Abstract
Increasing studies have shown protective effects of intermittent hypoxia on brain injury and heart ischemia. However, the effect of intermittent hypoxia on blood glucose metabolism, especially in diabetic conditions, is rarely observed. The aim of this study was to investigate whether intermittent hypoxia influences blood glucose metabolism in type 1 diabetic rats. Streptozotocin-induced diabetic adult rats and age-matched control rats were treated with intermittent hypoxia (at an altitude of 3 km, 4 h per day for 3 weeks) or normoxia as control. Fasting blood glucose, body weight, plasma fructosamine, plasma insulin, homeostasis model assessment of insulin resistance (HOMA-IR), pancreas β-cell mass, and hepatic and soleus glycogen were measured. Compared with diabetic rats before treatment, the level of fasting blood glucose in diabetic rats after normoxic treatment was increased (19.88 ± 5.69 mmol/L vs. 14.79 ± 5.84 mmol/L, p < 0.05), while it was not different in diabetic rats after hypoxic treatment (13.14 ± 5.77 mmol/L vs. 14.79 ± 5.84 mmol/L, p > 0.05). Meanwhile, fasting blood glucose in diabetic rats after hypoxic treatment was also lower than that in diabetic rats after normoxic treatment (13.14 ± 5.77 mmol/L vs. 19.88 ± 5.69 mmol/L, p<0.05). Plasma fructosamine in diabetic rats receiving intermittent hypoxia was significantly lower than that in diabetic rats receiving normoxia (1.28 ± 0.11 vs. 1.39 ± 0.11, p < 0.05), while there were no significant changes in body weight, plasma insulin and β-cell mass. HOMA-IR in diabetic rats after hypoxic treatment was also lower compared with diabetic rats after normoxic treatment (3.48 ± 0.48 vs. 3.86 ± 0.42, p < 0.05). Moreover, intermittent hypoxia showed effect on the increase of soleus glycogen but not hepatic glycogen. We conclude that intermittent hypoxia maintains glycemia in streptozotocin-induced diabetic rats and its regulation on muscular glycogenesis may play a role in the underlying mechanism.
Keywords: Intermittent hypoxia, Blood glucose metabolism, Type 1 diabetes, Glycogen
Introduction
Diabetes mellitus has become a worldwide health problem in recent years. In patients with diabetes mellitus, blood glucose is abnormally increased mainly because of impaired insulin secretion induced by beta-cell disfunction and/or increased insulin resistance in peripheral tissues. Hyperglycemia is a strong risk factor for microvascular and macrovasular complications, including retinopathy, nephropathy, neuropathy, and cardiovascular disorders, which subsequently threaten patients’ life quality and lifespan (Brownlee 2005; Klein et al. 1996; Roglic et al. 2005). Thus, blood glucose control is a primary element in the treatment of diabetes.
Accumulating evidence has indicated the role of hypoxia in protecting the heart and brain from ischemic injury (Anderson and Honigman 2011; Galle and Jones 2012). In recent years, hypoxia has also showed efficacy in glucose metabolism. It has been reported that people living at high altitude have lower blood glucose and insulin (Lindgärde et al. 2004). Hypobaric and hypoxic exposure has been proven to decrease blood glucose in healthy men and glycosylated hemoglobin in obese men (Brooks et al. 1991; Lippl et al. 2010), probably by increasing glucose utilization (Roberts et al. 1996). Moreover, in exoteric studies, hypoxia also stimulated glucose transportation in human and animal skeletal muscle (Azevedo et al. 1995; Gamboa et al. 2011). In our previous work, intermittent hypobaric hypoxia has been proven to induce proliferation of neural stem cells in the adult rat brain and increase glucose transport activity in cultured rat hippocampal neurons (Yu et al. 2008; Zhu et al. 2005).
However, the effect of intermittent hypobaric hypoxia on glucose metabolism in diabetic patients or animal models has been rarely reported. The aim of this study was to evaluate whether intermittent hypobaric hypoxia could influence blood glucose in streptozotocin-induced diabetic rats.
Materials and methods
Animals
Adult male Wistar rats, weighing 180 to 200 g, were housed at 22 ± 2 °C with a 12-h light/dark cycle while food and water were available ad libitum. Rats were weighed and randomly divided into four groups: (1) non-diabetes (normal control, CC, n = 7), (2) diabetes (DC, n = 17), (3) non-diabetes with intermittent hypoxia (CH, n = 11), and (4) diabetes with intermittent hypoxia (DH, n = 17). All treatments were approved by the Institutional Animal Care and Use Committee of the Academy of Military Medical Sciences.
Induction of diabetes
Diabetes was induced by a single injection of 1 % streptozotocin (STZ, Sigma–Aldrich, MO, USA, 60 mg/kg ip in 10 mM sodium citrate, pH 4.5) into rats after a 15-h fast. Age-matched rats in control groups were fasted and injected with sodium citrate alone. Seven days after STZ injection, non-fasting blood glucose was measured and animals were considered to be diabetic with blood glucose levels >16.7 mmol/L.
Intermittent hypoxia
Two groups of rats received intermittent hypoxic treatment in a chamber 4 h/day for 3 weeks, with a constant fraction of inspired oxygen of 14 %. The hypoxic and hypobaric environment in the chamber corresponded to that at an altitude of 3 km (Zhu et al. 2005). The other two groups of rats maintained in normoxic and normobaric environment. All rats were kept in normoxic condition for 24 h before receiving followed test or tissue collection.
Fasting blood glucose and intraperitoneal glucose tolerance test (IPGTT)
Fasting blood glucose (in whole blood) from snipped tails was tested in all rats before and after a 3-week hypoxic treatment with a blood glucose monitor (ACCU-CHECK Active, Roche Diagnostics). Six rats of each group were injected intraperitoneally with glucose solution (50 %, 2 g/kg body wt) after a 15-h fast (Hamamoto et al. 2001). Blood glucose was measured at 0, 15, 30, 60, 90, and 120 min after injection.
Plasma fructosamine, plasma insulin, and insulin resistance by homeostasis model assessment of insulin resistance (HOMA-IR)
Before tissue collection, rats were anesthetized by intraperitoneal injection of pentobarbital sodium (2 %, 60 mg/kg body wt). Blood were sampled from cardiac puncture, collected in tubes containing heparin, and centrifuged. Plasma was collected and stored at −80 °C. Plasma fructosamine was determined by the method described by Zhang et al. (Zhang et al. 2008) with modification. In brief, 100 μL of plasma was added to 2 mL of NBT (nitroblue tetrazolium) and kept at 37 °C for 15 min in water bath. Distilled water was used as blank and BSA (bovine serum albumin) was used as control. The absorbance was measured at 530 nm with a microplate reader.
Fasting plasma insulin was assayed with an immunoradioassay (IRA) kit (Beijing North Institute of Biological Technology, China) following the instructions (Zhou et al. 2009). Values for HOMA-IR index (fasting blood glucose (mmol/L) × fasting plasma insulin (μU/mL)/22.5) were calculated (Matthews et al. 1985).
Immunohistochemistry and measurement of β-cell mass
After rats were sacrificed, the pancreas were immediately removed, cut into two pieces, weighted, and fixed in 10 % formalin. Tissue samples were then washed and stored in 70 % ethanol. After that, tissue samples were embedded in paraffin and sectioned (5 μm). Tissue sections were dewaxed and rehydrated, followed by being rinsed in 0.1 M PBS containing 0.025 % Triton X-100. The sections were blocked in 5 % horse serum for 90 min at room temperature, serially incubated in mouse anti-insulin antibody (1:500, Millipore, MA, USA) overnight at 4 °C, biotinylated anti-mouse immunoglobulin G (1:500, Vector Laboratories, CA, USA) for 1 h, avidin/biotin complex (ABC, Vector Laboratories, CA, USA) for 30 min and solution containing 0.02 % 3, 3′ -diaminobenzidine tetrahydrochloride (DAB) for 10 min. The nuclei were counterstained with hematoxylin.
β-cell mass in pancreas was measured as described before with modification (Finegood et al. 2001). Briefly, sections were imaged using an Olympus microscope (IX71) attached with a DP71 camera and Image-pro Express. Cross-sectional area of β-cells (brown) and area of all tissue (brown and blue) were measured in all fields per section. β-cell mass (mg) per piece was calculated as the cross-sectional area of β-cells/total tissue and multiplied by the weight of the tissue piece before fixation. Total β-cell mass per animal was the sum of the products from the two pancreas pieces.
Hepatic and muscular glycogen
After rats were sacrificed, hepatic tissue and soleus were isolated immediately, weighted and stored at −80 °C. Hepatic and muscular glycogen was determined with a commercial kit (Nanjing Jiancheng Institute of Bioengineering, Jiangsu, China) (Liu et al. 2012).
Statistical analysis
Data are expressed as mean ± SD. Values of HOMA-IR were transformed for statistical analysis because of their skewed distributions. One-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test was used for multiple comparisons. Kruskal-Wallis test followed by Games-Howell test was also used as appropriate. Two-way analysis of variance was also used to analyze values of hepatic and soleus glycogen. A p value less than 0.05 was considered statistically significant.
Results
Intermittent hypoxia maintained fasting blood glucose in diabetic rats
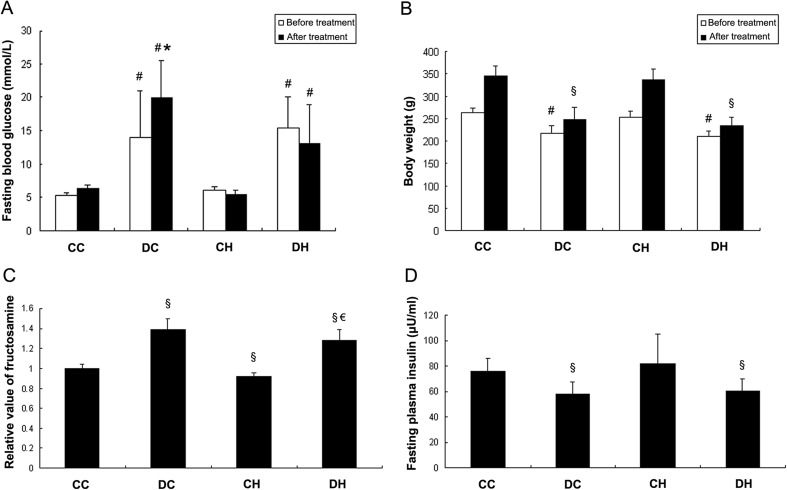
The diabetic model was induced by 7-day STZ injection, fasting blood glucose of rats in the DC group and the DH group was both increased (14.09 ± 6.89 mmol/L vs. 5.40 ± 0.39 mmol/L, p < 0.05; 15.5 ± 4.66 mmol/L vs. 5.4 ± 0.39 mmol/L, p < 0.05, Fig. 1a) while body weight decreased (217.18 ± 16.65 g vs. 264.00 ± 10.36 g, p < 0.05; 211.12 ± 12.37 g vs. 264.00 ± 10.36 g, p < 0.05, Fig. 1b) compared with rats in the CC group as control. After 3-week treatment, fasting blood glucose of rats in the DC group and in the DH was still increased compared with the CC group before treatment (19.88 ± 5.69 mmol/L vs. 5.40 ± 0.39 mmol/L, p < 0.05; 13.14 ± 5.77 mmol/L vs. 5.40 ± 0.39 mmol/L, p < 0.05, Fig. 1a). And fasting blood glucose of rats in the CH group did not change compared with the control group at baseline (5.46 ± 0.67 mmol/L vs. 5.40 ± 0.39 mmol/L, p > 0.05, Fig. 1a). Moreover, when compared with the DC group before treatment, fasting blood glucose in the DC group after treatment was significantly increased (19.88 ± 5.69 mmol/L vs. 14.09 ± 6.89 mmol/L, p < 0.05), while it was not changed in the DH group receiving hypoxic treatment (13.14 ± 5.77 mmol/L vs. 14.09 ± 6.89 mmol/L, p > 0.05). And fasting blood glucose in the DH group after hypoxic treatment was also lower than that in the DC group after normoxic treatment (13.14 ± 5.77 mmol/L vs. 19.88 ± 5.69 mmol/L, p < 0.05). Meanwhile, although body weights in both diabetic groups were still decreased compared with the CC group after treatment (247.65 ± 28.48 g vs. 345.43 ± 22.17 g, p < 0.05; 233.47 ± 20.85 g vs. 345.43 ± 22.17 g, p < 0.05, Fig. 1b), intermittent hypoxia did not affect body weight in diabetic and non-diabetic rats (233.47 ± 20.85 g vs. 247.65 ± 28.48 g, p > 0.05; 337.82 ± 23.71 g vs. 345.43 ± 22.17 g, p > 0.05). The data suggested that intermittent hypoxia could maintain fasting blood glucose level from progressive impairment in diabetic rats after injection of STZ.
Fig. 1.
Effect of hypoxia on fasting blood glucose (a), body weight (b), plasma fructosamine (c), and plasma insulin (d) of diabetic and non-diabetic rats. Compared with diabetic rats in the DC group before treatment, fasting blood glucose in diabetic rats treated with normoxia (DC) was increased while it was not changed in diabetic rats treated with intermittent hypoxia (DH). Plasma fructosamine in the DH group was lower than that in the DC group, while body weight and plasma insulin did not change. # p < 0.05 vs. the CC group before treatment; *p < 0.05 vs. the DC group before treatment; § p < 0.05 vs. the CC group after treatment; € p < 0.05 vs. the DC group
Intermittent hypoxia decreased plasma fructosamine and HOMA-IR in diabetic rats
To further investigate how intermittent hypoxia effect plasma glucose level, fasting plasma fructosamine was measured in rats after hypoxia or normoxia treatment. The data showed that plasma fructosamine level was higher in the DC group and the DH group than the control group (1.39 ± 0.11vs.1 ± 0.04, p < 0.05; 1.28 ± 0.11 vs.1 ± 0.04, p < 0.05, Fig. 1c). After 3-week intermittent hypoxia, plasma fructosamine in the DH group was lower than in the DC group that is in normoxic condition (1.28 ± 0.11 vs. 1.39 ± 0.11, p < 0.05, Fig. 1c), while plasma fructosamine in the CH group was also lower than the CC group (0.92 ± 0.03 vs. 1 ± 0.04, p < 0.05, Fig. 1c).
Fasting plasma insulin was also assessed. The results indicated that plasma insulin was lower in the DC group and in the DH group when compared with the control group (57.61 ± 10.20 μU/mL vs. 75.59 ± 10.60 μU/mL, p < 0.05; 60.31 ± 9.53 μU/mL vs. 75.59 ± 10.60 μU/mL, p < 0.05, Fig. 1d). However, plasma insulin in the DH group was not different with that in the DC group (60.31 ± 9.53 μU/mL vs. 57.61 ± 10.20 μU/mL, p > 0.05, Fig. 1d). And plasma insulin in the CH group was not different with that in the CC group (82.23 ± 23.20 μU/mL vs. 75.59 ± 10.60 μU/mL, p > 0.05, Fig. 1d).
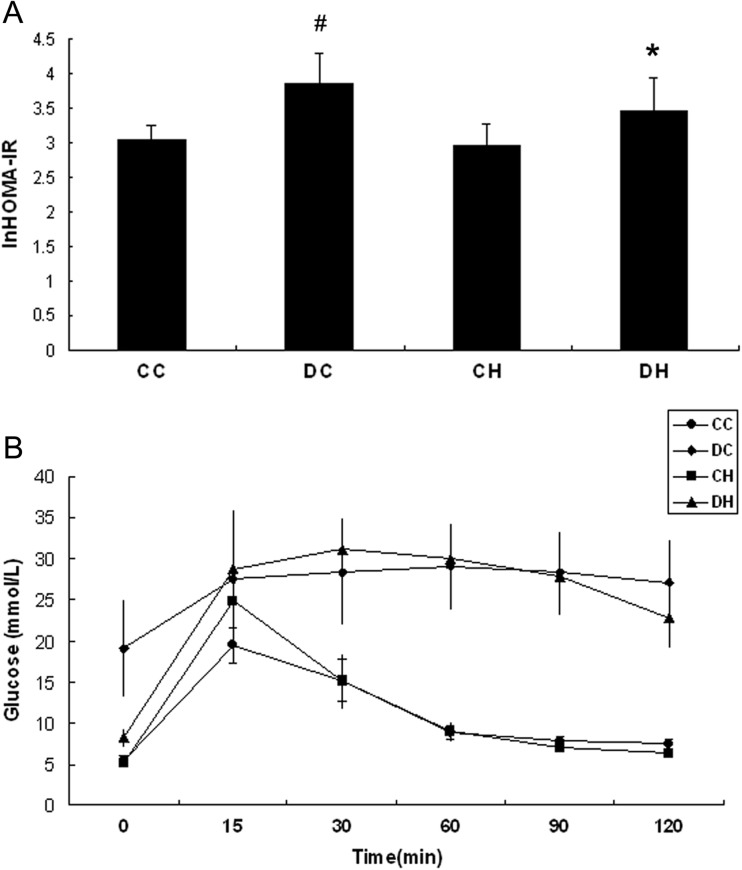
We next investigated the insulin resistance, and HOMA-IR was calculated and analyzed. HOMA-IR was increased obviously in the DC group (3.86 ± 0.42 vs. 3.03 ± 0.22, p < 0.05, Fig. 2a) but not in the DH group (3.48 ± 0.48 vs. 3.03 ± 0.22, p > 0.05, Fig. 2a) compared with the CC group. Meanwhile, HOMA-IR in the DH group was lower significantly than in the DC group (3.48 ± 0.48 vs. 3.86 ± 0.42, p < 0.05, Fig. 2a). While the plasma insulin was not different between the DH group and the DC group, it was proposed that glucose uptake was increased in diabetic rats after intermittent hypoxia.
Fig. 2.
Effect of hypoxia on HOMA-IR (a) and IPGTT (b) of diabetic and non-diabetic rats. HOMA-IR of diabetic rats treated with intermittent hypoxia (DH) was lower compared with diabetic rats in normoxia (DC), while IPGTT did not show difference after hypoxic treatment in diabetic or non-diabetic rats. # p < 0.05 vs. the CC group; *p < 0.05 vs. the DC group
In the IPGTT test, although blood glucose was lower in the DH group (n = 6) than the DC group (n = 6) at the beginning (p < 0.05, Fig. 2b), its level in the DH group was not different with that in the DC group at each of the following time points. One hundred twenty minutes after injection, blood glucose in DH group declined but not significantly compared with the DC group (p > 0.05, Fig. 2b). The blood glucose was elevated obviously in the CH group compared with the CC group at the 15-min time point and declined to the same level as in the CC group at the following time points. It needs to be mentioned that plasma insulin was not measured, which increased the limitation of the test.
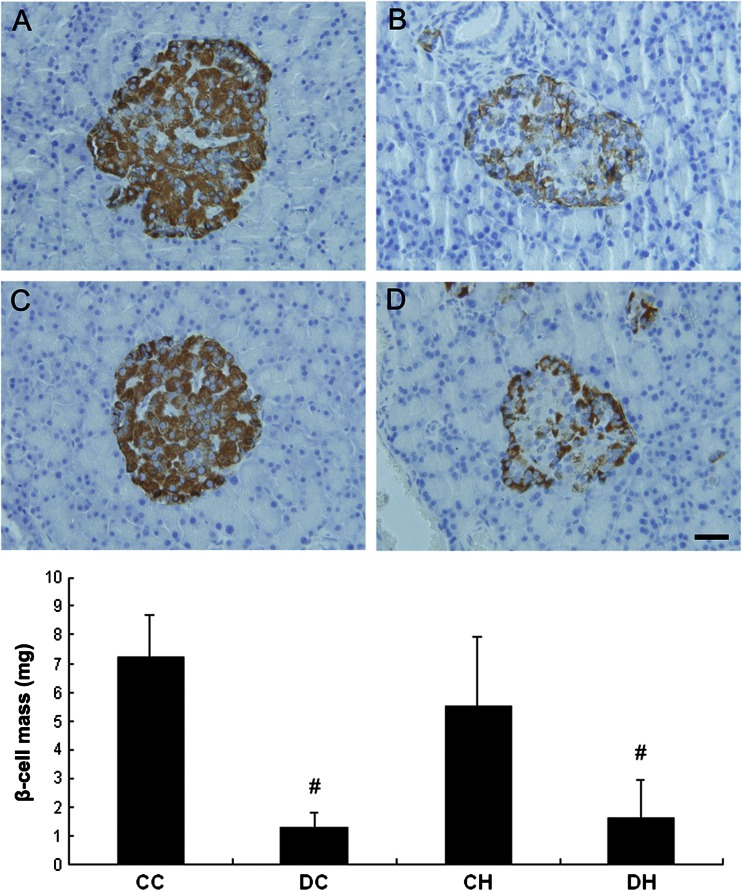
β-cell mass did not change after intermittent hypoxia
Finally, the pancreases were processed for immunohistochemistry of insulin and β-cell mass analysis after hypoxia treatment. β-cell mass in the DC group and the DH group was decreased compared with the CC group (1.30 ± 0.51 mg vs. 7.23 ± 1.47 mg, p < 0.05; 1.65 ± 1.26 mg vs. 7.23 ± 1.47 mg, p < 0.05, Fig. 3). After hypoxic treatment, β-cell mass in the DH group was not different with that in the DC group (1.65 ± 1.26 mg vs. 1.30 ± 0.51 mg, p > 0.05), neither with β-cell mass in the CH group as compared with the CC group (5.51 ± 2.44 mg vs. 7.23 ± 1.47 mg, p > 0.05).
Fig. 3.
Effect of hypoxia on pancreas β-cell mass of diabetic and non-diabetic rats. β-cell mass showed no difference after hypoxic treatment in diabetic or non-diabetic rats. a CC group, b DC group, c CH group, and d DH group; # p < 0.05 vs. the CC group; Bar: 30 μm
Intermittent hypoxia increased soleus glycogen but not hepatic glycogen
Hepatic and soleus glycogen was also determined in all rats after hypoxic or normoxic treatment. As shown in Fig. 4a, hepatic glycogen in each group was similar, while soleus glycogen in the DC, CH, and DH groups was higher than in the CC group. Through two-way analysis of variance, it was shown that both induction of diabetes (p < 0.05, two-way ANOVA, Fig. 4b) and hypoxia (p < 0.05, two-way ANOVA, Fig. 4b) showed evident effect on the increase of soleus glycogen, although their interaction did not show such effect (p= 0.056, two-way ANOVA, Fig. 4b).
Fig. 4.
Effect of hypoxia on hepatic glycogen (a) and soleus glycogen (b) of diabetic and non-diabetic rats. Hepatic glycogen was identical in each group, while both induction of diabetes (p < 0.05, two-way ANOVA) and hypoxia (p < 0.05, two-way ANOVA) showed effect on increasing soleus glycogen. # p < 0.05 vs. the CC group; § p < 0.05, two-way ANOVA
Discussion
In the current study, we evidenced that intermittent hypobaric hypoxia maintained glycemia in type 1 diabetic rats. Furthermore, intermittent hypoxia showed influence on the increasement of soleus glycogen but not on hepatic glycogen. Thus, intermittent hypoxia may maintain blood glucose through its influence on muscular glycogenesis in type 1 diabetes.
Intermittent hypoxia has been previously studied and focused on its role in metabolic disorders, cardiovascular morbidity, and neurological abnormality, because of its association with obstructive sleep apnea (as reviewed in (Drager and Jun 2010; Lal et al. 2012; Prabhakar and Semenza 2012)). On the other hand, increasing evidences also show favorable action of intermittent hypoxia. Burtscher et al. have proven intermittent hypoxia increase exercise tolerance in patients with chronic obstructive pulmonary disease (Burtscher et al. 2009). Intermittent hypoxia has also been reported to protect cardiovascular system from post-ischemia through increasing coronary vasculature and providing more efficient metabolism (Anderson and Honigman 2011), as well as promote hippocampal neurogenesis in adult rats (Zhu et al. 2005, 2010).
In our study, intermittent hypoxia maintained fasting blood glucose and lowered HOMA-IR of diabetic rats without a change in plasma insulin concentration. The effect of intermittent hypoxia on glucose metabolism of diabetic patients or animal models has been rarely reported. Mackenzie et al. have demonstrated hypoxic exposure (O2 = 14.6 %, 60 min), lower blood glucose, and improve peripheral insulin sensitivity in type two diabetic patients (Mackenzie et al. 2011, 2012). In our experiment, however, plasma insulin was not changed while HOMA-IR was lower in diabetic rats in hypoxic condition compared with diabetic rats in normoxic condition. It is not clear that whether insulin sensitivity of diabetic rats was affected by intermittent hypoxia. A more previous study has indicated that hypoxia (in vitro incubation, 95 % N2/5 % CO2, 60 min) accelerates glucose transportation in skeletal muscle of non-insulin-dependent diabetic patients (Azevedo et al. 1995). And the affection of hypoxia and insulin on glucose transportation are addictive (Mackenzie et al. 2012). Azevedo et al. have deduced that it is insulin-signaling defect that leads to insulin resistant in muscles while the glucose transport effector system still functional in diabetic patients (Azevedo et al. 1995). Similar to muscle exercise, hypoxia has been proven to induce translocation of glucose transporter 4 (GLUT4) from interior pools to the surface of plasma membrane through a calcium-dependent but not insulin-dependent pathway and result in glucose uptake in skeletal muscles (Cartee et al. 1991; Youn et al. 1991). Muscle exercise has been proven to increase glucose transportation in insulin-resistant skeletal muscle of rats and patients (Perseghin et al. 1996; Ruzzin and Jensen 2005). In other studies, hypoxia also increased GLUT4 protein expression in skeletal muscles (Dill et al. 2001; Xia et al. 1997). Skeletal muscle is the major position for glucose disposal and plays a important role in keeping glucose homeostasis in whole body (DeFronzo et al. 1981). GLUT4 is the main glucose transporter isoform expressed in skeletal muscle. Several studies have demonstrated that elevated GLUT4 expression or translocation to membrane was related to increased glycogen content in skeletal muscles (Holmes et al. 1999; McCoy et al. 1996). On the other hand, Young et al. showed high-altitude exposure (4300 m, 18 days) and decreased skeletal muscle glycogen utilization (Young et al. 1982). Thus, it could be deduced that hypoxia affects both glycogen synthesis and glycogen degradation. As indicated by Pescador et al., hypoxia not only promoted glycogen synthesis through up-regulating the glycogen synthase expression but also repressed glycogen degradation by reducing glycogen phosphorylase activity (Pescador et al. 2010). The potential mechanism for the raise of soleus glycogen content of diabetic rats after hypoxia in our study requires advanced investigation.
Although numerous work has been carried out to investigate the role of hypoxia in metabolism change, various hypoxia strategies applied in different experiments leaded to divergent results. The protocol in the current study was used according to our previous work (Zhu et al. 2005), showing its favorable affection on neurogenesis in adult rat brain. On the other hand, intermittent hypoxic protocols have also been applied to mimic the clinical disorder of sleep apnoea, which usually obtained much lower oxygen concentration. Under these relatively serious conditions, intermittent hypoxia has been found to increase blood glucose, impair glucose tolerance, and lead to other metabolic dysfunctions (Lee et al. 2013; Rafacho et al. 2013). Whether it is the difference in the pattern of hypoxia that causes diverse effect on glucose metabolism needs advanced attention.
In conclusion, our work has shown that intermittent hypobaric hypoxia helps maintain glycemia from advanced impairment and lower HOMA-IR in type 1 diabetic rats. Meanwhile, muscular glycogenesis may play a role in blood glucose disposal by hypoxia. The underlying mechanism by which intermittent hypobaric hypoxia influences glucose metabolism and its effect on type 2 diabetes need further investigation.
Acknowledgments
This study was supported by the National Basic Research Programs of China (2011CB910800 and 2012CB518200).
Compliance with ethical standards
All treatments were approved by the Institutional Animal Care and Use Committee of the Academy of Military Medical Sciences.
Contributor Information
Ming Fan, Phone: 8610-66932333, Email: fanmingchina@126.com.
Lingling Zhu, Phone: 8610-66931315, Email: linglingzhuamms@126.com.
References
- Anderson J, Honigman B. The effect of altitude-induced hypoxia on heart disease: do acute, intermittent, and chronic exposures provide cardioprotection? High Alt Med Biol. 2011;12:45–55. doi: 10.1089/ham.2010.1021. [DOI] [PubMed] [Google Scholar]
- Azevedo JJ, Carey J, Pories W, Morris P, Dohm G. Hypoxia stimulates glucose transport in insulin-resistant human skeletal muscle. Diabetes. 1995;44:695–698. doi: 10.2337/diab.44.6.695. [DOI] [PubMed] [Google Scholar]
- Brooks G, et al. Increased dependence on blood glucose after acclimatization to 4,300 m. J Appl Physiol. 1991;70:919–927. doi: 10.1063/1.349599. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Burtscher M, et al. Intermittent hypoxia increases exercise tolerance in patients at risk for or with mild COPD. Respir Physiol Neurobiol. 2009;165:97–103. doi: 10.1016/j.resp.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Cartee G, Douen A, Ramlal T. Stimulation of glucose transport in skeletal muscle by hypoxia. J Appl Physiol. 1991;70:1593–1600. doi: 10.1152/jappl.1991.70.4.1593. [DOI] [PubMed] [Google Scholar]
- DeFronzo R, Jacot E, Jequier E, Maeder E, Wahren J, Felber J. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Dill R, Chadan S, Li C, Parkhouse W. Aging and glucose transporter plasticity in response to hypobaric hypoxia. Mech Ageing Dev. 2001;122:533–545. doi: 10.1016/S0047-6374(01)00216-0. [DOI] [PubMed] [Google Scholar]
- Drager L, Jun J, Polotsky V. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab. 2010;24:843–851. doi: 10.1016/j.beem.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegood D, McArthur M, Kojwang D, Thomas M, Topp B, Leonard T, Buckingham R. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes. 2001;50:1021–1029. doi: 10.2337/diabetes.50.5.1021. [DOI] [PubMed] [Google Scholar]
- Galle A, Jones N. The neuroprotective actions of hypoxic preconditioning and postconditioning in a neonatal rat model of hypoxic-ischemic brain injury. Brain Res. 2012 doi: 10.1016/j.brainres.2012.12.026. [DOI] [PubMed] [Google Scholar]
- Gamboa J, Garcia-Cazarin M, Andrade F. Chronic hypoxia increases insulin-stimulated glucose uptake in mouse soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2011;300:R85–R91. doi: 10.1152/ajpregu.00078.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto Y, et al. Recovery of function and mass of endogenous beta-cells in streptozotocin-induced diabetic rats treated with islet transplantation. Biochem Biophys Res Commun. 2001;287:104–109. doi: 10.1006/bbrc.2001.5563. [DOI] [PubMed] [Google Scholar]
- Holmes B, Kurth-Kraczek E, Winder W. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol. 1999;87:1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein B, Moss S. Relation of glycemic control to diabetic microvascular complications in diabetes mellitus. Ann Intern Med. 1996;124:90–96. doi: 10.7326/0003-4819-124-1_Part_2-199601011-00003. [DOI] [PubMed] [Google Scholar]
- Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest. 2012;141:1601–1610. doi: 10.1378/chest.11-2214. [DOI] [PubMed] [Google Scholar]
- Lee E, et al. Time-dependent changes in glucose and insulin regulation during intermittent hypoxia and continuous hypoxia. Eur J Appl Physiol. 2013;113:467–478. doi: 10.1007/s00421-012-2452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgärde F, Ercilla M, Correa L, Ahrén B. Body adiposity, insulin, and leptin in subgroups of Peruvian Amerindians. High Alt Med Biol. 2004;5:27–31. doi: 10.1089/152702904322963663. [DOI] [PubMed] [Google Scholar]
- Lippl F, Neubauer S, Schipfer S, Lichter N, Tufman A, Otto B, Fischer R. Hypobaric hypoxia causes body weight reduction in obese subjects. Obesity (Silver Spring) 2010;18:675–681. doi: 10.1038/oby.2009.509. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. Intrauterine growth retardation increases the susceptibility of pigs to high-fat diet-induced mitochondrial dysfunction in skeletal muscle. PLoS One. 2012;7:e34835. doi: 10.1371/journal.pone.0034835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie R, Maxwell N, Castle P, Brickley G, Watt P. Acute hypoxia and exercise improve insulin sensitivity (S(I) (2*)) in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2011;27:94–101. doi: 10.1002/dmrr.1156. [DOI] [PubMed] [Google Scholar]
- Mackenzie R, Maxwell N, Castle P, Elliott B, Brickley G, Watt P. Intermittent exercise with and without hypoxia improves insulin sensitivity in individuals with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:E546–E555. doi: 10.1210/jc.2011-2829. [DOI] [PubMed] [Google Scholar]
- Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McCoy M, Proietto J, Hargreaves M. Skeletal muscle GLUT-4 and postexercise muscle glycogen storage in humans. J Appl Physiol. 1996;80:411–415. doi: 10.1152/jappl.1996.80.2.411. [DOI] [PubMed] [Google Scholar]
- Perseghin G, et al. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- Pescador N, et al. Hypoxia promotes glycogen accumulation through hypoxia inducible factor (HIF)-mediated induction of glycogen synthase 1. PLoS One. 2010;5:e9644. doi: 10.1371/journal.pone.0009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar N, Semenza G. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafacho A, Gonçalves-Neto L, Ferreira F, Protzek A, Boschero A, Nunes E, Zoccal D. Glucose homoeostasis in rats exposed to acute intermittent hypoxia. Acta Physiol (Oxf) 2013 doi: 10.1111/apha.12118. [DOI] [PubMed] [Google Scholar]
- Roberts A, Reeves J, Butterfield G, Mazzeo R, Sutton J, Wolfel E, Brooks G. Altitude and beta-blockade augment glucose utilization during submaximal exercise. J Appl Physiol. 1996;80:605–615. doi: 10.1152/jappl.1996.80.2.605. [DOI] [PubMed] [Google Scholar]
- Roglic G, et al. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care. 2005;28:2130. doi: 10.2337/diacare.28.9.2130. [DOI] [PubMed] [Google Scholar]
- Ruzzin J, Jensen J. Contraction activates glucose uptake and glycogen synthase normally in muscles from dexamethasone-treated rats. Am J Physiol Endocrinol Metab. 2005;289:E241–E250. doi: 10.1152/ajpendo.00587.2004. [DOI] [PubMed] [Google Scholar]
- Xia Y, Warshaw J, Haddad G. Effect of chronic hypoxia on glucose transporters in heart and skeletal muscle of immature and adult rats. Am J Physiol. 1997;273:1734–1741. doi: 10.1152/ajpregu.1997.273.5.R1734. [DOI] [PubMed] [Google Scholar]
- Youn J, Gulve E, Holloszy J. Calcium stimulates glucose transport in skeletal muscle by a pathway independent of contraction. Am J Physiol. 1991;260:C555–C561. doi: 10.1152/ajpcell.1991.260.3.C555. [DOI] [PubMed] [Google Scholar]
- Young A, Evans W, Cymerman A, Pandolf K, Knapik J, Maher J. Sparing effect of chronic high-altitude exposure on muscle glycogen utilization. J Appl Physiol. 1982;52:857–862. doi: 10.1152/jappl.1982.52.4.857. [DOI] [PubMed] [Google Scholar]
- Yu S, et al. Hypoxic preconditioning up-regulates glucose transport activity and glucose transporter (GLUT1 and GLUT3) gene expression after acute anoxic exposure in the cultured rat hippocampal neurons and astrocytes. Brain Res. 2008;1211:22–29. doi: 10.1016/j.brainres.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lian B, Cui F. Effect of FeSO4 treatment on glucose metabolism in diabetic rats. Biometals. 2008;21:685–691. doi: 10.1007/s10534-008-9153-8. [DOI] [PubMed] [Google Scholar]
- Zhou J, et al. Protective effect of berberine on beta cells in streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Eur J Pharmacol. 2009;606:262–268. doi: 10.1016/j.ejphar.2008.12.056. [DOI] [PubMed] [Google Scholar]
- Zhu L, et al. Neurogenesis in the adult rat brain after intermittent hypoxia. Brain Res. 2005;1055:1–6. doi: 10.1016/j.brainres.2005.04.075. [DOI] [PubMed] [Google Scholar]
- Zhu X, et al. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci. 2010;30:12653–12663. doi: 10.1523/JNEUROSCI.6414-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]