Summary
The systemic inflammatory response is a challenge in the management of paediatric patients undergoing cardiac surgery. Although multi‐factorial, a contribution by the lectin pathway of complement activation has been postulated. We therefore investigated the changes in serum levels of mannose binding lectin (MBL) and activities of MBL–MBL‐associated serine protease (MASP)‐1 and MBL–MASP‐2 complexes immediately before and during surgery, throughout the first postoperative day and at discharge from the hospital. These changes were analysed in relation to postoperative complications. Blood samples were obtained from 185 children with congenital heart disease undergoing surgical correction with the use of cardiopulmonary bypass: preoperatively (MBL‐1), 15 min after initiation of cardiopulmonary bypass (CPB) (MBL‐E), 30 min (MBL‐2), 4 h (MBL‐3), 12 h (MBL‐4) and 24 h (MBL‐5) post‐CPB and at discharge from hospital (MBL‐K). Alterations in serum MBL levels were calculated as a ratio of its serum level at subsequent time‐points (MBL‐2, ‐3, ‐4, ‐5) to the preoperative (MBL‐1) value. Decreases in MBL and MBL–MASP complexes were observed in all samples, correlating with a decrease in C4 and increase in C4a, confirming activation of the lectin pathway. Changes in MBL levels between children with an uncomplicated postoperative course and those suffering from infection or low cardiac output syndrome did not differ significantly, but significant differences were observed between the SIRS and non‐SIRS groups. Paediatric cardiac surgery with the use of cardiopulmonary bypass activates the complement system via the lectin pathway and the latter contributes to the development of the post‐bypass systemic inflammatory response.
Keywords: cardiopulmonary bypass, complement, immunology, inflammation systemic, paediatric
Introduction
Despite progress in the perfusion technique (circuit miniaturization, heparin‐coated circuits, ultrafiltration) and the introduction of anti‐inflammatory pharmacological strategies (steroids), the systemic inflammatory response syndrome (SIRS) is still a challenge in the management of paediatric patients undergoing cardiac surgery 1, 2, 3, 4. Postoperative SIRS presents with a wide range of clinical manifestations from transient fever to cardiovascular collapse and organ dysfunction, prolonging stay in intensive care and increasing costs 3, 4. Post‐bypass inflammation is a complex phenomenon involving multiple interdependent cellular and humoral pathways, among which the complement system plays a central role 5, 6. The complement system consists of approximately 40 plasma or cell surface proteins 7. Three major activation pathways have been described: the classical pathway (activated by antigen–antibody complexes); the alternative pathway (activated by microbial surface structures enhancing spontaneous C3 hydrolysis); and the lectin pathway (activated by ficolins and some collectins). Some of the most biologically active products of complement activation, C3a, iC3b, C5a and C5b‐9, are potent mediators involved in inflammatory injury.
There are three critical moments for complement activation by CPB: first, during the contact of heparinized blood with the membranes of the circuit; secondly, after reperfusion of the ischaemic myocardium; and thirdly, after heparin–protamine complex production. Previous studies have confirmed activation via classical and alternative pathways (CP and AP). CPB membranes and myocardial reperfusion activate both CP and AP, whereas protamine–heparin complexes, endotoxins, C‐reactive protein and apoptotic cells activate complement via the classical pathway 6.
The role of the lectin pathway is perhaps still underestimated, although its involvement in ischaemia/reperfusion injury and in the development of cardiovascular disease has been reported. In particular, mannose‐binding lectin (MBL) has been implicated 8. Mannose‐binding lectin belongs to the collectin family, a group of C‐type lectins also possessing a collagen‐like helical domain 9, 10. MBL binds with the highest affinity to D‐mannose, N‐acetyl‐D‐glucosamine and L‐fucose, which allows specific recognition of numerous polysaccharides and glycoconjugates such as bacterial lipopolysaccharides, capsular polysaccharides, fungal mannans, etc. Moreover, it recognizes some phospholipids, Neisseria outer membrane proteins and DNA of apoptotic cells. The plasma concentration of MBL can increase up to threefold in response to infection. After binding of the MBL–MASP complex to its target structure, conformational changes lead to the activation of MASPs which, in consequence, makes the cleavage of C4 and C2 possible and thus the initiation of the complement cascade. In spite of a different initiation mechanism, the lectin pathway resembles the classical pathway (CP), forming the same convertases for C3 and C5 components. Circulating plasma levels of MBL are, to a large extent, determined genetically. Nevertheless, individuals carrying the same genotype exhibit a wide range of concentrations 11.
Lectin pathway activation during CPB was first reported by Marcheix et al. 12, who demonstrated that serum MBL concentration had decreased remarkably both after 30 min and after 240 min post‐CPB, presumably as a consequence of MBL consumption. Simultaneously, the levels of activated C3a and C4a increased during the first 30 min. Decreasing C5a and increasing membrane attack complex (C5b‐9) concentrations were observed. Other studies performed by Bilgin et al. 13 suggested that high serum MBL contributed to the development of sterile SIRS after cardiac surgery. At the same time, there are studies proving that MBL can be a potent factor involved in the development of the systemic inflammatory response in intensive care unit (ICU) patients 14, 15.
This topic has been addressed mainly in adult patients, yet children are generally more susceptible to the inflammatory response to CPB because of various factors including haemodilution, longer surgical time, changes in temperature, deep hypothermic circulatory arrest, lower perfusion rates, higher shear stress of blood cell components, higher metabolic requirements and transfusion of blood products 5. Furthermore, the innate immune response is particularly important during childhood, due to immaturity of adaptive immunity.
Our previous study 16 suggested a strong contribution of high preoperative levels of serum MBL (associated with high MBL‐producing genotypes) in the development of SIRS after paediatric cardiac surgery. Here we report data concerning lectin pathway activation during paediatric CPB.
Patients and methods
Patients
In total, 185 children, aged 3 months to 17 years (mean = 3 years and 4 months) with congenital heart disease on whom cardiac surgery with the use of cardiopulmonary bypass was performed, were recruited to this prospective study. They represented the majority of the cohort analysed previously in relation to preoperative MBL concentrations 16. Mean basic Aristotle score (BAS) was 7·62 [standard deviation (s.d.) = 2·03; median = 8]. Mean CPB time was 85·9 min (± 42·40), mean aorta cross‐clamping time was 42·9 min (± 27·24). The approval of the local ethics committee and written informed parental consent were obtained. The procedures performed were: atrial septal defect repair (n = 16), atrioventricular septal defect repair (n = 10), bidirectional cavopulmonary anastomosis (bidirectional Glenn) (n = 26), pulmonary atresia correction with homograft (n = 11), hybrid approach stage II procedure (n = 3), double outlet of right ventricle–intraventricular repair (n = 2), Rastelli procedure (n = 3), Ross procedure (n = 8), definitive correction of tetralogy of Fallot (n = 19), ventricular septal defect repair (n = 25), mitral valvuloplasty (n = 5), valve replacement (n = 6) and Fontan operation‐external conduit (50 patients). The cardiopulmonary bypass circuit consisted of Capiox®FX05 or FX15 (Terumo) x‐coated oxygenator with integrated arterial filter, non‐coated circuit (Chalice®), non‐pulsatile roller pump Stockert SIII. Leucocyte‐depleted red blood cells were added to the priming solution. Methylprednisolone (30 mg per kg body weight) was administered to all children aged less than 1 year at the induction of anaesthesia. The combination of on‐bypass ultrafiltration and post‐bypass modified ultrafiltration was performed.
The postoperative course was observed and documented until hospital discharge. Patients were screened for symptoms of postoperative complications: infection, systemic inflammatory response (consensus definition) 17 and organ dysfunction 17. Symptoms of SIRS were observed during the first 3 postoperative days; patients with tachycardia due to anxiety, increased doses of inotropic agents and tachyarythmias were not included. All patients with suspected precocious infection were excluded. Cardiovascular status was assessed using the vasoactive inotropic score (VIS) 18. Low cardiac output syndrome (LCOS) was defined according to criteria proposed by Hoffman et al. 19.
Exclusion criteria were: age less than 3 months, preoperative mechanical ventilation, preoperative infection or organ dysfunction and death during surgery
Median hospital stay until discharge or death was 11 days (range = 1–95).
Blood samples
Blood samples were collected in tubes without anti‐coagulant (for separation of serum to quantify MBL, MBL–MASPs complexes, C5a, C4) and in ethylenediamine tetraacetic acid (EDTA) tubes (for quantification of C4a) and stored at −80°C until testing. Samples were obtained immediately before surgery (MBL‐1), 15 min after initiation of CPB (MBL‐E) and 30 min post‐CPB (5 min after protamine administration) (MBL‐2). Further samples were obtained at 4 (MBL‐3), 12 (MBL‐4) and 24 (MBL‐5) h post‐CPB and in survivors just before hospital discharge (point MBL‐K; mean 14·3 postoperative day).
Samples for measurement of complement components, C4, C4a and C5a were taken preoperatively (point 1 = MBL‐1), 30 min post‐CPB (point 2 = MBL‐2) and 24 h post‐bypass (point 3 = MBL‐5).
MBL quantification and determination of MBL–MASP activities
Serum MBL concentration/activity was measured by enzyme‐linked immunosorbent assay (ELISA) based on solid‐phase mannan binding and detection using murine monoclonal (HYB131‐1; BioPorto, Hellerup, Denmark) anti‐human MBL antibody. The complementary forms of MBL activity, MBL–MASP‐2 complex activity 20 and MBL–MASP‐1 activity 21, were measured using an ELISA and a fluorescence [time‐resolved immunofluorometric assay (TRIFMA)] method, respectively. Alterations in serum MBL were calculated as a ratio of its serum levels at subsequent time‐points (MBL‐2, ‐3, ‐4, ‐5) to the preoperative (MBL‐1) value. Rabbit polyclonal anti‐human C4c antibodies (Dako, Glostrup, Denmark) and corresponding murine horseradish peroxidase (HRP)‐conjugated secondary antibodies (Dako) were used for detection of activated C4c fragment (for estimation of MBL–MASP‐2 complex activity). VPR‐AMC peptide (Bachem, Bubendorf, Switzerland) was used as the substrate for MASP‐1 22.
C4a determination in plasma
Quantitive determination of complement C4a was performed using complement C4a des Arg (human) ELISA kit (Enzo Biochem, New York, NY, USA).
C4 determination in sera
Measurement of C4 was performed using Vitros Chemistry Products C4 reagent in conjunction with Vitros Chemistry Products Calibrator Kit 20 and Vitros Chemistry Products FS Diluent Pack 2 on the Vitros 5,1 FS/4600 Chemistry and Vitros 5600 Integrated Systems (Ortho Clinical Diagnostics, Raritan, NJ, USA).
C5a determination in plasma
Analysis was performed using human complement C5a DuoSet ELISA kit (R&D Systems, Abingdon, UK), according to the manufacturer's recommendations.
Statistical analysis
Statistical parameters were calculated using the Statistica software package (StatSoft, Kraków, Poland). Data that were not distributed normally were presented as medians and the significance of the differences between medians was computed by the Mann–Whitney U‐test. Successive measurements were compared using the Wicoxon matched‐pairs test. P‐values < 0·05 were considered statistically significant.
Results
Thirty‐five patients (18·9%) fulfilled the criteria of CPB‐SIRS. Seven manifested fever, elevated leucocyte count and mild cardiovascular collapse (VIS ≤ 10) with no need for prolonged ICU stay. The remaining 28 children required significant inotropic support (VIS > 10) and LCOS was observed in 22 patients. Pulmonary infiltration with tachypnoea and need for oxygen supplementation was observed in eight patients, while 23 patients required mechanical ventilation for more than 24 h. Acute respiratory distress syndrome was diagnosed in one patient. Nineteen children met the criteria of renal dysfunction and renal replacement therapy was necessary in 12 patients; hepatic dysfunction was observed in 14 patients.
Infection was documented in 11 patients during the immediate postoperative period (up to the 7th day) and in 33 patients in total during total their hospital stay.
Changes in serum MBL levels and MBL–MASP activities during and after CPB
Serum MBL, MBL–MASP‐1 and MBL–MASP‐2 were measured in 185 patients. Thirty‐two patients (17·3%) had preoperative serum levels of MBL < 100 ng/ml and were excluded from further analyses. In the remaining 153 patients, we observed a significant decrease in MBL at all time‐points compared with the pre‐bypass level (Table 1). As would be expected, similar patterns were also observed for both MBL–MASP complexes. The largest decrease was observed in the first 15 min from by‐pass (sample MBL‐E) which was enhanced by blood dilution. These changes coincided with a decrease in C4 and a rise in C4a levels within 30 min post‐bypass (Fig. 1). C5a levels did not change significantly (Fig. 2).
Table 1.
Changes of mannose‐binding lectin (MBL) serum levels and corresponding MBL–MBL‐associated serine protease (MASP) activities during and after surgery.
| MBL concentration | MBL–MASP‐1 activity | MBL–MASP‐2 activity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (ng/ml) | (mU/ml) | (mU/ml) | |||||||
| Sample | Median | Mean | P * | Median | Mean | P * | Median | Mean | P * |
| 1 | 1246 | 1538 | 436 | 561 | 451 | 578 | |||
| E | 630 | 830 | <0·0001 | 281 | 341 | <0·0001 | 272 | 327 | <0·0001 |
| 2 | 964 | 1156 | <0·0001 | 359 | 426 | <0·0001 | 338 | 392 | <0·0001 |
| 3 | 1066 | 1353 | 0·04 | 415 | 545 | 0·004 | 411 | 489 | 0·95 |
| 4 | 1147 | 1327 | 0·02 | 409 | 530 | 0·11 | 370 | 456 | 0·08 |
| 5 | 888 | 1054 | 0·0003 | 335 | 445 | 0·24 | 314 | 388 | 0·008 |
| K | 1621 | 1829 | 0·002 | 518 | 721 | 0·67 | 465 | 557 | 0·003 |
*Median values compared with those of sample 1, using Wicoxon's matched‐pairs test.
Figure 1.
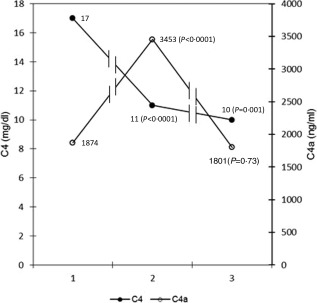
Serum levels of C4 and C4a measured: preoperatively [point 1: mannose‐binding lecton (MBL)‐1], 30 min (point 2: MBL‐2) and 24 h (point 3: MBL‐5) post‐ cardiopulmonary bypass (CPB). Median values from 20 patients were used.
Figure 2.
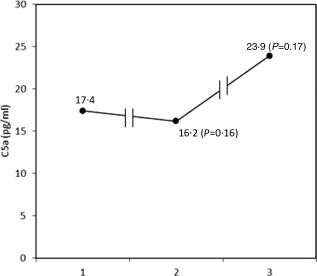
C5a median levels (n = 24) measured: preoperatively [point 1: mannose‐binding lectin (MBL)‐1], 30 min (point 2: MBL‐2) and 24 h (point 3: MBL‐5) post‐cardiopulmonary bypass (CPB).
Changes in serum MBL concentration expressed as median ratios of the values at 30 min post‐bypass to the pre‐operative values (MBL‐2/MBL‐1) were analysed in relation to postoperative complications (Table 2). No difference between children with an uncomplicated postoperative course and those suffering from infection, organ failure or low cardiac output syndrome was found. However, a statistically significant difference was observed with SIRS (Table 2). Indeed, a similar relationship with SIRS was found for all samples (MBL‐3/MBL‐1; MBL‐4/MBL‐1; MBL‐5/MBL‐1) taken during the first 24 h post‐bypass (Table 3, Fig. 3).
Table 2.
Changes in serum mannose‐binding lectin (MBL) level 30 min after weaning from cardiopulmonary bypass (CPB) (ratio MBL2/1) in relation to postoperative complications*.
| Complication | Yes | No | P |
|---|---|---|---|
| Infection | 0·65 | 0·78 | 0·62 |
| SIRS | 0·58 | 0·82 | 0·03 |
| LCOS | 0·82 | 0·77 | 0·35 |
| Renal insufficiency | 0·79 | 0·77 | 0·74 |
| Liver dysfunction | 0·58 | 0·78 | 0·63 |
| MODS | 0·78 | 0·77 | 0·91 |
*Decreases in serum MBL are expressed as the median ratio of values obtained at 30 min post‐bypass to the preoperative values (MBL‐2/MBL‐1). Only patients with preoperative values > 100 ng/ml were included. The significance of the differences was determined by the Mann–Whitney U‐test. LCOS = low cardiac output syndrome; MODS = multiple organ dysfunction syndrome.
Table 3.
Changes in serum mannose‐binding lectin (MBL) during the first 24 postoperative hours* in systemic inflammatory response (SIRS) and non‐SIRS patients.
| Ratios | SIRS | P | |
|---|---|---|---|
| Yes | No | ||
| MBL‐2/1 | 0.58 | 0.82 | 0.03 |
| MBL‐3/1 | 0.68 | 0.99 | 0.001 |
| MBL‐4/1 | 0.71 | 0.92 | 0.004 |
| MBL‐5/1 | 0.57 | 0.79 | 0.004 |
*Decreases in serum MBL were calculated as the median ratios of the median values obtained at the times indicated (see Methods section) to the corresponding presurgery (MBL‐1) values. The significance of the differences was then determined by the Mann–Whitney U‐test. The deepest decrease in MBL levels was observed in SIRS patients. Only patients with preoperative MBL values > 100 ng/ml were included.
Figure 3.
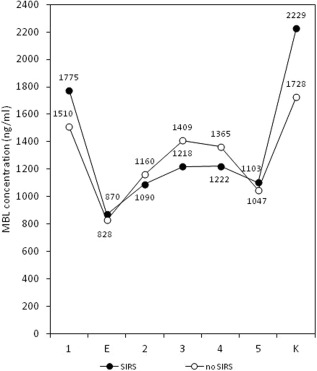
Serum mannose‐binding lectin (MBL) levels alterations during and after bypass surgery in systemic inflammatory response (SIRS) (n = 35) and non‐SIRS (n = 118) patients (mean values).
Discussion
Cardiac surgery using cardiopulmonary bypass stimulates an inflammatory response. More than 100 mechanisms participate in this phenomenon 5, 6, 23, but most studies of biomarkers have not investigated correlations between levels of markers and clinical outcome. The complement system may be a key mechanism in the host response to CPB, and that could include the lectin pathway. Its potent activator, mannose‐binding lectin, plays an important role in the development of cardiovascular disease and ischaemia–reperfusion injury 8. Lectin pathway involvement in the reaction to CPB during adult cardiac surgery was reported originally by Hoedemaekers et al. 24 and Marcheix et al. 12, but in the first report the authors did not examine the clinical relevance of this phenomenon, while in the second no association with postoperative complications was observed. Then Billgin et al. 13 suggested that fresh frozen plasma transfusion to MBL‐deficient patients led to the development of postoperative sterile SIRS. More recently, Hein et al. 25 demonstrated lectin pathway activation during cardiopulmonary bypass by measuring the residual capacity for complement activation mediated by MBL and ficolins which was parallel to the increase of C3a. The phenomenon was observed independently of the oxygenator/circuit type (heparin‐ or physio‐coated).
The potential role of the lectin pathway has not been investigated previously in a paediatric population, where mechanisms of innate immunity play an especially important role. Meanwhile, despite anti‐inflammatory strategies (coated circuits, steroids, leucocyte‐depleted red cells, ultrafiltration), SIRS still remains a challenge. The incidence of post‐bypass SIRS in paediatric patients is estimated at between 8·9 and 30·5% 3, 4, 26, depending on the criteria applied. It is associated with longer time of mechanical ventilation, longer time in ICU and total hospital stay, organ injury and death. In view of those considerations, we were prompted to investigate the potential role of the lectin pathway in complement‐mediated SIRS activation.
We observed a decrease in serum MBL at all time‐points during the surgery and first postoperative day. The deep decrease in MBL level 15 min after the beginning of CPB was enhanced by haemodilution, but in all samples taken on the day of surgery MBL remained lower than preoperatively. In numerous previous studies 12, 24, 25, 27, 28, complement activation was demonstrated by increasing serum levels of C3a but that does not indicate which route was responsible for its generation, as it is common to all pathways. As our research focused on the lectin pathway, we decided to measure C4a levels – a product of lectin and classical, but not alternative, pathways. We found that the drop in MBL levels was accompanied by concomitant changes in C4 and C4a levels, although classical pathway activation could have contributed. Conversely, lectin pathway activation could have been mediated partly by ficolins and/or other collectins. We failed to observe any increase in C5a levels, in agreement with previous studies 12, 27, 28, 29.
As mentioned, we used poly‐2‐methoxyethylacrylate (PMEA)‐coated (x‐coating) CPB oxygenator (TERUMO®) and non‐coated circuit (CHALICE®). The safety of x‐coated circuits has been postulated 30 but, conversely, other reports suggest the generation of inflammation despite their use 31. The efficacy of different types of circuit coatings in preventing an inflammatory response is still under investigation. In the above‐mentioned study, Hein et al. 25 confirmed lectin pathway activation, independently of the type of circuits used (heparin‐ or physio‐ coatings), and provided evidence for ficolin‐2 depletion during cardiopulmonary bypass using a heparin‐coated circuit. However, they did not find any significant decrease in MBL serum level, which was a remarkable phenomenon in our study. This discrepancy could have been caused by differences in tubings employed (uncoated in our study) and/or the difference in the numbers of patients examined. Furthermore, we excluded children with very low MBL serum level (<100 ng/ml) from the analysis. Although MBL is known to take part in ischaemia/reperfusion injury 32, we failed to prove its influence. The changing ratios for patients after surgery using aorta cross‐clamping and, without it, did not differ significantly, although we found a trend towards greater change in the former 30 min after weaning from CBP (P = 0·065) (not shown). The potential mechanism is still MBL‐dependent activation of complement on the surface of the cardiopulmonary bypass circuit. Nevertheless, the aim of our study was not to investigate the safety of specific circuits but to study the contribution of lectin pathway activation to SIRS development, using CPB as a model for generation of sterile inflammation.
Our results showed the deepest decrease in MBL levels in patients presenting with symptoms of post‐bypass sterile SIRS, strongly suggesting MBL involvement in the development of systemic inflammation. This is in agreement with our earlier observations, that postoperative SIRS is related to higher preoperative serum MBL levels and high MBL‐producing genotypes 16. Similar observations were reported by Smithson et al. for adult ICU patients 15.
Our study has at least two limitations. First, it is sometimes difficult to distinguish between sterile and infectious inflammation, so to avoid misinterpretation of the data we excluded all doubtful cases. Secondly, MBL levels can be influenced by haemodilution, which is especially significant immediately after the commencement of CPB. Some authors have used albumin level or haematocrit as a dilution indicator. It is not an issue in the paediatric population, because albumin and red blood cells are added to a priming solution. We omitted it deliberately, as complement activation was confirmed by measuring C4 and C4a and we analysed relative change of MBL levels between time‐points. A significant change was found only in the SIRS group.
Conclusions
Paediatric cardiac surgery with the use of cardiopulmonary bypass activates the complement system via the lectin pathway. Lectin pathway activation contributes to the development of post‐bypass systemic inflammatory response.
Disclosure
The authors have no disclosures to declare.
Acknowledgements
This study was supported by the Polish National Science Centre, grant no. UMO‐2011/03/B/NZ6/00052. The authors thank Dr David C. Kilpatrick for critical reading of the manuscript and helpful discussion and Mrs Jadwiga Ciechowicz from the Medical University of Lodz for her help with the statistical analysis.
References
- 1. Durandy Y. Minimizing systemic inflammation during cardiopulmonary bypass in the pediatric population. Artif Organs 2014; 38:11–8. [DOI] [PubMed] [Google Scholar]
- 2. Scrascia G, Rotunno C, Guida P et al Perioperative steroids administration in pediatric cardiac surgery: a meta‐analysis of randomized controlled trials. Pediatr Crit Care Med 2014; 15:435–42. [DOI] [PubMed] [Google Scholar]
- 3. Soares LC, Ribas D, Spring R, Silva JM, Miyague NI. Clinical profile of systemic inflammatory response after pediatric cardiac surgery with cardiopulmonary bypass. Arq Bras Cardiol 2010; 94:127–33. [DOI] [PubMed] [Google Scholar]
- 4. Güvener M, Korun O, Demirtürk OS. Risk factors for systemic inflammatory response after congenital cardiac surgery. J Card Surg 2015; 30:92–6. [DOI] [PubMed] [Google Scholar]
- 5. Kozik DJ, Tweddell JS. Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg 2006; 81:S2347–54. [DOI] [PubMed] [Google Scholar]
- 6. Stahl GL, Shernan SK, Smith PK, Levy JH. Complement activation and cardiac surgery: a novel target for improving outcomes. Anesth Analg 2012; 115:759–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Merle NS, Church SE, Fremeaux‐Bacchi V, Roumenina LT. Complement system part I ‐ molecular mechanisms of activation and regulation. Front Immunol 2015; 6:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pągowska‐Klimek I, Cedzyński M. Mannan‐binding lectin in cardiovascular disease. Biomed Res Int 2014; 2014:616817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turner MW. Mannose‐binding lectin: the pluripotent molecule of the innate immune system. Immunol Today 1996; 17:532–40. [DOI] [PubMed] [Google Scholar]
- 10. Kilpatrick DC. Mannan‐binding lectin and its role in innate immunity. Transfus Med 2002; 12:335–52. [DOI] [PubMed] [Google Scholar]
- 11. Cedzyński M, Świerzko AS, Kilpatrick D. Factors of the lectin pathway of complement activation and their clinical associations in neonates. J Biomed Biotechnol 2012; 2012:363246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marcheix B, Carrier M, Martel C et al Effect of pericardial blood processing on postoperative inflammation and the complement pathways. Ann Thorac Surg 2008; 85:530–5. [DOI] [PubMed] [Google Scholar]
- 13. Bilgin YM, Brand A, Berger SP. Mannose‐binding lectin is involved in multiple organ dysfunction syndrome after cardiac surgery: effects of blood transfusions. Transfusion 2008; 48:601–8. [DOI] [PubMed] [Google Scholar]
- 14. Garred P, Strom JJ, Quist L, Taaning E, Madsen HO, Association of mannose‐binding lectin polymorphisms with sepsis and fatal outcome, in patients with systemic inflammatory response syndrome. J Infect Dis 2003; 188:1394–403. [DOI] [PubMed] [Google Scholar]
- 15. Smithson A, Perello R, Aibar J et al Genotypes coding for low serum levels of mannose‐binding lectin are under represented among individuals suffering from noninfectious systemic inflammatory response syndrome. Clin Vaccine Immunol 2010; 17:447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pągowska‐Klimek I, Świerzko AS, Michalski M et al Mannose‐binding lectin (MBL) insufficiency protects against the development of systemic inflammatory response after pediatric cardiac surgery. Immunobiology 2016; 221:175–81. [DOI] [PubMed] [Google Scholar]
- 17. Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8. [DOI] [PubMed] [Google Scholar]
- 18. Gaies MG, Gurney JG, Yen AH et al Vasoactive‐inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010; 11:234–8. [DOI] [PubMed] [Google Scholar]
- 19. Hoffman TM, Wernovsky G, Atz AM et al Prophylactic intravenous use of milrinone after cardiac operation in pediatrics (PRIMACORP) study. Am Heart J 2002; 143:15–21. [DOI] [PubMed] [Google Scholar]
- 20. Cedzynski M, Szemraj J, Swierzko AS et al Mannan‐binding lectin (MBL) insufficiency in children with recurrent infections of the respiratory system. Clin Exp Immunol 2004; 136:304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Świerzko AS, Szala A, Sawicki S et al Mannose‐binding lectin (MBL) and MBL‐associated serine protease‐2 (MASP‐2) in women with malignant and benign ovarian tumours. Cancer Immunol Immunother 2014; 11:1129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Presanis J, Hajela K, Ambrus G, Gal P, Sim RB. Differential substrate and inhibitor profiles for human MASP‐1 and MASP‐2. Mol Immunol 2003; 40:921–9. [DOI] [PubMed] [Google Scholar]
- 23. Hall R. Identification of inflammatory mediators and their modulation by strategies for the management of the systemic inflammatory response during cardiac surgery. J Cardiothorac Vasc Anesth 2013; 27:983–1033. [DOI] [PubMed] [Google Scholar]
- 24. Hoedemaekers C, van Deuren M, Sprong T et al The complement system is activated in a biphasic pattern after coronary artery bypass grafting. Ann Thorac Surg 2010; 89:710–6. [DOI] [PubMed] [Google Scholar]
- 25. Hein E, Munthe‐Fog L, Thiara AS, Fiane AE, Mollnes TE, Garred P. Heparin‐coated cardiopulmonary bypass circuits selectively deplete the pattern recognition molecule ficolin‐2 of the lectin pathway in vivo . Clin Exp Immunol 2015; 179:294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seghaye MC, Vasquez‐Jimenez J, Qing M et al Clinical relevance of the systemic inflammatory reaction to cardiac operations in children In: Baykut D, Krian A, eds. Current perspectives of the extracorporeal circulation. Berlin/Heidelberg: Springer‐Verlag, 2000:117–25. [Google Scholar]
- 27. Tárnok A, Hambsch J, Emmrich F et al Complement activation, cytokines, and adhesion molecules in children undergoing cardiac surgery with or without cardiopulmonary bypass. Pediatr Cardiol 1999; 20:113–25. [DOI] [PubMed] [Google Scholar]
- 28. Chenoweth DE, Cooper SW, Hugli TE, Stewart RW, Blackstone EH, Kirklin JW. Complement activation during cardiopulmonary bypass: evidence for generation of C3a and C5a anaphylatoxins. N Engl J Med 1981; 304:497–503. [DOI] [PubMed] [Google Scholar]
- 29. Hetland G, Moen O, Bergh K et al Both plasma‐ and leukocyte‐associated C5a are essential for assessment of C5a generation in vivo . Ann Thorac Surg 1997; 63:1076–80. [DOI] [PubMed] [Google Scholar]
- 30. Suzuki Y, Daitoku K, Minakawa M, Fukui K, Fukuda I. Poly‐2‐ methoxyethylacrylate‐coated bypass circuits reduce activation of coagulation system and inflammatory response in congenital cardiac surgery. J Artif Organs 2008; 11:111–6. [DOI] [PubMed] [Google Scholar]
- 31. Itoh H, Ichiba S, Ujike Y et al A prospective randomized trial comparing the clinical effectiveness and biocompatibility of heparin‐coated circuits and PMEA‐coated circuits in pediatric cardiopulmonary bypass. Perfusion 2015. Jul 30. pii:0267659115598217. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32. Busche MN, Pavlov V, Takahashi K, Stahl GL. Myocardial ischemia and reperfusion injury is dependent on both IgM and mannose‐binding lectin. Am J Physiol Heart Circ Physiol 2009; 297:H1853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


