Abstract
Bacterial infections typically elicit a strong Heat Shock Response (HSR) in host cells. However, the gastric pathogen Helicobacter pylori has the unique ability to repress this response, the mechanism of which has yet to be elucidated. This study sought to characterize the underlying mechanisms by which H. pylori down-modulates host HSP expression upon infection. Examination of isogenic mutant strains of H. pylori defective in components of the type IV secretion system (T4SS), identified the secretion substrate, CagA, to be essential for down-modulation of the HSPs HSPH1 (HSP105), HSPA1A (HSP72), and HSPD1 (HSP60) upon infection of the AGS gastric adenocarcinoma cell line. Ectopic expression of CagA by transient transfection was insufficient to repress HSP expression in AGS or HEK293T cells, suggesting that additional H. pylori factors are required for HSP repression. RT-qPCR analysis of HSP gene expression in AGS cells infected with wild-type H. pylori or isogenic cagA-deletion mutant found no significant change to account for reduced HSP levels. In summary, this study identified CagA to be an essential bacterial factor for H. pylori-mediated suppression of host HSP expression. The novel finding that HSPH1 is down-modulated by H. pylori further highlights the unique ability of H. pylori to repress the HSR within host cells. Elucidation of the mechanism by which H. pylori achieves HSP repression may prove to be beneficial in the identification of novel mechanisms to inhibit the HSR pathway and provide further insight into the interactions between H. pylori and the host gastric epithelium.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-016-0680-x) contains supplementary material, which is available to authorized users.
Keywords: Helicobacter pylori, CagA, Heat shock proteins, Heat shock response, Gastric cancer
Introduction
Heat shock proteins (HSPs) are a highly conserved protein family that functions as molecular chaperones to maintain the structural and functional integrity of cellular proteins. Upon disruption to protein homeostasis by proteotoxic insults such as heat stress, radiation, proteotoxic chemicals, reactive oxygen species, and infection, the production of HSPs is rapidly induced to recover proteostasis and promote cell survival. Induction of HSP expression is achieved by the activation of the transcription factor heat shock factor-1 (HSF1) and collectively, this response to proteotoxic stress is termed the heat shock response (HSR). HSPs function to maintain proteostasis by “holding” and “folding” proteins to promote their correct conformation, prevent mis-folded protein aggregates, and target damaged proteins for degradation (Morimoto 2008). Importantly, the scope of HSP functions extend well beyond their chaperoning roles to the regulation of many cellular processes including but not limited to apoptosis, autophagy, senescence, inflammation, and immunity (Calderwood et al. 2012; Murphy 2013).
Most bacterial infections studied to date induce HSP expression with numerous studies showing activation of the HSR upon infection by bacterial pathogens such as S. typhimurium, L. pneumophila, E. coli, and S. aureus (Axsen et al. 2009; Shen et al. 2009; Zheng et al. 2004). In contrast to the upregulation of host-cell HSP expression associated with such bacterial infections, the gastric pathogen, Helicobacter pylori, has a novel ability to down-modulate host HSP expression upon acute infection of gastric epithelial cells (Axsen et al. 2009; Baek et al. 2004; Konturek et al. 2001; Pierzchalski et al. 2006; Targosz et al. 2006; Yeo et al. 2004). H. pylori is a Gram-negative, spiral bacterium that infects 50 % of humans globally. Infection with this bacterium is the strongest known individual risk factor for gastric cancer, which is the third leading cause of cancer-related death worldwide (Ferlay et al. 2012; Herrera and Parsonnet 2009). In addition to gastric cancer, H. pylori infection is also a major risk factor for the development of chronic gastritis, mucosa associated lymphoid tissue lymphoma (MALT) as well as gastric and duodenal ulcers (Bayerdorffer et al. 1997; Kuipers 1997). One of the major virulence factors of H. pylori strains is a 40-kb cytotoxin associated gene pathogenicity island abbreviated to cagPAI, which encodes a type IV secretion system (T4SS). This macromolecular complex belongs to a family of conserved secretion apparatuses that enable bacterial factors such as DNA and proteins to be translocated into host cells (Alvarez-Martinez and Christie 2009). For H. pylori, the cytotoxin CagA is the only T4SS protein substrate identified so far to be translocated into host cells upon infection (Asahi et al. 2000; Backert et al. 2000; Odenbreit et al. 2000; Stein et al. 2000). Upon translocation into the host cell, CagA undergoes tyrosine phosphorylation by host kinases at a number of Glu-Pro-Ile-Tyr-Ala (EPIYA) motifs in its carboxyl region (Asahi et al. 2000; Higashi et al. 2002; Odenbreit et al. 2000; Stein et al. 2000). Up to 20 host-binding partners of CagA have been identified, with CagA phosphorylation sites being highly promiscuous for host cell kinases (Backert et al. 2010). Studies have demonstrated complex activation of host signaling pathways by translocated CagA involving both CagA EPIYA phosphorylation-dependent and -independent mechanisms (Backert et al. 2010). To date, the bacterial factors responsible for the repression of HSP expression upon acute H. pylori infection in vitro remains unclear, with previous studies providing evidence both for and against a potential role for CagA in mediating this effect (Axsen et al. 2009; Targosz et al. 2006).
This study seeks to examine the underlying mechanisms by which H. pylori represses HSP expression. In doing so, this study aims to potentially identify novel mechanisms for inhibition of the HSR pathway, providing insight for both the therapeutic intervention of HSR and the pathogenic events of H. pylori infection.
Materials and methods
Mammalian cell culture
Human gastric adenocarcinoma cell line, AGS, or human embryonic kidney cell line, HEK-293T, were grown in RPMI or Dulbecco’s modified Eagle’s medium (DMEM), respectively. Both growth media were supplemented with 10 % FBS (Thermo Scientific) and for HEK293T, 1 % antimycotics/antibiotics (Gibco cat. 15240-062). Cells were incubated at 37 °C, 5 % CO2. Brightfield images of cell lines and/or treated cells were obtained to examine cell morphology using an Eclipse TE-2000-U (Nikon) with 200× magnification.
H. pylori culture
H. pylori strain P12, previously designated strain 888-0, was isolated from a duodenal ulcer patient and contains both the vacA cytotoxin and a functional cagPAI (Fischer et al. 2010; Haas et al. 1993). H. pylori isogenic mutants P12∆cagL (Gorrell et al. 2013), P12∆cagL/cagL (Gorrell et al. 2013), P12∆cagPAI (Odenbreit et al. 2001; Selbach 2002) and P12∆cagA (kindly provided by S. Backert, Erlangen, Germany) were cultured and maintained as described previously (Gorrell et al. 2013). Broth cultures were shaken at 120 rpm overnight (approximately 16 h) prior to the day of infection and cell cultures were infected with H. pylori cultures that had attained an OD550nm of 0.5-2.0.
Infection of AGS cells with isogenic mutant strains of H. pylori
The human gastric adenocarcinoma cell line AGS was cultured for infection experiments and inoculated with H. pylori liquid culture as described previously (Gorrell et al. 2013). AGS cells were plated in a 6-well dish (1 × 105/well) or a 10-cm dish (7.5 × 105/well) 48 h prior to infection in RPMI media supplemented with 10 % heat inactivated FBS. AGS cells were infected at a multiplicity of infection (MOI) of 50 with an equal volume of BHI broth added to the non-infected control.
Transient transfection
Both AGS and HEK293T cells were transfected at 70–90 % confluency with the expression vector pSP65/SRα-CagA-HA and pSP65/SRα-PR-CagA-HA to transiently express HA-tagged wild-type CagA (derived from H. pylori strain NCTC11637) and the HA-tagged phosphorylation-resistant CagA mutant, PR-CagA-HA (Higashi et al. 2002). Growth media was replaced with antibiotic free media, supplemented with 10 % FBS 2–3 h prior to transfection. Cells were transfected using Lipofectamine LTX and Plus reagent (Life Technologies) as per the manufacturer’s protocol. Approximately 12–16 h later, cells were washed once with 1× PBS and full growth media was added.
Generation of stable cell lines
HEK293T cells were transiently transfected for virus production with the retroviral packaging vector pCL-Ampho (IMGENEX) and retroviral expression vectors using Lipofectamine LTX and plus reagent (Life Technologies) according to the manufacturer’s instructions. Cell media was replaced 16 h following transfection, 24 h later the virus-containing media was collected and filtered using a 0.45-μm filter. AGS cells were transduced with retroviral overexpression pBABEpuro-IRES-EGFP vectors expressing wild-type human HSF1 (HSF1WT) or a constitutively active mutant of HSF1 (HSF1ΔRDT) (Fujimoto et al. 2005; Nguyen et al. 2013). GFP-positive transductants were selected by FACS as previously described (Lang et al. 2012).
Western blot analysis
For analysis of lysates of AGS or HEK293T cells, Western blot analysis was performed as outlined previously (Lang et al. 2012). For the protein sample preparation of H. pylori-inoculated cells, cell media was removed and equal volumes (approximately 100 μl for 6-well dish) of 2× Laemmli buffer heated to 95 °C was added to each sample well. Cells were scraped and the sample collected and heated at 95 °C for 5 min, from which time it was stored at −20 °C. Protein samples prepared in RIPA lysis buffer (10–15 μg of protein) or Laemmli buffer (15–25 μl of sample) were loaded onto a 4–20 % Tris-glycine polyacrylamide gradient gel (NuSep), after which transfer and immunoblotting were performed as described previously (Lang et al. 2012). Antibodies used for immunoblotting: CagA (Santa Cruz, sc-25766), HSF1 (Enzo, ADI-SPA-901), HSF1 p-Ser326 (Epitomics, #2043-1), HSPA1A (Abcam, ab47455), HSPD1 (Thermo Scientific, MS120P1), HSPH1 (Santa Cruz, sc-6241), actin (BD Biosciences, 612656), anti-HA (Covance, MMS-101P). Chemiluminescent signals were captured by exposure to an X-ray film (GE healthcare or Fuji Film) and the resultant signals quantified by densitometry using the ImageJ software (NIH). Densitometric data from multiple experiments were converted to mean fold change relative to actin expression before normalization to the control sample.
RT-qPCR analysis of gene expression
Total RNA was isolated using the Agilent Absolutely Total RNA extraction kit according to manufacturer’s instructions. Total RNA was quantified by spectrophotometry and 1 μg of RNA was reverse-transcribed using iScript Select cDNA Synthesis Kit (Bio-Rad) according to the manufacturer’s instructions. cDNA (10 ng) was added to the qPCR reaction using SYBR Green PCR Master Mix (Life Technologies, catalog no. 4309155). Samples were loaded into the thermal cycler Rotorgene3000 with cycle conditions as follows; hold time: 10 min, 95 °C, Cycle: [95 °C, 15 sec; 60 °C, 60 sec] for 40 cycles. Data were analyzed using the LinRegPCR software, as outlined previously (Ruijter et al. 2009). Gene expression of human HSPH1, HSPA1A, HSPD1, HSF1, and CXCL8 were normalized to that of the house keeping gene RPL32, and represented as fold change relative to the non-infected BHI control sample. Primer sequences for these genes are: HSPH1 5′-TGCAATACTTTCCCCGGCAT-3′ (forward) and 5′-ACAAAGCGGCCTATTTTTGCT-3′ (reverse); HSPA1A 5′-GAGGCGTACCTGGGCTACCC-3′ (forward) and 5′-GTTGAGCCCCGCGATCACAC-3′ (reverse); HSPD1 5′-CCGACGACCTGTCTCGCC-3′ (forward) and 5′-TGTTCTTCCCTTTGGCCCCAT-3′ (reverse); HSF1 5′-CTGGCCATGAAGCATGAGAATG-3′ (forward) and 5′-TGCACCAGTGAGATCAGGAACT-3′ (reverse); RPL32 5′-CAGGGTTCGTAGAAGATTCAAGGG-3′ (forward) and 5′-CTTGGAGGAAACATTGTGAGCGATC-3′ (reverse); and CXCL8 5′-CAGAGACAGCAGAGCACACA-3′ (forward) and 5′-GGCAAAACTGCACCTTCACA-3′ (reverse).
Immunocytochemistry
Cells were cultured on 13 mm coverslips in a 24-well plate. Prior to fixation, cells were rinsed twice in PBS followed by the addition of 4 % paraformaldehyde for 15 min at 37 °C. Cells were permeabilized with 0.1 % Triton-X for 10 min at room temperature (RT) and washed thrice in PBS prior to immunostaining. Permeabilized cells were blocked with 10 % FBS/PBS for 30 min at RT. Primary antibodies, anti-HA (Covance, MMS-101P), and anti-HSF1 (Enzo ADI-SPA-901) were added at 1:1000 dilution and incubated overnight at 4 °C. Unbound antibody was removed by washing with PBS and an appropriate fluorophore-conjugated secondary antibody (Life technologies) was added at a dilution of 1:2500 and incubated for 1 h at RT. Unbound secondary antibody was removed by washing with PBS. DAPI (4′,6-diamidino-2-phenylindole, Life Technologies) was included as a nuclear stain.
Results
H. pylori-mediated repression of host HSPs expression is CagA-dependent
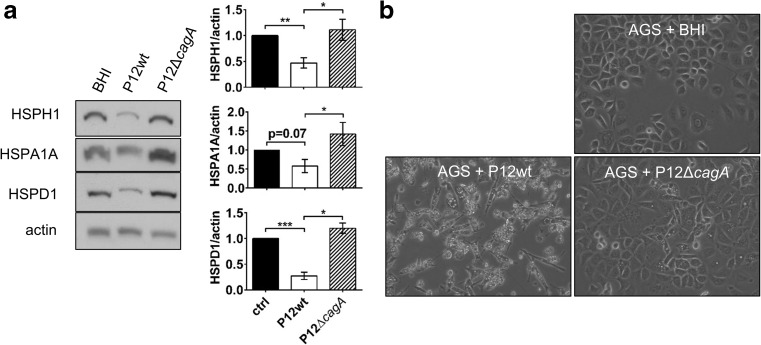
Given that CagA is one of the major virulence factors of H. pylori and the sole known protein delivered by the cagPAI-encoded T4SS into the host cell during infection, we investigated the importance of CagA for the altered HSP expression previously observed upon H. pylori infection in vitro (Axsen et al. 2009; Baek et al. 2004; Konturek et al. 2001; Pierzchalski et al. 2006; Targosz et al. 2006; Yeo et al. 2004). Wild-type H. pylori strain P12 (P12wt) and its CagA-deficient isogenic mutant (P12ΔcagA) were used to infect the gastric adenocarcinoma cell line AGS to model H. pylori infection of gastric epithelia in vitro (Backert et al. 2001; Kwok et al. 2002; Kwok et al. 2007). Following 24 h of infection, HSP expression was quantitated by Western blot analysis. Co-culture of AGS cells with H. pylori P12wt induced a significant reduction in protein expression levels of HSPH1 (HSP105), HSPD1 (HSP60), and HSPA1A (HSP72). In contrast, the P12ΔcagA mutant did not reduce expression of the aforementioned HSPs (Fig. 1a). H. pylori P12wt translocated CagA into the host cell under the experimental conditions used as shown by cell scattering and the acquisition of a ‘hummingbird’ cell morphology upon infection of AGS gastric epithelial cells with P12wt (Fig. 1b). The ‘hummingbird’ effect has previously been shown to be dependent upon an intact cagPAI and phosphorylation of translocated CagA (Backert et al. 2001; Segal et al. 1999). Consistent with this, the P12ΔcagA mutant did not induce ‘hummingbird’ morphology in infected AGS cells (Fig. 1b). All together, these results suggest that translocated CagA triggers down-modulation of host HSP expression.
Fig. 1.
H. pylori-mediated host HSP repression is CagA-dependent. a Western blot protein-expression analysis of chaperone proteins HSPH1 (HSP105), HSPA1A (HSP72), and HSPD1 (HSP60) in AGS cells 24 h post-inoculation (p.i.) with H. pylori P12wt, P12ΔcagA mutant (MOI: 50), or culture broth control (BHI). Densitometric analysis of mean fold change in expression normalized to BHI control from pooled experiments shown on right ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, n = 5 b Micrographs of AGS cell morphology upon co-culture with H. pylori P12wt or P12ΔcagA mutant at 24 h p.i
Repression of host HSP expression requires an intact T4SS
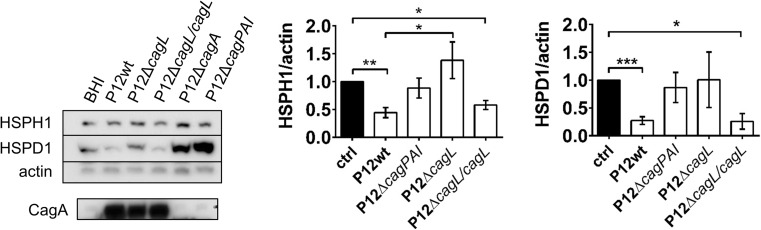
To further examine a role for CagA translocation in HSR suppression, AGS cells were inoculated with H. pylori P12wt or deletion mutants lacking either the cagL gene (P12ΔcagL) or the entire cagPAI (P12ΔcagPAI). Deletion of cagL abolishes T4SS function, and deletion of cagPAI leads to deletion of the cagA gene and genes encoding the T4SS (Fischer et al. 2001; Kwok et al. 2007). In support of CagA translocation playing an essential role in H. pylori down-modulating host-cell HSP expression, inoculation with either the ΔcagL or ΔcagPAI mutants did not reduce HSPH1, HSPA1A, or HSPD1 protein expression (Fig. 2). When T4SS function was restored by re-introducing cagL into the ΔcagL strain, protein expression of HSPH1 and HSPD1 were significantly reduced to levels comparable with those observed in response to P12wt (Fig. 2). Together, these findings indicated that an intact T4SS, able to translocate CagA, is required for H. pylori-mediated repression of host HSP expression.
Fig. 2.
H. pylori-mediated down-modulation of host HSPH1 and HSPD1 protein levels requires an intact T4SS. Western blot protein expression analysis of chaperone proteins following inoculation of AGS cells with H. pylori P12wt (as in Fig. 1) or isogenic mutant strains, P12ΔcagL (n = 5), P12ΔcagL/cagL rescue (n = 4) or P12ΔcagPAI (n = 6) of H. pylori P12, 24 h p.i. The expression of CagA in the various H. pylori strains as detected by Western blot is also shown. Densitometric analysis of mean fold change in expression normalized to BHI control (ctrl) from pooled experiments shown on right (mean ± SEM), *p < 0.05, **p < 0.01, ***p < 0.001
CagA is required but not sufficient for the down-modulation of host-cell HSP expression
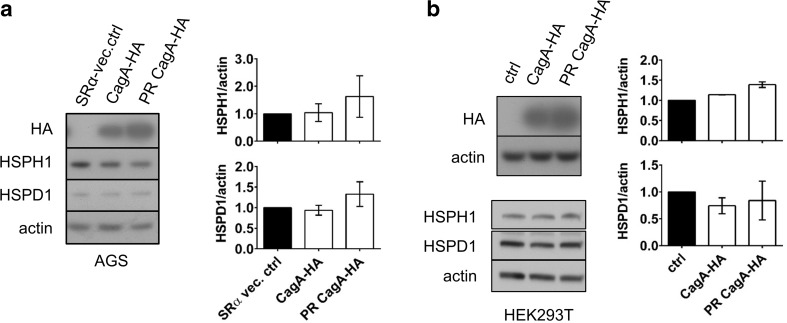
To determine whether CagA was the sole factor responsible for H. pylori-mediated HSP repression, AGS cells were transiently transfected with expression vectors encoding HA-tagged CagA (CagA-HA) or a HA-tagged phosphorylation-resistant CagA mutant (PR-CagA-HA). The latter carries a tyrosine to alanine residue substitution in the EPIYA motifs, which contain the tyrosine phosphorylation sites in CagA (Higashi et al. 2002). We also examined the effect of ectopic CagA expression in HEK293T cells that show higher transfection efficiency than AGS cells, and greater gene expression from the SV40 promoter due to the presence of the T-antigen in this cell line. Ectopic expression of CagA-HA or PR-CagA-HA did not reduce HSP expression in either AGS (Fig. 3a) or HEK293T (Fig. 3b) cells. Taken together these results suggest that while HSP down-modulation by H. pylori infection requires CagA translocation, this effect may be dependent upon additional bacterial factors in conjunction with translocated CagA to achieve HSP repression in the host cell.
Fig. 3.
Expression of cagA is not sufficient for down-modulation of HSPs in AGS or HEK293T cells. a AGS cells were transfected with 1.5 μg of cagA-expression vector DNA or SRα vector control. Cell lysates were harvested 48 h post-inoculation and then analyzed by Western blot. Densitometry represents mean HSP/actin protein level ratios relative to that of the SRα vector control, ±SEM, n = 2. b HEK293T cells were transiently transfected with 4.0 μg of SRα expression vector containing CagA-HA or PR-CagA-HA, cell lysates were analyzed by Western blot 48 h following transfection. Densitometric analysis is shown on right with mean HSP levels normalized against actin control and expressed as fold change relative to non-transfected media control (ctrl) ±SEM, n = 2
HSF1 is activated upon infection of AGS cells with H. pylori
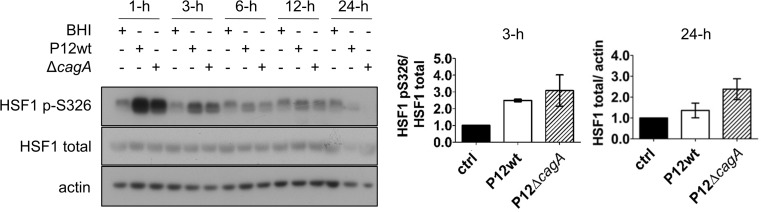
To investigate the underlying mechanism by which H. pylori down-modulates the level of host-cell HSPs, the activity and expression of the transcriptional regulator of HSP expression, HSF1, was examined during AGS co-culture with either the H. pylori P12wt or P12ΔcagA mutant. Within 1 h of infection with either strain, phosphorylation of HSF1 at Ser326 was dramatically increased (Fig. 4). Ser326 is a positive regulation site critical for HSF1 transcriptional activity (Chou et al. 2012; Guettouche et al. 2005). Level of Ser326 phosphorylation was also increased at 3 h post-infection with either strain compared to that of the non-infected BHI control (Fig. 4). However at later time points, levels of HSF1 phosphorylated Ser326 in P12wt- or P12ΔcagA-infected cells were comparable to that of the BHI control. In contrast, there was no variation in HSF1 total protein level relative to the BHI control during the 24 h of infection (Fig. 4). These data indicated that reduced HSP expression was not due to inhibition of HSF1 expression, or activation via phosphorylation.
Fig. 4.
Phosphorylation of HSF1 at Ser326 upon infection with H. pylori P12wt and P12ΔcagA mutant. Phosphorylation of HSF1 at Ser326 as well as HSF1 total levels within cell lysates of AGS infected with H. pylori P12wt or P12ΔcagA for 1, 3, 6, 12, and 24 h were examined by Western blot; representative blots are shown. Densitometric analysis of HSF1 p-Ser326/HSF1 mean expression ratio at 3-h p.i. (n = 2) and of HSF1/actin at 24-h p.i. (n = 4) is shown on right, ±SEM
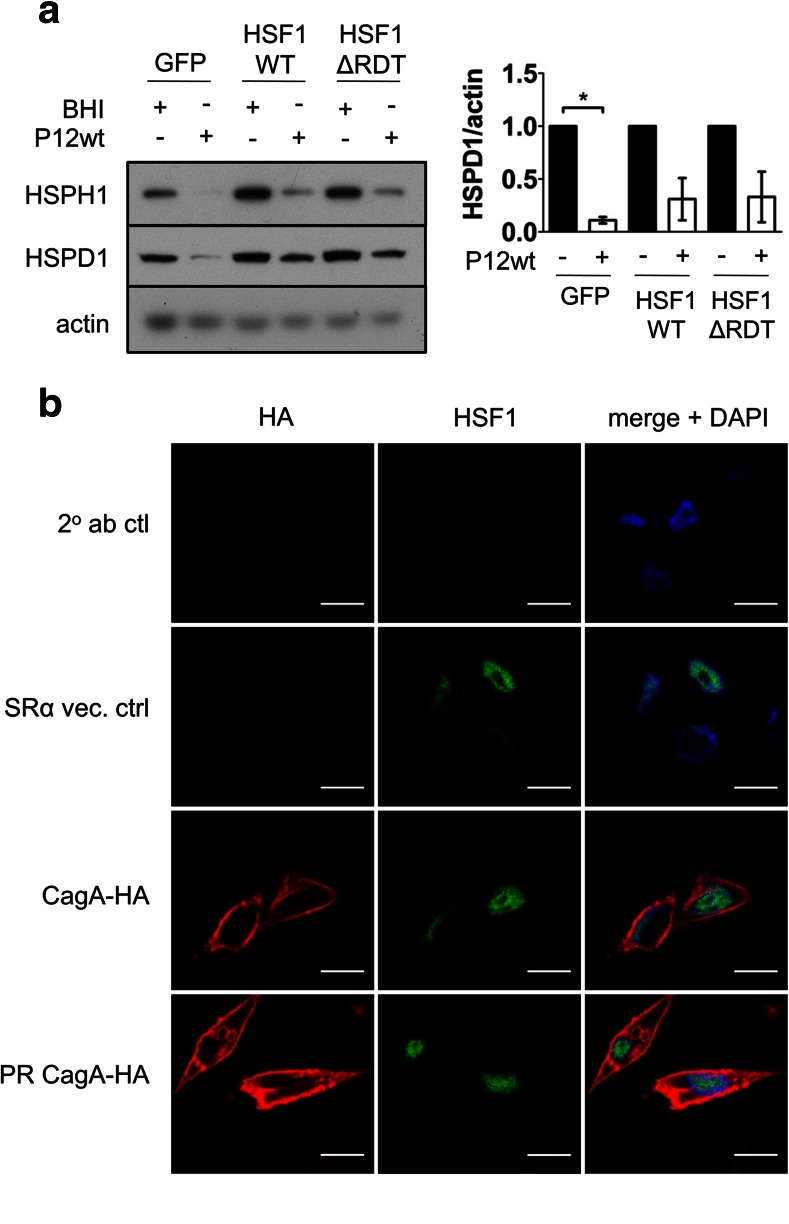
Overexpression of HSF1 partially rescues the reduction in HSP levels upon H. pylori infection of AGS cells
To further examine whether CagA targets HSF1 during H. pylori-mediated down-modulation of HSPs, AGS cells stably overexpressing human HSF1 (HSF1WT) or a constitutive active mutant (HSF1ΔRDT) were generated (Fig. S1b). Overexpression of HSF1WT or HSF1ΔRDT resulted in increased levels of the HSF1 transcriptional targets HSPH1 and HSPD1 (Fig. 5a). HSP levels were examined by Western blot analysis of cell lysates harvested following 24 h of infection with H. pylori P12wt. Consistent with previous infections, HSP expression was significantly down-modulated in P12wt-infected GFP control cells (Fig. 5a). Concurrently, HSP levels were also markedly reduced in the HSF1WT- and HSF1ΔRDT-overexpressing cell lines in response to infection with P12wt. However, the fold reduction in HSP expression was less than that of the AGS-GFP control-infected cells, indicating that while HSF1 overexpression partially rescued HSP expression, the primary mechanism for HSP repression by H. pylori is likely to be independent of HSF1 expression levels (Fig. 5a). In addition, the limited rescue effect observed upon overexpression of the constitutively active HSF1ΔRDT suggests that H. pylori-mediated HSP repression is also independent of altered HSF1 activation.
Fig. 5.
Overexpression of HSF1 partially rescues HSP repression upon H. pylori infection of AGS cells. a AGS cells overexpressing HSF1WT, HSF1ΔRDT, and the GFP control cell line were inoculated with H. pylori P12wt (MOI:50) or BHI broth, and HSP levels were analyzed 24 h post-infection by Western blot, n = 3. Densitometric analysis of mean fold change in HSPD1 levels ±SEM are shown on right expressed as a ratio of that of the BHI control for each given cell line, *p < 0.05. b CagA-expression vectors were used to transiently express CagA-HA or PR-CagA-HA in AGS cells. Immunostaining of AGS cells ectopically expressing CagA-HA and PR-CagA-HA using anti-HA and anti-HSF1 antibodies revealed no co-localization of CagA with endogenous HSF1. Top two rows represent secondary antibody control and empty SRα expression vector controls, respectively, n = 2. Scale bar = 25 μm
The molecular basis of the inhibitory effect upon HSP expression during H. pylori infection was further investigated by co-localization analysis of CagA and HSF1. AGS cells were transiently transfected with expression vectors encoding CagA-HA or PR-CagA-HA. Endogenous HSF1 was predominantly nuclear in the AGS cell line, whereas ectopically expressed CagA was localized to the cell periphery (Fig. 5b). The distribution of ectopically expressed CagA was in agreement with the previous observations that CagA upon translocation into or ectopic expression within the host cell was localized to the host-cell plasma membrane and cytoplasm (Backert et al. 2000; Higashi et al. 2002). The disparate cellular localisation of HSF1 and CagA indicated no direct interaction between the two molecules, and that the ectopic expression of CagA did not alter the nuclear localization of HSF1.
Activation of HSF1 upon infection of AGS cells with H. pylori does not result in elevated levels of HSP gene expression
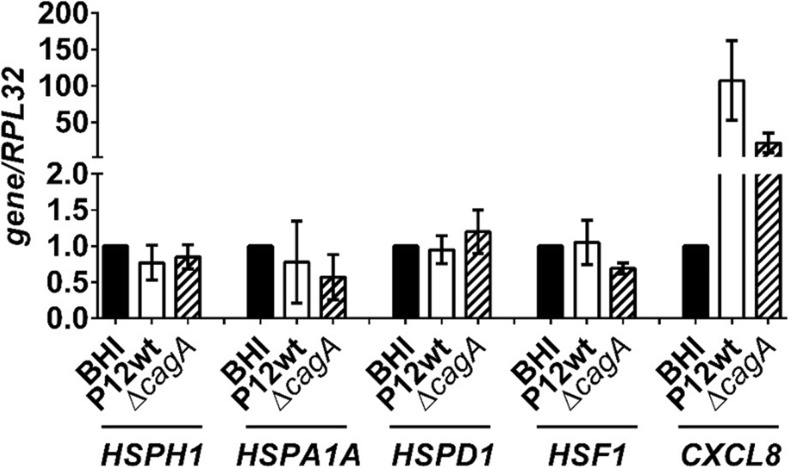
Our findings suggest that despite the strong phosphorylation of HSF1 within 3 h of infection with H. pylori P12wt and P12wtΔcagA mutant, HSP expression is reduced at 24 h post-infection. To determine whether H. pylori-mediated down-modulation of HSP levels was due to an inhibition of HSP gene transcription, RT-qPCR analysis of HSP mRNA levels was performed using samples collected at 4 h (data not shown) and 8 h post-infection with P12wt and P12ΔcagA (Fig. 6). Consistent with the constant relative HSF1 protein levels observed up to 24 h post-infection (Fig. 4), no change in HSF1 mRNA level was observed at 4 h (data not shown) or 8 h post-infection (Fig. 6). Despite significantly increased HSF1 phosphorylation up to 3 h post-infection (Fig. 4), and a robust infection indicated by upregulation of CXCL8, which encodes the proinflammatory chemokine interleukin-8 (Fig. 6), there was no concurrent increase in the mRNA levels of HSPH1, HSPA1A, or HSPD1 (Fig. 6). This suggests that the activation of these genes by HSF1 may be somewhat limited during H. pylori infection. Moreover, no significant reduction in HSP gene expression was observed at these time points to directly account for the reduced HSP expression observed within the cells at 24 h post-infection. Furthermore, no CagA-dependent effect upon gene expression was found. Thus, these results indicate that the CagA-dependent mechanism of HSP down-modulation is unlikely to be due to inhibition of HSP gene transcription.
Fig. 6.
H. pylori infection does not alter HSP or HSF1 gene expression. RT-qPCR expression analysis of genes HSPH1, HSPA1A, HSPD1, HSF1, and CXCL8 8 h p.i. of AGS cells with either H. pylori P12wt or P12ΔcagA mutant. Messenger RNA levels were normalized to that of the BHI control sample with RPL32 used as a reference gene. CXCL8 induction provided a positive control for robustness of infection, n = 3
Analysis of HSP expression upon inhibition of proteasome activity with H. pylori infection
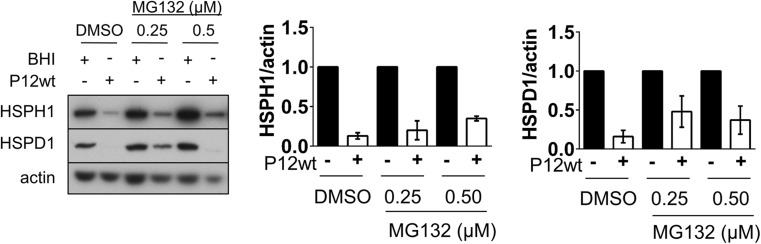
To examine whether reduced HSP levels observed at 24 h post-infection were mediated by enhanced proteasomal degradation of the HSPs, HSP levels in AGS inoculated with H. pylori P12wt and co-treated with the proteasome inhibitor MG132 were analyzed. In optimization of MG132 treatments, AGS cells were treated with a titration of MG132 concentrations for 24 h, with increasing levels of ubiquitinated protein seen up to MG132 concentration of 0.5 μM (Fig. S1a). Treatment of cells with MG132 at concentrations of 1.0 μM and higher caused increasing dose-dependent cellular toxicity (data not shown). While treatment with MG132 resulted in higher HSP expression in infected samples, this was matched by a comparable fold increase in HSP expression in the non-infected samples, likely due to the inherent HSR-activating properties of MG132 (Fig. 7). Accordingly, HSP levels were still substantially reduced in infected samples co-treated with MG132 compared to the non-infected BHI-inoculated cells. Although these data together do not exclude a contribution of proteasomal activity to HSP down-modulation, they strongly suggest that the primary mechanism of the H. pylori-mediated repression of HSP expression is not enhanced proteasomal degradation of HSPs.
Fig. 7.
MG132 treatment does not inhibit H. pylori-mediated HSP repression. AGS cells were treated with various concentrations of the proteasome inhibitor MG132 1 h prior to and during infection with H. pylori P12wt (MOI: 50) for a total of 24 h. Western blot analysis of protein expression following 24 h of combined infection and MG132 treatment is shown. Pooled densitometric values for HSPH1 (n = 2) and HSPD1 (n = 3) normalized to actin are shown on the right and represented as P12wt-infected expression relative to the non-infected BHI control sample for each respective MG132 concentration
Discussion
In addition to playing important roles in maintaining the integrity of cellular proteins and a cell’s response to proteotoxic stress, HSPs also function in inflammation, immune signaling, and antigen presentation (Calderwood et al. 2012; Henderson et al. 2010). The gastric pathogen H. pylori, the primary cause of chronic gastritis, peptic ulcer, MALT, and gastric cancer, has been demonstrated to down-modulate host-cell HSP expression upon infection (Axsen et al. 2009; Baek et al. 2004; Konturek et al. 2001; Pierzchalski et al. 2006; Targosz et al. 2006; Yeo et al. 2004). The mechanism of H. pylori-mediated host HSP repression is currently unknown, the elucidation of which may provide novel insight into host-pathogen interactions and may prove valuable in the effort to develop anti-HSP strategies for cancer treatment.
The current study demonstrated reduced protein level of a number of HSPs, namely HSPH1, HSPA1A, and HSPD1, upon acute infection of the human AGS gastric adenocarcinoma cell line AGS with H. pylori strain P12. A number of previous studies have reported repression of host HSP expression upon H. pylori infection (Axsen et al. 2009; Baek et al. 2004; Konturek et al. 2001; Pierzchalski et al. 2006; Targosz et al. 2006; Yeo et al. 2004). This study is the first to report this effect using the H. pylori strain P12 in AGS cells. This further suggests that HSP repression by H. pylori is unlikely to be limited to certain strains or host-cell models. Of the HSPs examined in this study, the down-modulation of HSPA1A upon H. pylori infection has been the most frequently reported (Axsen et al. 2009; Pierzchalski et al. 2006; Targosz et al. 2006; Yeo et al. 2004). Axsen et al. reported a mild down-modulation of HSPD1 at 6 and 12 h in AGS but not KatoIII cells upon infection with the H. pylori strain J166. However, in contrast to this study, Axsen et al., found no significant reduction in protein expression of HSPD1 after 24 h of infection (Axsen et al. 2009). Yeo et al did not observe down-modulation of HSPD1 but repression of HSPB1 (HSP27) upon H. pylori infection; in contrast, Axsen et al. reported HSPB1 expression to be unaffected (Axsen et al. 2009; Yeo et al. 2004). The variable findings regarding which specific HSPs are repressed may be due to numerous experimental factors including strain-specific effects, the host-cell models used, the multiplicity of H. pylori infection used, as well as inconsistent infection periods between studies. However, although studies to date, including the current study, reveal variations in the individual HSPs repressed following H. pylori infection, reports are consistent in that unlike infection with other bacterial species, H. pylori infection results in some level of HSP repression rather than HSP upregulation.
This study identified for the first time that CagA acted as a bacterial factor essential for H. pylori-mediated repression of host HSP expression. In contrast, Axsen et al found reduced HSP expression upon H. pylori infection to be independent of CagA and the cagPAI (Axsen et al. 2009). Using semi-quantitative RT-PCR, Targosz et al., found that infection of human gastric MKN7 cells with cagA − vacA − H. pylori strain 326 also resulted in reduction of HSPA1A gene expression up to 72 h post-infection; however, this effect was more prominent during infection with cagA + vacA + H. pylori, suggesting that both CagA-dependent and independent mechanisms exist to mediate HSP repression (Targosz et al. 2006). This is in agreement with findings from the current study where no HSP-gene induction (i.e., levels higher than the non-infected BHI control) was observed upon H. pylori infection irrespective of whether the strain used for infection expressed CagA or not. Given that upon infection with either wild-type H. pylori or a CagA-deficient mutant there was a strong phosphorylation of HSF1 at Ser326, indicative of HSF1 activation, we were surprised that we did not see an associated elevation in HSP gene expression. This observation suggested the possibility that a CagA-independent inhibition of HSP gene expression is also in operation.
Ectopic expression of cagA isolated from the H. pylori strain NCTC11637 was found to be insufficient to significantly reduce HSP expression in either HEK293T or the AGS gastric cell line, indicating that CagA may require additional bacterial factors to mediate HSP reduction. Further confirmation of this phenomenon could be achieved by experiments that examine HSP expression upon ectopic transfection of cagA in other gastric cell models or primary human gastric cells. Nevertheless, the effect of CagA upon HSP expression shown here is reminiscent of the interactions of CagA with its host-binding partners, the specificity and affinity of which appear to vary depending on whether the CagA is translocated from H. pylori during infection or ectopically expressed. Thus these findings further emphasize the importance of additional bacterial factors for CagA-mediated effects during H. pylori infection, reviewed in (Backert et al. 2010).
The current study investigated the potential of CagA targeting the primary transcriptional regulator of HSPs, HSF1, as a mechanism for H. pylori-mediated HSP repression. Data presented here demonstrated that the repression of HSP levels by H. pylori is mediated by a mechanism that is independent of any CagA-dependent alteration of HSF1 gene or protein expression. Gene expression levels of HSF1 were found to be unchanged 4 h (not shown) and 8 h post-infection, consistent with the report from Pierzchalski et al. where RT-PCR analysis of HSF1 gene expression was constant during H. pylori infection of KatoIII cells at time points examined up to 48 h (Pierzchalski et al. 2006). Moreover, the current study found that HSF1 protein levels remained constant relative to the non-infected control over 24 h, a time period at which HSP levels were significantly reduced in the infected host AGS cells. Together these findings indicate that the observed reduction in HSP expression is not due to a reduction in HSF1 expression. This is further supported by the finding that overexpression of wild-type human HSF1 or a constitutively active mutant of HSF1ΔRDT was unable to significantly rescue H. pylori-mediated repression of HSP expression.
Interestingly, within 1 and 3 h of H. pylori P12wt infection of AGS cells, HSF1 phosphorylation at Ser326 was markedly increased. Phosphorylation of this serine residue has been shown to occur in response to proteotoxic stressors including heat stress, proteasome inhibition with MG132 and heavy metal exposure (CdCl2) and single amino acid substitution of this residue dramatically reduces HSF1 transcriptional activity (Chou et al. 2012; Guettouche et al. 2005). This is the first study to identify phosphorylation of HSF1 at Ser326 during bacterial infection. Of note, infection with H. pylori P12wt or its isogenic ΔcagA mutant both induced phosphorylation of host-cell HSF1 to similar levels, indicating that CagA is not essential for this effect during H. pylori infection. Of note, the marked increase in HSF1 phosphorylation at Ser326 did not result in increased HSP gene expression at 4 h (not shown) or 8 h post-infection, suggesting a potential restriction of HSF1 transcriptional activity that prevented enhanced expression of HSPs. Both Axsen et al. and Targosz et al. demonstrated CagA-independent down-modulation of HSP and gene expression respectively (Axsen et al. 2009; Targosz et al. 2006), while the current study found that the ability of H. pylori P12 to down-modulate HSP levels was dependent on cagA, cagL, and an intact cagPAI. The finding that HSP and mRNA levels were not increased upon infection with either wild-type H. pylori or T4SS-defective mutants, despite apparent HSF1 activation, supports the possibility of CagA-independent restriction of HSP expression at the transcriptional level by H. pylori. Pierzchalski et al. proposed loss of HSF1 DNA-binding activity as a mechanism for the down-modulation of HSP levels during H. pylori infection upon formation of STAT1/HSF1 and STAT3/HSF1 complexes (Pierzchalski et al. 2006). Whether the formation of these complexes is indeed responsible for the observed down-modulation of HSP expression upon H. pylori infection remains to be investigated; if true this may present a potential mechanism for the apparent restriction of HSP gene expression despite increased phosphorylation of HSF1 at the Ser326 site.
No CagA-dependent effect upon HSP gene expression was observed, suggesting translocated CagA may impact upon HSP expression at a post-transcriptional level. Yeo et al., provided evidence to suggest that down-modulation of HSPA1A protein level occurs within 2 h of H. pylori infection, which points to a more direct mode of HSP repression, independent of altered HSP gene expression (Yeo et al. 2004). Experiments within this study in which proteasome activity was inhibited with MG132 found no dramatic alteration in the fold repression of HSP expression upon H. pylori infection. However, while treatment with MG132 demonstrated proteasome inhibitory activity (Fig. S1a), complete proteasome inhibition was not confirmed. Further experiments would be needed to clarify any role for proteasomal degradation during HSP reduction by H. pylori.
Nevertheless, the data presented suggest a CagA-dependent block of HSP mRNA translation and/or altered HSP mRNA processing. Recently, acute H. pylori infection has also been shown to repress HSPA5 (Grp78/BiP) expression and the unfolded protein response (UPR). Despite significantly increased HSPA5 gene expression upon infection and chemical activation of the UPR with tunicamycin, acute H. pylori infection was shown to repress both basal and tunicamycin-induced protein levels of HSPA5 (Baird et al. 2013). The increased production of host-cell proteins such as IL-8 upon infection (Brandt et al. 2005), suggests that any translational repression mediated by H. pylori is possibly specific to HSP transcripts. Specific repression of translation of HSP mRNAs has been described in other contexts; for example under basal conditions, translation of chicken reticulocyte HSP70 mRNA is restricted through a reduced elongation rate (Theodorakis et al. 1988). In addition, injection of IFN-γ and TNF-α, inflammatory factors that are also associated with H. pylori infection, were found to preferentially inhibit HSP translation without altering mRNA levels in a mouse model of colitis (Hu et al. 2007). Whether H. pylori infection plays a role in specific HSP repression via a mechanism similar to the previously mentioned contexts remains to be seen.
Conclusions
While the importance of CagA to the cytotoxicity of H. pylori infection and the development of disease are well established, it remains largely unclear how H. pylori benefits from translocating CagA into host cells (Backert et al. 2010). The current study has identified CagA to be a major factor for H. pylori-mediated HSP down-modulation upon infection. In addition to the HSPs previously known to be repressed by H. pylori, expression of HSPH1 has been identified for the first time in this study to be down-modulated by H. pylori, further highlighting an apparent universal repression of stress-associated proteins by this pathogen. Given the immune-regulatory roles of HSPs, we hypothesize in agreement with Axsen et al., that H. pylori may benefit from CagA-dependent acute repression of HSP levels by modulation of the host immune response and thereby aiding the establishment of infection (Axsen et al. 2009). While CagA was shown to be essential for H. pylori-mediated repression of HSP expression, expression of CagA alone was not sufficient for this effect, highlighting the importance of additional bacterial factors. A specific mechanism for the repression of HSP expression has yet to be clearly identified; however, the data presented reveals an ‘uncoupling’ of HSP mRNA and protein expression similar to that previously reported (Baird et al. 2013). Future studies that identify the mechanism by which H. pylori mediates the repression of stress proteins may reveal a novel method to inhibit the HSR pathway; a strategy with significant potential for the treatment of numerous human cancers. Characterization and perturbation of H. pylori-mediated HSP repression will further our understanding of H. pylori-host interactions and may provide important insights into the pathogenesis of gastric diseases caused by H. pylori infection.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(GIF 78 kb)
Acknowledgments
National Health and Medical Research Council of Australia R Douglas Wright Fellowship No. 395525 (JP); Australian Postgraduate Award (BL); Monash Micro Imaging (MMI) for microscopy support. H. pylori strains P12, P12∆cagPAI, and P12ΔcagA were kindly provided by Prof. Steffen Backert (Friedrich-Alexander University, Erlangen, Germany). HEK293T cells were kindly provided by Prof. Tony Tiganis (Dept. Biochemistry and Molecular Biology, Monash University).
Contributor Information
Terry Kwok, Email: terry.kwok@monash.edu.
John T. Price, Email: john.price@vu.edu.au
References
- Alvarez-Martinez CE, Christie PJ. Biological diversity of prokaryotic type IV secretion systems. Microbiol Mol Biol Rev: MMBR. 2009;73:775–808. doi: 10.1128/MMBR.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi M, et al. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000;191:593–602. doi: 10.1084/jem.191.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axsen WS, Styer CM, Solnick JV. Inhibition of heat shock protein expression by Helicobacter pylori. Microb Pathog. 2009;47:231–236. doi: 10.1016/j.micpath.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backert S, et al. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell Microbiol. 2000;2:155–164. doi: 10.1046/j.1462-5822.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- Backert S, Moese S, Selbach M, Brinkmann V, Meyer TF. Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Mol Microbiol. 2001;42:631–644. doi: 10.1046/j.1365-2958.2001.02649.x. [DOI] [PubMed] [Google Scholar]
- Backert S, Tegtmeyer N, Selbach M. The versatility of Helicobacter pylori CagA effector protein functions: the master key hypothesis. Helicobacter. 2010;15:163–176. doi: 10.1111/j.1523-5378.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- Baek H, Lim JW, Kim H, Kim JM, Kim JS, Jung HC, Kim KH. Oxidative-stress-related proteome changes in Helicobacter pylori-infected human gastric mucosa. Biochem J. 2004;379:291–299. doi: 10.1042/bj20031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird M, et al. The unfolded protein response is activated in Helicobacter-induced gastric carcinogenesis in a non-cell autonomous manner. Lab Investig. 2013;93:112–122. doi: 10.1038/labinvest.2012.131. [DOI] [PubMed] [Google Scholar]
- Bayerdorffer E, Miehlke S, Neubauer A, Stolte M. Gastric MALT-Lymphoma and Helicobacter pylori Infection. Aliment Pharmacol Ther. 1997;11(Suppl 1):89–94. doi: 10.1046/j.1365-2036.11.s1.12.x. [DOI] [PubMed] [Google Scholar]
- Brandt S, Kwok T, Hartig R, Konig W, Backert S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci U S A. 2005;102:9300–9305. doi: 10.1073/pnas.0409873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK, Murshid A, Gong J. Heat shock proteins: conditional mediators of inflammation in tumor immunity. Front Immunol. 2012;3:75. doi: 10.3389/fimmu.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SD, Prince T, Gong J, Calderwood SK. mTOR is essential for the proteotoxic stress response, HSF1 activation and heat shock protein synthesis. PLoS One. 2012;7:e39679. doi: 10.1371/journal.pone.0039679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J et al. (2012) GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC Cancer Base No. 11 [Internet]. International Agency for Research on Cancer; 2013., Lyon, France
- Fischer W, Puls J, Buhrdorf R, Gebert B, Odenbreit S, Haas R. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol Microbiol. 2001;42:1337–1348. doi: 10.1046/j.1365-2958.2001.02714.x. [DOI] [PubMed] [Google Scholar]
- Fischer W, et al. Strain-specific genes of Helicobacter pylori: genome evolution driven by a novel type IV secretion system and genomic island transfer. Nucleic Acids Res. 2010;38:6089–6101. doi: 10.1093/nar/gkq378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Takaki E, Hayashi T, Kitaura Y, Tanaka Y, Inouye S, Nakai A. Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J Biol Chem. 2005;280:34908–34916. doi: 10.1074/jbc.M506288200. [DOI] [PubMed] [Google Scholar]
- Gorrell RJ, et al. A novel NOD1- and CagA-independent pathway of interleukin-8 induction mediated by the Helicobacter pylori type IV secretion system. Cell Microbiol. 2013;15:554–570. doi: 10.1111/cmi.12055. [DOI] [PubMed] [Google Scholar]
- Guettouche T, Boellmann F, Lane WS, Voellmy R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005;6:4. doi: 10.1186/1471-2091-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas R, Meyer TF, van Putten JPM. Aflagellated mutants of Helicobacter pylori generated by genetic transformation of naturally competent strains using transposon shuttle mutagenesis. Mol Microbiol. 1993;8:753–760. doi: 10.1111/j.1365-2958.1993.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Henderson B, Calderwood SK, Coates AR, Cohen I, van Eden W, Lehner T, Pockley AG. Caught with their PAMPs down? The extracellular signalling actions of molecular chaperones are not due to microbial contaminants. Cell Stress Chaperones. 2010;15:123–141. doi: 10.1007/s12192-009-0137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera V, Parsonnet J. Helicobacter pylori and gastric adenocarcinoma. Clin Microbiol Infect. 2009;15:971–976. doi: 10.1111/j.1469-0691.2009.03031.x. [DOI] [PubMed] [Google Scholar]
- Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatkeyama M. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA. Protein Sci. 2002;295:683–686. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- Hu S, et al. Translational inhibition of colonic epithelial heat shock proteins by IFN-gamma and TNF-alpha in intestinal inflammation. Gastroenterology. 2007;133:1893–1904. doi: 10.1053/j.gastro.2007.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek JW, et al. Heat shock protein 70 (HSP70) in gastric adaptation to aspirin in Helicobacter pylori infection. J Physiol Pharmacol. 2001;52:153–164. [PubMed] [Google Scholar]
- Kuipers EJ. Helicobacter pylori and the risk and management of associated diseases: gastritis, ulcer disease atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1997;11(Suppl 1):71–88. doi: 10.1046/j.1365-2036.11.s1.5.x. [DOI] [PubMed] [Google Scholar]
- Kwok T, Backert S, Schwarz H, Berger J, Meyer TF. Specific entry of Helicobacter pylori into cultured gastric epithelial cells via a zipper-like mechanism. Infect Immun. 2002;70:2108–2120. doi: 10.1128/IAI.70.4.2108-2120.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok T, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449:862–866. doi: 10.1038/nature06187. [DOI] [PubMed] [Google Scholar]
- Lang BJ, et al. Heat stress induces epithelial plasticity and cell migration independent of heat shock factor 1. Cell Stress Chaperones. 2012;17:765–778. doi: 10.1007/s12192-012-0349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ME. The HSP70 family and cancer. Carcinogenesis. 2013;34:1181–1188. doi: 10.1093/carcin/bgt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CH, Lang BJ, Chai RC, Vieusseux JL, Kouspou MM, Price JT (2013) Heat shock factor 1 impacts both positively and negatively upon cellular clonogenic growth depending upon p53 status Biochem J 452 doi:10.1042/BJ20130098 [DOI] [PubMed]
- Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by Type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- Odenbreit S, Gebert B, Puls J, Fischer W, Haas R. Interaction of Helicobacter pylori with professional phagocytes: role of the cag pathogenicity island and translocation, phosphorylation and processing of CagA. Cell Microbiol. 2001;3:21–31. doi: 10.1046/j.1462-5822.2001.00088.x. [DOI] [PubMed] [Google Scholar]
- Pierzchalski P, Krawiec A, Ptak-Belowska A, Baranska A, Konturek SJ, Pawlik WW. The mechanism of heat-shock protein 70 gene expression abolition in gastric epithelium caused by Helicobacter pylori infection. Helicobacter. 2006;11:96–104. doi: 10.1111/j.1523-5378.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, Moorman AF. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal ED, Cha J, Lo J, Falkow S, Tompkins LS. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci U S A. 1999;96:14559–14564. doi: 10.1073/pnas.96.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbach M. Functional analysis of the Helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect Immun. 2002;70:665–671. doi: 10.1128/IAI.70.2.665-671.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, et al. Targeting eEF1A by a Legionella pneumophila effector leads to inhibition of protein synthesis and induction of host stress response. Cell Microbiol. 2009;11:911–926. doi: 10.1111/j.1462-5822.2009.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci U S A. 2000;97:1263–1268. doi: 10.1073/pnas.97.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targosz A, Pierzhalski P, Krawiec A, Szczyrk U, Brzozowski T, Konturek SJ, Pawlik WW. Helicobacter pylori inhibits expression of heat shock protein 70 (HSP70) in human epithelial cell line importance of cagA protein. J Physiol Pharmacol. 2006;57:265–278. [PubMed] [Google Scholar]
- Theodorakis NG, Banerji SS, Morimoto RI. HSP70 mRNA translation in chicken reticulocytes is regulated at the level of elongation. J Biol Chem. 1988;263:14579–14585. [PubMed] [Google Scholar]
- Yeo M, et al. Restoration of heat shock protein 70 suppresses gastric mucosal inducible nitric oxide synthase expression induced by Helicobacter pylori. Proteomics. 2004;4:3335–3342. doi: 10.1002/pmic.200400951. [DOI] [PubMed] [Google Scholar]
- Zheng L, He M, Long M, Blomgran R, Stendahl O. Pathogen-induced apoptotic neutrophils express heat shock proteins and elicit activation of human macrophages. J Immunol. 2004;173:6319–6326. doi: 10.4049/jimmunol.173.10.6319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(GIF 78 kb)