Abstract
Background
Predictors of worse outcomes (rebleeding, surgery and death) of peptic ulcer bleeding (PUB’s) are essential indicators because of significant morbidity and mortality. rates of PUB’s. However those have been rarely infrequently reported since changes in medical therapy (proton poump inhibitors-PPI) and application of newer endoscopic hemostasis.
Aim
Our purposes were to determine: 1) independent risk factors of 30-day rebleeding, surgery, and death and 2) whether ulcer size is an independent predictor of major outcomes in patients with severe PUB’s after successful endoscopic hemostasis and treatment with optimal medical (high dose IV PPI) vs. prior treatment (high dose IV histamine 2 antagonists – H2RA’s).
Methods
A large prospectively followed population of patients hospitalized with severe PUB’s between 1993 and 2011 at two US tertiary care academic medical centers, stratified by stigmata of recent hemorrhage (SRH) was studied. Using multivariable logistic regression analyses, independent risk factors of each outcome (rebleeding, surgery, and death) up to 30 days were analyzed. Effects of medical treatment (H2RA patients 1993–2005 vs. PPI’s 2006–2011) were also analysed.
Results
1264 patients were included. For ulcers ≥10mm, the odds of 30-day rebleeding increased 6% per each 10% increase in ulcer size (OR 1.06, 95%CI 1.02–1.10, p=0.0053). Other risk factors of 30-day rebleeding were major SRH, inpatient start of bleeding, and prior GI bleeding. Major SRH and ulcer size ≥10 mm were predictors of 30-day surgery. Risk factors of 30-day death were major SRH, inpatient bleeding, any initial platelet transfusion or fresh frozen plasma transfusion ≥2 units. Among patients with major SRH and outpatient start of bleeding, larger ulcer size was also a risk factor for death (OR 1.08 per 10% increase in ulcer size, 95%CI 1.02–1.14, p=0.0095). Ulcer size was a significant independent variable for both time periods.
Conclusions
Ulcer size is a risk factor and should be carefully recorded at initial endoscopy to improve patient triage and management.
Keywords: peptic ulcer, bleeding, outcomes, ulcer size, stigmata of recent hemorrhage
INTRODUCTION
The incidence of peptic bleeding ulcer (PUB) ranges between 19.4 to 57.0 per 100,000 individuals and mortality has not significantly decreased, despite the decreasing incidence of peptic ulcer, strategies to eradicate against Helicobacter pylori infection, and prophylaxis against ulceration from non-steroidal anti-inflammatory drugs (NSAIDs’)1. Endoscopic hemostasis is the standard treatment in high-risk peptic bleeding ulcer, along with high dose PPI’s2. Successful initial hemostasis can be achieved in over 90% of patients. However, there is still a rather high rate of rebleeding (10 to 20%) as well as deaths (5 to 10%)3 particularly in unselected patients. Identifying risk factors which could help predict rebleeding in PUB and potentially change treatments to improve outcomes is warranted. That could help identify a high-risk subgroup of patients which needs more aggressive endoscopic and medical treatment and follow-up to reduce short-term rates of rebleeding and death.
Several studies reported that ulcer size is an independent risk of rebleeding for gastroduodenal ulcer1,4–12. However, these studies were performed 7–21 years ago, included a small number of patients relative to the incidence of PUB in the general population, were selective and often excluded patients with severe co-morbidities, and did not use optimal medical and endoscopic therapies as is now recommended by the international consensus for the management of patients with nonvariceal upper gastrointestinal bleeding2,3. With changes in medical therapy (high dose IV PPI’s) and newer endoscopic hemostasis in the last fifteen years (such as thermal coagulation or hemoclipping rather than epinephrine injection alone), independent risk factors for rebleeding have infrequently been reported for PUB’s. Moreover, much less is known about ulcer size as a predictor of surgery and death for PUB’s. Nor have the potential effects of medical treatments (high dose IV H2RA’s vs. PPI’s) after successful endoscopic hemostasis been reported for these important clinical outcomes.
In patients with severe PUB’s, our purposes were to determine: 1) independent risk factors of 30-day rebleeding, surgery, and death, 2) whether ulcer size alone or in combination with other risk factors is an independent predictor of major outcomes, and 3) to determine whether there was a difference between patients treated with high dose PPI’s vs. H2RA’s after successful endoscopic hemostasis.
METHODS
This two center prospective study was approved by the institutional review boards of the University of California, Los Angeles Medical Center and the Veterans Affairs Greater Los Angeles Medical Center. Data were collected prospectively and reviewed retrospectively.
Patients
A large prospectively population of consecutive patients hospitalized with severe peptic ulcer bleeding between January 1st 1993 and December 20th 2011 at two tertiary care academic medical centers (UCLA Ronald Reagan Medical Center and the VA West Los Angeles Medical Hospital), were studied. Before the availability of IV PPI’s in our hospitals, those between 1993–2005 were treated with high dose H2RA’s - Ranitidine or Famotidine (50 mg bolus and 6.25 mg/hr) for 72 hours and then oral PPI BID. Those from 2006–2011 received treated high dose intravenous PPI (80 mg IV bolus then 8mg/hour pantoprazole). PUB’s were stratified by stigmata of recent hemorrhage (SRH) and treated with endoscopic hemostasis. The inclusion criteria were 1) age up to 18 years, 2) a UGIH defined as the presence of signs or symptoms of UGI hemorrhage (hematemesis and/or melena), a decrease in hemoglobin from baseline of ≥ 2g/dl, and/or transfusions of ≥ 2 units of packed red blood cell (PRBC), and 3) the cause of the UGIH was an ulcer seen at the upper GI endoscopy. These same criteria were also used to define ulcer rebleeding. The pre-endoscopic exclusion criteria were: 1) patients unable to provide written informed consent; 2) patients with mental impairment, inability, or refusal to follow instructions; 3) patients having an unstable medical or surgical problem precluding urgent endoscopy; and 4) patients having a history of alcoholism or drug abuse hindering compliance and reliability. The endoscopic exclusions were: non-ulcer lesions (including varices, erosions, neoplasia, Mallory Weiss tears or esophagitis) or no UGI lesion found (in esophagus, stomach, or duodenum).
Endoscopic Treatments, Rebleeding, Retreatment and Surgery
During initial endoscopy all patients with major stigmata of ulcer hemorrhage (SRH) – spurting arterial bleeding (Forrest – F - IA), non-bleeding visible vessel (FIIA) adherent clot (FIIB), and oozing bleeding (FIB) were treated with thermal coagulation (heater probe or multipolar probe-MPEC) or hemoclipping with or without epinephrine injection. In one RCT, some patients were treated with epinephrine injection or sclerotherapy alone. A second look endoscopy was not performed except for clinically significant rebleeding. If severe rebleeding occurred (using the same criteria of severity as the index bleed), patients had a second endoscopy and retreatment of PUB’s with major SRH. Those with an additional rebleed were referred for ulcer surgery. Surgery was defined as the requirement of non-endoscopic hemostasis for rebleeding after endoscopic (e.g. initial failure to control ulcer bleeding or rebleeding after two endoscopic treatment sessions) or pharmaceutical treatment failure.
Ulcer size estimate and classification for analysis
The maximum dimension (length or width) of ulcers was estimated with open biopsy forceps, a thermal probe, an open snare, or another accessory of known dimensions. In case of more than one ulcer, either the ulcer with the SRH or if no SRH and more than one ulcer were present, the largest one was used in the size classification and analysis. In case of more than one ulcer with the same SRH, the largest one was included in the analysis. In case of a large ulcer with less severe SRH together with a smaller ulcer with more severe SRH, the smaller ulcer was included in the analysis.
Data Collected
The database provided key baseline information such as demographics, history of prior bleeds and transfusions, medications, symptoms and signs of UGI hemorrhage at presentation, co-morbidities, routine laboratory results, and endoscopic findings and treatment. Outcomes (length of hospitalization, transfusion requirement, rebleeding rate, surgery, and death) had been prospectively assessed and recorded until patient discharge and then at 30 days after endoscopic diagnosis by a research study coordinator and were all reviewed by the data managers and PI (D.J.) for accuracy and completeness.
The CURE Hemostasis prognosis score is a composite score of 6 items, which are: 1) age more than 65 years; 2) hypotension or shock on presentation for UGI bleeding; 3) any major organ comorbidity, 4) any severe comorbidities, 5) rebleeding during the hospitalization (prior to the index endoscopy); and 6) RBC transfusions of more than 5 units for initial resuscitation. Originally this score was created by the CURE Hemostasis Group to help risk stratify patients with severe UGI hemorrhage, to triage to level of medical care on presentation, and to compare with other prognosis scores, in order to predict the risk of rebleeding, need for endoscopic or other intervention, and mortality up to 30 days13,14.
Statistical analysis
All data were deidentified and entered into data files by an experienced data manager. SAS software, version 9.2, (SAS Institute, Cary, NC) was used for data management and analyses. p<0.05 was considered significant. All tests were two sided. All analyses were performed in consultation with biostatisticians.
We considered 3 outcomes - rebleeding, death and surgery at 30 days - and 29 potential predictors in addition to ulcer size including gender, melena in last 30 days, hematemesis in past 30 days, dyspepsia, hemodynamic instability (syncope, shock or hypotension), inpatient bleed status; any aspirin, NSAID, anticoagulant, antiplatelet use; alcohol use, albumin, prior UGI bleeding, cirrhosis, H2RA or PPI treatment before hemorrhage, PPT>35s, platelet count <50,000/mm3, major SRH, endoscopic hemostasis treatment, successful initial hemostasis, any initial transfusion (RBC, FFP, platelet), location of ulcer, age, CURE hemostasis prognosis score, hemoglobin count, PTT in seconds; and number of RBC, FFP, and platelet units transfused.
For the bivariate analyses, the p values for comparing rebleeding, death or surgery at 30 days versus binary covariates were computed using the Fisher’s exact test. The p values for comparing continuous variables versus rebleeding, death or surgery were computed using the non-parametric Wilcoxon rank sum test, as most continuous variables did not follow the normal distribution.
For the multivariate analyses, multiple backward stepwise logistic regression with a p < 0.25 main effect retention criterion and a p < 0.15 interaction retention criterion were used to simultaneously evaluate all covariates. Five pre - specified interactions with ulcer size were considered: 1) stigmata of recent hemorrhage, 2) platelet transfusion at baseline, 3) fresh frozen plasma transfusion at baseline, 4) red blood cell transfusion at baseline, 5) inpatient bleed. The potential nonlinear relation between log ulcer size versus log odds was assessed by splines yielding a final piecewise linear spline with a knot at 10 mm. Thus separate odds ratio for ulcers ≤ 10 mm vs. > 10 mm are reported. We report the adjusted OR’s and 95% CI’s as well as the concordance (C) statistic.
For missing data, ten variables had missing values: ulcer size (17.6%), cirrhosis (22.3%), prior GI bleed (20.1%), PTT (23.1%), initial RBC transfusion (2.1%), initial FFP transfusion (2.2%), initial platelet transfusion (1.9%), successful initial hemostasis (1.2%), any aspirin use (0.4%) and prognostic score (0.5%). Missing values were imputed using Markov Chain Monte Carlo (MCMC) regression imputation. Final model results were compared using all patients with imputed ulcer size and the covariates (Model I) data versus the corresponding subset with non missing ulcer size (Model II). We decided to report Model II, which presents a better clinical significance with a comparable concordance statistic (C) and accuracy than the Model I.
We also computed the odds ratios for the relationship between ulcer size and the 30 day outcomes separately for period I (1993–2005) and for period II (2006–2011) by including the appropriate interaction terms into the corresponding logistic regression models while controlling for the covariates previously identified using the backwards procedure for variable selection and p<0.15 as the retention criterion.
RESULTS
Between 1993 and 2011, 1264 patients were admitted for a bleeding ulcer or developed inpatient ulcer hemorrhage, documented by endoscopy. 801 patients were at UCLA Ronald Reagan Medical Center, and 463 at the VA West Los Angeles Medical Center.
Characteristics of studied population
Demographics are summarized in Table 1.
Table 1.
Baseline clinical characteristics of study patients
| No. Patients (%) n=1264 |
|
|---|---|
| Age, mean±SD | 61.4 years ± 16.5 |
| Sex (M/F) | 940/324 (74.4%/25.6%) |
| Inpatient, no. (%) | 319 (25.2%) |
| Hematemesis, no. (%) | 521 (41.2%) |
| Melena, no. (%) | 988 (78.2%) |
| NSAIDs, no. (%) | 315 (24.9%) |
| Anticoagulation, no. (%) | 146 (11.6%) |
| Antiplatelet agent, no. (%) | 25 (2%) |
| Aspirin, no. (%) | 468 (37.0%) |
| Severity of bleeding, no. (%) | |
| Hypotension | 372 (29.4%) |
| Shock | 126 (10.0%) |
| Syncope | 170 (13.5%) |
| Platelet count, median (IQR) | 202500 (140000, 273000) |
| PTT, median (IQR) | 26 (23, 30) |
| Hemoglobin count, median (IQR) | 7.8 (6.8, 8.8) |
| Initial Transfusion of packed Red blood cell, median (IQR) | 3 (2, 5) |
| Initial Transfusion of platelet units, no. (%) | 269 (21%) |
| Initial Transfusion of fresh frozen plasma, no. (%) | 116 (9%) |
| Location, no. (%) | |
| Esophageal | 61 (4.8%) |
| Gastric Pylorus | 576 (45.6%) |
| Duodenal | 72 (5.7%) |
| Anastomotic | 524 (41.5%) |
| Other/Unknown | 23 (1.8%) |
| 8 (0.6%) | |
Endoscopic findings and treatment
688 (54.9%) patients had major SRH at the time of initial endoscopy and all of them received high dose IV H2RA or PPI therapy after successful endoscopic hemostasis. 126 (10%) had active arterial bleeding, 95 (7.5%) had oozing, 312 (24.7%) had non-bleeding visible vessel, and 155 (12.3%) had adherent clot. These were all treated endoscopically. The others had either minor SRH such as spots for 110 (8.7%) or clean ulcers for 466 (36.9%) patients. None of the latter patients had endoscopic hemostasis nor treatment with high dose IV PPI’s or H2RA’s.
A total of 44.4% (561) of patients received endoscopic hemostatic treatment. Initial hemostasis was achieved in more than 95% of cases. Most of patients (510/561) received a thermal coagulation (e.g. multipolar probe, 146 [26.0%] or heater probe, 89 [15.9%]) or combination endoscopic hemostasis treatment (e.g. epinephrine injection plus either multipolar probe coagulation or hemoclipping, 275 [49.0%]). 10 (1.8%) patients had hemoclips alone. A few patients received injection alone, 40 (7.1%) had epinephrine injection and one (0.2%) had sclerotherapy.
Outcomes
The mean number of RBC units transfused was 4.5 ± 5.1, mean units of fresh frozen plasma was 1.4 ± 3.7, and the mean units of platelets was 1.0 ± 5.3. Patients stayed at hospital a median time of 4 days (range 0–30).
224 (17.7 %) patients rebled within 30 days. 84 (6.7 %) patients underwent surgery within 30 days, and 91 (7.2 %) patients died.
Independent risk factors of 30 day ulcer rebleeding
Results of bivariate analysis for ulcer rebleeding up to 30 days are shown in Table 2. For PUB rebleeding, Table 3 summarizes the predictors of 30-day rebleed risk with their adjusted odds ratios and 95% CI from the multivariate analysis. The following factors were associated with a higher risk (odds ratio) of PUB rebleeding: ulcer size, stigmata of recent hemorrhage, inpatient bleed, higher CURE hemostasis prognosis score, and prior UGI bleed. The following factors were associated with lower odds of PUB rebleeding: successful initial endoscopic hemostasis, female gender, any aspirin use and endoscopic hemostatic treatment. Also, treatment in period II (2006–2011) resulted in a significantly lower rebleed rate than period I (1993–2005). However, aspirin use did not reach significance (p=0.0705).
Table 2.
Bivariate analysis of risk factors for ulcer rebleeding within 30 days comparing patients with and without rebleeding
| Rebleeding | No Rebleeding | p value | |
|---|---|---|---|
| History of hematemesis in the past 30 days | 49.1% | 39.5% | 0.0088 |
| Syncope, shock or hypotension | 50.9% | 37.8% | 0.0004 |
| Female gender | 18.8% | 27.1% | 0.0089 |
| Inpatient status | 42.9% | 21.4% | <0.0001 |
| Any Aspirin | 29.1% | 38.9% | 0.0060 |
| Any NSAIDs’ | 19.7% | 26.2% | 0.0498 |
| Low albumin | 76.0% | 53.4% | <0.0001 |
| Prior UGI bleeding | 53.8% | 34.9% | <0.0001 |
| Cirrhosis | 19.9% | 12% | 0.0127 |
| PTT>35 seconds | 64.6% | 52.5% | 0.0033 |
| Major SRH | 87.1% | 47.4% | <0.0001 |
| Endoscopic hemostatic therapy | 62.5% | 36.4% | <0.0001 |
| Successful initial hemostasis | 91.9% | 98.7% | <0.0001 |
| RBC, FFP and/or platelet transfusion | 91.0% | 80.7% | <0.0001 |
| CURE hemostasis prognosis score, median (IQR) | 3 (2, 4) | 2 (1, 3) | <0.0001 |
| Platelet count, median (IQR) | 185000 (106000, 273000) |
207000 (145000, 273000) |
0.0333 |
FFP: fresh frozen plasma, HP: helicobacter pylori, NSAIDs’: non-steroidal anti- inflammatory drugs, PPI: proton pomp inhibitor, PTT: prothrombin time, RBC: red blood cell, SRH: stigmata of recent hemorrhage.
Table 3.
Multivariate logistic analysis of risk factors for ulcer rebleeding at 30 days
| Odds Ratio (95%CI) | p Value | |
|---|---|---|
| Major SRH | 7.29 (4.24–12.53) | 0.0000 |
| Inpatient bleed | 1.63 (1.13–2.36) | 0.0089 |
| CURE hemostasis prognosis score | 1.56 (1.37–1.79) | 0.0000 |
| Prior UGI bleeding | 1.45 (1.02–2.06) | 0.0362 |
| *Ulcer size(per 10% increase) for ulcers ≥10 mm | 1.07 (1.03–1.11) | 0.0013 |
| Any aspirin use | 0.74 (0.52–1.06) | 0.0989 |
| Female (vs male) gender | 0.63 (0.42–0.94) | 0.0240 |
| Endoscopic hemostatic therapy | 0.54 (0.35–0.85) | 0.0070 |
| Time period 2006–2011 vs 1993–2005 | 0.41 (0.25–0.65) | 0.0001 |
| Successful initial hemostasis | 0.32 (0.14–0.70) | 0.0044 |
for ulcers > 10 mm. These were no significant covariate x time or ulcer size x time interaction effects.
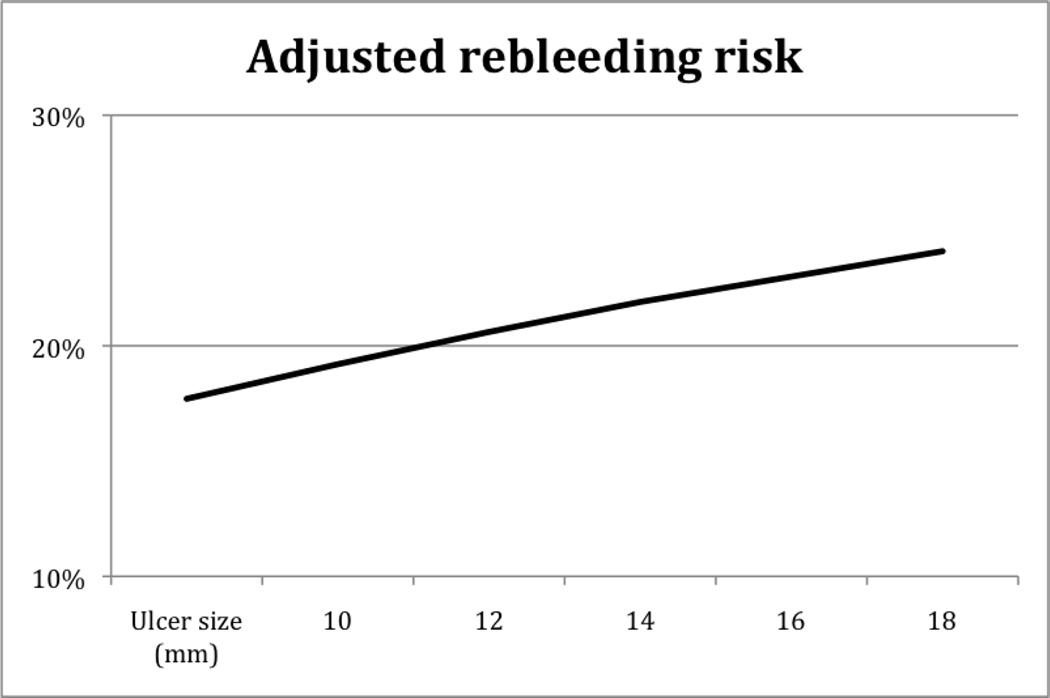
The median size of ulcers was 10mm. For ulcers 10 mm or larger, the odds of 30 day rebleeding increased 6% per each 10% increase in ulcer size (OR 1.06, 95%CI 1.02–1.10, p=0.0053). To translate this into a clinically relevant application, refer to Figure 2. This details the increasing risk of rebleeding for each 2 mm of ulcer size from the multivariable analysis. As examples, a 10 mm ulcer had a rebleeding risk of 17.7%, 14 mm ulcer a 20.6% risk, and a 20 mm ulcer a 24.1% risk.
Figure 2.
Adjusted risk of rebleeding as a function of ulcer size from multivariable logistics model
Independent risk factors of 30 day surgery
Results of bivariate analysis for risk factors of 30 day surgery are shown in Table 4. In the multivariate analysis with adjusted odds ratios, ulcer size (OR 95%CI 1.04 [1.00–1.08] per each 10% increase, p=0.0360), presence of major stigmata of recent hemorrhage (OR 95%CI 39.21 [8.95–171.71], p<0.0001), and units RBC’s at baseline per unit transfused (OR 95% CI 1.09 [1.00–1.18] p=0.0430) were significant risk factors of 30-day surgery. Other potential risk factors that had a higher odds ratio of surgery were prior UGIB and cirrhosis although they did not reach statistical significance. The following factors were significantly protective: successful initial hemostatis (OR 95%CI 0.22 [0.09–0.52], p=0.0006), aspirin (OR 95%CI 0.40 [0.22–0.75], p=0.0038), initial endoscopic hemostatic treatment (OR 95%CI 0.36 [0.20–0.64], p=0.0005), and later time period (OR 95% CI 0.49 [0.24–1.00] p=0.0493). There were no significant covariate × time or ulcer size × time interaction effects. The logistic regression model had a concordance statistic (C) of 85% with nominal sensitivity 83% and specificity 74%.
Table 4.
Bivariate analysis of risk factors for ulcer surgery within 30 days
| Surgery | No Surgery | p value | |
|---|---|---|---|
| Hematemesis | 53.6% | 40.3% | 0.0214 |
| Inpatient status | 35.7% | 24.5% | 0.0268 |
| Any aspirin use | 17.9% | 38.6% | 0.0001 |
| Low albumin | 74.2% | 57.1% | 0.0102 |
| Prior UGI bleeding | 53.5% | 37.3% | 0.0078 |
| Cirrhosis | 24.1% | 12.6% | 0.0223 |
| PPI treatment at admission | 46.4% | 34.2% | 0.0325 |
| Major SRH | 96.4% | 51.4% | <0.0001 |
| Endoscopic hemostatic therapy | 61.9% | 39.6% | <0.0001 |
| Successful initial hemostasis | 84.1% | 98.5% | <0.0001 |
| Any RBC, FFP and/or platelet transfusion | 96.4% | 81.5% | <0.0001 |
| CURE hemostasis prognosis score, median (IQR) | 3 (2,4) | 2 (1, 3) | <0.0001 |
| Ulcer size, Median (IQR) | 14 (7.5, 20) |
10 (7, 15) |
0.0066 |
FFP: fresh frozen plasma, HP: helicobacter pylori, NSAIDs’: non-steroidal anti- inflammatory drugs, PPI: proton pomp inhibitor, PTT: prothrombin time, RBC: red blood cell, SRH: stigmata of recent hemorrhage.
Independent risk factors of 30 day death
Results of bivariate analysis for risk factors of 30 day death are shown in Table 5. In the multivariate analysis with adjusted odds ratios, the following factors were significant predictors of mortality within 30 days: major stigmata of recent hemorrhage (OR 95%CI 3.02 [1.27–7.20], p=0.0126), inpatient bleed (OR 95%CI 3.78 [2.09–6.82], p<0.00001), higher CURE prognosis score (OR 95%CI 1.48 [1.17–1.89], p=0.0013), any platelet initial transfusion (OR 95%CI 3.63 [1.88–7.00], p=0.0001), and initial transfusion of more than 2 units of FFP (OR 95%CI 3.74 [1.80–7.80], p=0.0004). Cirrhosis also was associated with a higher odds of death but did not reach significance (p=0.1193). The following factors were significant protective factors of mortality: higher initial RBC transfusion (OR 95%CI 0.84 [0.75–0.94], p=0.0023) and presence of hemodynamic instability at the admission (syncope/shock/hypotension) (OR 95%CI 0.54 [0.3–0.95], p=0.0341). The logistic regression model had a concordance statistic (C) 88% with nominal sensitivity 80% and specificity 80%.
Table 5.
Bivariate analysis of risk factors for death within 30 days
| Death | No Death | p value | |
|---|---|---|---|
| History of melena in the past 30 days | 64.8% | 79.2% | 0.0023 |
| Inpatient status | 61.5% | 22.4% | <0.0001 |
| Any Aspirin | 21.1% | 38.4% | 0.0009 |
| Any NSAIDs’ | 9.9% | 26.2% | 0.0002 |
| Low albumin | 92.4% | 54.6% | <0.0001 |
| Cirrhosis | 32.8% | 11.9% | <0.0001 |
| PPI treatment at admission | 46.2% | 34.2% | 0.0229 |
| PTT>35s | 82.7% | 52.3% | <0.0001 |
| Major SRH | 81.3% | 52.3% | <0.0001 |
| Endoscopic hemostatic therapy | 64.8% | 39.2% | <0.0001 |
| Any RBC, FFP and/or platelet transfusion | 96.7% | 81.4% | <0.0001 |
| Ulcer size, median (IQR) | 10 (8, 20) | 10 (7, 15) | 0.0253 |
| CURE hemostasis prognosis score, median (IQR) | 4 (3, 4) | 2 (1, 3) | <0.0001 |
| Platelet count, median (IQR) | 117000 (53000, 179000) |
211000 (148000, 278000) |
<0.0001 |
FFP: fresh frozen plasma, NSAIDs’: non-steroidal anti-inflammatory drugs, PPI: proton pomp inhibitor, PTT: prothrombin time, RBC: red blood cell, SRH: stigmata of recent hemorrhage.
We found significant interactions between 1) ulcer size and stigmata of recent hemorrhage and 2) ulcer size and inpatient PUB bleeds after adjusting for covariates, as shown by Figure 1. Among patients with major SRH and outpatient start of bleeding, larger ulcer size was a risk factor for death (OR 1.08 per each 10% increase in ulcer size > 10 mm, 95%CI 1.02–1.14, p=0.0095). Among patients with SRH and inpatient PUB bleeds, the adjusted risk of death was 11.2% at 25th percentile of ulcer size versus 12.7% at 75th percentile of ulcer size. Whereas for patients with SRH and outpatient bleed, the adjusted risk of death was 3.6% at 25th percentile of ulcer size versus 6.3% at 75th percentile of ulcer size. This was an almost 2 fold increase in the risk of death due to the increase in ulcer size and its interaction with SRH and inpatient vs. outpatient start of PUB.
Figure 1.
30-day adjusted percent of death as a function of ulcer size, inpatient or outpatient start of bleeding, and major stigmata of recent hemorrhage.
There were 1023 patients in time period I (1993–2005) treated with IV H2RA’s after successful hemostasis and 241 patients in period II (2006–2011) treated with IV PPI’s. In the multivariable logistic analysis of the relationship between ulcer size and 30 day outcomes, ulcer size was a significant independent variable for both period I and II. Therefore, the relationship of ulcer size and major outcomes did not depend upon the type of IV medical treatment used.
DISCUSSION
During the last two decades, there has been a substantial improvement in bleeding peptic ulcer treatment2,3. This includes medical treatment with high dose IV PPI’s which plays a role in gastric acid inhibition as well as enhanced platelet aggregation15 and improvement in endoscopic hemostasis. However, the rate of death remains between 5 to 10%. Further data are necessary to independently identify the predictors of bad outcomes for peptic ulcer bleeding in order to stratify patients who could benefit from more intensive endoscopic and medical treatments to potentially reduce rates of rebleeding, surgery and death. Although substantial data have been published on the topic of risk stratification in UGIB, these data are difficult to extrapolate to patients managed by current guidelines for endoscopic and medical therapy2,3. Previous studies performed in 1990s and early 2000s and reassessed by Elmunzer et al4, and Garcia-Iglesia et al16 in two meta analyses identified hemodynamic instability, hemoglobin value, transfusion, comorbid illness, active bleeding, large ulcer size, posterior duodenal ulcer, and lesser gastric curvature ulcer as independent risk factors of rebleeding after endoscopic therapy4,16. A substantial number of the studies included had patients treated with epinephrine alone as endoscopic hemostasis and some did not utilize high dose IV PPI’s after successful endoscopic hemostasis. Therefore, these studies were not designed with optimal medical and endoscopic therapy as is now recommended by an international consensus2 and a recent PUB guideline3. Furthermore, they reported risk factors of 30-day rebleeding without analyzing risks factors of 30 day surgery and death. Therefore new evidence-based data for managing patients after successful initial endoscopic hemostasis with currently recommended techniques IV and PPI therapy are warranted.
In our study of a large number of patients using multivariable analyses, the independent predictors of 30-day rebleeding were ulcer size, major SRH, inpatient bleed, and history of UGI bleeding. The independent predictors of 30-day surgery were major SRH and ulcer size. The risk factors for 30-day death were major SRH, inpatient bleed, and initial transfusion of platelets or fresh frozen plasma. The relationship between ulcer size and 30 day outcomes was similar for the two time periods. This indicates that the improvement in IV medical therapy (with high dose PPI) did not change ulcer size as an important risk factor for major 30 day outcomes. However, there was a significant reduction in 30 day rebleeding rate for recently treated patients (period II) than period I (table 3). This difference may be mostly due to H2RA than oral PPI (part of period I) as suggested by previous study17,18.
The presence of these risk factors could help physicians triage patients to a higher level of care and a clinical consideration for a more aggressive initial endoscopic therapy and post-hemostasis medical care strategies. For example, more aggressive initial endoscopic therapy could include triple therapy (epinephrine, MPEC, and hemoclipping) with or without Doppler probe monitoring of underlying arterial blood flow to achieve definitive hemostasis19 during the initial endoscopy.
In the overall cohort or patients, ulcer size was not an independent risk factor of death, as was suggested by two previous studies20,21. However among the subset of patients with major SRH and outpatient start of ulcer bleeding, increasing ulcer size was a predictor of death. In addition, there was a significant interaction between ulcer size and major SRH so that if both were present mortality increased – figure 1.
Concerning protective factors in our study, aspirin was protective for 30 day surgery and tended to be a protective factor for rebleeding. This finding may appear unusual22 but was already reported23. It may be explained that in the case of peptic ulcer bleeding due to aspirin, aspirin can be discontinued immediately after the initial bleed to improve outcomes related to platelet aggregation and arterial clotting such as ulcer rebleeding and need for surgery.
Routine second-look endoscopy remains controversial and would lead to a large number of unnecessary procedures24,25. A second endoscopy was only performed for clinically significant rebleeding in the currently reported CURE study. However, the international Nonvariceal UGI Bleeding Consensus Group in 2010 concluded that second-look endoscopy would be of statistical benefit but only in select high-risk patients, although this patient subset was not clearly defined2. Performing risk stratification based on information available after the index endoscopy could help select patients who are most likely to benefit from second-look endoscopy (and retreatment if SRH were present), thereby potentially improving clinical outcomes while limiting costs. We found that ulcer size was a risk factor of 30-day rebleeding. Based upon our results, a second look endoscopy ought to be considered in the subgroup of patients with large ulcers. As a potential guideline (Figure 2), patients with ulcers and major SRH of 14 mm would have about 21% risk whereas those 20 mm would have a 24% risk. However, a prospective study would be necessary to define the best threshold of ulcer size for predicting a higher rebleeding rate, so that the appropriate subgroups could be chosen for second look endoscopy while excluding lower risk patients who would not benefit.
Co-morbidities were reported to be a risk factor of death in patients with peptic bleeding ulcer. In a systematic review and meta-analysis of 16 studies by Leontiadis et al, the risk of death (30-day or in-hospital mortality) was significantly greater in PUB patients with than in those without comorbidity (RR: 4.44; 95% confidence interval (CI): 2.45–8.04)26. In this review, hepatic disease had higher odds of death (4.04 [2.38–6.85]) than diabetes, respiratory and cardiac diseases. We did not analyze each comorbidity, however we found that cirrhosis had an arithmetically (not significant) higher odds of surgery and death within 30 days. We also report that the CURE hemostasis prognosis score was a predictor of rebleeding and death. In this score, the presence of any major organ system comorbidity and its severity are included. Inpatient start of bleed was also a significant risk factor of 30-day rebleeding and death in our study.
Our results for PUB deaths and RBC transfusion are contrary to those reported recently by Villanueva27. In our study initial RBC transfusion (per unit) for resuscitation was protective (OR 0.84 CI 0.75–0.94). In their RCT of non-variceal and variceal patients, Villanueva reported that limiting RBC transfusions significantly improved early survival especially in good risk cirrhotic and non-ulcer, non-variceal subgroups27. The differences in results of the two studies are that our ulcer patients included an unselected patient population, with both inpatient and outpatient start of hemorrhage, PUB patients with severe co-morbidities, and a much greater number of PUB patients. Because of these differences, we believe that our results about the relationship between RBC transfusions and death are more clinically relevant and generalizable to PUB patients currently seen in large hospitals than those in the highly selected RCT of Villanueva which had many clinically relevant exclusion criteria27.
The principal limitation of our study is the measurement of ulcer size. An accurate and validated tool for ulcer measurement is not yet available in endoscopy, so ours was an approximation, with the use of a biopsy forcep or other accessories of known dimensions. However, these are only tools available now in clinical practice. One other potential limitation was that the numbers of patients treated in period 1 (H2RA era) were greater than period II (PPI era). However, in our multivariable analysis, the relationship of ulcer size and outcomes remained.
CONCLUSIONS
This study of prospectively collected data highlights predictors of recurrent bleeding, surgery and death in patients with severe peptic bleeding ulcer treated with successful endoscopic hemostasis therapy and currently recommended doses of PPI’s. Increasing ulcer size was an independent risk factor of PUB rebleeding and surgery in the subset with ulcers 10 mm or larger. Other risk factors for 30 day PUB rebleeding were major SRH, inpatient bleed, and history of prior UGI bleeding. Protective factors which decreased PUB deaths were initial hemodynamic instability and increased initial RBC transfusions. Combined with major SRH, increasing ulcer size was also a predictor of death. Ulcer size should be recorded at initial endoscopy and used to identify patients with high risk ulcers and to help improve strategies for patient triage, management, and outcomes.
Acknowledgments
This study was partially funded by NIH-NIDDK 41301 CURE Center Grant – Human Studies CORE and a Veterans Administration Clinical Merit Review Grant (D. Jensen, MD).
Marine Camus, MD received a grant from the Philippe Foundation for supporting her research exchange program between France and the United States
Abbreviations
- CURE
Center for Ulcer Research and Education
- H2RA
histamine 2 receptor- antagonist
- FFP
fresh frozen plasma
- NSAID
non-steroidal anti-inflammatory drug
- PPI
proton pump inhibitor
- PRBC
packed red blood cells
- PTT
pro-thrombin time
- PUB
peptic bleeding ulcer
- SRH
stigmata of recent hemorrhage
- UGIH
upper gastrointestinal hemorrhage
Footnotes
Authorship statement:
- Guarantor of the article: Pr Dennis M Jensen
- Author contributions:
Marine Camus: analysis and interpretation of data, drafting of the manuscript
Thomas O. Kovacs: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content
Dennis M. Jensen: study concept and design, acquisition of data, study supervision analysis and interpretation of data, drafting of the manuscript; revision of manuscript.
Mary Ellen Jensen: acquisition of data, analysis and interpretation of data
Daniela Markovic: statistical analysis, critical revision of the manuscript for important intellectual content.
Jeffrey Gornbein: statistical analysis and its interpretation
- All authors approved the final version of the manuscript
- Authors’ declaration of personal interests: none
- Declaration of funding interests:
References
- 1.Lau JY, Sung J, Hill C, et al. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84:102–113. doi: 10.1159/000323958. [DOI] [PubMed] [Google Scholar]
- 2.Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101–113. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 3.Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345–360. doi: 10.1038/ajg.2011.480. [DOI] [PubMed] [Google Scholar]
- 4.Elmunzer BJ, Young SD, Inadomi JM, et al. Systematic review of the predictors of recurrent hemorrhage after endoscopic hemostatic therapy for bleeding peptic ulcers. Am J Gastroenterol. 2008;103:2625–2632. doi: 10.1111/j.1572-0241.2008.02070.x. [DOI] [PubMed] [Google Scholar]
- 5.Lo C-C, Hsu P-I, Lo G-H, et al. Comparison of hemostatic efficacy for epinephrine injection alone and injection combined with hemoclip therapy in treating high-risk bleeding ulcers. Gastrointest Endosc. 2006;63:767–773. doi: 10.1016/j.gie.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 6.Villanueva C, Balanzó J, Espinós JC, et al. Prediction of therapeutic failure in patients with bleeding peptic ulcer treated with endoscopic injection. Dig Dis Sci. 1993;38:2062–2070. doi: 10.1007/BF01297086. [DOI] [PubMed] [Google Scholar]
- 7.Brullet E, Calvet X, Campo R, et al. Factors predicting failure of endoscopic injection therapy in bleeding duodenal ulcer. Gastrointest Endosc. 1996;43:111–116. doi: 10.1016/s0016-5107(06)80110-0. [DOI] [PubMed] [Google Scholar]
- 8.Brullet E, Campo R, Calvet X, et al. Factors related to the failure of endoscopic injection therapy for bleeding gastric ulcer. Gut. 1996;39:155–158. doi: 10.1136/gut.39.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong SKH, Yu L-M, Lau JYW, et al. Prediction of therapeutic failure after adrenaline injection plus heater probe treatment in patients with bleeding peptic ulcer. Gut. 2002;50:322–325. doi: 10.1136/gut.50.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung IK, Kim EJ, Lee MS, et al. Endoscopic factors predisposing to rebleeding following endoscopic hemostasis in bleeding peptic ulcers. Endoscopy. 2001;33:969–975. doi: 10.1055/s-2001-17951. [DOI] [PubMed] [Google Scholar]
- 11.Hu M-L, Wu K-L, Chiu K-W, et al. Predictors of rebleeding after initial hemostasis with epinephrine injection in high-risk ulcers. World J Gastroenterol. 2010;16:5490–5495. doi: 10.3748/wjg.v16.i43.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu PWY, Joeng HKM, Choi CLY, et al. Predictors of peptic ulcer rebleeding after scheduled second endoscopy: clinical or endoscopic factors? Endoscopy. 2006;38:726–729. doi: 10.1055/s-2006-925179. [DOI] [PubMed] [Google Scholar]
- 13.Gralnek IM, Jensen DM, Gornbein J, et al. Clinical and economic outcomes of individuals with severe peptic ulcer hemorrhage and nonbleeding visible vessel: an analysis of two prospective clinical trials. Am J Gastroenterol. 1998;93:2047–2056. doi: 10.1111/j.1572-0241.1998.00590.x. [DOI] [PubMed] [Google Scholar]
- 14.Savides TJ, Jensen DM. GI Bleeding. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. pathophysiology/Diagnosis/Management. 10th. Philadelphia: Elsevier Saunders; 2015. pp. 297–335. [Google Scholar]
- 15.Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev. 2010:CD002094. doi: 10.1002/14651858.CD002094.pub2. [DOI] [PubMed] [Google Scholar]
- 16.García-Iglesias P, Villoria A, Suarez D, et al. Meta-analysis: predictors of rebleeding after endoscopic treatment for bleeding peptic ulcer. Aliment Pharmacol Ther. 2011;34:888–900. doi: 10.1111/j.1365-2036.2011.04830.x. [DOI] [PubMed] [Google Scholar]
- 17.Tsoi KK, Hirai HW, Sung JJ. Meta-analysis: comparison of oral vs. intravenous proton pump inhibitors in patients with peptic ulcer bleeding. Aliment Pharmacol Ther. 2013;38:721–728. doi: 10.1111/apt.12441. [DOI] [PubMed] [Google Scholar]
- 18.Chen CC, Lee JY, Fang YJ, Hsu SJ, Han ML, Tseng PH, Liou JM, Hu FC, Lin TL, Wu MS, Wang HP, Lin JT. Randomised clinical trial: high-dose vs. standard-dose proton pump inhibitors for the prevention of recurrent haemorrhage after combined endoscopic haemostasis of bleeding peptic ulcers. Aliment Pharmacol Ther. 2012;35:894–903. doi: 10.1111/j.1365-2036.2012.05047.x. [DOI] [PubMed] [Google Scholar]
- 19.Jensen DM, Ohning GV, Kovacs TOG, Ghassemi KA, Jutabha R, Duali GS, Machicado GA. Doppler Endoscopic Probe as a guide to risk stratification and definitive hemostasis of peptic ulcer bleeding. Gastrointest Endosc. 2015 doi: 10.1016/j.gie.2015.07.012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu PWY, Ng EKW, Cheung FKY, et al. Predicting mortality in patients with bleeding peptic ulcers after therapeutic endoscopy. Clin Gastroenterol Hepatol. 2009;7:311–316. doi: 10.1016/j.cgh.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Chow LW, Gertsch P, Poon RT, et al. Risk factors for rebleeding and death from peptic ulcer in the very elderly. Br J Surg. 1998;85:121–124. doi: 10.1046/j.1365-2168.1998.00665.x. [DOI] [PubMed] [Google Scholar]
- 22.Lin CC, Hu HY, Luo JC, Peng YL, Hou MC, Lin HC, Lee FY. Risk factors of gastrointestinal bleeding in clopidogrel users: a nationwide population-based study. Aliment Pharmacol Ther. 2013;38:1119–1128. doi: 10.1111/apt.12483. [DOI] [PubMed] [Google Scholar]
- 23.Hasselgren G, Blomqvist A, Eriksson S, et al. Short and long term course of elderly patients with peptic ulcer bleeding--analysis of factors influencing fatal outcome. Eur J Surg. 1998;164:685–691. doi: 10.1080/110241598750005570. [DOI] [PubMed] [Google Scholar]
- 24.Marmo R, Rotondano G, Bianco MA, et al. Outcome of endoscopic treatment for peptic ulcer bleeding: Is a second look necessary? A meta-analysis. Gastrointest Endosc. 2003;57:62–67. doi: 10.1067/mge.2003.48. [DOI] [PubMed] [Google Scholar]
- 25.Messmann H, Schaller P, Andus T, et al. Effect of programmed endoscopic follow-up examinations on the rebleeding rate of gastric or duodenal peptic ulcers treated by injection therapy: a prospective, randomized controlled trial. Endoscopy. 1998;30:583–589. doi: 10.1055/s-2007-1001360. [DOI] [PubMed] [Google Scholar]
- 26.Leontiadis GI1, Molloy-Bland M, Moayyedi P, Howden CW. Effect of comorbidity on mortality in patients with peptic ulcer bleeding: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:331–345. doi: 10.1038/ajg.2012.451. [DOI] [PubMed] [Google Scholar]
- 27.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Eng J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]