Abstract
Treatment with (neo)adjuvant chemotherapy for breast cancer, as currently given, causes cell damage by induction of double-strand DNA breaks. Because BRCA1 and BRCA2 proteins play a role in the repair of DNA damage, the efficacy of (neo)adjuvant chemotherapy may be increased in BRCA1/2-associated breast cancer patients. As a downside, acute chemotherapy-related toxicity may also be increased. We selected all female patients who were treated at the Erasmus MC Cancer Institute, with (neo)adjuvant chemotherapy for primary or locoregional recurrence of breast cancer (PBC/LR) between January 1, 2004 and December 31, 2014. The primary outcome was the relative total dose intensity (RTDI), calculated for anthracyclines and taxanes separately. Secondary outcomes were the occurrence of febrile neutropenia, delay in chemotherapy administration, and switch to another chemotherapy regimen due to toxicity. In total, 701 patients treated for PBC/LR were eligible for data analyses, among which 85 BRCA1/2 mutation carriers (n = 67 BRCA1 and n = 18 BRCA2). The mean RTDI for anthracyclines was not significantly different between both groups (98.7 % in the BRCA1/2, 96.6 % in the sporadic group, p = 0.27). Also the mean RTDI for taxanes was not significantly different between the groups (93.6 % in the BRCA1/2-associated, 90.0 % in the sporadic group, p = 0.12). Linear regression analysis revealed no significant effect of BRCA1/2 mutation carriership on the RTDIs. No significant differences were found in the percentages of patients presenting with febrile neutropenia, having a delay in chemotherapy administration or switching to an altered chemotherapy regimen. Additionally, the odds ratios showed no significant effect of BRCA1/2 mutation carriership on the secondary outcome variables. (Neo)adjuvant chemotherapy-related toxicity was not different between BRCA1/2-associated and sporadic breast cancer patients suggesting that the DNA damage repair mechanism of non-cancer cells with only one normal copy of either the BRCA1 or BRCA2 gene is sufficiently functional to handle acute chemotherapy-associated toxicity.
Keywords: Chemotherapy, BRCA mutation, Toxicity, Breast cancer, Dose intensity
Introduction
Carriers of a germline BRCA1 or BRCA2 (BRCA1/2) mutation face an increased lifetime risk of developing breast cancer, estimated to range from 47 to 66 % for BRCA1 mutation carriers and from 40 to 57 % for BRCA2 mutation carriers [1, 2].
Carriers of a germline BRCA1/2 mutation, by definition, have one allele with a mutation in the BRCA1/2 gene, while the gene on the other allele is intact. In normal cells, it seems that enough BRCA1 or BRCA2 protein is present for adequate functioning of cells in the various tissues of these women. However, BRCA1/2-associated breast cancers often have lost the wild-type allele through somatic alterations during tumor development. As a consequence, there is no functional BRCA1 or BRCA2 protein in these tumor cells. Since BRCA1 and BRCA2 proteins are essential in the repair of double-strand DNA breaks (DSBs) by homologous recombination [3, 4], treatments which cause DSBs might be more effective in BRCA1/2-associated than in sporadic breast cancer patients, which tumor cells mostly have an intact homologous recombination repair system. The platinum derivates carboplatin and cisplatin, both strong inducers of DSBs, indeed showed higher efficacy in BRCA1/2-associated compared to sporadic breast cancer patients [5–7]. Although less pronounced, anthracyclines are also known to induce indirect DSBs by inhibiting topoisomerases, causing DNA interstrand cross-links and the generation of free radicals [8]. Accordingly, several clinical studies have shown increased sensitivity for anthracycline-containing chemotherapy in BRCA1/2 mutation carriers [9–11].
An important question is whether acute toxicity due to (neo)adjuvant chemotherapy is different in BRCA1/2 mutation carriers treated for breast cancer when compared with sporadic breast cancer patients. Since (neo)adjuvant chemotherapy induces massive DNA damage also in normal cells, one might argue that the amount of functional BRCA1 or BRCA2 protein in mutation carriers is too low to repair all the DNA damage created, compared to sporadic breast cancer patients, resulting in more toxicity. Thus far two studies investigated the acute toxicity of (neo)adjuvant chemotherapy in BRCA1/2-associated, compared to sporadic breast cancer patients, with inconsistent results [12, 13]. In the retrospective study of Shanley et al., comparing 62 BRCA1/2 mutation carriers with breast cancer to 62 matched sporadic breast cancer cases, a large proportion of patients (80/124; 65 %) was treated with older chemotherapy regimens without anthracyclines, while no patient was treated with taxanes. In BRCA2 mutation carriers, less hematologic toxicity and dose alterations were observed compared to both BRCA1-associated and sporadic breast cancer patients, while no differences were seen for BRCA1-associated versus sporadic patients [12]. In the study by Huszno et al., comparing 41 BRCA1/2-associated with 229 breast cancer patients without a BRCA1/2 mutation, all patients were treated with an anthracycline-based regimen and also patients treated with taxanes were included [13]. It was found that the proportion of patients with neutropenia at the planned start date of the second chemotherapy cycle was significantly higher in breast cancer patients with a BRCA1/2 mutation compared to patients without a BRCA1/2 mutation. Twelve patients (4.5 %), all in the group of patients without a BRCA1/2 mutation, required early termination of treatment due to chemotherapy toxicity, mostly because of grade 3-4 neutropenia. Nausea and vomiting were seen more often in patients without a BRCA1/2 mutation. There were no differences in the other investigated variables (anemia, diarrhea, and mucositis).
Nowadays, standard (neo)adjuvant chemotherapy regimens for breast cancer contain both anthracycline (either epirubicin or doxorubicin) and taxanes (either paclitaxel or docetaxel). In view of the sparse available data on toxicity of taxanes and currently used chemotherapy regimens in BRCA1/2 mutation carriers, we performed a larger single-center retrospective cohort study to examine potential differences in (neo)adjuvant chemotherapy-associated toxicity between BRCA1/2-associated and sporadic breast cancer patients.
Patients and methods
Patient population
For this retrospective cohort study, we selected from the hospital pharmacy prescription registry all female patients who were treated at the Erasmus MC Cancer Institute, Rotterdam, The Netherlands, with adjuvant or neoadjuvant chemotherapy for primary breast cancer or local/locoregional recurrence (PBC/LR). Further eligibility criteria concerned: chemotherapy regimen consisting of anthracyclines and/or taxanes and chemotherapy treatment started between January 1, 2004 and December 31, 2014. Patients who were previously treated with chemotherapy for either breast or another invasive cancer were not excluded, but subgroup analyses were performed with the exclusion of these patients, since pre-treated patient might have increased hematologic toxicity. Patients treated with (neo)adjuvant chemotherapy twice in the time period of the study were included for both episodes of chemotherapy treatment. Eleven PBC/LRs were excluded because of missing data concerning chemotherapy administration, leaving a total of 704 PBC/LRs (in 701 patients) eligible for the primary analysis (Fig. 1).
Fig. 1.
Study population
For eligible patients, data on tumor characteristics (type of histology, differentiation grade, estrogen receptor status, progesterone receptor status, HER2 status and stage) and treatment details (surgery, radiotherapy, and/or chemotherapy) were retrieved. We also collected specific data on chemotherapy treatment (treatment regimen, dosing, delays, alterations, and complications). Data on mutation status were collected from the institutional database of the family cancer clinic. Patients not tested for a BRCA1/2 mutation were considered as sporadic breast cancer patients.
Chemotherapy regimens
During the time period of the study, the chemotherapy regimens were not different for BRCA1/2 mutation carriers and sporadic patients. Patients were treated with systemic therapy based on the national guidelines. For patients with HER2-negative breast cancer, the standard regimens at start of the study contained anthracyclines but no taxanes. From July 2008 till the end of the study, standard regimen for node-positive patients included taxanes (3-weekly docetaxel), while for node-negative patients, taxanes (3-weekly docetaxel) were included in the standard regimen from October 2011 onwards. Patients with HER2-positive breast cancer were treated with anthracyclines and no taxanes till August 2006. Trastuzumab was added to this regimen from September 2005 onwards. From August 2006 till the end of the study, the standard regimen contained anthracyclines and taxanes (weekly paclitaxel) in combination with trastuzumab.
Some patients were treated with other schemes because of participation in a clinical trial, prior chemotherapy treatment, or comorbidities. Standard G-CSF (granulocyte colony-stimulating factor) prophylaxis was only used for six cycles of TAC (docetaxel, doxorubicin, and cyclophosphamide) and dose-dense regimens (AC, doxorubicin/cyclophosphamide, given every 2 weeks). In case of febrile neutropenia or persisting neutropenia at planned start of next chemotherapy cycle, G-CSF was added to the next treatment cycle. In case of febrile neutropenia or persisting neutropenia at planned start of next chemotherapy cycle in patients treated with G-CSF, dose reduction was considered. Furthermore, dose reduction and/or dose delay were considered based on the severity of hematological and non-hematological toxicities.
Toxicity outcomes
Primary outcome was the relative total dose intensity (RTDI), a measure of delivered (actual) total dose intensity (ATDI; i.e. administered dose over the total time course of treatment), relative to the planned total dose intensity (PTDI). The RTDI, therefore, expresses the effect of reductions, delays, as well as premature discontinuations of a treatment. The RTDI was calculated separately for anthracyclines and taxanes.
RTDI was calculated based on an adaptation of the formula described by Loibl et al. [14], and defined as the ratio of the ATDI and the PTDI, expressed as a percentage:
The ATDI was defined as the actual total dose intensity over the real treatment duration, expressed as percentage/day. In case of permanent treatment discontinuation, the remaining cycles were calculated with the planned length and zero dose:
The PTDI was defined as the planned total dose intensity over the entire treatment duration, expressed as %/day:
The secondary outcomes were the occurrence of one or more episodes of febrile neutropenia, of one or more delays in chemotherapy administration (either due to anthracycline-related toxicity or taxane-related toxicity) and of switch to another chemotherapy regimen.
Statistical analyses
We evaluated characteristics of patients, tumors and chemotherapy regimens, as well as outcome variables by comparing patients with proven BRCA1/2-associated breast cancer (BRCA1/2 group) with those with sporadic breast cancer (sporadic group). For categorical variables, Pearson’s Chi square test was used to test for significant differences between the two groups, and the two-sample Wilcoxon rank-sum (Mann–Whitney) test was used for differences between continuous variables.
To quantify the effect of carrying a BRCA1/2 gene mutation on the RTDI of anthracyclines and taxanes, we performed univariate linear regression analyses. To estimate the effect of mutation carriership on the other endpoints (i.e. a delay in administration of chemotherapy, febrile neutropenia, and an alteration of the chemotherapy scheme due to toxicity), we used logistic regression models to obtain odds ratios (ORs) and accompanying 95 % confidence intervals (CIs), using treatments for PBC/LRs in sporadic patients as the reference group. To adjust for other variables, we fitted multivariate regression models. We considered age at start of chemotherapy, previous chemotherapy, radiotherapy before chemotherapy, neoadjuvant chemotherapy, and number of administered chemotherapy cycles as potential confounders. We incorporated a variable in a regression model if (1) there was a significant difference in the median or in the distribution of the respective variable between the BRCA1/2-associated and the sporadic group and (2)—for linear regression models—univariate analysis of the respective variable showed a significant association with the outcome, or—for logistic regression models—the likelihood ratio test showed that the model including the respective variable was significantly different from the model without the variable.
All p values were two-sided, and a significance level α = 0.05 was used. Analyses were performed with STATA (version 13.1; StataCorp, College Station, TX, USA).
Results
In total, 701 patients were eligible for data analyses, of whom one BRCA1 mutation carrier and two sporadic patients were treated with two separate episodes of (neo)adjuvant chemotherapy for a PBC/LR during the study period. Tables 1 and 2 depict the patient and tumor characteristics, and the treatment features, respectively. 85 patients (12 %) were BRCA1/2 mutation carriers (n = 67 BRCA1 and n = 18 BRCA2). The median age at start of chemotherapy was significantly lower in the BRCA1/2 group compared to the sporadic group (38 years [range 21–64] vs. 51 years [range 23–77], respectively, p < 0.001). PBC/LRs in BRCA1/2 mutation carriers more often showed high differentiation grade (Bloom/Richardson grade 3), triple-negative tumors and negative lymph node status compared to PBC/LRs in sporadic patients. For a total of 492 PBC/LRs (70 %), treatment with both anthracycline- and taxane-containing chemotherapy was applied, while chemotherapy consisted of anthracyclines with no taxanes for 193 PBC/LRs (27 %) and for 19 PBC/LRs (3 %) of taxanes with no anthracyclines. In the BRCA1/2 group, more patients were previously treated with chemotherapy for breast cancer or for another invasive malignancy (13 vs. 5 % in the sporadic group, p = 0.004; Table 2).
Table 1.
Patient and tumor characteristics
| BRCA1/2 mutation carriers (n = 85) | Sporadic patients (n = 616) |
p value | |
|---|---|---|---|
| Year of birth, median (range) | 1971 (1942–1990) | 1957 (1936–1987) | <0.001 |
| Year of birth, n (%) | |||
| 1930–1939 | 0 (0) | 8 (1) | <0.001 |
| 1940–1949 | 4 (5) | 130 (21) | |
| 1950–1959 | 15 (18) | 205 (33) | |
| 1960–1969 | 18 (21) | 188 (31) | |
| 1970–1979 | 30 (35) | 66 (11) | |
| 1980–1989 | 17 (20) | 19 (3) | |
| 1990–1999 | 1 (1) | 0 (0) | |
| Ethnicity, n (%) | |||
| East Asian | 0 (0) | 17 (3) | 0.33 |
| Black | 6 (7) | 36 (6) | |
| White | 79 (93) | 557 (90) | |
| Other | 0 (0) | 6 (1) | |
| BRCA mutation, n (%) | |||
| BRCA1 | 67 (79) | – | – |
| BRCA2 | 18 (21) | – | – |
| PBC/LRs in BRCA1/2 mutation carriers (n = 86) | PBC/LRs in sporadic patients (n = 618) | ||
|---|---|---|---|
| Age at start chemotherapy, median (range) | 38 (21–64) | 51 (23–77) | <0.001 |
| Age at start chemotherapy, n (%) | |||
| 20–29 years | 12 (14) | 15 (2) | <0.001 |
| 30–39 years | 34 (40) | 61 (10) | |
| 40–49 years | 23 (27) | 179 (29) | |
| 50–59 years | 12 (14) | 221 (36) | |
| 60–69 years | 5 (6) | 136 (22) | |
| 70–79 years | 0 (0) | 6 (1) | |
| Body surface area (m2), median (range) | 1.8 (1.4–2.3) | 1.8 (1.4–2.6) | 0.41 |
| Histologic subtype, n (%) | |||
| Ductal | 74 (88) | 521 (86) | 0.005 |
| Lobular | 1 (1) | 58 (10) | |
| Other | 9 (11) | 30 (5) | |
| Unknown | 2 | 9 | |
| Histologic grade (Bloom/Richardson), n (%) | |||
| 1 | 4 (5) | 48 (8) | <0.001 |
| 2 | 13 (16) | 265 (46) | |
| 3 | 64 (79) | 269 (46) | |
| Unknown | 5 | 36 | |
| Receptor status, n (%) | |||
| Triple-negative | 51 (60) | 101 (17) | <0.001 |
| Estrogen receptor positive | 32 (37) | 466 (75) | <0.001 |
| HER2-positive | 4 (5) | 135 (22) | <0.001 |
| Lymph node status, n (%) | |||
| N0 | 55 (65) | 228 (38) | <0.001 |
| N1 | 21 (25) | 254 (43) | |
| N2 | 4 (5) | 78 (13) | |
| N3 | 4(5) | 37 (6) | |
| Unknown | 2 | 21 | |
PBC/LR Primary breast cancer or local/locoregional recurrence
Table 2.
Features of (neo)adjuvant chemotherapy and other treatments
| PBC/LRs in BRCA1/2 mutation carriers (n = 86) | PBC/LRs in sporadic patients (n = 618) | p value | |
|---|---|---|---|
| Planned chemotherapy regimen, n (%) | |||
| Containing both anthracyclines and taxanes | 49 (57) | 443 (72) | <0.001 |
| 3 × FE100C/3 × D | 46 (53) | 290 (47) | |
| 4 × AC/12 × P | 1 (1) | 103 (17) | |
| 6 × TAC | 1 (1) | 40 (6) | |
| Other | 1 (1) | 10 (2) | |
| Containing anthracyclines and no taxanes | 30 (35) | 163 (26) | |
| 5 × FE90C | 19 (22) | 86 (14) | |
| 6 × FE90C | 7 (8) | 60 (10) | |
| 4 × AC | 1 (1) | 13 (2) | |
| Other | 3 (3) | 4 (1) | |
| Containing taxanes and no anthracyclines | 7 (8) | 12 (2) | |
| Dose-dense regimens, n (%) | 3 (3) | 1 (0.2) | <0.001 |
| Regimens with standard G-CSF prophylaxis, n (%) | 4 (5) | 41 (7) | 0.48 |
| Regimens with weekly chemotherapy, n (%) | 1 (1) | 104 (17) | <0.001 |
| Number of 3-weekly chemotherapy cycles, median (range) | 6 (3–10) | 6 (1–8) | 0.14 |
| Previous chemotherapy, n (%) | 11 (13) | 31 (5) | <0.01 |
| Adjuvant radiotherapy before chemotherapy, n (%) | 2 (2) | 87 (14) | <0.01 |
| Neoadjuvant chemotherapy, n (%) | 10 (12) | 81 (13) | 0.70 |
PBC/LR primary breast cancer or local/locoregional recurrence, 3 × FE100C/3 × D three cycles of 3-weekly fluorouracil 500 mg/m2, epirubicin 100 mg/m2 and cyclophosphamide 500 mg/m2, followed by three cycles of docetaxel 100 mg/m2, 4 × AC/12 × P four cycles of 3-weekly doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2, followed by 12 cycles of weekly paclitaxel 80 mg/m2, 6 × TAC six cycles of 3 weekly docetaxel 75 mg/m2, doxorubicin 50 mg/m2 and cyclophosphamide 500 mg/m2, 5 × FE90C five cycles of 3-weekly fluorouracil 500 mg/m2, epirubicin 90 mg/m2 and cyclophosphamide 500 mg/m2, 6 × FE90C six cycles of 3-weekly fluorouracil 500 mg/m2, epirubicin 90 mg/m2 and cyclophosphamide 500 mg/m2, 4 × AC four cycles of 3-weekly doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2, G-CSF granulocyte colony-stimulating factor
Primary outcome
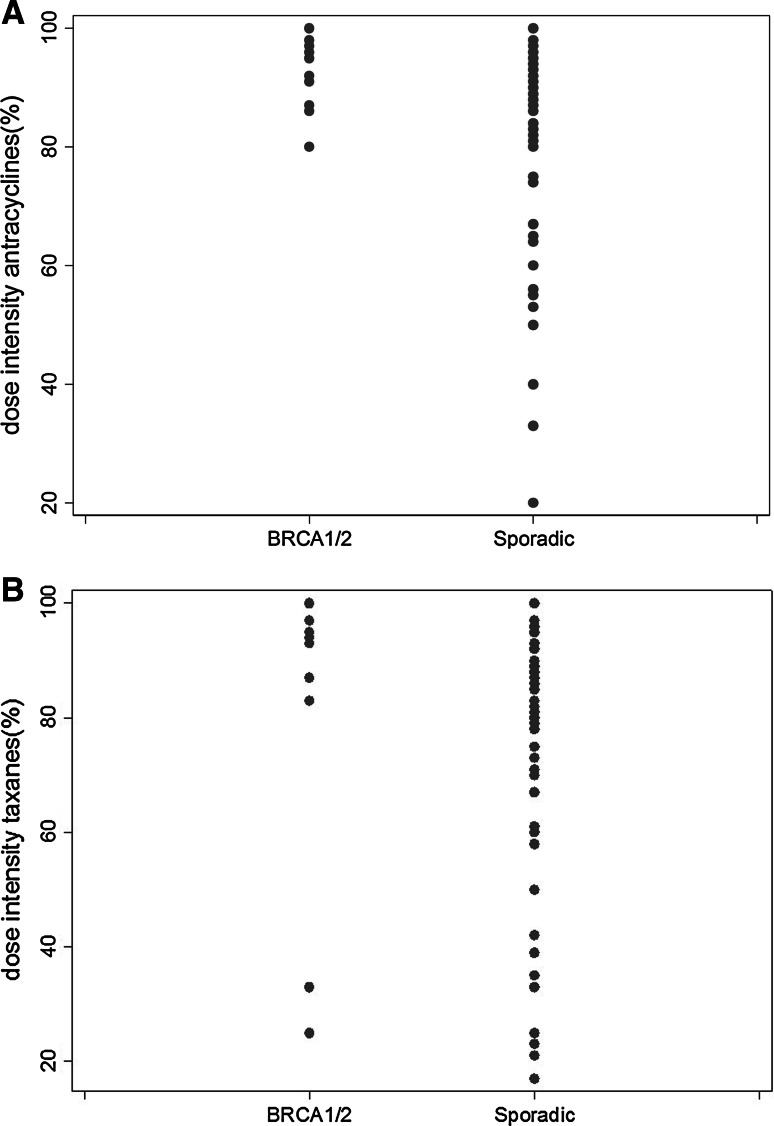
The mean RTDI for anthracyclines was high, without significant differences between the BRCA1/2 and the sporadic groups (98.7 and 96.6 %, respectively, p = 0.27; Table 3). The mean RTDI for taxanes was slightly lower than for anthracyclines, but again without significant differences between the two groups (93.6 % in the BRCA1/2 group and 90.0 % in the sporadic group, p = 0.12; Table 3). As illustrated in Fig. 2, BRCA1/2 mutation carriers showed less variability in the RTDI than sporadic patients. As shown in Table 4, the linear regression models revealed no significant effect of BRCA1/2 mutation carriership on the RTDIs.
Table 3.
Primary and secondary outcome variables
| PBC/LRs in BRCA1/2 mutation carriers (n = 86) | PBC/LRs in sporadic patients (n = 618) | p value | |
|---|---|---|---|
| Mean relative total dose intensity, % (SD) | |||
| Anthracyclines | 98.7 (3.7) | 96.6 (10.5) | 0.27 |
| Taxanes | 93.6 (17.6) | 90.0 (19.9) | 0.12 |
| Febrile neutropenia, n (%) | 18 (21) | 107 (17) | 0.42 |
| Delay of chemotherapy administration, n (%) | |||
| Because of anthracyclines | 12 (15) | 90 (15) | 0.97 |
| Because of taxanes | 2 (4) | 46 (10) | 0.13 |
| Alteration of chemotherapy scheme, n (%) | 8 (9) | 65 (11) | 0.73 |
PBC/LR Primary breast cancer or local/locoregional recurrence, SD standard deviation
Fig. 2.
Relative total dose intensity (%) for a anthracyclines and b taxanes, separately for BRCA1/2-associated and sporadic breast cancer patients
Table 4.
Linear regression analyses for mean relative total dose intensity
| Univariate model | Multivariate model | |||
|---|---|---|---|---|
| Coefficient (SE) | p value | Coefficient (SE) | p value | |
| Mean RTDI anthracyclines (%) | ||||
| BRCA1/2 versus sporadic | 1.69 (1.08) | 0.12 | Not applicablec | |
| Age at start chemotherapy | −0.05 (0.03) | 0.12 | ||
| Previous chemotherapya | −1.09 (1.84) | 0.55 | ||
| Radiotherapy before chemotherapyb | −1.16 (1.00) | 0.25 | ||
| Mean RTDI taxanes (%) | ||||
| BRCA1/2 versus sporadic | 3.94 (2.98) | 0.19 | 3.33 (2.97) | 0.26 |
| Age at start chemotherapy | −0.10 (0.08) | 0.22 | – | – |
| Previous chemotherapya | 6.71 (4.17) | 0.11 | – | – |
| Radiotherapy before chemotherapyb | −6.77 (2.75) | 0.01 | −6.50 (2.76) | 0.02 |
RTDI Relative total dose intensity, SE standard error
a Versus no previous chemotherapy
b Versus no radiotherapy before chemotherapy
c None of the variables were associated with the outcome variable
Secondary outcomes
As shown in Table 3, no significant differences between the BRCA1/2-associated and sporadic groups were found in the percentage of patients presenting with febrile neutropenia (21 and 17 %, respectively, p = 0.42), having a delay in chemotherapy administration due to chemotherapy toxicity (for anthracyclines: 15 % in both groups, p = 0.97; for taxanes: 4 % in the BRCA1/2-associated, and 10 % in the sporadic group, p = 0.13) or switching to an altered chemotherapy regimen due to chemotherapy toxicity (9 % in the BRCA1/2-associated and 11 % in the sporadic group, p = 0.73). Additionally, the ORs yielded by logistic regression showed no significant effect of BRCA1/2 mutation carriership on the secondary outcome variables (Table 5).
Table 5.
Logistic regression analyses for secondary outcome variables
| Univariate model odds ratio (95 % CI) |
Multivariate model odds ratio (95 % CI) |
|
|---|---|---|
| Febrile neutropenia | 1.27 (0.71–2.27) | 1.11 (0.59–2.07)a |
| Delay of chemotherapy administration | ||
| Because of anthracyclines | 0.99 (0.50–1.97) | Not applicableb |
| Because of taxanes | 0.36 (0.08–1.54) | Not applicableb |
| Alteration of chemotherapy scheme | 0.80 (0.33–1.93) | Not applicableb |
Sporadic breast cancer patients as references versus breast cancer patients with a BRCA1/2 mutation
CI confidence interval
a Adjusted for age at start chemotherapy. The other variables did not meet the criteria for incorporation in the multivariate model
b No variables did meet the criteria for incorporation in the multivariate model as described in the methods section
Subgroup analyses
To exclude effect modification by differences in treatment regimens between the two groups on the outcome variables, we performed analyses with exclusion of certain chemotherapy regimens. Exclusion of the patients being treated with regimens administered with standard G-CSF prophylaxis (n = 4 treated with dose-dense regimens; n = 41 treated with TAC), with regimens consisting of weekly chemotherapy administration (n = 105) or with regimens containing taxanes with no anthracyclines (n = 19) did not significantly influence the results of both primary and secondary outcome variables (data not shown). Febrile neutropenia was then found in 25 % of the BRCA1/2-associated and in 20 % of the sporadic group, p = 0.57.
Excluding the patients who were previously treated with adjuvant chemotherapy for breast cancer or for another invasive cancer (n = 42) also did not significantly influence the results of both primary and secondary outcome variables (data not shown). When taking the BRCA1/2-associated and the sporadic group together, the RTDI was not significantly different between patients previously versus not previously treated with chemotherapy (for anthracyclines RTDI: 96.8 % in both groups, p = 0.80; for taxanes RTDI: 95.8 vs. 90.1 %, p = 0.20).
Discussion
In this single-center retrospective cohort study, we found no differences in RTDI of (neo)adjuvant chemotherapy (both for anthracyclines and taxanes) between BRCA1/2-associated and sporadic breast cancer patients. Furthermore, we found no differences in the occurrence of febrile neutropenia, in delay of chemotherapy administration or in alteration of the chemotherapy regimen due to toxicity between the two groups. Our observations on the absence of increased acute toxicity due to (neo)adjuvant chemotherapy in BRCA1/2 mutation carriers, compared to sporadic breast cancer patients, suggest that the DNA damage repair mechanism of non-cancer cells with only one normal copy of either the BRCA1 or BRCA2 gene is sufficiently functional to handle acute chemotherapy-associated toxicity.
Our results have to be interpreted in the light of the two previously published studies on chemotherapy-associated toxicity in BRCA1/2 mutation carriers. Huszno et al. found more neutropenia at the planned date of the second chemotherapy cycle in BRCA1/2-associated (n = 41) than in sporadic breast cancer patients (n = 229) [13]. It is unclear what the clinical relevance of this finding is, since they did not mention the proportion of patients needing dose reductions, experiencing delay in chemotherapy administration and febrile neutropenia. We choose to use more clinically relevant outcome measures such as dose intensity which is likely to be associated with efficacy [15] and febrile neutropenia that might have consequences for the subsequent cycle. The data of the study of Shanley et al., not finding increased chemotherapy-associated toxicity in BRCA1/2-associated (n = 62) compared to sporadic breast cancer patients (n = 62) [12], are hardly comparable to our study observations, since a large part of their patients were treated with older chemotherapy regimens.
To the best of our knowledge, our study is the largest published on this topic so far. We did not find any differences in clinically relevant toxicity measures after treatment with anthracyclines and/or taxanes between BRCA1/2-associated and sporadic breast cancer patients. In both previous studies, as well as in our study, age at the start of chemotherapy was significantly lower in the BRCA1/2 group than in the sporadic group. Although increased risk of myelosuppression at increased age of administration has been previously reported [16], in our study no difference was seen in mean RTDI comparing BRCA1/2 mutation carriers aged >50 years to BRCA1/2 mutation carriers younger than 50 years (data not shown).
In the BRCA1/2 group more patients were previously treated with adjuvant chemotherapy than in the sporadic group, mainly for an earlier primary breast cancer. Since there is a maximum cumulative dose for anthracyclines, a relevant proportion of these patients did receive a non-anthracycline-containing regimen. One might expect increased toxicity when patients are treated for a second time with chemotherapy. Leaving out all pre-treated patients, however, did not influence the results, and comparing previously treated patients with non-previously treated patients (irrespective of BRCA1/2 mutation status) showed no significant differences in the RTDI, suggesting that previous treatment with chemotherapy does not increase acute chemotherapy-related toxicity.
In the BRCA1/2-associated group, fewer patients were treated with weekly chemotherapy regimens and with regimens containing standard G-CSF prophylaxis. However, exclusion of patients treated with these regimens did not significantly influence the results. The percentage of patients presenting with febrile neutropenia in the sporadic group increased in the subgroup analyses, compared to the percentage found in the primary analysis, which might be explained by the fact that in the sporadic group a larger proportion of patients were treated with regimens containing standard G-CSF prophylaxis.
We are aware of a number of shortcomings to be mentioned. Despite the fact that our study is the largest published on this topic with inclusion of 86 PBC/LRs in BRCA1/2-associated patients, being 12 % of the total study group, this number is still quite low. The numbers were too small to perform useful analyses for BRCA1 and BRCA2 mutation carriers separately, which would be of interest since BRCA1 and BRCA2 proteins have different roles in the DNA repair mechanism and the cell cycle. Nevertheless, it is unlikely that a clinically relevant difference will be found with higher numbers of patients, since the RTDI, especially for anthracycline is very high. In contrast to the study of Huszno et al., not all our patients were tested for a BRCA1/2 mutation, but we expect, if any, only a small proportion of BRCA1/2 mutation carriers in the sporadic subgroup, since at our institution (and in The Netherlands) patients are already tested with a low suspicion of BRCA1/2 mutation carriership. Further, in the current study, we did not include non-hematologic toxicity as an outcome, since it is well known that these outcome variables are more prone to inter-observer variability and are less clinically relevant when they do not lead to dose delay or dose reduction [17]. For the same reason, we did not include hematologic laboratory values measured at planned start of a new cycle, since these are only relevant when they lead to dose reduction, delay in chemotherapy administration, or alteration of chemotherapy regimen. It could have been of scientific interest to compare neutrophil nadir levels between BRCA1/2 mutation carriers and sporadic patients. Unfortunately, due to the retrospective nature of our data, these data are lacking.
Recent data showed increased efficacy of platinum derivates in patients with triple-negative breast cancer and/or a BRCA1/2 mutation, leading to incorporation of carboplatin in standard (neo)adjuvant chemotherapy regimens in this population [5–7]. These studies did not report on differences in toxicity between BRCA1/2 mutation carriers and sporadic breast cancer patients. In our study, the number of patients treated with carboplatin was very low and no conclusions can be drawn hereon. Poly ADP-ribose polymerase (PARP) inhibitors are an important new class of targeted anti-cancer drugs which induce DSBs in tumors with homologous recombination deficiency due to, for example, a mutation in one of the BRCA genes. Recently, the first PARP inhibitor has been approved for the treatment of BRCA1/2-associated ovarian cancer, while trials in early and metastatic breast cancer are ongoing. Lederman et al. compared toxicity of the PARP inhibitor olaparib in BRCA1/2 mutation carriers and sporadic patients with ovarian cancer and found no differences in toxicity [18]. Both platinum derivates and PARP inhibitors have a much higher capacity to induce DSBs, compared to anthracyclines. Therefore, further research on the toxicity of these regimens in BRCA1/2 mutation carriers compared to sporadic patients is warranted, especially since these drugs will be increasingly used in the treatment of BRCA1/2-associated breast cancer.
Conclusion
In conclusion, there seems no clinically relevant difference in toxicity of anthracycline- and taxane- containing (neo)adjuvant chemotherapy regimens for BRCA1/2-associated compared to sporadic breast cancer patients, which suggests that the DNA damage repair mechanism of non-cancer cells with only one normal copy of either the BRCA1 or BRCA2 gene is sufficiently functional to handle acute chemotherapy-associated toxicity.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Antoniou A, Pharoah PDP, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jasin M. Homologous repair of DNA damage and tumorigenesis: the BRCA connection. Oncogene. 2002;21:8981–8993. doi: 10.1038/sj.onc.1206176. [DOI] [PubMed] [Google Scholar]
- 4.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/S0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 5.Tutt A, Ellis P, Kilburn L, Symposium San Antonio Breast Cancer, et al. Abstract S3-01: the TNT trial: a randomized phase III trial of carboplatin (C) compared with docetaxel (D) for patients with metastatic or recurrent locally advanced triple negative or BRCA1/2 breast cancer. Cancer Res. 2014;75(S3–01):2015. [Google Scholar]
- 6.von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomized phase 2 trial. Lancet Oncol. 2014;15:747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 7.Byrski T, Huzarski T, Dent R, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2014;147:401–405. doi: 10.1007/s10549-014-3100-x. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy RD, Quinn JE, Mullan PB, et al. The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst. 2004;96:1659–1668. doi: 10.1093/jnci/djh312. [DOI] [PubMed] [Google Scholar]
- 9.Kriege M, Seynaeve C, Meijers-Heijboer H, et al. Sensitivity to first-line chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27:3764–3771. doi: 10.1200/JCO.2008.19.9067. [DOI] [PubMed] [Google Scholar]
- 10.Fourquet A, Stoppa-Lyonnet D, Kirova YM, et al. Clinical response to induction chemotherapy or radiotherapy related to BRCA1/2 mutation status. Am J Clin Oncol. 2009;32:127–131. doi: 10.1097/COC.0b013e31817f9e1c. [DOI] [PubMed] [Google Scholar]
- 11.Arun B, Bayraktar S, Liu DD, et al. Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J Clin Oncol. 2011;29:3739–3746. doi: 10.1200/JCO.2011.35.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanley S, McReynolds K, Ardern-Jones A, et al. Acute chemotherapy-related toxicity is not increased in BRCA1 and BRCA2 mutation carriers treated for breast cancer in the United Kingdom. Clin Cancer Res. 2006;12:7033–7038. doi: 10.1158/1078-0432.CCR-06-1246. [DOI] [PubMed] [Google Scholar]
- 13.Huszno J, Budryk M, Kolosza Z, et al. The influence of BRCA1/BRCA2 mutations on toxicity related to chemotherapy and radiotherapy in early breast cancer patients. Oncology. 2013;85:278–282. doi: 10.1159/000354834. [DOI] [PubMed] [Google Scholar]
- 14.Loibl S, Skacel T, Nekljudova V, et al. Evaluating the impact of relative total dose intensity (RTDI) on patients’ short and long-term outcome in taxane- and anthracycline-based chemotherapy of metastatic breast cancer—a pooled analysis. BMC Cancer. 2011;11:131. doi: 10.1186/1471-2407-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw. 2009;7:99–108. doi: 10.6004/jnccn.2009.0009. [DOI] [PubMed] [Google Scholar]
- 16.Dees EC, O’Reilly S, Goodman SN, et al. A prospective pharmacologic evaluation of age-related toxicity of adjuvant chemotherapy in women with breast cancer. Cancer Invest. 2000;18:521–529. doi: 10.3109/07357900009012191. [DOI] [PubMed] [Google Scholar]
- 17.Brundage MD, Pater JL, Zee B. Assessing the reliability of tow toxicity scales: implications for interpreting toxicity data. J Natl Cancer Inst. 1993;85:1138–1148. doi: 10.1093/jnci/85.14.1138. [DOI] [PubMed] [Google Scholar]
- 18.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcome by BRCA status in a randomized phase 2 trial. Lancet Oncol. 2014;15:852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]