Abstract
Breast cancer guidelines advise sentinel lymph node biopsy (SLNB) in patients with ductal carcinoma in situ (DCIS) on core biopsy at high risk of invasive cancer or in case of mastectomy. This study investigates the incidence of SLNB and SLN metastases and the relevance of indications in guidelines and literature to perform SLNB in order to validate whether SLNB is justified in patients with DCIS on core biopsy in current era. Clinically node negative patients diagnosed from 2004 to 2013 with only DCIS on core needle biopsy were selected from a national database. Incidence of SLN biopsy and metastases was calculated. With Fisher exact tests correlation between SLNB indications and actual presence of SLN metastases was studied. Further, underestimation rate for invasive cancer and correlation with SLN metastases was analysed. 910 patients were included. SLNB was performed in 471 patients (51.8 %): 94.5 % had pN0, 3.0 % pN1mi and 2.5 % pN1. Patients undergoing mastectomy had 7 % SLN metastases versus 3.5 % for breast conserving surgery (BCS) (p = 0.107). The only factors correlating to SLN metastases were smaller core needle size (p = 0.01) and invasive cancer (p < 0.001). Invasive cancer was detected in 16.7 % by histopathology with 15.6 % SLN metastases versus only 2 % in pure DCIS. SLNB showed metastases in 5.5 % of patients; 3.5 % in case of BCS (any histopathology) and 2 % when pure DCIS was found at definitive histopathology (BCS and mastectomy). Consequently, SLNB should no longer be performed in patients diagnosed with DCIS on core biopsy undergoing BCS. If definitive histopathology shows invasive cancer, SLNB can still be considered after initial surgery.
Keywords: DCIS, Sentinel lymph node biopsy, Incidence, Treatment
Introduction
Since 1989, population-based breast cancer screening was gradually implemented in the Netherlands, aiming at reduced breast cancer mortality by early breast cancer detection. This has also led to an increased detection rate of (asymptomatic) calcifications on mammography, in particular since the introduction of digital mammography. Calcifications can be a sign of ductal carcinoma in situ (DCIS). DCIS is considered a non-obligate precursor lesion of breast cancer [1, 2]. DCIS represents 15–25 % of all (pre-) malignant lesions detected by screening [1].
Treatment of DCIS consists of breast conserving surgery (BCS), frequently followed by radiotherapy, or mastectomy, depending on size of the area with DCIS and breast, grade and preference of the patient. Indications for a sentinel lymph node biopsy (SLNB) are based on the risk for invasive breast cancer. According to literature, invasive breast cancer is found in up to 30 % of excision specimens after diagnosis of pure DCIS on core biopsy. [3–21] The indications vary among Dutch, English and American breast cancer guidelines and include a solid mass on imaging, extensive calcifications, lesion larger than 25 mm on imaging, a palpable mass, high-grade DCIS, or age below 55 years [22–24].
For the treatment of invasive breast cancer, there is a trend towards minimizing invasive staging and treatment of the axilla in clinically node negative patients with limited sentinel lymph node (SLN) metastases [25, 26]. Several independent randomized clinical trials are even investigating whether SLNB can be safely omitted in clinically node negative invasive breast cancer patients treated with breast conserving therapy (BCT) [27, 28]. If de-escalation of invasive axillary treatment is already considered to be safe for minimal SLN metastases in case of invasive breast cancer, this might be even truer for axillary management in case of DCIS. Here we investigate the incidence of SLNB, SLN metastases and relevance of indications in guidelines and literature to perform SLNB in order to validate whether SLNB is justified in patients with DCIS on core biopsy in current era
Methods
Study design and patients
The acquisition of informed consent for this retrospective study was waived by the medical ethics committee of Maastricht University Medical Centre, Canisius-Wilhelmina Hospital, Catharina Hospital and the Netherlands Cancer Institute. All consecutive patients preoperatively diagnosed with and treated for DCIS in one centre from 2004 to 2013, and from 2008 to 2013 in the other centres, were selected from the national pathological PALGA database (‘Nationwide network and registry of histo- and cytopathology in the Netherlands’) and considered for inclusion [29]. Clinically node negative patients were included. Patients with ipsilateral invasive breast cancer were excluded. The following data were extracted from the medical records of all patients: radiology outcomes, core needle biopsy methods, surgical procedures and pathology reporting on tumour type, grade, size, and (sentinel) lymph nodes.
Radiological and surgical techniques
Core needle biopsy was generally performed stereotactic in case of calcifications and ultrasound- or MR-guided in case of a mass lesion without calcifications. To confirm the presence of calcifications, a radiograph of the biopsies was performed [30]. Surgical treatment of DCIS consisted of BCS or mastectomy, depending on size of the area with DCIS and breast, grade and preference of the patient. The SLN was identified using the triple technique, consisting of lymphoscintigraphy, blue dye, and a gamma probe. Lymph nodes that were radioactive, blue-stained, or suspicious for malignancy at palpation were removed.
Pathological techniques
Core biopsies and SLN’s were routinely processed and stained with haematoxylin and eosin. SLNs were sliced with a maximum thickness of 5 mm. Each paraffin block was step sectioned at 250–500-µm intervals at three levels and stained with haematoxylin and eosin. If no metastasis was detected with haematoxylin and eosin, immunohistochemical staining was performed with anti-cytokeratin antibody MNF116 or AE1CK18. Each lymph node was categorized as benign (pN0), isolated tumour cells (pN0(i+)) (<0.2 mm), micrometastasis (pN1mi) (0.2–2.0 mm) or macrometastasis (pN1-3) (>2.0 mm) [23].
The surgical breast specimen was inked according to a generally agreed colour coding system, sliced with a maximal thickness of 5 mm and routinely processed. DCIS and, if present, invasive breast cancer was classified into grade I, II or III according to the modified Bloom-Richardson grading system.
Statistical analyses
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS), version 22.0 (IBM Corporation, Armonk, NY, USA). The incidence of the performance of SLNB and if performed SLN metastases in DCIS patients was calculated. Furthermore, a correlation between the indications in the guidelines and literature (mastectomy, solid mass on imaging, extensive microcalcifications, lesion >25 mm on imaging, palpable mass, high-grade DCIS, age <55, core needle biopsy method, core needle biopsy size) for performing an SLNB with the actual presence of SLN metastases was analysed. Also, the incidence of invasive breast cancer in definitive histopathology and the correlation between an invasive component and SLN metastases was evaluated. All correlations were analysed with the Fisher exact test. Subsequent logistic regression analysis was not performed due to the small amount of significant correlations. To examine the consequences of omission of SLNB on systemic therapy indication, the number of patients receiving systemic therapy was studied as was the number of times SLN metastases were the sole indicator to start systemic therapy. A p value of <0.05 was considered statistically significant. Descriptive categorical data are presented as proportions and absolute numbers. Continuous variables are presented as means with standard deviations (SD).
Results
A total of 1251 patients were considered for inclusion. Subsequently, 341 patients were excluded for the following reasons: 171 had suspicion for an ipsilateral invasive carcinoma, 167 were incorrectly coded and 3 objected to use their medical record for research purposes. Finally, 910 patients were included. Table 1 summarizes all patient and diagnostic characteristics.
Table 1.
Patient demographics and tumour characteristics of all 910 patients and divided per centre
| MUMC N = 154 | CWZ N = 171 | NKI N = 428 | CZE N = 157 | Total N = 910 | |
|---|---|---|---|---|---|
| Median age in years (range) | 60 (33–84) | 58 (32–81) | 55 (27–90) | 58 (30–80) | 57 (27–90) |
| DCIS biopsy (%) | |||||
| Grade 1 | 31 (20.1) | 26 (15.2) | 110 (25.7) | 33 (21.0) | 200 (22.0) |
| Grade 2 | 67 (43.5) | 45 (26.3) | 189 (44.2) | 47 (29.9) | 348 (38.2) |
| Grade 3 | 56 (36.4) | 100 (58.5) | 129 (30.1) | 77 (49.1) | 362 (39.8) |
| Palpable mass (%) | 27 (17.5) | 12 (7.0) | 40 (9.3) | 1 (0.6) | 80 (8.8) |
| Mammography (%) | |||||
| No abnormalities | 7 (4.5) | 5 (2.9) | 9 (2.1) | 1 (0.6) | 22 (2.4) |
| Calcifications | 117 (76.0) | 160 (93.6) | 360 (84.1) | 146 (93.0) | 783 (86.0) |
| Mass | 13 (8.5) | – | 10 (2.3) | 3 (1.9) | 26 (2.9) |
| Calcification and mass | 17 (11.0) | 6 (3.5) | 49 (11.5) | 7 (4.5) | 79 (8.7) |
| Ultrasound (%) | |||||
| In total performed | 154 (100) | 154 (90.1) | 295 (68.9) | 157 (100) | 761 (83.6) |
| No abnormalities | 101 (65.6) | 118 (76.6) | 128 (43.4) | 134 (85.4) | 481 (63.3) |
| Benign lesion | 1 (0.6) | 3 (2.0) | 4 (1.4) | 1 (0.6) | 9 (1.2) |
| Suspect lesion | 32 (20.8) | 16 (10.4) | 124 (42.0) | 19 (12.1) | 191 (25.1) |
| Other | 20 (13.0) | 17 (11.0) | 39 (13.2) | 3 (1.9) | 79 (10.4) |
| MRI (%) | |||||
| In total performed | 105 (68.2) | 10 (5.8) | 285 (66.6) | 9 (5.7) | 409 (44.9) |
| No abnormalities | 17 (16.2) | – | 103 (36.1) | 3 (33.3) | 123 (30.0) |
| Non mass like enhanced | 64 (61.0) | 7 (70.0) | 157 (55.1) | 5 (55.6) | 233 (57.0) |
| Massa enhanced | 19 (18.0) | 1 (10.0) | 21 (7.4) | 1 (11.1) | 42 (10.3) |
| Asymmetry | 5 (4.8) | 2 (20.0) | 4 (1.4) | – | 11 (2.7) |
| Operation (%) | |||||
| Lumpectomy | 66 (42.9) | 100 (58.5) | 223 (52.1) | 126 (80.3) | 515 (56.6) |
| Mastectomy | 88 (57.1) | 71 (41.5) | 205 (47.9) | 31 (19.7) | 395 (43.4) |
MUMC Maastricht University Medical Centre, Maastricht, CWZ Canisius-Wilhelmina Hospital, Nijmegen, NKI Netherlands Cancer Institute, Amsterdam, CZE Catharina Hospital, Eindhoven, DCIS Ductal carcinoma in situ, MRI Magnetic Resonance Imaging
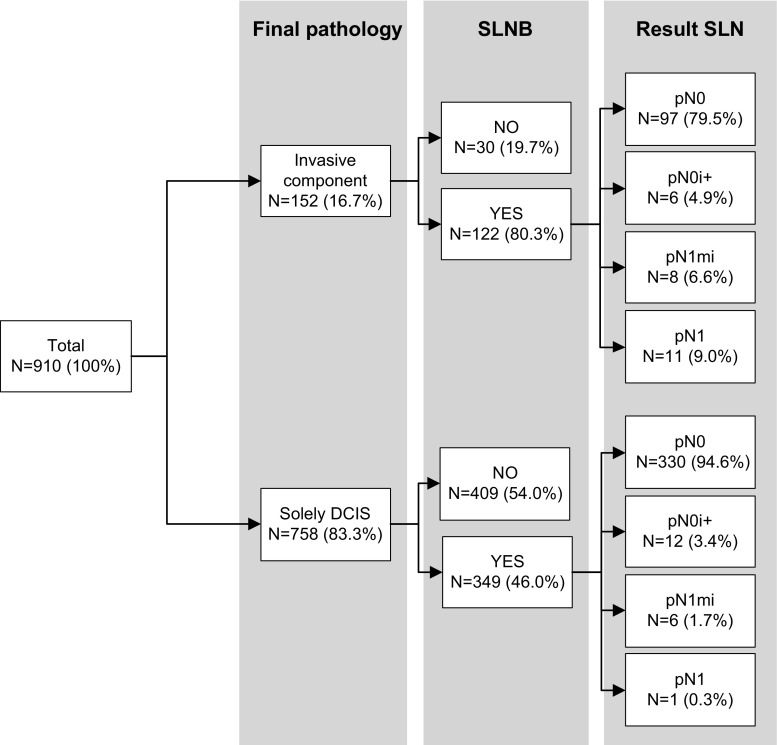
An SLNB was performed in 471 patients (51.8 %) (Fig. 1). The SLN showed no metastases in 427 patients (90.7 %), isolated tumour cells in 18 (3.8 %), micrometastases in 14 (3.0 %), and macrometastases in 12 (2.5 %) (Table 2). Of the 26 patients with SLN (micro)metastases (Table 3), 14 patients (53.8 %) underwent completion axillary lymph node dissection (cALND). In 9 out of 14 patients (64.3 %), no additional lymph node metastases were found, in one an additional micrometastasis and in four macrometastases. Final histopathology, of all axillary surgery, showed pN0 in 427 (90.7 %), pN0i+ in 18 (3.8 %), pN1mi in 12 (2.5 %), and ≥pN1 in 14 patients (3.0 %).
Fig. 1.
Results of the sentinel lymph node biopsy. DCIS Ductal carcinoma in situ. SLNB sentinel lymph node biopsy. pN0 No metastases. pN0i + Isolated tumour cells. pN1mi micrometastasis. pN1 1–3 macrometastases
Table 2.
Pathology results of SLNB and excision specimen of the 910 patients
| MUMC N = 154 | CWZ N = 171 | NKI N = 428 | CZE N = 157 | Total N = 910 | |
|---|---|---|---|---|---|
| SLNB | |||||
| In total performed (%) | 105 (68.2) | 76 (44.4) | 177 (41.4) | 113 (72.0) | 471 (51.8) |
| No metastases | 92 (87.6) | 70 (92.2) | 159 (89.8) | 106 (93.8) | 427 (90.7) |
| Isolated tumour cells | 11 (10.5) | 3 (3.9) | 3 (1.7) | 1 (0.9) | 18 (3.8) |
| Micrometastases | 2 (1.9) | 3 (3.9) | 8 (4.5) | 1 (0.9) | 14 (3.0) |
| Macrometastases | – | _ | 7 (4.0) | 5 (4.4) | 12 (2.5) |
| Final pathology | |||||
| DCIS excision (%) | |||||
| No residual | 6 (3.9) | 12 (7.0) | 37 (8.6) | 13 (8.3) | 68 (7.5) |
| DCIS grade 1 | 17 (11.0) | 15 (8.8) | 94 (22.0) | 24 (15.3) | 150 (16.5) |
| DCIS grade 2 | 55 (35.7) | 44 (25.7) | 164 (38.3) | 34 (21.6) | 297 (32.6) |
| DCIS grade 3 | 76 (49.4) | 100 (58.5) | 133 (31.1) | 86 (54.8) | 395 (43.4) |
| Invasive cancer (%) | 27 (17.5) | 24 (14.0) | 75 (17.5) | 26 (16.6) | 152 (16.7) |
| IDC grade 1 | 9 (33.3) | 7 (29.2) | 26 (34.7) | 7 (26.9) | 49 (32.2) |
| IDC grade 2 | 10 (37.1) | 11 (45.8) | 27 (36.0) | 12 (46.2) | 59 (38.8) |
| IDC grade 3 | 4 (14.8) | 4 (16.7) | 12 (16.0) | 2 (7.7) | 22 (14.5) |
| Other | 4 (14.8) | 2 (8.3) | 10 (13.3) | 5 (19.2) | 22 (14.5) |
MUMC Maastricht University Medical Centre, Maastricht, CWZ Canisius-Wilhelmina Hospital, Nijmegen, NKI Netherlands Cancer Institute, Amsterdam, CZE Catharina Hospital, Eindhoven, SLNB Sentinel lymph node biopsy, DCIS Ductal carcinoma in situ, IDC Invasive ductal carcinoma
Table 3.
Radiological and pathological findings for patients with a positive sentinel lymph node
| Patient | Age (years) | Palpable mass | Mammographic | DCIS grade biopsy | pN-status SLNB | Operation | Final pathology | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass | Size (mm) | DCIS grade | DCIS (mm) | Invasion | Invasion (mm) | pN-status (SNLB + cALND*) | ||||||
| 1 | 53 | Yes | No | 10 | 2 | pN1mi | Mast. | 2 | ǂ | Yes | 6 | pN1mi |
| 2 | 51 | No | No | 10 | 2 | pN1 | Mast. | 3 | ǂ | Yes | 9 | pN1 |
| 3 | 56 | No | No | 76 | 1 | pN1 | Mast. | 1 | 70 | Yes | 6 | pN1 |
| 4 | 64 | No | Yes | 40 | 3 | pN1 | Mast. | 3 | 40 | No | pN2 | |
| 5 | 60 | No | Yes | 10 | 2 | pN1 | Lump. | 1 | ǂ | Yes | 10 | pN1 |
| 6 | 46 | Yes | Yes | 50 | 2 | pN1 | Mast. | 3 | ǂ | Yes | 15 | pN1 |
| 7 | 34 | Yes | No | 70 | 3 | pN1mi | Mast. | 3 | 90 | Yes | 5 | pN1mi |
| 8 | 43 | Yes | No | 52 | 3 | pN1 | Mast. | 3 | 35 | Yes | 13 | pN1 |
| 9 | 56 | No | No | 41 | 3 | pN1mi | Lump. | 3 | 40 | Yes | 9 | pN1mi |
| 10 | 53 | No | No | 25 | 2 | pN1mi | Lump. | 2 | 25 | No | pN1mi | |
| 11 | 49 | No | No | 56 | 2 | pN1mi | Mast. | 2 | 50 | Yes | 11 | pN1 |
| 12 | 35 | No | No | 120 | 3 | pN1mi | Mast. | 2 | 100 | No | pN1mi | |
| 13 | 39 | No | No | 80 | 2 | pN1 | Mast. | 2 | 90 | Yes | 7 | pN1 |
| 14 | 51 | No | No | 20 | 2 | pN1mi | Mast. | 3 | 6 | Yes | 26 | pN1mi |
| 15 | 69 | No | Yes | 15 | 1 | pN1mi | Lump. | 1 | 30 | Yes | 9 | pN1mi |
| 16 | 51 | No | No | ǂ | 2 | pN1mi | Mast. | 3 | 60 | No | pN1mi | |
| 17 | 53 | No | No | ǂ | 3 | pN1mi | Mast. | 3 | ǂ | No | pN1mi | |
| 18 | 57 | No | No | ǂ | 3 | pN1mi | Mast. | 3 | ǂ | No | pN1mi | |
| 19 | 58 | Yes | Yes | 20 | 2 | pN1mi | Lump. | 2 | 39 | Yes | 4 | pN1mi |
| 20 | 54 | No | No | 80 | 2 | pN1mi | Mast. | 3 | 105 | Yes | 90 | pN3a |
| 21 | 60 | No | No | 21 | 3 | pN1 | Mast. | 3 | ǂ | Yes | 4 | pN1 |
| 22 | 68 | No | No | 60 | 3 | pN1 | Mast. | 3 | 8 | Yes | 7 | pN1 |
| 23 | 59 | No | No | ǂ | 3 | pN1 | Lump. | 3 | 20 | Yes | 14 | pN1 |
| 24 | 59 | No | No | 20 | 2 | pN1 | Lump. | 3 | 20 | Yes | 4 | pN1 |
| 25 | 71 | No | No | 31 | 2 | pN1 | Mast. | 3 | 100 | Yes | 25 | pN1 |
| 26 | 67 | No | No | 12 | 3 | pN1mi | Mast. | 3 | 55 | No | – | pN1mi |
Yrs years, US ultrasound, DCIS Ductal carcinoma in situ, SLNB sentinel lymph node biopsy, Mast. Mastectomy, Lump. Lumpectomy, cALND completion axillary lymph node dissection
* If performed
All guidelines include a mastectomy as an indication to perform an SLNB. A mastectomy was performed in 395 patients (43.4 %) and BCS in 515 (56.6 %). Of the patients treated with mastectomy, 68.6 % underwent an SLNB. SLN metastases were found in 7 % of patients; of which 3.7 % micrometastases and 3.3 % macrometastases. After cALND, final results were 3.0 % pN1mi and 4.0 % ≥pN1. Of the patients treated with BCS, 38.8 % underwent SLNB. In 3.5 % a metastasis was detected in the SLN, of which 2 % micrometastases and 1.5 % macrometastases. Final histopathology results did not change pN-status following cALND in BCS patients. The difference in SLN results between patients undergoing mastectomy and patients undergoing BCS was not statistically significant (p = 0.107) (Table 3).
Other indications to perform an SLNB known before surgery like a palpable tumour, mass on mammography, tumour larger than 25 mm, high-grade DCIS, age below 55 and core biopsy method (stereotactic versus sonographically) were not significantly correlated to SLN metastases (Tables 4). The only variable correlated to SLN metastases was smaller core needle size (p = 0.010). Core needle size was known in 336 patients. Upstaging to invasive breast cancer occurred in 28 out of 207 (13.5 %) patients diagnosed with a large 9–11G needle and 26 out of 129 (20.2 %) diagnosed with a smaller 14–19G needle. This difference was not statistically significant (p = 0.074). The indication ‘extensive microcalcifications on preoperative imaging’ was not studied, since the extent of calcifications was unknown for the patients in our cohort (only yes/no).
Table 4.
Independent predictors of SLN metastases of the 471 patients undergoing an SLNB
| Odds ratio | 95 % Confidence interval | p value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age (≤55 vs. >55 years) | 0.846 | 0.384 | 1.867 | 0.691 |
| Palpable tumour | 1.881 | 0.679 | 5.210 | 0.211 |
| Size tumour (≤25 vs. >25 mm) | 1.193 | 0.495 | 2.878 | 0.435 |
| Mass on mammography | 1.558 | 0.566 | 4.290 | 0.377 |
| High-grade DCIS | 0.686 | 0.308 | 1.526 | 0.421 |
| Biopsy method (stereotactic vs. sonographic) | 1.909 | 0.682 | 5.343 | 0.207 |
| Size core needle (14–18 vs. 9–11 gauge) | 7.244 | 1.444 | 36.353 | 0.010 |
| Surgery (lumpectomy vs. mastectomy) | 2.079 | 0.857 | 5.045 | 0.107 |
| Invasive ductal carcinoma | 9.012 | 3.686 | 22.038 | <0.001 |
SLN sentinel lymph node, SLNB sentinel lymph node biopsy, DCIS ductal carcinoma in situ
In 152 patients (16.7 %), the initial DCIS on core biopsy was upstaged to invasive cancer in definitive histopathology (Table 2). An SLNB was performed in 80.3 % of these patients and showed metastases in 15.6 % of which 4.9 % pN1mi and 10.7 % pN1 (Table 5). In 349 out of 758 patients (46.0 %) with pure DCIS in definitive histopathology an SLNB was performed and showed metastases in 2 % of which 1.7 % pN1mi and 0.3 % pN1 (Table 5). Invasive breast cancer was positively correlated to SLN metastases (p < 0.001) (Table 4). Numbers are too low to perform further analysis on these findings.
Table 5.
Axillary status of patients that underwent axillary surgery (SLNB ± cALND)
| No metastases | Micrometastases | Macrometastases | Total | |
|---|---|---|---|---|
| DCIS (%) | 342 (97.9) | 6 (1.7) | 1 (0.3) | 349 |
| Invasive carcinoma (%) | 103 (84.4) | 6 (4.9) | 13 (10.7) | 122 |
| Total (%) | 445 (94.5) | 12 (2.5) | 14 (3.0) | 471 |
SLNB sentinel lymph node biopsy, cALND completion axillary lymph node dissection, DCIS Ductal carcinoma in situ
Systemic therapy was indicated for 105 patients (11.5 %), according to current Dutch guidelines, because of tumour characteristics. In 17 of these patients (1.9 %) this indication was solely based on the presence of SLN metastases.
Discussion
The aim of this study was to investigate the incidence of SLN metastases in patients with pure DCIS on core biopsy and explore whether this justifies current recommended indications for performing SLNB as published in current guidelines and literature. In our cohort, SLNB was performed in 51.8 % of patients and showed 5.5 % SLN metastases, of which 3.0 % pN1mi and 2.5 % pN1. Final histopathology after breast surgery, SLNB and cALNDs, showed 2.5 % pN1mi and 3.0 % pN1, and invasive breast cancer in 16.7 %.
Our results are consistent with the recent studies of Francis and Prendeville [17, 19]. Francis showed 2.9 % pN1mi and 2.4 % pN1 and 21 % invasive breast cancer [17], Prendeville found 0.5 % pN1, no micrometastases and 30 % invasive breast cancer [19]. The differences with this study are that Francis only included patients that underwent SLNB and that Prendeville had a study population of only 296 patients.
The English, Dutch, and American breast cancer guidelines all advice an SLNB in patients undergoing a mastectomy, since it cannot be performed afterwards [22–24] Further, mastectomy is often performed in case of a large diameter of a lesion or calcifications on mammography, which is suggested to correlate with an elevated risk of invasive carcinoma [1]. In our cohort, an SLNB was performed in only 68.6 % of patients undergoing a mastectomy, and in 38.8 % in case of BCS. A positive SLN was detected in 7.0 versus 3.5 %, respectively, though there was no significant correlation between type of breast surgery and the presence of SLN metastases (p = 0.107), nor between lesion size >25 mm and SLN metastases (p = 0.435).
Other indications to perform an SLNB according to the guidelines and literature where: a solid mass on imaging, extensive calcifications, lesion larger than 25 mm on imaging, smaller core needle size, a palpable mass, high-grade DCIS, or age below 55 years [18, 20, 22, 23]. These indications are based on the risk for invasive breast cancer. In our study, only a smaller core needle size (p = 0.010) and invasive carcinoma as final diagnosis (p < 0.001) were positively correlated to SLN metastases. The correlation between smaller core needle size and SLN metastases might be attributed to a higher rate of upstaging to invasive breast cancer in patients were a needle with a smaller core was used. When a large core needle (9–11G) was used, only 13.5 % was upstaged, compared to 20.2 % for small core needle. Though this difference was not statistically significant (p = 0.074), these numbers show us that the upstaging rate is lower with a modern, large core needle.
The correlation between invasive carcinoma at final histopathology and SLN metastases was to be expected. Also, in 2 % of the patients with pure DCIS at final histopathology, SLN metastases were found. To our opinion, these cases probably had occult invasion.
In invasive breast cancer, there is a trend to minimize invasive management of the axilla. Patients with invasive breast cancer in the ACOSOG Z0011 (pN1) and IBCSG 23-01 (pN1mi) trials, randomized for watchful waiting after a positive SLN, were likely to have residual lymph nodal disease in 11–27 %. [25, 26] Even though these lymph nodes were not surgically removed, overall survival was not affected and regional recurrence rate low. This implies that the remaining 5.5 % SLN metastases in our cohort will not affect overall survival and regional recurrence rate. The main difference with ACOSOG Z0011 is that those patients were mostly treated with adjuvant systemic therapy (97 %) and always underwent BCS with radiotherapy. Other arguments for safely omitting SLNB in patients with DCIS on core biopsy could be selected from the NSABP B-04 trial. [31] Only half of remaining nodal metastases became clinically relevant. Further, a delayed ALND, in case lymph nodes became clinically positive during follow-up, did not affect overall survival and disease-free survival, in patients with invasive breast cancer whom were not treated with adjuvant systemic or radiation therapy [31]. Multiple trials are now investigating the safety of omitting SLNB in clinically node negative invasive breast cancer patients treated with BCT [27, 28]. DCIS so far escaped from this trend to minimize invasive staging and treatment of the axilla.
Biology, loco-regional and systemic therapy all have a role in survival and (regional) recurrence. In our cohort, radiotherapy was administrated in case of BCS and rarely in case of mastectomy. Systemic therapy was indicated in 11.5 % of patients based on the presence of primary invasive breast cancer characteristics and/or nodal metastases. If no SLNB would have been performed at all in this population, the indication for systemic therapy would have been missed in 1.9 % of patients.
Our study is limited by its retrospective design. In 48.2 % an SLNB was not performed. Unfortunately, the exact reason for (not) performing an SLNB could not be retrieved. Further, the low event rate of SLN metastases limits the opportunity to perform comprehensive (prognostic) analyses. This low event rate also implicates that SLNB is of limited value in patients with DCIS in current era [32]. The study of Broekhuizen et al. already demonstrated that the survival of DCIS patients is not affected by SLN micrometastases [33].
In conclusion, the SLNB contained a metastasis in 5.5 %, of which 2.5 % macrometastases. In patients undergoing a mastectomy, 7.0 % had SLN metastases. For BCS this was 3.5 %. None of the guidelines indications to perform an SLNB was correlated to SLN metastases. The presence of an invasive component in the excision specimen however is significantly correlated to SLN metastases, with a chance of 15.6 % of SLN metastases in case of invasive cancer versus only 2 % in case of pure DCIS at final histopathology.
To our opinion, SLNB should no longer be performed in patients diagnosed with DCIS on core biopsy in case they are treated with BCS. SLNB can still be performed afterwards, if final histopathology reveals invasive breast cancer. This way, DCIS patients are not needlessly put at risk for complications and unnecessary medical costs.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
L. M. van Roozendaal and B. Goorts have contributed equally to this work.
References
- 1.van Deurzen CH, Hobbelink MG, van Hillegersberg R, van Diest PJ. Is there an indication for sentinel node biopsy in patients with ductal carcinoma in situ of the breast? A review. Eur J Cancer. 2007;43(6):993–1001. doi: 10.1016/j.ejca.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Bundred N, Dixon JM. Carcinoma in situ. BMJ. 2013;347:f3289. doi: 10.1136/bmj.f3289. [DOI] [PubMed] [Google Scholar]
- 3.Silverstein MJ, Rosser RJ, Gierson ED, Waisman JR, Gamagami P, Hoffman RS, Fingerhut AG, Lewinsky BS, Colburn W, Handel N. Axillary lymph node dissection for intraductal breast carcinoma–is it indicated? Cancer. 1987;59(10):1819–1824. doi: 10.1002/1097-0142(19870515)59:10<1819::AID-CNCR2820591023>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 4.Intra M, Veronesi P, Mazzarol G, Galimberti V, Luini A, Sacchini V, Trifiro G, Gentilini O, Pruneri G, Naninato P, Torres F, Paganelli G, Viale G, Veronesi U. Axillary sentinel lymph node biopsy in patients with pure ductal carcinoma in situ of the breast. Arch Surg. 2003;138(3):309–313. doi: 10.1001/archsurg.138.3.309. [DOI] [PubMed] [Google Scholar]
- 5.Klauber-DeMore N, Tan LK, Liberman L, Kaptain S, Fey J, Borgen P, Heerdt A, Montgomery L, Paglia M, Petrek JA, Cody HS, Van Zee KJ. Sentinel lymph node biopsy: is it indicated in patients with high-risk ductal carcinoma-in situ and ductal carcinoma-in situ with microinvasion? Ann Surg Oncol. 2000;7(9):636–642. doi: 10.1007/s10434-000-0636-2. [DOI] [PubMed] [Google Scholar]
- 6.Cox CE, Nguyen K, Gray RJ, Salud C, Ku NN, Dupont E, Hutson L, Peltz E, Whitehead G, Reintgen D, Cantor A (2001) Importance of lymphatic mapping in ductal carcinoma in situ (DCIS): why map DCIS? Am Surg 67(6):513–519; discussion 519–521 [PubMed]
- 7.Kelly TA, Kim JA, Patrick R, Grundfest S, Crowe JP. Axillary lymph node metastases in patients with a final diagnosis of ductal carcinoma in situ. Am J Surg. 2003;186(4):368–370. doi: 10.1016/S0002-9610(03)00276-9. [DOI] [PubMed] [Google Scholar]
- 8.Lara JF, Young SM, Velilla RE, Santoro EJ, Templeton SF. The relevance of occult axillary micrometastasis in ductal carcinoma in situ: a clinicopathologic study with long-term follow-up. Cancer. 2003;98(10):2105–2113. doi: 10.1002/cncr.11761. [DOI] [PubMed] [Google Scholar]
- 9.Pendas S, Dauway E, Giuliano R, Ku N, Cox CE, Reintgen DS. Sentinel node biopsy in ductal carcinoma in situ patients. Ann Surg Oncol. 2000;7(1):15–20. doi: 10.1007/s10434-000-0015-z. [DOI] [PubMed] [Google Scholar]
- 10.Veronesi P, Intra M, Vento AR, Naninato P, Caldarella P, Paganelli G, Viale G. Sentinel lymph node biopsy for localised ductal carcinoma in situ? Breast. 2005;14(6):520–522. doi: 10.1016/j.breast.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Zavotsky J, Hansen N, Brennan MB, Turner RR, Giuliano AE. Lymph node metastasis from ductal carcinoma in situ with microinvasion. Cancer. 1999;85(11):2439–2443. doi: 10.1002/(SICI)1097-0142(19990601)85:11<2439::AID-CNCR19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 12.Camp R, Feezor R, Kasraeian A, Cendan J, Schell S, Wilkinson E, Copeland E, Lind S. Sentinel lymph node biopsy for ductal carcinoma in situ: an evolving approach at the University of Florida. Breast J. 2005;11(6):394–397. doi: 10.1111/j.1075-122X.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 13.Cserni G. Sentinel lymph node biopsy as a tool for the staging of ductal carcinoma in situ in patients with breast carcinoma. Surg Today. 2002;32(2):99–103. doi: 10.1007/s005950200000. [DOI] [PubMed] [Google Scholar]
- 14.Farkas EA, Stolier AJ, Teng SC, Bolton JS, Fuhrman GM (2004) An argument against routine sentinel node mapping for DCIS. Am Surg 70(1):13–17; discussion 17-18 [PubMed]
- 15.Rahusen FD, Meijer S, Taets van Amerongen AH, Pijpers R, van Diest PJ. Sentinel node biopsy for nonpalpable breast tumors requires a preoperative diagnosis of invasive breast cancer. Breast J. 2003;9(5):380–384. doi: 10.1046/j.1524-4741.2003.09503.x. [DOI] [PubMed] [Google Scholar]
- 16.Mittendorf EA, Arciero CA, Gutchell V, Hooke J, Shriver CD. Core biopsy diagnosis of ductal carcinoma in situ: an indication for sentinel lymph node biopsy. Current surgery. 2005;62(2):253–257. doi: 10.1016/j.cursur.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Francis AM, Haugen CE, Grimes LM, Crow JR, Yi M, Mittendorf EA, Bedrosian I, Caudle AS, Babiera GV, Krishnamurthy S, Kuerer HM, Hunt KK. Is sentinel lymph node dissection warranted for patients with a diagnosis of ductal carcinoma in situ? Ann Surg Oncol. 2015 doi: 10.1245/s10434-015-4547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brennan ME, Turner RM, Ciatto S, Marinovich ML, French JR, Macaskill P, Houssami N. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011;260(1):119–128. doi: 10.1148/radiol.11102368. [DOI] [PubMed] [Google Scholar]
- 19.Prendeville S, Ryan C, Feeley L, O’Connell F, Browne TJ, O’Sullivan MJ, Bennett MW. Sentinel lymph node biopsy is not warranted following a core needle biopsy diagnosis of ductal carcinoma in situ (DCIS) of the breast. Breast. 2015;24(3):197–200. doi: 10.1016/j.breast.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Fahrbach K, Sledge I, Cella C, Linz H, Ross SD. A comparison of the accuracy of two minimally invasive breast biopsy methods: a systematic literature review and meta-analysis. Arch Gynecol Obstet. 2006;274(2):63–73. doi: 10.1007/s00404-005-0106-y. [DOI] [PubMed] [Google Scholar]
- 21.Meijnen P, Oldenburg HS, Loo CE, Nieweg OE, Peterse JL, Rutgers EJ. Risk of invasion and axillary lymph node metastasis in ductal carcinoma in situ diagnosed by core-needle biopsy. Br J Surg. 2007;94(8):952–956. doi: 10.1002/bjs.5735. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman SA, Harris EE, Bailey L, Chadha M, Dutton SC, Freedman GM, Goyal S, Halyard MY, Horst KC, Novick KL, Park CC, Suh WW, Toppmeyer D, Zook J, Haffty BG (2015) ACR appropriateness criteria(R) ductal carcinoma in situ. Oncology 29(6):446–458, 441–460 [PubMed]
- 23.NABON (2012) Richtlijn Mammacarcinoom (Dutch breast cancer guideline)
- 24.Harnett A, Smallwood J, Titshall V, Champion A, Guideline Development G. Diagnosis and treatment of early breast cancer, including locally advanced disease–summary of NICE guidance. BMJ. 2009;338:b438. doi: 10.1136/bmj.b438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Hunt KK, Morrow M, Ballman K (2010) Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 252(3):426–432, discussion 432–423. doi:10.1097/SLA.0b013e3181f08f32 [DOI] [PMC free article] [PubMed]
- 26.Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, Gentilini O, Mastropasqua MG, Mazzarol G, Massarut S, Garbay JR, Zgajnar J, Galatius H, Recalcati A, Littlejohn D, Bamert M, Colleoni M, Price KN, Regan MM, Goldhirsch A, Coates AS, Gelber RD, Veronesi U, International Breast Cancer Study Group Trial i (2013) Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol 14 (4):297–305. doi:10.1016/S1470-2045(13)70035-4 [DOI] [PMC free article] [PubMed]
- 27.Gentilini O, Veronesi U. Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: sentinel node vs Observation after axillary UltraSouND) Breast. 2012;21(5):678–681. doi: 10.1016/j.breast.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Roozendaal L (2014) Omitting sentinel node procedure in breast cancer patients undergoing breast conserving therapy
- 29.Casparie ea (2007) PALGA: Dutch pathology registry
- 30.Ellis IO, Humphreys S, Michell M, Pinder SE, Wells CA, Zakhour HD, Pathology UKNCCfBS, European Commission Working Group on Breast Screening P (2004) Best practice no 179. Guidelines for breast needle core biopsy handling and reporting in breast screening assessment. J Clin Pathol 57(9):897–902. doi:10.1136/jcp.2003.010983 [DOI] [PMC free article] [PubMed]
- 31.Fisher B, Montague E, Redmond C, Deutsch M, Brown GR, Zauber A, Hanson WF, Wong A. Findings from NSABP protocol no. B-04-comparison of radical mastectomy with alternative treatments for primary breast cancer. I. Radiation compliance and its relation to treatment outcome. Cancer. 1980;46(1):1–13. doi: 10.1002/1097-0142(19800701)46:1<1::AID-CNCR2820460102>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Elshof LE, Tryfonidis K, Slaets L, van Leeuwen-Stok AE, Skinner VP, Dif N, Pijnappel RM, Bijker N, Rutgers EJ, Wesseling J. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ—the LORD study. Eur J Cancer. 2015;51(12):1497–1510. doi: 10.1016/j.ejca.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Broekhuizen LN, Wijsman JH, Peterse JL, Rutgers EJ. The incidence and significance of micrometastases in lymph nodes of patients with ductal carcinoma in situ and T1a carcinoma of the breast. Eur J Surg Oncol. 2006;32(5):502–506. doi: 10.1016/j.ejso.2006.02.006. [DOI] [PubMed] [Google Scholar]