Abstract
Objective:
In this report, we present a rare case of avascular necrosis (AVN) in an otherwise healthy 42-year-old male patient treated with low dose oral corticosteroids for his bronchitis. A systematic review of the literature related to AVN and corticosteroids was performed.
Case Report:
Forty-two-year-old male with no underlying conditions predisposing him to AVN who had been treated four years before for chronic bronchitis with two courses of oral prednisone therapy presented with bilateral AVN of the hips.
Methods:
An OVID database search of the terms “low total dose,” “corticosteroids,” and “avascular necrosis” was performed. Two PubMed searches of various permutations of “low-dose,” “corticosteroids,” “avascular necrosis,” and “osteonecrosis” were also performed. Results were then narrowed to relevant articles.
Results:
Median total dose of oral corticosteroids in patients with AVN in reviewed articles was 981 mg, with lowest reported association at 105 mg. Median duration of therapy was 16 days with shortest course of six days.
Conclusion:
There is emerging data linking AVN with corticosteroid doses previously thought to be safe. After reviewing the relevant literature, it is our consensus to inform all patients regarding AVN before oral corticosteroid use.
Keywords: Glucocorticoids, avascular necrosis of femoral head, osteonecrosis, otolaryngology, clinical practice patterns
Avascular necrosis (AVN) is a rare but serious disorder in which interruption of blood supply results in bone tissue death and destruction of the articular surface of joints, most commonly the hip. AVN carries high morbidity accounting for an estimated 10% of all United States hip arthroplasties,1 whereas AVN with subsequent fracture of the femoral head carries an in-hospital mortality rate of 1%–10%2–4 and a one year mortality rate ranging from 12% to 37%,5,6 depending on patient age and sex. The male-to-female ratio of AVN is approximately 4:1, with mean age of onset occurring between the fourth and fifth decade.2 Although AVN is strongly associated with several risk factors, including underlying rheumatologic conditions such as systemic lupus erythematosis, bisphosphonate use, radiation exposure, and heavy alcohol consumption,7,8 systemic corticosteroid use has been implicated in almost one-third of all cases of AVN.1,9,10 Several studies have investigated a proposed dose-dependent relationship between corticosteroid use and AVN with varied results. Cumulative total dose relationships have been reported to range from 440 to 9000 mg with relative risks (RRs) of AVN of 6 and 8.8 for the respective doses.11–13 Controversy exists among studies examining duration of corticosteroid treatment. Although some have shown that AVN can occur with therapy ranging from 2813 to 700 days,11 others found no risk associated with long-term treatment with corticosteroids.14,15
CASE REPORT
Previous studies looking at cumulative total dose relationships between oral steroids and AVN often fail to differentiate between patients receiving solely oral corticosteroids versus those who have also been administered intravenous (IV) corticosteroids. In this case report, we discuss a 42-year-old male who sustained AVN of his right hip after low total dose corticosteroid treatment. This patient presented to our department with worsening of his right hip pain, which had been present for four years. There was no history of trauma. The patient had sustained a lower back injury three years before, and his worsening hip pain had previously been attributed to this. His past medical history was significant for an umbilical hernia and asthma controlled with an albuterol inhaler as required; it was negative for any history of autoimmune disease. Family history was significant for hypertension in both parents and negative for autoimmune diseases. He had a 15 pack-year smoking history and no history of alcohol abuse. Upon further investigation, it was found that he had been treated for a chronic bronchitis exacerbation four years before with two short courses of oral prednisone therapy, which included: 40 mg per day for seven days, 20 mg per day for two days, and 10 mg per day for two days followed by a two week break. His second course consisted of 60 mg per day for four days, 40 mg per day for four days, 20 mg per day for four days, and 10 mg per day for four days. Total duration of therapy was 27 days, and total cumulative dose of prednisone was 860 mg.
Physical exam revealed reduced range of passive and active motion of the right hip. Bilateral plain films and magnetic resonance imaging of the hips were obtained due to concern of AVN. Final impression of the magnetic resonance imaging was read as bilateral AVN of the hip, with the right hip having had a more acute phase with marrow involvement than the left.
One month later, he elected to undergo right hip replacement. The hip arthroplasty was performed with a femoral prosthesis without complication. He was discharged four days postoperatively to begin physical therapy and rehabilitation of the joint. One year after the procedure, he was able to walk without any detectable limp and regained nearly full range of pain-free motion in right hip, whereas the left hip never progressed to the point of needing replacement.
METHODS
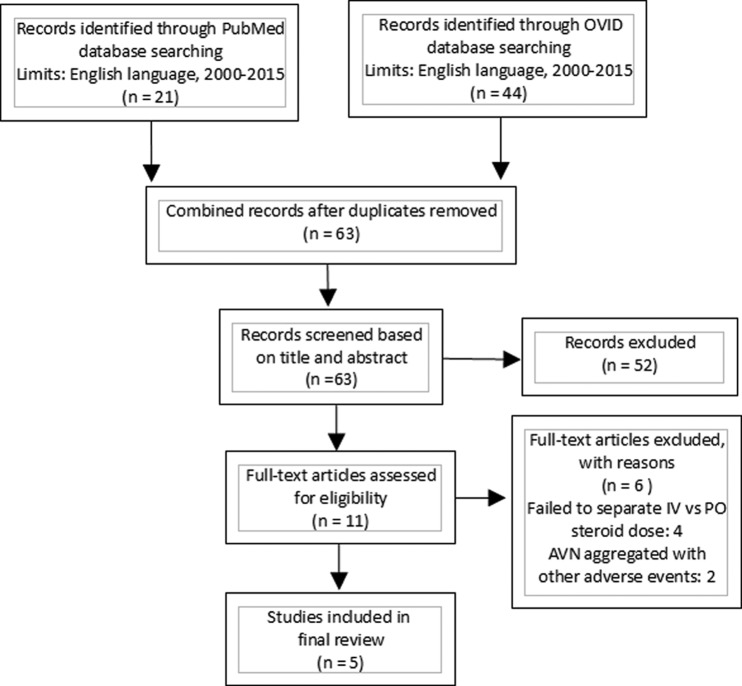
A search of the PubMed and OVID databases for English language publications between 2000 and 2015 was performed (Fig. 1). OVID key search terms included “low total dose” and “corticosteroids” or “low total dose steroids” and “avascular necrosis.” PubMed search terms included “low dose” and “corticosteroids” or “low dose” and “steroids” and “avascular necrosis.” Articles published before this date, not of the English language, lacking in detail regarding duration and dosing of oral steroid or relating to IV steroid administration only were excluded. Data collected and analyzed included demographic data, documented risk factors for AVN, routes of steroid administration, cumulative steroid dose, total duration of therapy, and incidence of AVN. For the purposes of this review, total dose and cumulative dose refer to total amounts of corticosteroids prescribed for a single treatment episode spanning less than three months. None of the literature found in our review had access to a centralized pharmacologic reporting system for patients analyzed, therefore “life-time” cumulative dose of corticosteroids and its relation to AVN would be impossible to quantify.
Figure 1.
Flow chart explanation of systematic literature review.
RESULTS
Forty-six articles were identified on the initial database search. Five full-length articles met the inclusion criteria for this study. These articles consisted of one retrospective cohort study,16 one case-control study,13 and three case reports.17–19 A total of 10,825 total instances of AVN of the hip were reported across these five articles, with an overwhelming majority occurring in the Dilisio study.16 A total of 123/10,825 cases of AVN of the hip were associated with oral corticosteroid use (1.14%), and one case was associated with inhaled corticosteroid use. Of the total instances of AVN found in our review of the literature, 5705/10,825 were male (52.7%) and 5120/10,825 were female (47.3%). The median reported age for AVN secondary to oral corticosteroid use was 51.5 years with a range of 29–69 years. The indications for corticosteroid use were reported in four articles for a total of 14 patients and included asthma 4/14 (28.6%) 3/14 (21.4%) for autoimmune diseases, 2/14 (14.3%) chronic bronchitis, and 1/14 (7.1%) each for migraine with aura, postchemotherapy nausea, cerebral edema, gout, and acute transplant rejection. Durations of corticosteroid therapy reported in the articles included: 6 days, 11 days, 21 days, 44 days, and 20 years. Excluding the 20-year case report, median duration of oral corticosteroid therapy associated with AVN in our literature review was 16 days. Reported cumulative doses of oral or inhaled corticosteroids associated with subsequent development of AVN (converted to prednisone equivalent) ranged from 105 to 1160 mg, with median cumulative dose of 981 mg.
DISCUSSION
The patient in our case report fits many of the demographic characteristics of AVN (male, 40–50 years old), although his lack of underlying comorbidities or chronic medications and the delayed presentation of AVN make this an unusual case. Our patient's total cumulative dose of 860 mg of prednisone coincides with other high-risk dose ranges in the literature13 which fall between 440 and 1290 mg, although it contrasts with more recent claims suggesting more than 1000 mg as the threshold for concern.20 One possible limitation in interpreting the study by Bauer et al.13 that produced the 440- to 1290-mg dose range is its exclusion of any patient who presented with AVN more than three years after receiving oral prednisone therapy, as this would have excluded our patient who was diagnosed with AVN more than four years after his courses of oral prednisone. Among the two studies which calculated RR of AVN among their patients with documented corticosteroid use, both found increased risk of AVN with oral corticosteroids compared with controls.13,16 Dilisio, whose study involved patients with isolated oral corticosteroid exposure, found a RR of 1.378 for AVN (95% confidence interval [CI], 1.069–1.697) at total doses as low as 105 mg (prednisone equivalent), and RR of 3.704 (95% CI, 2.806–4.602) for patients who had received at least 210 mg (prednisone equivalent). Bauer's nested case-control reported RR 0 (95% CI, 0–5) for total doses of oral prednisone less than 430 mg, RR 6 (95% CI, 1–43) for total doses between 440 and 1290 mg, and an increased but undefined RR for total doses more than 1290 mg. Our case report highlights an important concern for those generally considered low risk for AVN as prescriptive practices become increasingly dependent on the use of prednisone for a multitude of diseases spanning each subspecialty within otolaryngology.
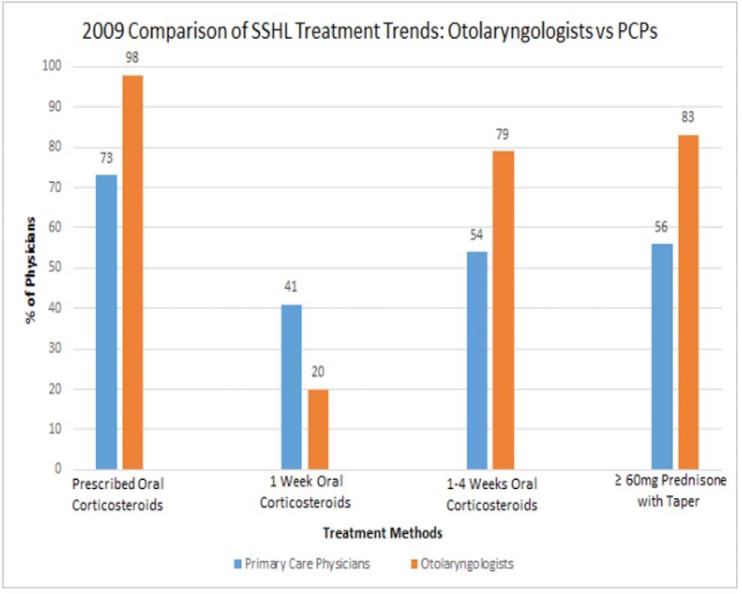
The use of oral corticosteroids in the United States has been increasing over the past 15 years, with prescriptions for oral prednisone accounting for a majority of the increase. A review of national databases collected by the Centers for Disease Control and Prevention cited a 13% increase in prescriptions of oral prednisone across all specialties for adults from 2000 to 2009, whereas the increase in prescriptions for children during the same time period was even higher at 38%.21 With respect to otolaryngology, current medical management of rhinosinusitis, sudden sensorineural hearing loss (SSHL), and many other diseases rely heavily on oral corticosteroid therapy as a key treatment modality for both children and adults.22,23 Recent attempts to compare prescriptive practices of corticosteroids between primary care physicians and otolaryngologists are sparse, but initial reports indicate significantly higher rates of corticosteroid prescriptions by otolaryngologists (Fig. 2).24–26 In addition to this variability in practice trends, it appears that for certain conditions such as SSHL, there is significant variability within the field of otolaryngology itself, with otologists choosing corticosteroid therapy at the highest frequency.27
Figure 2.
Comparison of otolaryngologist versus primary care physician corticosteroid use in SSHL.
Limitations in our study include a sparsity of literature analyzing isolated use of oral corticosteroids, with the clear majority having been published before 2000. Only one of the articles included in our review specifically looked at patients who had solely received oral corticosteroids,16 with three of the five articles, including patient populations who had either unknown previous histories of IV corticosteroid use or at least one documented instance of IV corticosteroid administration. We included one case report that involved isolated use of inhaled corticosteroids.17 Furthermore, several patients from the case reports had complicated clinical pictures due to underlying malignancy and concurrent use of chemotherapy with corticosteroids,18 remote past medical history of hip fracture,17 or lack of data collected on comorbidities,16 making it difficult to isolate corticosteroids as the sole risk factor for AVN.
Although it is difficult to assess the motivations behind increasing oral corticosteroid use in the field of otolaryngology as a whole, recent surveys have raised concerns regarding practitioners' reliance on anecdotal experience as opposed to evidence provided in the academic literature. A recent survey of factors affecting prescriptions of corticosteroids revealed that 74% of respondents cited previous experience and 59% cited familiarity as very important factors, with less than 50% of respondents citing academic literature, side effect profile, or empiric data as even moderately important factors influencing their choice of corticosteroid use.28 This is particularly concerning as a recent review of medical malpractice and corticosteroid use found that 65% of allegations cited negligence or improper use of corticosteroids as reason for filing, with another 36% citing lack of informed consent. The most common complication of corticosteroid use cited by plaintiffs was AVN, affecting 39% of plaintiffs after corticosteroid therapy.29 Recent systematic reviews of oral corticosteroid monotherapy in acute sinusitis, SSHL, and chronic rhinosinusitis without nasal polyps have questioned its utility and found its value to be unclear, placing its position as standard therapy in these conditions in jeopardy.30–32 Regarding informed consent and oral corticosteroid use, a recent randomized controlled trial found that patients had great difficulty recalling discussions of adverse risks of prednisone use as little as two weeks after the discussion took place, with median number of adverse risks recalled ranging from 1 to 2.33 The current state of the literature regarding oral corticosteroid use in common otolaryngologic problems remains unclear, the use of corticosteroids among practitioners remains highly variable, and practitioners may be overestimating patients' ability to recall the myriad of risks associated with corticosteroid use.
CONCLUSION
Ultimately, the rarity of development of AVN from oral corticosteroids limits our understanding of this disease and its risks factors to case reports and a few cohort studies. The risk of AVN from high cumulative doses of IV corticosteroids is more established and therefore warrants informed consent when appropriate. Due to the paucity of literature on the subject, more research is needed regarding the isolated use of oral corticosteroids and risk of AVN. Emerging reports of AVN after total doses of corticosteroids previously thought safe have necessitated a need for complete consensus among otolaryngologists regarding informed consent of AVN before oral corticosteroid use. After a systematic review of the literature, we advocate that a discussion about AVN should be included in every patient encounter before a course of oral corticosteroids, highlighting both the rarity and severity of such a complication. Patients undergoing high dose oral steroids, even at total doses less than 1000 mg, who have other potential risk factors would especially benefit from this discussion. This includes: underlying high-risk medical conditions for AVN (systemic lupus erythematosis, bisphosphonate therapy, alcoholism, etc.), male gender in their forties or fifties, or any previous treatment with corticosteroids.
ACKNOWLEDGMENTS
Consent: Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Author contributions P. J. Kennedy Jr. was involved in writing and revision of case report and review of literature; A. Bassiouni and J. Brunworth were involved in editing of case report, determining methods for review of literature, and revisions; and A. Psaltis and J. Antisdel were involved with interpretation of review of literature as well as revision of discussion and conclusion.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Powell C, Chang C, Naguwa SM, et al. Steroid induced osteonecrosis: An analysis of steroid dosing risk. Autoimmun Rev 9:721–743, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frost SA, Nguyen ND, Black DA, et al. Risk factors for in-hospital post-hip fracture mortality. Bone 49:553–558, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Alzahrani K, Gandhi R, Davis A, Mahomed N. In-hospital mortality following hip fracture care in southern Ontario. Can J Surg 53:294–298, 2010. [PMC free article] [PubMed] [Google Scholar]
- 4. Alvarez-Nebreda ML, Jiménez AB, Rodríguez P, Serra JA. Epidemiology of hip fracture in the elderly in Spain. Bone 42:278–285, 2008. [DOI] [PubMed] [Google Scholar]
- 5. LeBlanc ES, Hillier TA, Pedula KL, et al. Hip fracture and increased short-term but not long-term mortality in healthy older women. Arch Intern Med 171:1831–1837, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Panula J, Pihlajamäki H, Mattila VM, et al. Mortality and cause of death in hip fracture patients aged 65 or older: A population-based study. BMC Musculoskelet Disord 12:105, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seamon J, Keller T, Saleh J, Cui Q. The pathogenesis of nontraumatic osteonecrosis. Arthritis 2012:601763, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: A caution. J Bone Joint Surg Br 89:349–353, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Ninomiya S. An epidemiological survey of idiopathic avascular necrosis of the femoral head in Japan. Annual Report of Japanese Investigation Committee for Intractable Disease, 1989. [Google Scholar]
- 10. Shigemura T, Nakamura J, Kishida S, et al. Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: Prospective MRI study. Rheumatology (Oxford) 50:2023–2028, 2011. [DOI] [PubMed] [Google Scholar]
- 11. Aaron RK, Voisinet A, Racine J, et al. Corticosteroid-associated avascular necrosis: Dose relationships and early diagnosis. Ann NY Acad Sci 1240:38–46, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Santori FS, Santori N, Piccinato A. Avascular Necrosis of the Femoral Head: Current Trends. New York, NY: Springer Science & Business Media, 2004. [Google Scholar]
- 13. Bauer M, Thabault P, Estok D, et al. Low-dose corticosteroids and avascular necrosis of hip and knee. Pharmacoepidemiol Drug Saf 9:187–191, 2000. [DOI] [PubMed] [Google Scholar]
- 14. Shigemura T, Nakamura J, Kishida S, et al. Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: Prospective MRI study. Rheumatology 50:2023–2028, 2011. [DOI] [PubMed] [Google Scholar]
- 15. Nakamura J, Ohtori S, Sakamoto M, et al. Development of new osteonecrosis in systemic lupus erythematosus patients in association with long-term corticosteroid therapy after disease recurrence. Clin Exp Rheumatol 28:13–18, 2010. [PubMed] [Google Scholar]
- 16. Dilisio M. Osteonecrosis following short-term, low-dose oral corticosteroids: A population-based study of 24 million patients. Orthopedics 37:631–636, 2014. [DOI] [PubMed] [Google Scholar]
- 17. Kisielinski K, Niedhart C, Schneider U, Niethard FU. Osteonecrosis 15 years after femoral neck fracture and long-term low-dose inhaled corticosteroid therapy. Joint Bone Spine 71:237–239, 2004. [DOI] [PubMed] [Google Scholar]
- 18. van den Berkmortel F, de Wit R, de Rooy J, DeMulder P. Osteonecrosis in patients with testicular tumours treated with chemotherapy. Neth J Med 62:23–26, 2004. [PubMed] [Google Scholar]
- 19. Hussain A, Young WB. Steroids and aseptic osteonecrosis (AON) in migraine patients. Headache 47:600–604, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Richards R. Short-term corticosteroids and avascular necrosis: Medical and legal realities. Cutis 80:343–348, 2007. [PubMed] [Google Scholar]
- 21. Lee T, Lee G, Smith M. The prescribing patterns of prednisone in the US. Poster session presented at Pharmacology and Pharmacotherapeutics, 2012 American College of Allergy, Asthma & Immunology Annual Scientific Meeting; 8–13 Nov., 2012 Anaheim, CA, 2012. [Google Scholar]
- 22. Ozturk F, Bakirtas A, Ileri F, Turktas I. Efficacy and tolerability of systemic methylprednisolone in children and adolescents with chronic rhinosinusitis: A double-blind, placebo-controlled randomized trial. J Allergy Clin Immunol 128:348–352, 2011. [DOI] [PubMed] [Google Scholar]
- 23. Subramanian HN, Schechtman KB, Hamilos DL. A retrospective analysis of treatment outcomes and time to relapse after intensive medical treatment for chronic sinusitis. Am J Rhinol 16:303–312, 2002. [PubMed] [Google Scholar]
- 24. Shemirani NL, Schmidt M, Friedland DR. Sudden sensorineural hearing loss: An evaluation of treatment and management approaches by referring physicians. Otolaryngol Head Neck Surg 140:86–91, 2009. [DOI] [PubMed] [Google Scholar]
- 25. Lee LN, Bhattacharyya N. Regional and specialty variations in the treatment of chronic rhinosinusitis. Laryngoscope 121:1092–1097, 2011. [DOI] [PubMed] [Google Scholar]
- 26. Thompson SK, Doerr TD, Hengerer AS. Infectious mononucleosis and corticosteroids: Management practices and outcomes. Arch Otolaryngol Head Neck Surg 131:900–904, 2005. [DOI] [PubMed] [Google Scholar]
- 27. Coelho DH, Thacker LR, Hsu DW. Variability in the management of idiopathic sudden sensorineural hearing loss. Otolaryngol Head Neck Surg 145:813–817, 2011. [DOI] [PubMed] [Google Scholar]
- 28. Govil N, Rafii BY, Paul BC, et al. Glucocorticoids for vocal fold disease: A survey of otolaryngologists. J Voice 28:82–87, 2014. [DOI] [PubMed] [Google Scholar]
- 29. Nash JJ, Nash AG, Leach ME, Poetker DM. Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg 144:10–15, 2011. [DOI] [PubMed] [Google Scholar]
- 30. Venekamp RP, Thompson MJ, Hayward G. Systemic corticosteroids for acute sinusitis. Cochrane Database Syst Rev 3:CD008115, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wei BPC, Stathopoulos D, O'Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev 7:CD003998, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poetker DM, Jakubowski LA, Lal D, et al. Oral corticosteroids in the management of adult chronic rhinosinusitis with and without nasal polyps: An evidence-based review with recommendations. Int Forum Allergy Rhinol 3:104–120, 2013. [DOI] [PubMed] [Google Scholar]
- 33. Glicksman JT, Sherman I, Rotenberg BW. Informed consent when prescribing medication: A randomized controlled trial. Laryngoscope 124:1296–1300, 2014. [DOI] [PubMed] [Google Scholar]