Summary
Dry eye is an allegedly autoimmune disorder for which the initiating mechanisms and the targeted antigens in the ocular surface are not known, yet there is extensive evidence that a localized T helper type 1 (Th1)/Th17 effector T cell response is responsible for its pathogenesis. In this work, we explore the reconciling hypothesis that desiccating stress, which is usually considered an exacerbating factor, could actually be sufficient to skew the ocular surface's mucosal response to any antigen and therefore drive the disease. Using a mouse model of dry eye, we found that desiccating stress causes a nuclear factor kappa B (NF‐κB)‐ and time‐dependent disruption of the ocular surface's immune tolerance to exogenous ovalbumin. This pathogenic event is mediated by increased Th1 and Th17 T cells and reduced regulatory T cells in the draining lymph nodes. Conversely, topical NF‐κB inhibitors reduced corneal epithelial damage and interleukin (IL)‐1β and IL‐6 levels in the ocular surface of mice under desiccating stress. The observed effect was mediated by an augmented regulatory T cell response, a finding that highlights the role of mucosal tolerance disruption in dry eye pathogenesis. Remarkably, the NF‐κB pathway is also involved in mucosal tolerance disruption in other ocular surface disorders. Together, these results suggest that targeting of mucosal NF‐κB activation could have therapeutic potential in dry eye.
Keywords: conjunctival tolerance, desiccating stress, dry eye, mucosal tolerance, ocular surface
Introduction
Dry eye disease (DED), a highly prevalent disorder, is characterized by a dysfunctional tear film and ocular surface 1. Although it was initially ascribed a minor role, inflammation is now considered to be the main underlying mechanism in the pathophysiology of DED and is the target of most current treatment options 2. Hallmarks of mucosal inflammation are commonly observed in DED, such as increased proinflammatory cytokine levels in the tear film 3, 4, epithelial apoptosis 5 and leucocyte infiltration 6. In addition, a key role of the adaptive immune response in DED is supported by the clinical impact of cyclosporin A treatment 7 and the fact that adoptive transfer of CD4+ T cells reproduces the mucosal disease 8.
The conjunctiva, as any other highly exposed mucosal surface, is challenged continuously by environmental factors and is loaded with both exogenous and endogenous antigens, yet it remains uninflamed and thus fully functional in most individuals. The regulatory mechanisms behind this protective stance ultimately derive from mucosal tolerance 9, 10, 11; that is, the suppression of a potentially harmful adaptive immune response against innocuous antigens. A recent hypothesis put forth that DED is a localized form of mucosal autoimmune disease, triggered when the ocular surface's immune balance is overthrown by still uncharacterized factors, perhaps challenging environmental conditions 12. Supporting this hypothesis, desiccating stress (DS) is sufficient to induce pathogenic T helper type 1 (Th1) 5 and Th17 13 T cell responses in a widely studied mouse model of DED 8. It remains to be determined whether the T cells involved are specific for some as‐yet undefined autoantigens, or if they actually represent the generalized disruption of the protective tolerance mechanisms to any mucosal antigen.
Regarding its potential role in DED pathogenesis, mucosal tolerance is known to be affected in another ocular surface disorder, eye drop preservative toxicity 10 and, more importantly, that restoration of this immune function can protect mice from the disease 11. Mucosal tolerance is governed by the epithelial lining, which exerts a tolerogenic or immunogenic sway at the initiation of adaptive immune responses 14. In this process, epithelial nuclear factor kappa B (NF‐κB) pathway signalling plays a decisive role in the downstream immune outcome 11, 14, 15, 16, thus affording an opportunity to control the mucosal epithelium's influence by pharmacological inhibition. In the ocular surface, the epithelial NF‐κB pathway acts as a central regulator of inflammation in several corneal and conjunctival disorders 17. For this work, we favoured the possibility that DS initiates DED by triggering a generalized disruption of ocular surface immune tolerance, instead of a specific autoimmune response towards corneal and conjunctival antigens. Therefore, we set out to assess whether mucosal tolerance is indeed affected in a DS‐induced murine model of DED and if it is involved in disease development.
Materials and methods
Mice
BALB/c and C3H mice (8–12 weeks old), which were bred and maintained in our conventional animal facility, were used for the in‐vivo experiments. All experiments were approved by the Institute of Experimental Medicine Animal Ethics Committee and adhered to the Association for Research in Vision Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Reagents and antibodies
All reagents were from Sigma‐Aldrich (Buenos Aires, Argentina) unless specified otherwise. Fluorochrome‐tagged antibodies were from BioLegend (San Diego, CA, USA) and ImmunoTools (Friesoythe, Germany). Grade V ovalbumin (OVA) was used in all experiments.
DS model
Mice were subjected to DS by subcutaneous (s.c.) injection of 0·5 mg scopolamine hydrobromide (Boehringer Ingelheim, Buenos Aires, Argentina) three times a day (9 a.m., 1 p.m. and 5 p.m.), and by housing in a perforated cage to allow forced air to flow from a fan for 12 h a day (9 a.m.−9 p.m.). For some experiments, either 5 µl/eye of phosphate‐buffered saline, 0·1 mM pyrrolidine dithiocarbamate (PDTC) or 0·5 mM sulphasalazine (SSZ) were instilled on both eyes every time the mice were injected.
OVA instillation and immunization for delayed‐type hypersensitivity (DTH) assays
Mice under DS were instilled on both eyes, once or twice per day at the indicated time‐points with 5 µl/eye of 2 mg/ml OVA. Immunization and DTH assays were performed as described previously 11 at the time‐points indicated.
Assessment of tear production and of corneal surface damage and irregularity
Tear production was measured by wetting of phenol‐red impregnated filter paper, and corneal surface damage was assessed by fluorescein uptake and graded by the National Eye Institute scoring system, as described elsewhere 18, 19.
Eye explants and cells from eye‐draining lymph nodes
After euthanasia, the entire eye globe with the tarsal conjunctiva still attached was excised under aseptic conditions with the aid of a dissection microscope, as described elsewhere 10. Both explants from each animal were pooled, washed three times with phosphate‐buffered saline (PBS) and then cultured in 1 ml of medium without serum. Supernatants were collected after 24 h for further analysis. For analysis of eye‐draining lymph node cells, submandibular lymph nodes were excised and rendered into a cell suspension by mechanical dissociation and sieving through wire mesh. For some experiments, inguinal lymph nodes were also collected as controls. For functional experiments, CD3+ T cells were isolated by negative selection with magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Purity [assessed by fluorescence activated cell sorter (FACS)] was > 95% for all experiments.
Cell lines and cultures
Cell cultures were performed in RPMI‐1640 medium supplemented with 10% fetal calf serum, 10 mM glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and 5 × 10−5 M 2‐mercaptoethanol in a humidified incubator with 5% CO2 at 37°C.
Enzyme‐linked immunosorbent assay (ELISA)
Interleukin (IL)‐1β and IL‐6 concentrations in supernatants were determined with commercial ELISA kits according to the manufacturer's instructions (BD Biosciences, Buenos Aires, Argentina).
Local adoptive transfer (LAT) assays
T cells from the submandibular lymph nodes of mice under DS were mixed with T cells from OVA‐immunized mice and OVA‐pulsed antigen‐presenting cells (T cell‐depleted splenocytes from naive mice) at a 1 : 1 : 1 ratio, and 35 µl of the resulting cell suspension containing a total of 3 × 106 cells were injected into the footpads of naive mice. Footpad thickness was recorded before and 24 h after cell injection by a masked observer, and swelling calculated accordingly.
In‐vitro expansion of OVA‐specific T cells. T cells from the submandibular lymph nodes of mice under DS were cultured for 7 days at a density of 5 × 106 cells/ml in the presence of an equal number of mitomycin‐treated, T cell‐depleted splenocytes as a source of antigen‐presenting cells and 100 µg/ml OVA. After Ficoll gradient centrifugation to remove dead cells (> 60% at the end of the culture period), cells were stimulated and processed as indicated for intracellular cytokine staining.
Immunostaining and flow cytometry
For antigen staining, cells were washed in PBS with 0·5% bovine serum albumin, incubated for 15 min with 5 µg/ml 2·4G2 antibody (purified from ascites fluid) to block non‐specific binding to Fc receptors and then labelled with fluorochrome‐conjugated antibodies at previously titrated concentrations for 30 min at 4°C. Forkhead box protein 3 (FoxP3) staining was performed as indicated by the manufacturer (BioLegend). For intracellular cytokine staining, cells were first stimulated for 5 h with 50 ng/ml phorbol myristate acetate (PMA) and 1 µg/ml ionomycin in the presence of 10 µg/ml brefeldin A, then washed, fixed in 1% paraformaldehyde, permeabilized in 0·1% saponin and finally stained with the corresponding antibodies. For flow cytometry analysis, cells were washed thoroughly in PBS with 1 mM ethylenediamine tetraacetic acid before acquisition on a FACScalibur cytometer (Becton Dickinson, Buenos Aires, Argentina). Data were analysed with Flowing Software (Perttu Terho, Center for Biotechnology, Turku, Finland; www.flowingsoftware.com). Optimal compensation and gain settings, as well as viable cell gating, were determined as described previously 20. Overlaid histogram graphs are plotted in normalized form as a percentage of maximum for each histogram.
T cell proliferation assays
Single cell suspensions were labelled with carboxyfluorescein succinimidyl ester (CFSE), according to the manufacturer's instructions (BioLegend). For antigen proliferation assays, 2 × 105 cells were cultured for 3 days in 96‐well plates containing 100 µg/ml OVA. Soluble anti‐CD3 antibody was added to some cultures at the concentrations indicated.
Adoptive transfer
CD3+ T cells were isolated from the submandibular lymph nodes of mice treated as indicated in the text and pooled (each mouse yielding approximately 15 × 106 cells). Cells were then transferred to recipient mice by intraperitoneal injection of 15 × 106 cells/0·5 ml of phosphate‐buffered saline.
Statistical analysis
Student's t‐test and analysis of variance (anova) with Dunnett's post‐hoc test were used to compare means of two and three or more samples, respectively. Significance was set at P < 0·05 (two‐tailed tests) and calculations were performed using GraphPad Prism version 5 software (GraphPad Software, La Jolla, CA, USA).
Results
DS disrupts ocular mucosal tolerance in a NF‐κB‐dependent fashion
We initially explored the effect of DS on the ocular surface's immune homeostasis by employing a widely characterized murine model of DED 8. To this aim, mice were subjected to DS for 5 days by combining simultaneous daily injections of scopolamine to reduce tear secretion and exposure to a controlled air draft to increase tear evaporation. In this model, a pathogenic T cell response against corneal and conjunctival antigens ensues rapidly, which can sustain ocular surface damage after cessation of DS 8, 13, 21. For the first part of this work, as the antigens involved in DED's adaptive immune response are not known, we included ocular instillation of ovalbumin (OVA) at different time‐points to assess the antigen‐specific conjunctival immune response to a well‐characterized antigen 9, 10, 11 (Fig. 1a). As expected, we found that in non‐DS mice, ocular instillation of OVA markedly reduced in‐vitro antigen‐specific T cell proliferation and the in‐vivo DTH response after s.c. immunization with OVA in adjuvant (Fig. 1b,c). This phenomenon is referred to commonly as mucosal tolerance, and involves the induction of antigen‐specific regulatory T cells (Tregs) that suppress subsequent inflammation 9, 10. Interestingly, when OVA was administered from day 1 of DS, mice also developed comparably blunted DTH and T cell responses, suggesting that mucosal tolerance was not affected early by DS. However, when OVA instillation was delayed until day 4, only non‐DS mice developed suppressed DTH responses, whereas DS mice showed full DTH responses to antigen. As we have shown previously in another murine model of eye disease 11, ocular surface immune tolerance is dependent upon NF‐κB activation in conjunctival epithelial cells. Therefore, we tested whether topical NF‐κB inhibitors pyrrolidine dithiocarbamate (PDTC) and SSZ were able to prevent the disruption of immune homeostasis by DS. As depicted in Fig. 1c, topical instillation of either of the two inhibitors restored mucosal tolerance to OVA administered on days 4 and 5 to DS mice. NF‐κB inhibitors in and of themselves had little effect on conjunctival tolerance to OVA in non‐DS mice, as we have shown previously 11. To account for strain‐specific differences in the immune response, the experiments described above were performed separately in both BALB/c (Fig. 1) and C3H mice (data not shown), and as the results were comparable, all subsequent experiments were carried out with BALB/c mice. Together, these results show that DS induces a time‐dependent disruption of mucosal tolerance in mice, an event that is mediated by NF‐κB activation in the ocular surface.
Figure 1.
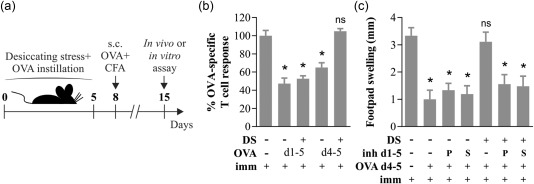
Desiccating stress (DS) disrupts ocular mucosal tolerance in a nuclear factor kappa B (NF‐κB)‐dependent fashion. (a) Experimental design for assessing the effect of DS on ocular surface immune tolerance. Mice subjected to normal environmental conditions or DS for 5 days were instilled ovalbumin (OVA) daily in both eyes from days 1 to 5 (d1–5) or on days 4 and 5 only (d4–5), then immunized (imm) subcutaneously (s.c.) with OVA + complete Freund's adjuvant (CFA) and assayed 1 week later. (b) In‐vitro T cell proliferation of splenocytes harvested on day 15 and stimulated with OVA over a 4‐day culture period, as assayed by carboxyfluorescein succinimidyl ester (CFSE) dilution. Results are expressed relative to the proliferative fraction observed in non‐instilled, OVA‐immunized mice. (c) In‐vivo delayed‐type hypersensitivity (DTH) assay (OVA‐induced footpad swelling) measured 48 h after challenge. For this experiment, NF‐κB inhibitors (inh d1–5) pyrrolidine dithiocarbamate (PDTC) (P) or sulphasalazine (S) were instilled daily from days 1 to 5 during DS induction, and OVA was instilled daily only on days 4 and 5. Results are shown as the difference between the antigen‐injected and phosphate‐buffered saline (PBS)‐injected footpads. Data are expressed as mean ± standard error of the mean (s.e.m.) (n = 3–5 experiments, 3–5 mice/group, BALB/c mice). *Indicates a statistically significant difference with the control group (immunized only), as determined by analysis of variance (anova) and Dunnett's post‐hoc test.
DS modifies the type of antigen‐specific T cells induced at the ocular mucosal surface
Once we had established that DS leads to a time‐dependent breakdown of ocular mucosal tolerance, we studied the antigen‐specific T cell response directly in the eye‐draining lymph nodes. To allow for appropriate comparisons, the experimental design was modified slightly to include either early (days 1–3, d1–3) or delayed OVA instillation (days 4–6, d4–6) relative to DS (Fig. 2a). As mucosal tolerance elsewhere relies upon antigen‐specific Tregs, we first tested if such cells were actually being induced in the eye‐draining lymph nodes of mice that received early or delayed OVA. Given the low frequency of antigen‐specific T cells in wild‐type mice, we resorted to the LAT assay due to its high sensitivity 22. For this experiment, T cells from the eye‐draining lymph nodes of DS, DS + OVA d1–3, and DS + OVA d4–6 mice were mixed with effector T cells from OVA‐immunized mice and OVA‐pulsed antigen‐presenting cells, and then injected into the footpads of naive mice. As shown in Fig. 2b, T cells from DS + OVA d1–3 mice suppressed the antigen‐specific DTH response significantly, whereas T cells from DS + OVA d4–6 mice had no effect compared to antigen‐naive DS mice. These findings confirmed that antigen‐specific Tregs were expanded in donor mice when OVA was administered on the first 3 days of DS, but not when administered later. Conversely, disruption of mucosal tolerance should implicate the induction of antigen‐specific effector T cells, and DS has been shown to favour Th1 and Th17 responses 5, 13. Therefore, we also tested for OVA‐specific interferon (IFN)‐γ‐ and IL‐17‐secreting T cells in the eye‐draining lymph nodes, and to overcome the low frequency of such cells we first expanded the rare antigen‐specific T cells in vitro. As depicted in Fig. 2c, after one round of OVA‐specific expansion both IFN‐γ+ and IL‐17+ CD4+ cells were increased significantly in the lymph node cultures of DS + VA d4–6 mice but not in DS + OVA d1–3 mice compared to antigen‐naive mice. This shows that antigen‐specific effector T cells were favoured after 3 days of DS, but not before. Moreover, the lack of expansion in DS + OVA d1–3 mice despite antigenic exposure is consistent with the detection of OVA‐specific Tregs by the LAT assay. These results show that DS induces a time‐dependent change in the antigen‐specific T cells induced at the ocular mucosal surface: from the basal‐state Treg‐controlled response to another dominated by the expansion of IFN‐γ‐ and IL‐17‐producing effector T cells.
Figure 2.
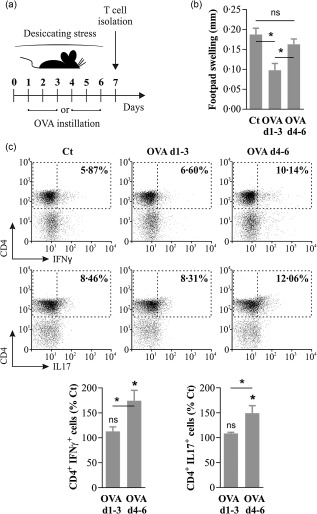
Effector T cells are favoured instead of regulatory T cells (Tregs) after 3 days of desiccating stress (DS). (a) Experimental design for assessing the effect of DS on the T cell response specific for an ocular surface antigen. Mice subjected to DS were instilled ovalbumin (OVA) daily in both eyes from days 1 to 3 (d1–3) or from days 4 to 6 (d4–6), and their T cells were isolated on day 7. (b) Local adoptive transfer assay for antigen‐specific suppression. T cells from the submandibular lymph nodes of either antigen‐naive mice (Ct), ovalbumin (OVA) d1–3 or OVA d4–6 mice were isolated and mixed with T cells from OVA + complete Freund's adjuvant (CFA)‐immunized mice and OVA‐pulsed antigen‐presenting cells, then injected into the footpads of naive mice. Swelling was measured 24 h later and expressed as the difference from preinjection footpad thickness. (c) Representative dot plots (top) and bar graphs (bottom) of cytokine production as assayed by intracellular flow cytometry in OVA‐expanded CD4+ T cells from Ct, OVA d1–3 and OVA d4–6 mice. Data are expressed as mean ± standard error of the mean (s.e.m.) (n = 3 experiments, 3 mice/group, BALB/c mice); *n.s. indicates a statistically significant or non‐significant difference, respectively, between the marked groups or with the control group, as determined by analysis of variance (anova) and Sidak's post‐hoc test.
Prevention of mucosal tolerance disruption at the ocular surface ameliorates DED
Mice under DS have markedly reduced tear secretion due to the effect of muscarinic blockade by scopolamine (for the first 5 days), but then fail to recover tear production rapidly because a CD4+ T cell‐dependent immune response develops against the cornea, conjunctiva and lacrimal glands 8, 21. We hypothesized that, if disruption of ocular surface immune tolerance was indeed a pathogenic event in DED and not a mere epiphenomenon of local inflammation, its prevention by NF‐κB inhibitor PDTC during DS should slow disease development. We assessed DED in mice by measuring corneal epithelial damage, tear production and proinflammatory cytokine secretion (IL‐1β and IL‐6), three parameters in clinical use 4. As shown in Fig. 3a, DS + inhibitor mice had lower fluorescein staining scores than DS mice on day 5, evidencing less corneal epithelial damage and DED severity 19. As expected, due to the muscarinic blockade, both DS and DS + inhibitor mice had reduced tear secretion during DS induction (Fig. 3b). Of note, during this period DS + inhibitor mice exhibited slightly less tear secretion than DS + mice, which could be ascribed to the lower extent of corneal damage and the associated reflex tear production in the former group. By contrast, DS + inhibitor mice recovered to baseline levels, on average, 3–4 days before DS mice. Compared with DS mice, tear production was increased significantly in DS + inhibitor mice after cessation of DS for up to 10 days. Regarding cytokine levels at the ocular surface, eye explants obtained on day 5 from DS mice produced higher IL‐1β and IL‐6 concentrations, which are also observed in DED patients 4 (Fig. 3c). By contrast, comparable explants from DS + inhibitor mice released near‐control levels of these proinflammatory cytokines. In other words, reduced corneal damage and inflammatory markers were observed in DS + inhibitor mice at a time‐point (day 5) in the disease model when there was almost no difference in tear levels and DS was constant, suggesting that a pathogenic host response was being modified by NF‐κB inhibition.
Figure 3.
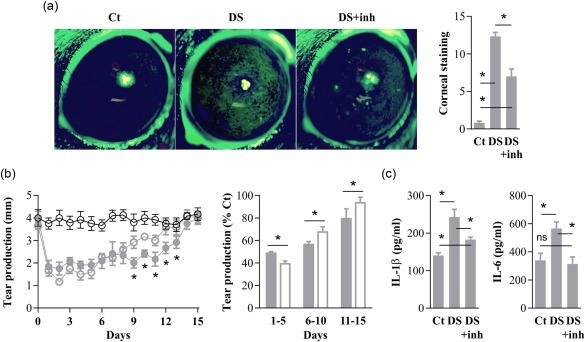
Topical nuclear factor kappa B (NF‐κB) inhibition improves desiccating‐stress (DS)‐induced dry eye. Mice were kept under no environmental stress (Ct) or exposed to DS with saline instillation DS or with topical NF‐κB inhibitor pyrrolidine dithiocarbamate (PDTC) (DS+inh) for 5 days. (a) Corneal epithelial integrity on day 5 in Ct, DS and DS + inh mice, as measured by corneal fluorescein staining. Representative photographs (left) of pooled data graded as described in Methods (right). (b) Daily tear production measurements in Ct mice (empty black circles), in DS mice (filled grey circles or bars) and in DS + inh mice (empty grey circles or bars). Representative experiment with absolute measurements (left) and all data (right) pooled and analysed in 5‐day time‐periods, expressed as relative to Ct mice. (c) Interleukin (IL)‐1β and IL‐6 production by eye explants obtained on day 5 from Ct, DS and DS + inh mice. Data are expressed as mean ± standard error of the mean (s.e.m.) (n = 3–5 experiments, 3–5 mice/group, BALB/c mice); *n.s. indicates a statistically significant or non‐significant difference, respectively, between the marked groups, as determined by analysis of variance (anova) and Sidak's post‐hoc test.
Prevention of mucosal tolerance disruption at the ocular surface blunts the development of a proinflammatory T cell response in the lymph nodes
As DED is mediated by an autoimmune T cell response 8, we examined the eye‐draining lymph nodes to determine if topical NF‐κB inhibitor PDTC was indeed affecting the T cell compartment. Day 5 of DS was selected as a representative time‐point because, as shown previously, environmental stress and tear production were comparable between DS and DS + inhibitor treatments. It should be noted that these experiments did not include OVA instillation because they were intended to examine the overall T cell response, including those T lymphocytes specific for ocular surface antigens. We observed no difference in the total number of CD3+ and CD3+CD4+ cells between control, DS and DS + inhibitor mice (data not shown). Nevertheless, T cells from DS mice proliferated more upon anti‐CD3 stimulation than those from control and DS + inhibitor mice (Fig. 4a), which could be interpreted as decreased regulatory T cell activity 23. In line with this finding, we detected in DS mice, but not in DS + inhibitor mice, higher numbers of activated CD4+ T cells (CD69+ and CD25+) and memory CD4+ T cells (CD62Llo CD44hi), and lower numbers of CD4+ FoxP3+ T cells than in control mice (Fig. 4b). These phenotypical differences are indeed suggestive of reduced number and activity of regulatory T cells in DS mice, as described previously 23, which in turn favour pathogenic Th1 and Th17 cell expansion 5, 23, 24, 25. Consistently, we detected increased IFN‐γ and IL‐17A production by CD4+ T cells in DS mice, but not in DS + inhibitor mice.
Figure 4.
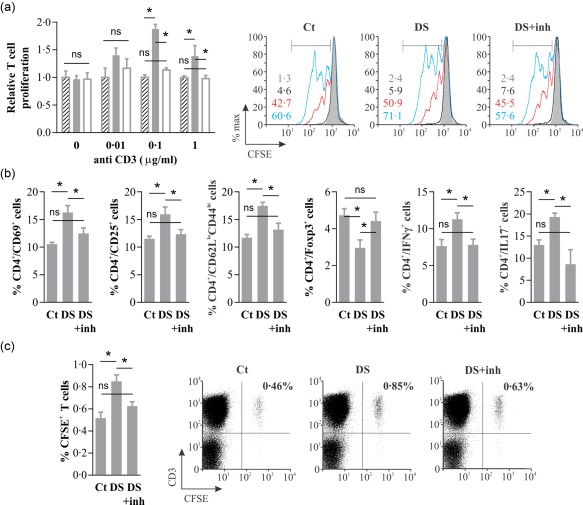
Topical nuclear factor kappa B (NF‐κB) inhibition prevents the development of a proinflammatory T cell response. Mice were kept under no environmental stress (Ct) or exposed to desiccating stress (DS) with saline instillation (DS) or with topical NF‐κB inhibitor pyrrolidine dithiocarbamate (PDTC) (DS + inh) for 5 days. (a) T cell proliferation in submandibular lymph node cells from Ct (hatched bars), DS (filled bars) and DS + inh (empty bars) mice on day 5, as induced by different anti‐CD3 antibody concentrations. Pooled data expressed relative to Ct mice (left). Representative experiment (right) with overlaid histograms from cultures with 0 (grey filled), 0·01 (black), 0·1 (red) and 1 (blue) µg/ml anti‐CD3 antibody, and the corresponding proliferative fraction shown in the same order. (b) Phenotype and cytokine production in CD4+ T cells obtained on day 5 from submandibular lymph nodes of Ct, DS and DS + inh mice. (c) Adoptive transfer of CD3+ T cells harvested from Ct, DS and DS + inh mice on day 5, labelled with carboxyfluorescein succinimidyl ester (CFSE) and injected into recipients (15 × 106 cells/mouse) that were subjected to mild desiccating stress (forced airflow without scopolamine) for 3 days. Pooled data (left) and representative dot plots (right) of cervical lymph node cells as analysed by fluorescence activated cell sorter (FACS). Data are expressed as mean ± standard error of the mean (s.e.m.) (n = 3–5 experiments, 3–5 mice/group, BALB/c mice); n.s. indicates a statistically significant or non‐significant difference, respectively, between the marked groups, as determined by analysis of variance (anova) and Sidak's post‐hoc test.
Lending further credit to the observed phenotypical and functional differences in the T cell response, we detected a significantly higher number of donor T cells in the cervical lymph nodes (and also in inguinal lymph nodes) of mice that were transferred adoptively with cervical lymph node cells from DS animals (Fig. 4c) and then subjected to DS themselves. Cagemates that were transferred adoptively with cells from DS + inhibitor mice showed less donor T cell homing to lymph nodes, being not significantly different from those that received cells from control mice (Fig. 4c). The same tendency was observed when donor CD4+ and CD8+ T cell migration was analysed separately (data not shown). These findings are consistent with memory T cell migration and in line with the increase in effector and effector memory T cells reported in the eye‐draining lymph nodes of DS mice 13.
Discussion
The conception of DED as the end result of an autoimmune response that is initiated by challenging environmental conditions and directed towards the ocular surface 12 poses intriguing questions, and at the same time opens new avenues for therapeutic intervention. Regarding the experimental proof, the implications of this hypothesis are twofold: on one hand, that there are protective mechanisms in place at the ocular surface under normal circumstances, and on the other hand that these processes are over‐run by a harsh environment and allow for an autoimmune response to ensue. Supporting the first claim, we have reported previously that under physiological conditions, the conjunctiva drives the expansion of suppressor T cells in the draining lymph nodes 10, and Siemasko et al. demonstrated the homeostatic role of CD4+FoxP3+CD25+ cells in the ocular surface 26. Contrastingly, evidence for the second implication was lacking, due to the unknown identity of the autoantigens involved in DED.
In this work, we show for the first time that DS (which typifies challenging environmental conditions) leads to a generalized disruption of ocular surface immune tolerance by using a different approach: the introduction of a known antigen to the ocular surface milieu (Fig. 1a). Antigen‐specific effector T cells were induced in mice under DS instead of regulatory T cells, which led to full‐blown DTH responses (Fig. 1b). Strikingly, mucosal tolerance disruption at the ocular surface was detected only if antigen administration was delayed for at least 3 days of DS, irrespective of whether it was continued beyond this point. This finding supports the pivotal role of antigen‐specific Tregs, which are induced early in the course of DS from naive T cells, and prevent any further development of effector T cells (Fig. 2). In other words, at least 3 days of DS were required to modify the imprint that the ocular surface exerts on migrating antigen‐presenting cells 27, which may indicate that a threshold of ocular surface damage must be surpassed in order for mucosal tolerance to be overcome. In line with this idea, the immune deviation at the ocular surface coincided in time with the early peak described for IFN‐γ secretion by conjunctival NK cells, which favours proinflammatory DC maturation 28. DS has been shown to induce early changes in the ocular surface epithelium that typify a stress response, which include increased retinoic acid early inducible gene 1 expression 29 and mitogen‐activated protein kinase activation 30. NF‐κB activation is a common downstream event in most epithelial stress responses 14, and increased NF‐κB signalling in the corneal and conjunctival epithelia has been reported in different models of ocular surface disorders 11, 31. The relevance of epithelial NF‐κB activation lies in the decisive role it plays in the mucosal immune outcome 14, 15, 16. Whereas reduced basal NF‐κB activity leads to tolerogenic conditioning of dendritic cells and induction of Tregs, increased epithelial NF‐κB signalling promotes immunogenic dendritic cell maturation and effector T cell generation in the lymph nodes 11, 16, 32. Consistently, our finding of DS‐induced mucosal tolerance disruption is also dependent upon local NF‐κB activation (Fig. 1c).
The observed skewing of the ocular surface's immune response towards an exogenous antigen could be regarded as an epiphenomenon of the already described and well‐characterized Th1 and Th17 responses against corneal and conjunctival antigens 5, 13. In other words, epithelial NF‐κB signalling could be a side effect of local NK cell and/or effector Th1 and Th17 cell activation, relatively independent of these key pathogenic events in DED onset. However, topical inhibition of NF‐κB activity under DS reduced disease signs markedly in the murine model, highlighting the importance of this step in DED progression (Fig. 3). This assertion is supported further by the observed changes in the underlying T cell response. Maintenance of mucosal tolerance at the ocular surface by reducing local NF‐κB activation led to increased regulatory T cell activity and decreased effector Th1 and Th17 responses (Fig. 4), which are ultimately responsible for the corneal and conjunctival damage that typifies DED. Of note, these changes were observed in the entire T cell compartment of the eye‐draining lymph nodes and were not restricted to OVA‐specific T cells, as there was no antigen instillation in those experiments. Our findings are consistent with the reported relevance of NF‐κB signalling in immune homeostasis in other mucosal sites. In asthma, a paradigmatic example of disrupted mucosal tolerance towards foreign antigens, NF‐κB inhibitors can prevent pathogenic T cell responses 33. Moreover, inflammatory bowel disease, which is caused by a dysregulated mucosal immune response towards commensal flora 34, is associated with loss of oral tolerance to fed antigens 35. Sulphasalazine and its analogues have been in clinical use for decades for local treatment of this disorder 36, and other NF‐κB inhibitors can ameliorate colitis in murine models 37. Regarding the relevance of NF‐κB pathway in ocular surface homeostasis, targeted disruption of IκBζ (a negative regulator of NF‐κB activity) in mice led to a marked inflammatory phenotype with loss of goblet cells and CD4+ cell infiltration 38. Also, a knock‐in mouse model of enhanced NF‐κB activity resulted in increased T cell activation and a Sjögren syndrome‐like phenotype 39 and, conversely, targeted loss of a NF‐κB regulator led to chronic intestinal mucosal inflammation 40. In line with these reports, alternative (anti‐inflammatory) NF‐κB activation induced by an oligonucleotide improved Sjögren's syndrome in another murine model 41. Finally, it should be mentioned that the DED model used in this work requires both scopolamine injections and forced air flow to reproduce a full‐blown eye disease. Systemic scopolamine is known to have effects per se on T cells 42 and endothelial cells 43, so our findings should be corroborated in other models of DED that do not employ this anticholinergic 44, 45, 46.
In summary, our findings show that DS disrupts the ocular surface's immune homeostasis, as evidenced by mucosal tolerance or lack thereof. Moreover, we also observed that topical modulation of NF‐κB activation leads to improvement of clinical markers of DED in mice exposed to DS, suggesting that disruption of the ocular surface's immune tolerance is an important pathogenic event. These findings provide experimental evidence for the dysregulated autoimmune hypothesis for DED 12. As mechanistic insight, we demonstrate that topical NF‐κB inhibitors can prevent the DS‐induced skewing in the mucosal immune response towards a Th1/Th17 phenotype, and thus block the development of the effector T cell response that causes DED. Thus, attempts at modulating mucosal tolerance could have a positive impact on DED.
Disclosure
All the authors have no disclosures to declare.
Acknowledgements
This study was funded by Agencia Nacional de Promoción Científica y Tecnológica (PICT 2013‐1436), Pan‐American Association of Ophthalmology, Fundación Alberto J. Roemmers, Fundación Allende.
References
- 1. Baudouin C, Aragona P, Messmer EM et al Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meeting. Ocul Surf 2013; 11:246–58. [DOI] [PubMed] [Google Scholar]
- 2. Calonge M, Enríquez‐de‐Salamanca A, Diebold Y et al Dry eye disease as an inflammatory disorder. Ocul Immunol Inflamm 2010; 18:244–53. [DOI] [PubMed] [Google Scholar]
- 3. Yoon K‐C, Jeong I‐Y, Park Y‐G, Yang S‐Y. Interleukin‐6 and tumor necrosis factor‐alpha levels in tears of patients with dry eye syndrome. Cornea 2007; 26:431–7. [DOI] [PubMed] [Google Scholar]
- 4. Jee D, Park SH, Kim MS, Kim EC. Antioxidant and inflammatory cytokine in tears of patients with dry eye syndrome treated with preservative‐free versus preserved eye drops. Invest Ophthalmol Vis Sci 2014; 55:5081–9. [DOI] [PubMed] [Google Scholar]
- 5. Zhang X, Chen W, De Paiva CS et al Desiccating stress induces CD4+ T‐cell‐mediated Sjögren's syndrome‐like corneal epithelial apoptosis via activation of the extrinsic apoptotic pathway by interferon‐γ. Am J Pathol 2011; 179:1807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reinoso R, Calonge M, Castellanos E et al Differential cell proliferation, apoptosis, and immune response in healthy and evaporative‐type dry eye conjunctival epithelia. Invest Ophthalmol Vis Sci 2011; 52:4819–28. [DOI] [PubMed] [Google Scholar]
- 7. Zhou XQ, Wei RL. Topical cyclosporine A in the treatment of dry eye: a systematic review and meta‐analysis. Cornea 2014; 33:760–7. [DOI] [PubMed] [Google Scholar]
- 8. Niederkorn JY, Stern ME, Pflugfelder SC et al Desiccating stress induces T cell‐mediated Sjögren's syndrome‐like lacrimal keratoconjunctivitis. J Immunol 2006; 176:3950–7. [DOI] [PubMed] [Google Scholar]
- 9. Egan RM. In vivo behavior of peptide‐specific T cells during mucosal tolerance induction: antigen introduced through the mucosa of the conjunctiva elicits prolonged antigen‐specific T cell priming followed by anergy. J Immunol 2000; 164:4543–50. [DOI] [PubMed] [Google Scholar]
- 10. Galletti JG, Gabelloni ML, Morande PE et al Benzalkonium chloride breaks down conjunctival immunological tolerance in a murine model. Mucosal Immunol 2013; 6:24–34. [DOI] [PubMed] [Google Scholar]
- 11. Guzmán M, Sabbione F, Gabelloni ML et al Restoring conjunctival tolerance by topical nuclear factor kappa B inhibitors reduces preservative facilitated allergic conjunctivitis in mice. Invest Ophthalmol Vis Sci 2014; 55:6116–26. [DOI] [PubMed] [Google Scholar]
- 12. Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol 2013; 32:19–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Y, Chauhan SK, Lee HS, Saban DR, Dana R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol 2014; 7:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the ‘epimmunome’. Nat Immunol 2010; 11:656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zaph C. Epithelial‐cell‐intrinsic IKK‐beta expression regulates intestinal immune homeostasis. Nature 2007; 446:552–6. [DOI] [PubMed] [Google Scholar]
- 16. Pasparakis M. Role of NF‐κB in epithelial biology. Immunol Rev 2012; 246:346–58. [DOI] [PubMed] [Google Scholar]
- 17. Lan W, Petznick A, Heryati S, Rifada M, Tong L. Nuclear factor‐κB: central regulator in ocular surface inflammation and diseases. Ocul Surf 2012; 10:137–48. [DOI] [PubMed] [Google Scholar]
- 18. Dursun D, Wang M, Monroy D et al A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci 2002; 43:632–8. [PubMed] [Google Scholar]
- 19. Chun YS, Park IK. Reliability of 4 clinical grading systems for corneal staining. Am J Ophthalmol 2014; 157:1097–102. [DOI] [PubMed] [Google Scholar]
- 20. Galletti JG, Cañones C, Morande PE et al Chronic lymphocytic leukemia cells bind and present the erythrocyte protein band 3: possible role as initiators of autoimmune hemolytic anemia. J Immunol 2008; 181:3674–83. [DOI] [PubMed] [Google Scholar]
- 21. Yoon K‐C, Ahn K‐Y, Choi W et al Tear production and ocular surface changes in experimental dry eye after elimination of desiccating stress. Invest Ophthalmol Vis Sci 2011; 52:7267–73. [DOI] [PubMed] [Google Scholar]
- 22. Jankowska‐Gan E, Hegde S, Burlingham WJ. Trans‐vivo delayed type hypersensitivity assay for antigen specific regulation. J Vis Exp 2013; e4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chauhan SK, El Annan J, Ecoiffier T et al Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol 2009; 182:1247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang X, Volpe EA, Gandhi NB et al NK cells promote Th‐17 mediated corneal barrier disruption in dry eye. PLOS ONE 2012; 7:e36822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng X, de Paiva CS, Li D‐Q, Farley WJ, Pflugfelder SC. Desiccating stress promotion of Th17 differentiation by ocular surface tissues through a dendritic cell‐mediated pathway. Invest Ophthalmol Vis Sci 2010; 51:3083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siemasko KF, Gao J, Calder VL et al In vitro expanded CD4+CD25+Foxp3+ regulatory T cells maintain a normal phenotype and suppress immune‐mediated ocular surface inflammation. Invest Ophthalmol Vis Sci 2008; 49:5434–40. [DOI] [PubMed] [Google Scholar]
- 27. Schaumburg CS, Siemasko KF, De Paiva CS et al Ocular surface APCs are necessary for autoreactive T cell‐mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol 2011; 187:3653–62. [DOI] [PubMed] [Google Scholar]
- 28. Chen Y, Chauhan SK, Saban DR, Sadrai Z, Okanobo A, Dana R. Interferon‐γ‐secreting NK cells promote induction of dry eye disease. J Leukoc Biol 2011; 89:965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coursey TG, Bohat R, Barbosa FL, Pflugfelder SC, de Paiva CS. Desiccating stress‐induced chemokine expression in the epithelium is dependent on upregulation of NKG2D/RAE‐1 and release of IFN‐γ in experimental dry eye. J Immunol 2014; 193:5264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luo L, Li D‐Q, Doshi A, Farley WJ, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP‐9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci 2004; 45:4293–301. [DOI] [PubMed] [Google Scholar]
- 31. Li D‐Q, Luo L, Chen Z, Kim H‐S, Song XJ, Pflugfelder SC. JNK and ERK MAP kinases mediate induction of IL‐1beta, TNF‐alpha and IL‐8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res 2006; 82:588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wullaert A, Bonnet MC, Pasparakis M. NF‐κB in the regulation of epithelial homeostasis and inflammation. Cell Res 2011; 21:146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El‐Hashim AZ, Renno WM, Abduo HT, Jaffal SM, Akhtar S, Benter IF. Effect of inhibition of the ubiquitin–proteasome‐system and IκB kinase on airway inflammation and hyperresponsiveness in a murine model of asthma. Int J Immunopathol Pharmacol 2011; 24:33–42. [DOI] [PubMed] [Google Scholar]
- 34. Xu X‐R, Liu C‐Q, Feng B‐S, Liu Z‐J. Dysregulation of mucosal immune response in pathogenesis of inflammatory bowel disease. World J Gastroenterol 2014; 20:3255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arrieta M‐C, Madsen KL, Field CJ, Meddings JB. Increasing small intestinal permeability worsens colitis in the IL‐10‐/‐ mouse and prevents the induction of oral tolerance to ovalbumin. Inflamm Bowel Dis 2015; 21:8–18. [DOI] [PubMed] [Google Scholar]
- 36. Weber CK, Liptay S, Wirth T, Adler G, Schmid RM. Suppression of NF‐kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology 2000; 119:1209–18. [DOI] [PubMed] [Google Scholar]
- 37. Shibata W, Maeda S, Hikiba Y et al Cutting edge: the IkappaB kinase (IKK) inhibitor, NEMO‐binding domain peptide, blocks inflammatory injury in murine colitis. J Immunol 2007; 179:2681–5. [DOI] [PubMed] [Google Scholar]
- 38. Ueta M, Hamuro J, Yamamoto M, Kaseda K, Akira S, Kinoshita S. Spontaneous ocular surface inflammation and goblet cell disappearance in I kappa B zeta gene‐disrupted mice. Invest Ophthalmol Vis Sci 2005; 46:579–88. [DOI] [PubMed] [Google Scholar]
- 39. Peng B, Ling J, Lee AJ et al Defective feedback regulation of NF‐kappaB underlies Sjogren's syndrome in mice with mutated kappaB enhancers of the IkappaBalpha promoter. Proc Natl Acad Sci USA 2010; 107:15193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang M, Lee AJ, Fitzpatrick L, Zhang M, Sun S‐C. NF‐kappa B1 p105 regulates T cell homeostasis and prevents chronic inflammation. J Immunol 2009; 182:3131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gilboa‐Geffen A, Wolf Y, Hanin G et al Activation of the alternative NFκB pathway improves disease symptoms in a model of Sjogren's syndrome. PLOS ONE 2011; 6:e28727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen Y, Chauhan SK, Lee HS et al Effect of desiccating environmental stress versus systemic muscarinic AChR blockade on dry eye immunopathogenesis. Invest Ophthalmol Vis Sci 2013; 54:2457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saeed RW, Varma S, Peng‐Nemeroff T et al Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med 2005; 201:1113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Turpie B, Yoshimura T, Gulati A, Rios JD, Dartt DA, Masli S. Sjögren's syndrome‐like ocular surface disease in thrombospondin‐1 deficient mice. Am J Pathol 2009; 175:1136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Contreras‐Ruiz L, Regenfuss B, Mir FA, Kearns J, Masli S. Conjunctival inflammation in thrombospondin‐1 deficient mouse model of Sjögren's syndrome. PLOS ONE 2013; 8:e75937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stevenson W, Chen Y, Lee S‐M et al Extraorbital lacrimal gland excision: a reproducible model of severe aqueous tear‐deficient dry eye disease. Cornea 2014; 33:1336–41. [DOI] [PubMed] [Google Scholar]


