya were randomized to provide integrated care (intervention arm) that included ANC, PMTCT, and HIV care and treatment in the same clinic; or standard of care (control arm) that included ANC, PMTCT, and referral for HIV care. All study sites were government health facilities that provided ANC, PMTCT, and HIV services with ≥ 20 new ANC clients per month. Facilities were stratified as “Health Center/Dispensary” (n = 8) or “Hospital” (n = 4) and randomly allocated 1:1 within strata to provide integrated or non-integrated services using ACluster software. The study enrolled HIV-positive pregnant women 18 years and older, not previously enrolled in HIV care, from June 2009 until March 2011 and followed each mother for one year after enrollment. Infants were followed until nine months after delivery. Although providers and patients could not be blinded, study investigators were blinded to study outcomes until the study was completed. A complete description of the study design and methods has been published previously16.
Study Intervention
At intervention sites ANC providers conducted combined ANC, PMTCT, and HIV care visits including enrollment in HIV care, management of HIV disease, management of opportunistic infections and HAART if eligible. The same clinician provided all antenatal and postpartum services including early infant diagnosis until a definitive pediatric HIV diagnosis was obtained or the child reached 18 months of age. At this time the woman and infant, if HIV infected, were referred for long-term care to the facility's HIV clinic.
At control sites routine ANC and PMTCT services were provided in the ANC as per National Guidelines. Once diagnosed, HIV infected women were encouraged to co-enroll in the facility's separate HIV clinic and provided a referral form to facilitate enrollment. Women presented separately at these clinics, and HIV clinic providers handled HIV enrollment, management of opportunistic infections, and management of HIV disease, including HAART if eligible.
Clinicians at each site received a week-long training and on- the-job mentorship with periodic refresher trainings on ANC, PMTCT, and HIV treatment, as well as instruction on clinic logistics to improve service utilization for both models. ANC nurses at intervention sites received additional training on HIV care and treatment including appropriate disease monitoring, treatment of opportunistic infections, prescription of ARVS, and management of patients on HAART.
National PMTCT and HIV treatment Protocols
ANC, PMTCT, and HIV services offered at each site followed Kenyan National Guidelines in both study arms, with the only difference being the clinic location and the provider of HIV care and treatment services for pregnant and postpartum women (ANC clinic versus the HIV clinic). National PMTCT protocols mandated that all HIV-positive women should receive ARVs for PMTCT, and that a combination of WHO clinical staging and CD4 testing was used to determine HAART eligibility. Over the course of the study design and implementation, the national PMTCT guidelines changed substantially. From 2007 until 2010 the National Guidelines recommended maternal zidovudine from 28 weeks gestation, a single dose of nevirapine for the mother during labor and for the infant within 72 hours of birth. In 2010 the PMTCT guidelines changed to begin maternal zidovudine from 14 weeks gestation and added maternal lamivudine and zidovudine for one week in the post partum period14. Infant prophylaxis regimens also changed in 2010 from single dose nevirapine with four weeks of zidovudine syrup to nevirapine monotherapy until cessation of breastfeeding.14 (See Table in supplemental digital content).
National Guidelines for HIV treatment recommended HAART initiation for HIV infected pregnant women at WHO clinical stage III or IV regardless of CD4 count, or WHO clinical stage I or II with a CD4 count ≤ 350/mm3 throughout the study period. According to National Guidelines, women were to receive WHO staging with CD4 testing at baseline and every six months thereafter. Adherence to HIV medications and ARVs was assessed by patient self-report on history during HIV follow up visits (for women on HAART) or at postpartum visits (for women not on HAART).
As part of the HIV care and treatment services substantial attempts were made to find patients who missed appointments for HIV follow up. A patient was defined as a “defaulter” once they had missed one visit. Defaulter tracing efforts included phone calls and home visits by community health assistants. A patient was defined as “lost-to-follow-up” if they missed at least one visit, three phone calls had been made, and two home visits were conducted at least two weeks apart in attempt to ask the patient to return to clinic. If infants had not come for HIV testing by 6 months of age then the defaulter-tracing program was also activated to find untested infants.
Study Objectives and Outcomes
The overall study objective was to assess the impact of integrating PMTCT and HIV care and treatment in the antenatal care setting on: HIV vertical transmission, maternal HIV treatment, infant HIV testing, patient enrollment, retention, adherence, and provider job satisfaction. The primary outcome for the study was vertical transmission of HIV. The secondary outcomes were mean change in maternal CD4 and WHO stage up to 1 year after enrollment, infant HIV testing uptake, HIV care enrollment rates, measures of patient retention, and adherence to HIV care compared between intervention and control sites. This manuscript focuses the primary outcomes. PMTCT service utilization, HIV care enrollment, and adherence outcomes are presented else where in this same issue by Turan J et al. Patient and provider satisfaction results were presented elsewhere by Vo B et. al.15
Specifically, the outcomes evaluated in this analysis were: change in maternal CD4 count from baseline to study closure, progression of WHO stage from baseline to study closure, composite clinical and immunological progression (WHO stage and CD4 count) from baseline until study closure, as well as ARV use, and time to HAART initiation. Infant outcomes evaluated in this analysis were: uptake of infant HIV testing, HIV incidence at six weeks and at study closure (approximately nine months of age), and HIV-free survival of infants at study closure.
Power Calculations and Sample Size
The SHAIP trial was powered to detect differences in vertical transmission rates. Power calculations for the trial were based on estimated differences in MTCT rates in the two study arms, accounting for stratification, based on prior published transmission rates using similar protocols for reference.16-18 With a sample size of 12 clusters, 591 HIV-positive women per study arm, and an average of 98 HIV-positive women per health facility, we calculated that we would have 80% power to be able to detect a minimum odds ratio of 2.02 for vertical transmission, assuming an intra-cluster correlation coefficient (ICC) of 0.01.
Data Collection and Data Monitoring Methods
Health facility staff members entered all patient socio-demographic and clinical data on paper encounter forms and registers as part of routine clinic care services. Data from paper records were entered into an electronic database, which employs the Open Medical Record System (OpenMRS®) platform. Study staff extracted data from the Open Medical Record System for each SHAIP participant until 12 months after study enrollment. Internal data quality monitoring was conducted monthly by the study team, and external data quality monitors conducted data quality monitoring semi-annually. Implementation fidelity was ensured through a mid-term integration assessment. The assessment demonstrated the integration scores of the facilities matched their study assignment, with some variations based on the availability of resources.19
Statistical Analysis
Statistical analysis was conducted using an intention-to-treat approach with all analyses adjusted for clustering of data within clinics. The comparison of the baseline characteristics in the intervention and control sites was done using cluster adjusted chi-square and t-tests. Logistic regression within a Generalized Estimating Equations (GEE) framework was used to analyze the dependent binary outcomes across the study arms, reporting the odds ratios (OR) and respective 95% confidence intervals (CI). Time to event outcomes were analyzed using cluster adjusted proportional Hazards Cox regression reporting hazard ratios (HR) and respective 95% confidence intervals.
Missing data due to patient attrition relative to the measurement of specific outcomes was a significant issue in this study because the study relied on routine health facility visits and program data. This especially affected analysis of postnatal outcomes and early infant diagnosis. We used two methods to deal with missing data. We employed inverse probability of censoring weighting (IPCW) to account for potential bias associated with missing outcome data, and modeled the probability of remaining in the sample (P(c)) based on calendar time and maternal characteristics at baseline. Data were weighted (1/(P(c)) so that the distribution of maternal characteristics was similar to the observed data at baseline. Additional variables with substantial missing data included dates of birth for infants and dates of death for infants who died. To estimate these dates we relied on existing baseline data on the woman's estimated date of delivery (EDD) on the study enrollment forms. For infant death, an estimate of the date of death was made based on the fact that the majority of infant deaths occur during the first month of life. Thus we estimated the date of death at one month following the infant's date of birth (or the EDD if date of birth was not available). Statistical analyses were conducted using Stata 12 for baseline, time-to-event, and initial bivariate analyses, and SAS 9.3 for IPCW analyses.
Ethical Approvals
This study was approved by the Committee on Human Research at the UCSF, the Ethical Review Committee at KEMRI and by the U.S. Centers for Disease Control and Prevention. All women participating in the study gave written informed consent for the use of their de-identified data in the study. Participation in the study did not require additional research activities beyond women's regular antenatal and HIV care.
Results
1172 pregnant women were enrolled in this study across 12 study facilities. The total catchment population in each arm of the study was similar and facility types were balanced in each arm in terms of size, level of care, and staffing, as described in our previous publication16. Baseline characteristics of the women were not statistically different between participants enrolled at intervention and control sites (Table 1).
Table 1. Comparison of baseline patient characteristics among intervention and control sites.
| Intervention (n=569) | Control (n=603) | P value | Number (%) with missing data | |
|---|---|---|---|---|
| Mean age in years (SE) | 25.0 (0.19) | 24.8 (0.18) | 0.582 | 6 (0.51) |
| Education, n (%) | 2 (0.17) | |||
| None or Some Primary | 481 (85%) | 533 (89%) | 0.372 | |
| Some Secondary or more | 84 (15%) | 68 (11%) | ||
| Marital status, n (%) | 10 (0.85) | |||
| Married | 472 (84%) | 500 (84%) | 0.986 | |
| Single/Separated/Divorced | 49 (8%) | 50 (8%) | ||
| Widowed | 43 (8%) | 48 (8%) | ||
| Median Gravidity (IQR) | 3 (2-4) | 3 (2-4) | 0.943 | 19 (1.62) |
| Median Parity (IQR) | 2 (1-3) | 2 (1-3) | 0.921 | 25 (2.13) |
| Mean Gestational Age, weeks (SE) | 26 (0.3) | 25.2 (0.3) | 0.100 | 10 (0.85) |
| WHO stage n (%) | 66 (5.63) | |||
| WHO Stage 1 | 339 (63%) | 455 (80%) | 0.403 | |
| WHO Stage 2 | 79 (15%) | 42 (7%) | ||
| WHO Stage 3 or 4 | 31 (6%) | 8 (1%) | ||
| Not Staged | 85 (15%) | 67 (12%) | ||
| Mean Baseline CD4 (SE) | 495 (19.87) | 523 (19.18) | 0.336 | 508 (43.34) |
| Baseline CD4 category | ||||
| ≤350 | 117 (34%) | 96 (30%) | 0.654 | |
| 351-499 | 79 (23%) | 83 (26%) | ||
| ≥500 | 146 (43%) | 143(44%) | ||
| Eligible for HAART | 127 (22%) | 87 (14%) | 0.278 | 0 |
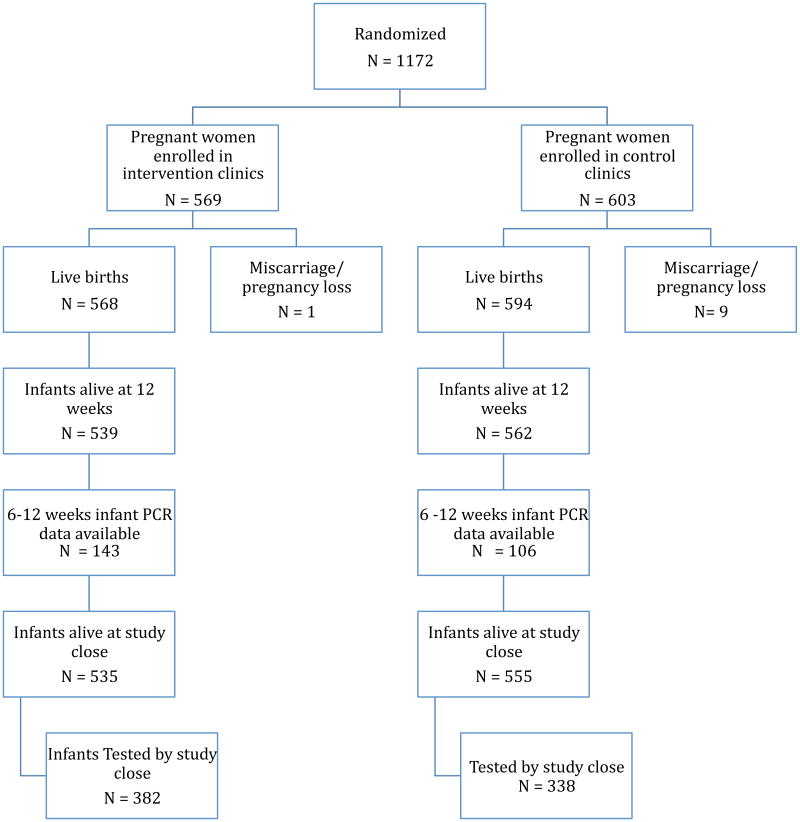
This study had significant attrition of both mothers and infants (Figure 1). All 1172 participants had baseline data collected. Among study participants who enrolled in HIV care, there was six month follow-up data for 313/611 (51.1%) women, twelve month follow up data for 256/611 (41.8%) women, and postpartum data for only 325/1172 (28%) of all women. Long term follow up data is not available for study participants who did not enroll in HIV care. Postnatal patient records were only available for a small number of study participants due to a variety of factors including; women not returning to the clinic following delivery, incorrect contact addresses limiting tracing efforts, and incomplete reporting by clinicians. Differences in baseline characteristics of women who where lost-to-follow-up and women who remained in the study did not reach statistical significance in any parameter compared (data not shown).
Figure 1. Diagram of study recruitment, enrollment, and infant follow up at intervention and control sites.
ARV use
At baseline 528/569 (92.8%) women received ARVs from the provider in the intervention clinics compared to 581/603 (96.4%) women at control clinics (OR 0.53, 95% CI 0.28 - 1.02). However, among the subgroup of women with data on self-reported ARV adherence (including women on HAART) collected at the postpartum visit (n=329), the proportion of women who reported completing the national PMTCT protocol and swallowed ARVs for PMTCT across the antepartum, intrapartum, and postpartum periods was very low in both arms and not significantly different in intervention 37/176 (21%) versus control clinics 23/153 (15%)(OR 1.72, 95% CI 0.85 - 3.48). In this same subgroup, the proportion of women who had reported swallowing only intrapartum and postpartum ARVs (incomplete PMTCT intervention) was lower in intervention [19/176 (11%)] compared to in control sites [60/153 (40%)], although this difference was not statistically significant (OR 0.39, 95% CI 0.11 - 1.36). Despite dispensing ARVs to a high proportion of women, 184/ 476 (39%) women reported after delivery (either at a postpartum visit or HIV follow up visit) that they had not swallowed any ARVs for PMTCT or HAART at any point during the study period (either antepartum, intrapartum, or postpartum); the proportion was not associated with study arm (OR 0.934, 95% CI 0.42 – 2.06).
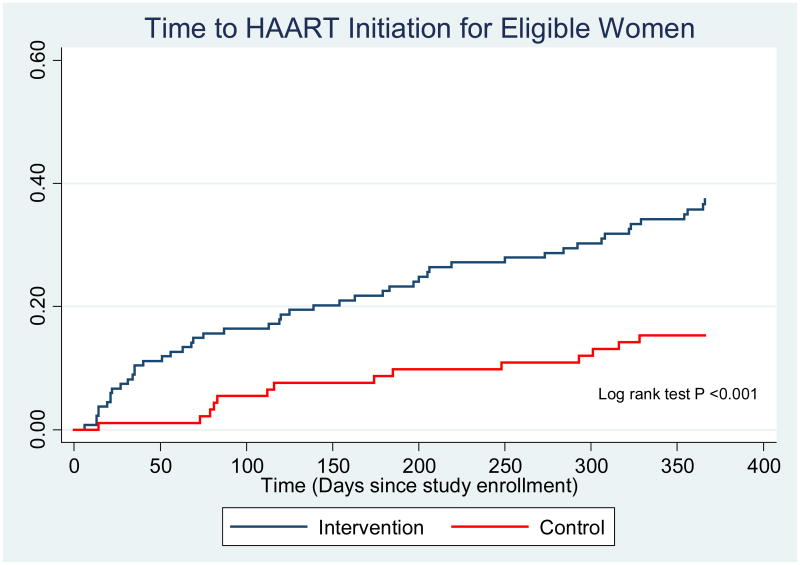
Among women who were eligible for HAART, only 88/239 (37%) women initiated HAART during pregnancy (defined as women who received at least two weeks of HAART prior to delivery),more initiated at intervention sites [66/143 (46%)] compared to control sites [22/96 (23%)](OR 2.37, 95% CI 1.06 - 5.27). In addition, the median time to HAART initiation, among eligible women, was shorter in the intervention arm [125 days (interquartile range (IQR) 35-273)] compared to the control arm [185 days (IQR 83-316)] (HR=2.74, 95% CI 1.56-4.80). (Figure 2)
Figure 2. Time to HAART Initiation for Eligible Women.
Maternal health outcomes
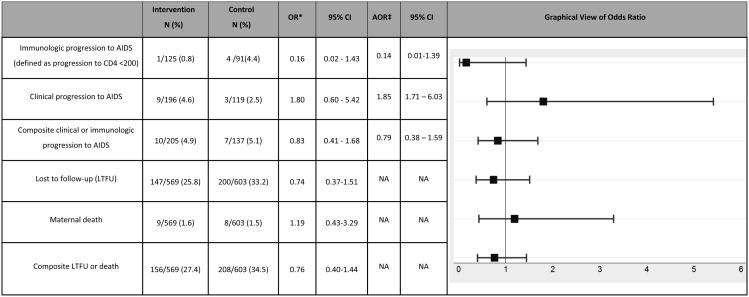
The maternal outcomes evaluated included changes in clinical and immunologic status between intervention and control sites. However, we only had both baseline and follow up absolute CD4 count on 216/1172 (18.4%) and WHO staging for both baseline and follow-up on 315/1172 (26.9%) of women. Among these women, we did not find significant differences between study arms in change in CD4 count, progression to WHO stage 3 or 4 disease, or in combined clinical/immunologic progression to AIDS from baseline to study close (Table 2).
Table 2. Comparison of maternal disease outcomes among intervention and control sites N= 1172.
OR are adjusted for cluster effects
Result obtained using Inverse probability of censoring weighting (IPCW)
Infant outcomes
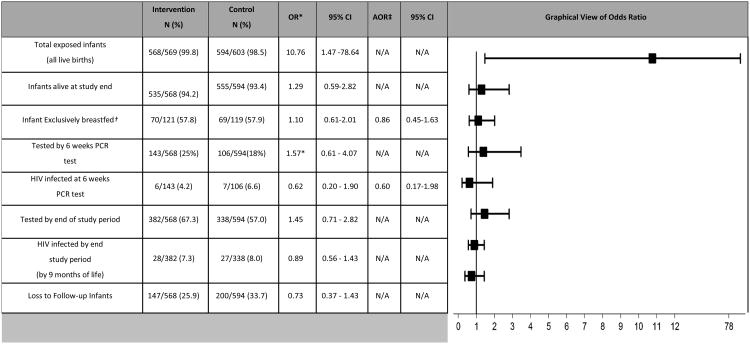
There was a high loss-to-follow-up rate of infants in both arms and as a result there were low levels of infant testing in both study arms. At six weeks more infants had undergone PCR testing in the intervention compared to control sites 43/568 (25%) vs. 106/594 (18%) (OR 1.57, 95% CI 0.61 - 4.07). By the end of the study, a slightly greater proportion of HIV-exposed infants had undergone HIV testing at intervention sites [382/568 (67.3%)] compared to control sites [338/594 (57.0%)](OR 1.45, 95% CI= 0.71-2.82), however these trends did not reach statistical significance.
Infant HIV-infection by six weeks of life (4.2% in intervention sites vs. 6.6% in control sites, OR 0.62, 95% CI 0.20–1.90) and at the end of follow up at nine months (7.3% in intervention sites vs. 8% in control sites, OR 0.89, 95% CI 0.56 - 1.43) did not differ by study arm (Table 3). We performed a sensitivity analysis to account for the substantial amount of missing infant HIV testing data due to low uptake of early infant diagnosis at the clinics. After adjusting for missing data the odds of infant infection at six weeks and at nine months showed similar odds as those obtained from the unadjusted analysis. Overall infant mortality rates and HIV-free survival did not differ significantly by study arm (Figures 3 & 4 in Supplemental Digital Content).
Table 3. Comparison of infant outcomes among intervention and control sites N=1172.
OR are adjusted for cluster effects
By patient report (out of 325 women with postpartum forms)
Result obtained using Inverse probability of censoring weighting (IPCW)
Multivariable analyses examining the impact of censoring showed that adjustment for loss-to-follow-up did not significantly change the results. Inverse probability of censoring weighting (IPCW) results were of similar direction, magnitude, and statistical significance to the initial analytical results for variables that had substantial missing data due to loss-to-follow-up including infant feeding method and infant HIV testing uptake. These results are presented in Table 3.
Discussion
To our knowledge, this study represents the first cluster randomized controlled trial with the specific aim to evaluate integrated ANC and HIV treatment for HIV infected pregnant women in sub-Saharan Africa. Currently, public health officials are looking for strategies to eliminate vertical transmission of HIV and, despite a lack of compelling data, integration of care has been proposed as a key strategy in the fight for eMTCT.1,4,20-22
This paper focuses on the infant transmission outcomes and maternal health outcomes associated with full integration of ANC, PMTCT, and HIV care. A separate manuscript published in this issue demonstrated that integration was associated with improved uptake of some key services along the PMTCT cascade. [Reference once published] In addition, we have previously found a high degree of both patient and provider acceptability with integration, and indications that providers prefer an integrated model of care in the ANC.15,23
In this study, over 90% of women were given ARVS at their first visit, however amongst women who came for postpartum visits, there was a substantial proportion of women (39%) who self-reported not taking ARVS. This suggests that there may still be substantial adherence barriers that have not been explained in this study.
Although the time to initiation of HAART among eligible pregnant women was shorter at intervention compared to control sites, based on the median time to HAART initiation (125 days) and the mean gestational age at enrollment (26 weeks), assuming a 40 week gestational period it appears that most women in both arms did not start HAART until the post-partum period. In addition, we did not find a statistically significant difference in vertical HIV transmission between study arms. Approximately half of women in Kenya, like many countries in the region, begin prenatal care in the third trimester of pregnancy.24 Therefore, even though some women were eligible to start HAART during pregnancy as per WHO and Kenyan National guidelines, most women did not initiate HAART until post-partum. This represents an enormous missed opportunity to further decrease mother-to-child transmission by using more effective regimens for sicker patients. Delay in HAART initiation may have been due to the need to draw baseline labs, including CD4 testing prior to HAART initiation, which can create significant delays in a rural setting where lab specimens must travel to a central lab for processing. (In study facilities CD4 results took approximately one month to return to facility). The delay in HAART initiation may have also been due to the clinic requirements for patients to undergo three adherence counseling sessions prior to HAART initiation which may also represent an undue visit burden on patients. The lengthy time to HAART initiation in both study arms, and the lack of difference in infant transmission rates between study arms, may serve as indirect evidence to support a more streamlined approach to HAART initiation during pregnancy whereby women who are diagnosed with HIV during pregnancy are started at the same visit on HAART without laboratory investigations (a strategy know as WHO Option B+).25 However, in order to implement Option B+ a significant increase in staff, clinical tools, adherence support, and community follow up systems will need to be in place to adequately follow the increased numbers of patients on HARRT.
In addition, this study may have failed to show a difference in vertical transmission due to a rapidly changing health policy environment regarding PMTCT. Over the course of the study national PMTCT protocols changed, which may have biased the results towards the null given that both intervention and control arms offered more effective PMTCT drug regimens during the latter half of the study.14 Improved PMTCT drug regimens are associated with decreased transmission rates7 and thus our study, although initially adequately powered to detect a difference in transmission may have become underpowered as national PMTCT regimens improved in Kenya.
This study also did not demonstrate any difference in maternal health outcomes over the course of the study period. This is likely due to the short follow up period of one year and the amount of missing data in both study arms. Given the healthy state and relatively high CD4 counts at enrollment in both arms, it was unlikely that any clinically significant differences in maternal morbidity or mortality could be demonstrated over such a short time period.
This study used patient data from a public HIV treatment program and the results inherently include all of the challenges and pitfalls of program implementation in a rural African setting. The challenges included training and retaining providers, incomplete record keeping, missing data, and problems with data quality. Other challenges included assuring that patients received care per national PMTCT protocols, as well as challenges in patient follow-up and retention in care. This study suffers from a high loss-to-follow-up rate (25-30%) in both arms. Although the data are imperfect, which is a major study weakness, they are also reflective of the real-world setting of programs in rural Africa and thus may have wider reaching validity and generalizability for program managers and implementers than that of a controlled clinical trial setting. As greater investments to healthcare systems and improvements to medical records systems are made in public health facilities, using patient records from public programs for research may become more feasible.
Despite limited evidence,20,22 integration of ANC, PMTCT and HIV services has been promoted by the WHO and Ministries of Health across much of sub-Saharan Africa as a strategy to help achieve the elimination of MTCT.4,26-32 In 2009, the NIH Office of AIDS Research identified as a top priority research on how to promote women's linkage to and retention in care at each step of the PMTCT cascade.33 This study demonstrates that although we are able to enroll more women in HIV care through integration, integration alone does not necessarily translate to improved maternal health outcomes or decreased rates of mother-to-child transmission of HIV, as compared to non-integrated services. If anything this study implies that integration may be necessary, but is not sufficient, in order to achieve eMTCT and more streamlined approaches to encourage rapid HAART initiation for all pregnant women should be developed, implemented, and rigorously evaluated.
Supplementary Material
Acknowledgments
The Investigator team would like to acknowledge all the Family AIDS Care and Education Services (FACES) staff, the SHAIP study staff, and Kenyan Ministries of Health staff from Migori, Rongo, and Nyatike Districts who participated in the care of women, without whom the study would not have been possible. In addition, we would like to acknowledge the Director of KEMRI for permission to publish this manuscript.
We thank the Kenyan women who participated in the study. We acknowledge the important logistical support of the KEMRI-UCSF Collaborative Group and especially Family AIDS Care and Education Services (FACES). We gratefully acknowledge the Director of KEMRI, the Director of KEMRI's Centre for Microbiology, and the Kenyan Ministry of Health for their support in conducting this research. We also thank John Oguda, Peter Manwari, George O'chieng, Pheobe Anyango, Kevin Owuor, Cinthia Blat, Jayne Kulzer, Nicole Schmidt, Katie Schwartz, Lisa Dillabaugh, Benard Otieno, and Evelyn Interis for their important contributions to this research. The project described was supported as a Public Health Evaluation by the President's Emergency Plan for AIDS Relief (PEPFAR)/Centers for Disease Control and Prevention (CDC). The content is solely the responsibility of the authors and does not necessarily represent the official views of PEPFAR or the CDC.
Sources of support: Funding: The research described was supported as a Public Health Evaluation by the President's Emergency Plan for AIDS Relief (PEPFAR)/U.S. Centers for Disease Control and Prevention (CDC). The study was funded under the CDC cooperative agreement number 5U2GPS001913-02. The funders had no role in data collection and analysis. The manuscript has received CDC clearance for journal submission. JMT was partially supported by award number K01MH081777 from the U.S. National Institute of Mental Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention or the U.S. National Institutes of Health.
Footnotes
Meetings at which parts of the data were presented (including title of conference, city, and date):
Integration for Impact, Reproductive Health and HIV Services in sub-Saharan Africa, September 12-14, 2012, Nairobi, Kenya.
7th IAS Conference on HIV Pathogenesis, Treatment and Prevention (IAS 2013), Kuala Lumpur, Malaysia, 30 June - 3 July 2013.
Conflict of Interest: The authors have declared that there are no competing interests.
Disclaimer: “The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention
References
- 1.World Health Organisation. PMTCT Strategic Vision 2010–2015: Preventing mother-to-child transmission of HIV to reach UNGASS and Millennium Development Goals. [Accessed December 4, 2012];2010 Available at: http://www.who.int/hiv/pub/mtct/strategic_vision.pdf.
- 2.WHO/UNAIDS/UNICEF. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. Geneva: WHO; 2010. [Google Scholar]
- 3.United Nations Secretary General. Global Strategy for Women's and Children's health: the partnership for Maternal and Child Health. 2010. p. 20. [Google Scholar]
- 4.Chi BH, Adler MR, Bolu O, et al. Progress, challenges, and new opportunities for the prevention of mother-to-child transmission of HIV under the US President's Emergency Plan for AIDS Relief. Journal of Acquired Immune Deficiency Syndromes. 2012;60(Suppl 3):S78–87. doi: 10.1097/QAI.0b013e31825f3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stringer JS, Sinkala M, Maclean CC, et al. Effectiveness of a city-wide program to prevent mother-to-child HIV transmission in Lusaka, Zambia. AIDS. 2005;19(12):1309–1315. doi: 10.1097/01.aids.0000180102.88511.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcos Y, Phelps BR, Bachman G. Community strategies that improve care and retention along the prevention of mother-to-child transmission of HIV cascade: a review. J Int AIDS Soc. 15(Suppl 2):17394. doi: 10.7448/IAS.15.4.17394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fowler MG, Lampe MA, Jamieson DJ, Kourtis AP, Rogers MF. Reducing the risk of mother-to-child human immunodeficiency virus transmission: past successes, current progress and challenges, and future directions. Am J Obstet Gynecol. 2007;197(3 Suppl):S3–9. doi: 10.1016/j.ajog.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 8.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic. 2010. p. 364. [Google Scholar]
- 9.Manzi M, Zachariah R, Teck R, et al. High acceptability of voluntary counselling and HIV-testing but unacceptable loss to follow up in a prevention of mother-to-child HIV transmission programme in rural Malawi: scaling-up requires a different way of acting. Trop Med Int Health. 2005;10(12):1242–1250. doi: 10.1111/j.1365-3156.2005.01526.x. [DOI] [PubMed] [Google Scholar]
- 10.Barker PM, Mphatswe W, Rollins N. Antiretroviral Drugs in the Cupboard are Not Enough: The Impact of Health Systems' Performance on Mother-to-Child Transmission of HIV. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011;56(2):e45. doi: 10.1097/QAI.0b013e3181fdbf20. [DOI] [PubMed] [Google Scholar]
- 11.National AIDS and STI Control Programme of the Ministry of Health. AIDS in Kenya. 7th. Nairobi, Kenya: NASCOP; 2005. [Google Scholar]
- 12.Ministry of Health K, editor. NASCOP. Annual program review. Nairobi, Kenya: 2011. [Google Scholar]
- 13.Lewis Kulzer J, Penner JA, Marima R, et al. Family model of HIV care and treatment: a retrospective study in Kenya. J Int AIDS Soc. 15(1):8. doi: 10.1186/1758-2652-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenya Ministry of Public Health and Sanitation, Kenya Ministry of Medical Services. National Recommendations for Prevention of Mother To Child Transmission of HIV, Infant & Young Child Feeding and Antiretroviral therapy for children, adults and adolescents. 2010. Jul 15, [Google Scholar]
- 15.Vo BN, Cohen CR, Smith RN, et al. Patient satisfaction with integrated HIV and antenatal care services in rural Kenya. AIDS Care. doi: 10.1080/09540121.2011.652357. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turan JM, Steinfeld RL, Onono M, et al. The study of HIV and antenatal care integration in pregnancy in Kenya: design, methods, and baseline results of a cluster-randomized controlled trial. PLoS One. 2012;7(9):e44181. doi: 10.1371/journal.pone.0044181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dabis F, Bequet L, Ekouevi DK, et al. Field efficacy of zidovudine, lamivudine and single-dose nevirapine to prevent peripartum HIV transmission. AIDS. 2005;19(3):309–318. [PMC free article] [PubMed] [Google Scholar]
- 18.Lallemant M, Jourdain G, Le Coeur S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351(3):217–228. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]
- 19.Steinfeld RL, Onono M, Cohen CR, Bukusi E. Assessing Integration of HIV services into antenatal care clinics accross different health facility contexts. Paper presented at: Integration for Impact Conference; Nairobi, Kenya. 2012. [Google Scholar]
- 20.Tudor Car L, Van-Velthoven MH, Brusamento S, et al. Integrating prevention of mother-to-child HIV transmission (PMTCT) programmes with other health services for preventing HIV infection and improving HIV outcomes in developing countries. Cochrane Database Syst Rev. 2011:CD008741. doi: 10.1002/14651858.CD008741.pub2. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organisation. Newborn and Child Health Services. World Health Education; 2006. Technical Consultation on the Integration of HIV Interventions into Maternal; p. 45. [Google Scholar]
- 22.Lindegren ML, Kennedy CE, Bain-Brickley D, et al. Integration of HIV/AIDS services with maternal, neonatal and child health, nutrition, and family planning services. Cochrane Database Syst Rev. 2012;9:CD010119. doi: 10.1002/14651858.CD010119. [DOI] [PubMed] [Google Scholar]
- 23.Winestone LE, Bukusi EA, Cohen CR, Kwaro D, Schmidt NC, Turan JM. Acceptability and feasibility of integration of HIV care services into antenatal clinics in rural Kenya: A qualitative provider interview study. Global public health. 2011 doi: 10.1080/17441692.2011.621964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenya National Bureau of Statistics, ICF Macro. Kenya Demographic and Health Survey 2008-09. Calverton, Maryland: KNBS and ICF Macro; 2010. [Google Scholar]
- 25.Thyssen A, Lange JH, Thyssen E, Reddi A. Toward an AIDS-Free Generation With Option B+: Reconceptualizing and Integrating Prevention of Mother to Child Transmission (PMTCT) With Pediatric Antiretroviral Therapy Initiatives. Journal of Acquired Immune Deficiency Syndromes. 2013;62(2):127–128. doi: 10.1097/QAI.0b013e3182749994. [DOI] [PubMed] [Google Scholar]
- 26.Pfeiffer J, Montoya P, Baptista AJ, et al. Integration of HIV/AIDS services into African primary health care: lessons learned for health system strengthening in Mozambique - a case study. J Int AIDS Soc. 2010;13:3. doi: 10.1186/1758-2652-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Black V, Hoffman R, Sugar C, Menon P, Venter F, Currier J. Safety and Efficacy of Initiating Highly Active Antiretroviral Therapy in an Integrated Antenatal and HIV Clinic in Johannesburg, South Africa. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2008 Nov;29(3):276–281. doi: 10.1097/QAI.0b013e318189a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etiebet MA, Fransman D, Forsyth B, Coetzee N, Hussey G. Integrating prevention of mother-to-child HIV transmission into antenatal care: learning from the experiences of women in South Africa. AIDS Care. 2004;16(1):37–46. doi: 10.1080/09540120310001633958. [DOI] [PubMed] [Google Scholar]
- 29.Freedman LP. Integrating HIV and maternal health services: will organizational culture clash sow the seeds of a new and improved implementation practice? Journal of Acquired Immune Deficiency Syndromes. 2011;57(Suppl 2):S80–82. doi: 10.1097/QAI.0b013e31821dba2d. [DOI] [PubMed] [Google Scholar]
- 30.Israel E, Kroeger M. Integrating Prevention of Mother-to-Child HIV Transmission into Existing Maternal, Child, and Reproductive Health Programs. [Accessed December 4, 2012];Technical Guidance Series. 2003 :22. Available at: http://www2.pathfinder.org/site/DocServer/Technical_Guidance_Series_3_PMTCTweb_01.pdf?docID=242.
- 31.Lambdin BH, Micek MA, Sherr K, et al. Integration of HIV Care and Treatment in Primary Health Care Centers and Patient Retention in Central Mozambique: A Retrospective Cohort Study. Journal of Acquired Immune Deficiency Syndromes. 2013 doi: 10.1097/QAI.0b013e3182840d4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazia G, Narayanan I, Warren C, et al. Integrating quality postnatal care into PMTCT in Swaziland. Global public health. 2009;4(3):253–270. doi: 10.1080/17441690902769669. [DOI] [PubMed] [Google Scholar]
- 33.Office of AIDS Research NIH, DHHS. Report from the Expert Consultation on Implementation Science Research: A Requirement for Effective HIV/AIDS Prevention and Treatment Scale-Up. Oakland CA: Pangaea Global AIDS Foundation; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.