Abstract
The extracellular α-amylase from the hyperthermophilic archaeum Pyrococcus furiosus (PFA) is extremely thermostable and of an industrial importance and interest. PFA aggregates and accumulates as insoluble inclusion bodies when expressed as a heterologous protein at a high level in Escherichia coli. In the present study, we investigated the roles of chaperones from P. furiosus in the soluble expression of recombinant PFA in E. coli. The results indicate that co-expression of PFA with the molecular chaperone prefoldin alone significantly increased the soluble expression of PFA. Although, co-expression of other main chaperone components from P. furiosus, such as the small heat shock protein (sHSP) or chaperonin (HSP60), was also able to improve the soluble expression of PFA to a certain extent. Co-expression of chaperonin or sHSP in addition to prefoldin did not further increase the soluble expression of PFA. This finding emphasizes the biotechnological potentials of the molecular chaperone prefoldin from P. furiosus, which may facilitate the production of recombinant PFA.
Keywords: Molecular chaperones, Prefoldin, Hyperthermophilic α-amylase, Soluble expression, Pyrococcus furiosus
Introduction
Molecular chaperones are defined as a functional class of unrelated families of proteins that assist the non-covalent assembly of other polypeptide-containing structures in vivo but are not components of these assembled structures when performing their normal biological functions (Ellis 1987). Molecular chaperones have diverse biological functions such as protecting cells from adverse environmental conditions, catalyzing solubility and refolding of stable protein aggregates (Ben-Zvi and Goloubinoff 2001), assisting folding of the newly synthesized polypeptide (Hartl and Hayer-Hartl 2002), and allowing the evolution of new protein functions and phenotypic traits (Tokuriki and Tawfik 2009; Rutherford and Lindquist 1998; Hartl et al. 2011). These functions render chaperones as promising candidates for biotechnological applications (de Marco 2007; Martinez-Alonso et al. 2009).
Numerous studies have demonstrated the use of molecular chaperone proteins to promote the soluble expression of heterogeneous proteins. Most molecular chaperone proteins reported to successfully promote soluble expressions are those of Escherichia coli. For examples, co-expression with the trigger factor (TF) alone was sufficient to prevent the aggregation of mouse endostatin, while overexpression of TF together with GroEL-GroES was more effective for human ORP150 and lysozyme (Nishihara et al. 2000). Overexpression of GroEL-GroES was also able to greatly increase the yield of soluble iron-regulatory protein 1 (Carvalho and Meneghini 2008) and to enhance the soluble expression of human interferon-γ (Yan et al. 2012). de Marco (2007) co-expressed eight combinations of E. coli molecular chaperones with a target protein in order to improve target protein’s soluble yield. Martinez-Alonso et al. (2009) achieved high-quality recombinant protein production by rehosting bacterial chaperones in eukaryotes.
Molecular chaperone systems of hyperthermophilic archaea have distinctive characteristics, compared to bacteria or eukarya (Lakanalamai et al. 2004). Hyperthermophilic archaea possess more compact genomes that encode reduced sets of heat shock proteins with chaperones that are more similar to eukaryotic chaperones. The chaperone systems in hyperthermophilic archaea are composed mainly of chaperonin, small heat shock protein (sHSP), and prefoldin (Lakanalamai et al. 2004). Chaperonins have an essential role in mediating the folding of newly synthesized proteins and assisting folding of stress-denatured proteins to their native state in an ATP-dependent manner (Zhang et al. 2010; Lund 2011). sHSP prevents aggregation of denatured proteins and in some cases promotes renaturation of proteins (Kim et al. 1998; Laksanalamai et al. 2001).
Prefoldin exists in all eukaryotes and archaea, but not in bacteria (Siegers et al. 1999). Archaeal prefoldin is a hexameric molecular chaperone complex containing two α-subunits and four β-subunits. Previous studies indicated that archaeal prefoldins were able to protect the aggregation of diverse heterogeneous proteins (Iizuka et al. 2008; Leroux et al. 1999; Laksanalamai et al. 2006; Okochi et al. 2002; Lundin et al. 2004; Hongo et al. 2012). Furthermore, overexpression of prefoldin from hyperthermophilic archaea was able to efficiently increase tolerance of E. coli hosts to different environmental stresses, such as the presence of an organic solvent (Okochi et al. 2008) or high temperature (Chen et al. 2010).
Enzymes from thermophilic organisms typically possess unique properties such as thermostability, salt tolerance, and pressure resistance (Atomi et al. 2011). The amylases, used in the starch industry, are the most widely utilized thermostable enzymes (Haki and Rakshit 2003; Emmanuel et al. 2000). The extracellular α-amylase from a hyperthermophilic archaeum Pyrococcus furiosus (PFA) has prominent enzyme properties and is significantly more thermostable compared to the thermostable α-amylase commonly used in the market (Dong et al. 1997; Jørgensen et al. 1997). When compared to the commonly used thermostable α-amylase (Taka-therm), PFA displayed a higher optimal temperature (near 100 °C) and thermostability, low optimal pH (5.5), and did not require Ca2+ for its activity and thermostability. Furthermore, the products were mainly G2–G7 with a minor amount of glucose (Dong et al. 1997). Due to the inherent difficulty to cultivate P. furiosus, sufficient production of this enzyme can only be achieved using recombinant expression. Previous studies indicated that recombinant PFA, when expressed at a high level, is always expressed as inclusion bodies (Dong et al. 1997; Wang et al. 2007). Several studies have tried to express PFA in its soluble form. Different methods have been attempted, including co-expression of PFA with thioredoxin along with culturing at 18 °C (Dong et al. 1997) and fusion expression of PFA with intein in E. coli (Grzybowska et al. 2004). However, these methods have resulted in poor yields of total soluble recombinant enzyme.
Chaperones from P. furiosus play an important role in assisting protein folding in various ways. In the present study, we rehosted the hyperthermophilic archaeon P. furiosus chaperone systems to bacteria cells and determined the optimal chaperone combination to maximize the soluble PFA yield. We have constructed a series of vectors encoding main components of P. furiosus chaperones and co-expressed them with PFA in E. coli. The results indicate that co-expression of P. furiosus molecular chaperone prefoldin could efficiently increase the soluble expression of recombinant PFA. This finding can lead to a more convenient production process for recombinant PFA and render it closer to its future industrial applications.
Material and methods
Bacterial strains and regents
E. coli strains DH5α and BL21(DE3) were used for plasmid preparation and protein expression, respectively. Restriction enzymes, T4 ligase, and other regents for gene manipulation were purchased from Takara Bio, Inc. (Shiga, Japan).
Constructions of recombinant plasmids
Genes encoding prefoldin, chaperonin (HSP60), sHSP, and extracellular α-amylase from P. furiosus (PFA) were PCR-amplified from plasmids constructed in previous studies (Chen et al. 2006, 2007, 2010, 2007). Primers used to amplify these genes were as follows: prefoldin: sense, 5′-GCCCATGGAAAACAATAAGGAAT-3′ (NcoI underlined); antisense, 5′-ACGCGTCGACGGAGCTCGAATTCTCATC-3′ (SalI underlined); HSP60: sense, 5′-ACTATCATATGGCCCAGTTAGCAGGC-3′ (NdeI underlined); antisense, 5′-CAGCTCGAGCTTGCTGGCAGCGATGAC-3′ (XhoI underlined); sHSP: sense, 5′-GCCCATGGTTCGTCGTATC-3′ (NcoI underlined); antisense, 5′-AACGTCGACTATTCAACTTT AACTTC-3′ (SalI underlined). PFA: sense, 5′-GAGGGAATTAATATGAAATACTTGG-3′ (PshBI underlined); antisense, 5′-GTGCTCGAGTGCGAACGCTAG-3′ (XhoI underlined). The amplified PCR fragments were double-digested with the appropriate restriction enzymes and then ligated into pACYCDuet-1 or pCDFDuet-1 vector. The constructs were confirmed by DNA sequencing. The recombinant plasmids were transformed into E. coli BL21(DE3) competent cells for expression.
Strain cultivation
All E. coli strains were cultured in 500-ml shake flasks containing 200 ml Luria-Bertani (LB) medium with corresponding antibiotics and shaking at 250 rpm, 37 °C, until OD600 = 0.6∼0.8. Subsequently, IPTG was supplemented in a final concentration of 1 mM and incubation was continued at 37 °C for 4 h to induce the expression of heterogeneous proteins. Osmolyte treatment was performed as described by de Marco et al. (2005) with minor modifications. Briefly, betaine and/or NaCl were added to final concentrations of 5 mM and 0.5 M, respectively, 30 min before IPTG induction. Cells were then harvested by centrifugation at 6000 rpm for 30 min. Supernatants were discarded completely, and pelleted cells were weighed as the cell wet weights.
Amylase activity assay
Harvested cells were resuspended in TE buffer (50 mM Tris–HCl, 100 mM NaCl, 10 mM EDTA, pH 8.0) and disrupted by ultrasonication at 200 W for 90 cycles on ice and centrifuged for 30 min at 16,500 rpm at 4 °C. The α-amylase activity was assayed according to Bernfeld and Wang et al. (Bernfeld 1955; Wang et al. 2007). Briefly, a 10 μl aliquot of suitably diluted enzyme was mixed with 490 μl sodium acetate buffer (50 mM, pH 5.5) containing 1 % soluble starch and incubated at 100 °C for 15 min. Hydrolysis was then stopped by cooling on ice. The reducing sugars formed were measured by the addition of DNS (3,5-dinitrosalicylic acid), using glucose as a standard. One unit of amylase was defined as the amount of enzyme required to produce reducing sugars equivalent to 1 μmol of glucose per minute.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Protein samples were also analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Protein samples were denatured in 2× SDS protein sample buffer at 100 °C for 5 min. The process was carried out using 4 % (w/v) stacking and 10 % (w/v) separating gels in a vertical slab gel apparatus, as described by Laemmli (1970). Subsequently, gels were stained with 0.05 % Coomassie Brilliant Blue R-250 for 4 h and destained in solution containing acetic acid, methanol, and water (1:3:6, v/v/v).
Results
Influences of chaperones from P. furiosus on the soluble expression of PFA
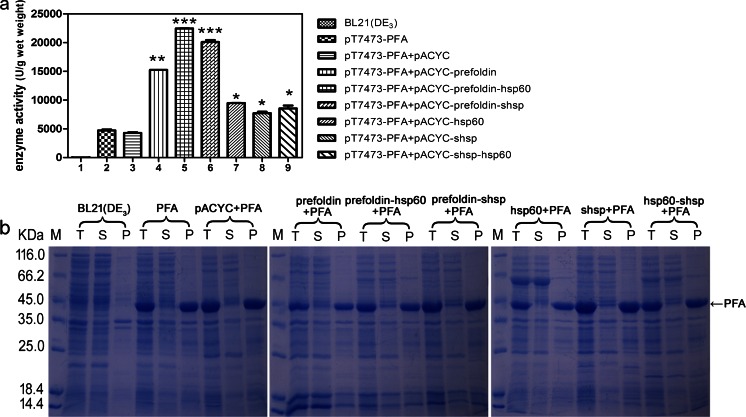
Hsp60 prefoldin and sHSP are three main components of the chaperone system in P. furiosus. In order to investigate whether these chaperone proteins can benefit the soluble expression of PFA, we cloned these chaperones (alone or in pairs) into pACYCDuet-1 plasmid and co-transformed them together with a PFA expressing plasmid pT7473-PFA into E. coli BL21(DE3). E. coli BL21(DE3) harboring the pT7473-PFA (designated as PFA) and E. coli BL21(DE3) harboring both pT7473-PFA and an empty pACYCDuet-1 plasmid (designated as PFA + pACYC) were used as controls. Following cell cultivation, IPTG induction, collection, and cell breakage by ultrasonication, supernatants were collected by centrifugation. The α-amylase activities in the supernatants were assayed under 100 °C. Samples were also analyzed by SDS-PAGE.
As presented in Fig. 1a, co-expression of each P. furiosus chaperone promoted the soluble expression of recombinant PFA to a certain degree. Co-expression of prefoldin, prefoldin and HSP60, or prefoldin and sHSP with PFA resulted in higher supernatant enzyme activity. However, SDS-PAGE results showed that the increase in soluble-expressed PFA bands was barely noticeable in all supernatant samples (Fig. 1b), which indicates that most of recombinant PFA remained as insoluble inclusion bodies despite of the significant increase in amylase activities in the supernatants, promoted by the co-expression P. furiosus chaperones.
Fig. 1.
Co-expression of chaperones originated from P. furiosus improved the soluble expression of recombinant PFA in E. coli. a Total PFA enzyme activity in supernatants. b SDS-PAGE analysis of the recombinant PFA expression in E. coli. All molecular chaperone genes were cloned into pACYCDuet-1 plasmid. M represents protein molecular weight marker. T total cells, S supernatant, P pellet. The values are representative results obtained from at least three experiments. Plots are given as mean ± SEM, and statistical significance is shown (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001)
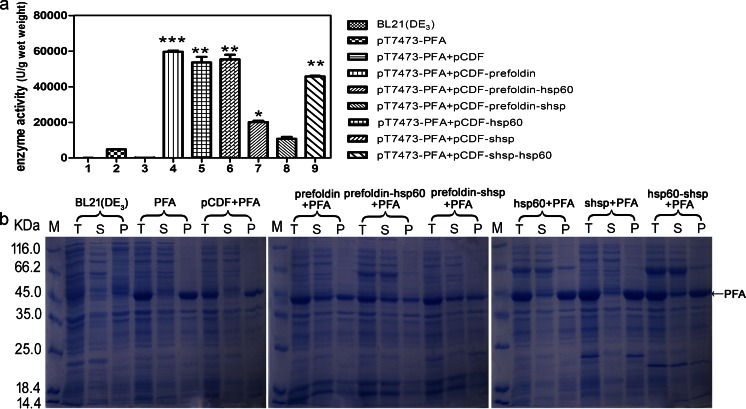
Increased expression of chaperones enhanced the soluble expression of PFA
Because co-expression of P. furiosus chaperones was able to improve the soluble expression of PFA, we next determined whether increased chaperone expressions can further enhance soluble PFA level. We therefore switched the vector used for chaperone expressions from pACYCDuet-1 to pCDFDuet-1, as the copy number of pCDFDuet-1 is two- to fourfolds higher than that of pACYCDuet-1 while maintaining the same promoter. We subsequently co-expressed the chaperone-expressing pCDFDuet-1 plasmids with pT7473-PFA. All subsequent experimental procedures were as described before. The results showed that by increasing the expression of P. furiosus chaperones, the soluble expression of PFA was increased. Strains harboring prefoldin displayed the highest supernatant enzyme activity, which was consistent with the pACYCDuet-1 expression system previously described. The α-amylase activity in the supernatant reached ∼60,000U/g wet weight when pCDF-prefoldin was co-expressed with pT7473-PFA (strain PFA + pCDF-prefoldin), which was nearly 15-folds higher than the control (strain PFA) (Fig. 2a). In addition, we observed a clear soluble PFA protein band in supernatant samples of strains harboring prefoldin using SDS-PAGE (Fig. 2b, lanes prefoldin + PFA S, prefoldin-hsp60 + PFA S, and prefoldin-shsp + PFA S). Additionally, co-expression of two chaperones, prefoldin and sHSP or prefoldin and HSP60, exhibited similar levels of soluble PFA increase as prefoldin alone, which indicates that the expression of prefoldin alone was adequate for promoting the soluble expression of PFA.
Fig. 2.
Increased expression of chaperones from P. furiosus further promoted the soluble expression of PFA. a Total PFA enzyme activity in supernatants. b SDS-PAGE analysis of the recombinant PFA expression in E. coli. All molecular chaperone genes were cloned into pCDFDuet-1 plasmid. M represents protein molecular weight marker. T total cells, S supernatant, P pellet. The values are representative results obtained from at least three experiments. Plots are given as mean ± SEM, and statistical significance is shown (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001)
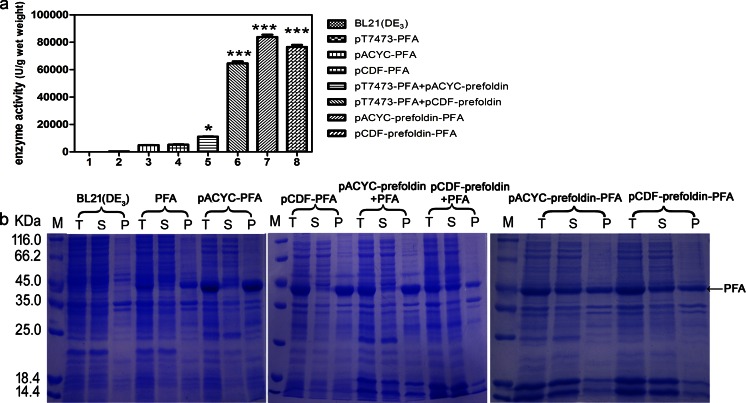
Co-expression of prefoldin and PFA in one plasmid
In order to simplify and facilitate the recombinant E. coli construction and protein expression, prefoldin and PFA-encoding genes were cloned into one plasmid, in pACYCDuet-1 or pCDFDuet-1 backbone. The constructed plasmids were designated as pACYC–prefoldin–PFA or pCDF–prefoldin–PFA and transformed them into E. coli BL21(DE3). pACYCDuet-1 or pACYCDuet-1 encoding PFA (designated as pACYC–PFA or pCDF–PFA) and the original PFA-expressing plasmid (pT7473–PFA) were used as controls. The enzyme activities in supernatants of pACYC–prefoldin–PFA and pCDF–prefoldin–PFA reached approximately 84,000 and 76,500 U/g wet weight, respectively (Fig. 3a), which have further improvements compared to the PFA + pCDF–prefoldin co-expression. By rough estimation via SDS-PAGE (comparing PFA protein band intensity in supernatant and pellet samples from the same strain, Fig. 3b lanes pACYC–prefoldin–PFA S to P or lanes pCDF–prefoldin–PFA S to P), over 50 % of recombinant PFA were expressed as soluble fraction in these E. coli BL21(DE3) strains that harbor prefoldin and PFA in a single plasmid.
Fig. 3.
Co-expression of prefoldin and PFA in one plasmid further promoted the soluble expression of PFA. a Total PFA enzyme activity in supernatants. b SDS-PAGE analysis of the recombinant PFA expression in E. coli. M represents protein molecular weight marker. T total cells, S supernatant, P pellet. The values are representative results obtained from at least three experiments. Plots are given as mean ± SEM, and statistical significance is shown (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001)
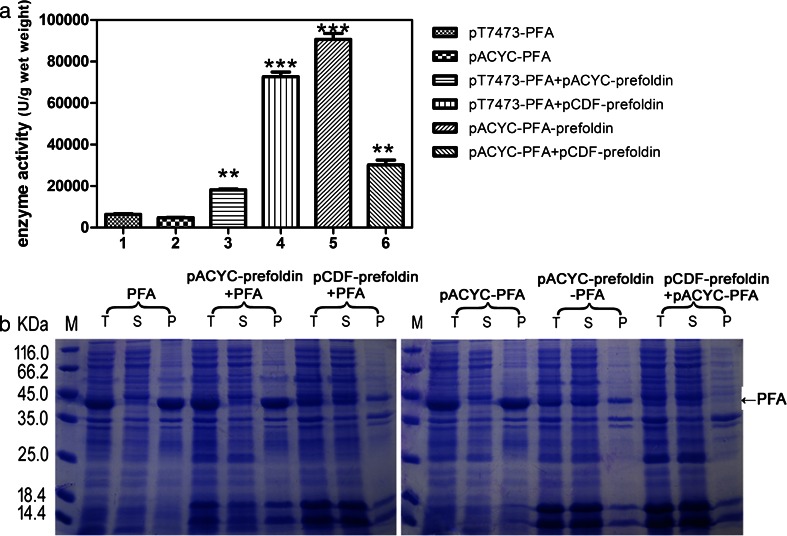
Influence of further increase of prefoldin expression levels on the expression of PFA
In order to investigate whether further increase of prefoldin expression can further help the total soluble PFA yield, we expressed PFA using a low copy number plasmid pACYC while prefoldin was expressed in a high copy number plasmid pCDF and subsequently co-transformed them into E. coli BL21(DE3) (designated as pCDF–prefoldin + pACYC–PFA). As presented in Fig. 4a, b, although most PFA were found in the soluble fraction, total PFA expression level and total amount of soluble PFA were significantly decreased. This result indicates that an appropriate expression level of profoldin was required.
Fig. 4.
Further overexpression of prefoldin eliminated insoluble expression of PFA but decreased the total PFA expression level. a Total PFA enzyme activity in supernatants. b SDS-PAGE analysis of the recombinant PFA expression in E. coli. M represents protein molecular weight marker. T total cells, S supernatant, P pellet. The values are representative results obtained from at least three experiments. Plots are given as mean ± SEM, and statistical significance is shown (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001)
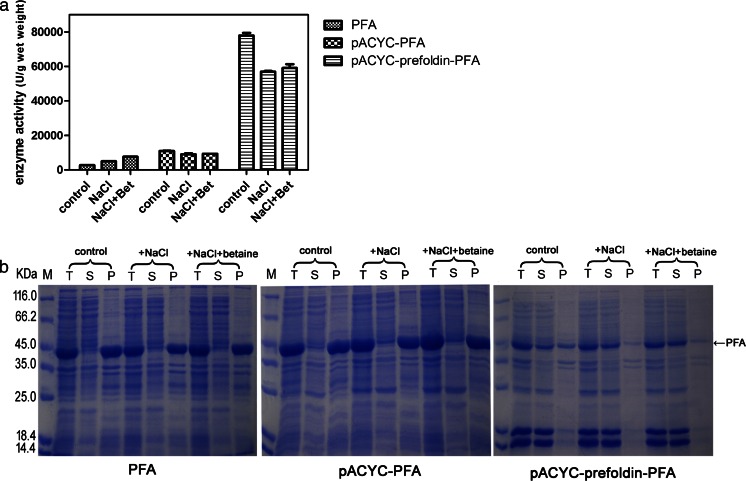
Influence of osmolyte treatment
Previous studies have reported that salt-induced accumulation of osmolytes could improve the soluble expression of recombinant proteins (de Marco et al. 2005). We have also determined the effect of osmolytes on the soluble expression of PFA. The results indicate that salt-induced accumulation of osmolytes indeed had some effect on the soluble expression of PFA (Fig. 5b, pACYC–prefoldin–PFA lanes + NaCl S to P and + NaCl + betaine S to P, compared with lanes control S to P). However, additional osmolyte treatment with the co-expression of chaperone prefoldin did not provide additional increase on the soluble expression of PFA (Fig. 5a).
Fig. 5.
Influence of osmolyte treatment on the soluble expression of PFA. a Total PFA enzyme activity in supernatants. b SDS-PAGE analysis of the recombinant PFA expression in E. coli. M represents protein molecular weight marker. T total cells, S supernatant, P pellet. The values are representative results obtained from at least three experiments. Plots are given as mean ± SEM
Discussion
The molecular chaperone systems of eukarya and bacteria are intricate, and the mechanism and functional co-operativity of the various molecular chaperones are even more complex. In contrast, the molecular chaperone machineries of hyperthermophilic archaea including P. furiosus are much more simplified. The simplified protein folding and chaperone system in hyperthermophilic archaea may contribute to a better understanding of the mechanisms of more complex chaperone systems. On the other hand, considering the original state of hyperthermophilic archaea evolution, chaperones from P. furiosus may have broader and unique functions for biotechnological applications.
In the present study, we have studied the effects of molecular chaperones from P. furiosus on the soluble expression of recombinant extracellular α-amylase PFA in E. coli. The results reveal that co-expression of prefoldin from P. furiosus alone remarkably enhanced the soluble expression of recombinant PFA in E. coli. Although co-expression of sHSP or chaperonin alone could also help to increase the soluble expression of PFA to a certain degree, the presence of chaperonin or sHSP in addition to prefoldin was not able to further improve the soluble expression of PFA.
The extracellular α-amylase from P. furiosus is a hyperthermostable enzyme with prominent enzyme properties of an industrial importance and interest. The lack of effective and adequate production techniques is one of the key impediments that hindered the biotechnological applications of PFA. In this report, we presented an efficient method for the soluble expression of recombinant PFA at a high expression level, which would be beneficial for future developments and industrial applications of this enzyme.
Acknowledgments
This work is supported by National Basic Research Program of China (973 Program) No. 2012CB721103.
Contributor Information
Yonghong Wang, Email: yhwang@ecust.edu.cn.
Yi Zhang, Email: yzhang@sibs.ac.cn.
References
- Atomi H, Sato T, Kanai T. Application of hyperthermophiles and their enzymes. Curr Opin Biotechnol. 2011;22(5):618–626. doi: 10.1016/j.copbio.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi AP, Goloubinoff P. Review: mechanisms of disaggregation and refolding of stable protein aggregates by molecular chaperones. J Struct Biol. 2001;135(2):84–93. doi: 10.1006/jsbi.2001.4352. [DOI] [PubMed] [Google Scholar]
- Bernfeld P. Amylases, α and β. Methods Enzymol. 1955;1:149–158. doi: 10.1016/0076-6879(55)01021-5. [DOI] [Google Scholar]
- Carvalho H, Meneghini R. Increased expression and purification of soluble iron-regulatory protein 1 from Escherichia coli co-expressing chaperonins GroES and GroEL. Braz J Med Bio Res. 2008;41(4):270–276. doi: 10.1590/S0100-879X2008005000009. [DOI] [PubMed] [Google Scholar]
- Chen HY, Chu ZM, Zhang Y, Yang SL. Over-expression and characterization of the recombinant small heat shock protein from Pyrococcus furiosus. Biotechnol Lett. 2006;28(14):1089–1094. doi: 10.1007/s10529-006-9058-y. [DOI] [PubMed] [Google Scholar]
- Chen HY, Chu ZM, Ma YH, Zhang Y, Yang SL. Expression and characterization of the chaperonin molecular machine from the hyperthermophilic archaeon Pyrococcus furiosus. J Basic Microbiol. 2007;47(2):132–137. doi: 10.1002/jobm.200610215. [DOI] [PubMed] [Google Scholar]
- Chen H, Yang LD, Zhang Y, Yang SL. Over-expression and characterization of recombinant prefoldin from hyperthermophilic archaeum Pyrococcus furiosus in E. coli. Biotechnol Lett. 2010;32:429–434. doi: 10.1007/s10529-009-0156-5. [DOI] [PubMed] [Google Scholar]
- de Marco A. Protocol for preparing proteins with improved solubility by co-expressing with molecular chaperones in Escherichia coli. Nat Protoc. 2007;2(10):2632–2639. doi: 10.1038/nprot.2007.400. [DOI] [PubMed] [Google Scholar]
- de Marco A, Vigh L, Diamant S, Goloubinoff P. Native folding of aggregation-prone recombinant proteins in Escherichia coli by osmolytes, plasmid- or benzyl alcohol-overexpressed molecular chaperones. Cell Stress Chaperones. 2005;10(4):329–339. doi: 10.1379/CSC-139R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Vieille C, Savchenko A, Zeikus JG. Cloning, sequencing, and expression of the gene encoding extracellular alpha-amylase from Pyrococcus furiosus and biochemical characterization of the recombinant enzyme. Appl Environ Microbiol. 1997;63(9):3569–3576. doi: 10.1128/aem.63.9.3569-3576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. Proteins as molecular chaperones. Nature. 1987;328(6129):378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- Emmanuel L, Stefan J, Bernard H, Abdel B. Thermophilic archaeal amylolytic enzymes. Enzyme Microb Technol. 2000;26:3–14. doi: 10.1016/S0141-0229(99)00142-8. [DOI] [Google Scholar]
- Grzybowska B, Szweda P, Synowiecki J. Cloning of the thermostable α-amylase gene from Pyrococcus woesei in Escherichia coli. Mol Biotechnol. 2004;26:101–109. doi: 10.1385/MB:26:2:101. [DOI] [PubMed] [Google Scholar]
- Haki GD, Rakshit SK. Developments in industrially important thermostable enzymes: a review. Bioresour Technol. 2003;89:17–34. doi: 10.1016/S0960-8524(03)00033-6. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295(5561):1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Hongo K, Itai H, Mizobata T, Kawata Y. Varied effects of Pyrococcus furiosus prefolding and P. furiosus chaperonin on the refolding reactions of substrate proteins. J Biochem. 2012;151(4):383–390. doi: 10.1093/jb/mvr141. [DOI] [PubMed] [Google Scholar]
- Iizuka R, Sugano Y, Ide N, Ohtaki A, Yoshida T, Fujiwara S, Imanaka T, Yohda M. Functional characterization of recombinant prefoldin complexes from a hyperthermophilic archaeon, Thermococcus sp. strain KS-1. J Mol Biol. 2008;377:972–983. doi: 10.1016/j.jmb.2008.01.070. [DOI] [PubMed] [Google Scholar]
- Jørgensen S, Vorgias CE, Antranikian G. Cloning, sequencing, characterization, and expression of an extracellular a-amylase from the hyperthermophilic archaeon Pyrococcus furiosus in Escherichia coli and Bacillus subtilis. J Biol Chem. 1997;268:16335–16342. doi: 10.1074/jbc.272.26.16335. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998;394(6693):595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lakanalamai P, Whitehead TA, Rbobb FT. Minimal protein-folding systems in hyperthermophilic archaea. Nat Rev Microbiol. 2004;2(4):315–324. doi: 10.1038/nrmicro866. [DOI] [PubMed] [Google Scholar]
- Laksanalamai P, Maeder DL, Robb FT. Regulation and mechanism of action of the small heat shock protein from the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol. 2001;183(17):5198–5202. doi: 10.1128/JB.183.17.5198-5202.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laksanalamai P, Pavlov AR, Slesarev AI, Robb FT. Stabilization of Taq DNA polymerase at high temperature by protein folding pathways from a hyperthermophilic archaeon, Pyrococcus furiosus. Biotechnol Bioeng. 2006;93:1–5. doi: 10.1002/bit.20781. [DOI] [PubMed] [Google Scholar]
- Leroux MR, Fandrich M, Klunker D, Siegers K, Lupas AN, Brown JR, Schiebel E, Dobson CM, Hartl FU. MtGimC, a novel archaeal chaperone related to the eukaryotic chaperonin cofactor GimC/prefoldin. EMBO J. 1999;18:6730–6743. doi: 10.1093/emboj/18.23.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund P. Insights into chaperonin function from studies on archaeal thermosomes. Biochem Soc Trans. 2011;39(1):94–98. doi: 10.1042/BST0390094. [DOI] [PubMed] [Google Scholar]
- Lundin VF, Stirling PC, Gomez-Rein J, Mwenifumbo JC, Obst JM, Valpuesta JM, Leroux MR. Molecular clamp mechanism of substrate binding by hydrophobic coiled-coil residues of the archaeal chaperone prefoldin. Proc Natl Acad Sci U S A. 2004;101:4367–4372. doi: 10.1073/pnas.0306276101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Alonso M, et al. Rehosting of bacterial chaperones for high-quality protein production. Appl Environ Microbiol. 2009;75(24):7850–7854. doi: 10.1128/AEM.01532-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara K, Kanemori M, Yanagi H, Yura T. Overexpression of trigger factor prevents aggregation of recombinant proteins in Escherichia coli. Appl Environ Microbiol. 2000;66:884–889. doi: 10.1128/AEM.66.3.884-889.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi M, Yoshida T, Maruyama T, Kawarabayasi Y, Kikuchi H, Yohda M. Pyrococcus prefoldin stabilizes protein-folding intermediates and transfers them to chaperonins for correct folding. Biochem Biophys Res Commun. 2002;291:769–774. doi: 10.1006/bbrc.2002.6523. [DOI] [PubMed] [Google Scholar]
- Okochi M, Kanie K, Kurimoto M, Yohda M, Honda H. Overexpression of prefoldin from hyperthermophilic archaeum Pyrococcus horokoshii OT3 endowed Escherichia coli with organic solvent tolerance. Appl Microbiol Biotechnol. 2008;79:443–449. doi: 10.1007/s00253-008-1450-1. [DOI] [PubMed] [Google Scholar]
- Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- Siegers K, Waldmann T, Leroux MR, Grein K, Shevchenko A, Schiebel E, Hartl FU. Compartmentation of protein folding in vivo: sequestration of non-native polypeptide by the chaperonin-GimC system. EMBO J. 1999;18:75–84. doi: 10.1093/emboj/18.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuriki N, Tawfik DS. Chaperonin overexpression promotes genetic variation and enzyme evolution. Nature. 2009;459:668–671. doi: 10.1038/nature08009. [DOI] [PubMed] [Google Scholar]
- Wang LS, Zhou Q, Chen H, Chu Z, Lu J, Zhang Y, et al. Efficient solubilization, purification of recombinant extracellular alpha-amylase from Pyrococcus furiosus expressed as inclusion bodies in Escherichia coli. J Ind Microbiol Biotechnol. 2007;4(3):187–192. doi: 10.1007/s10295-006-0185-1. [DOI] [PubMed] [Google Scholar]
- Yan X, Hu S, Guan YX, Yao SJ. Coexpression of chaperonin GroEL/GroES markedly enhanced soluble and functional expression of recombinant human interferon-gamma in Escherichia coli. Appl Microbiol Biotechnol. 2012;93(3):1065–1074. doi: 10.1007/s00253-011-3599-2. [DOI] [PubMed] [Google Scholar]
- Zhang J, Baker ML, Schroder GF, Douglas NR, et al. Mechanism of folding chamber closure in a group II chaperonin. Nature. 2010;463(7279):379–383. doi: 10.1038/nature08701. [DOI] [PMC free article] [PubMed] [Google Scholar]