Abstract
Characterized by low serum testosterone levels and diverse symptoms, male hypogonadism is a common condition. Current medical treatment focuses on testosterone supplementation using multiple modalities such as injections, gels and pellets. Interestingly, while testosterone is considered an anabolic androgenic steroid, it has not been saddled with the social stigma that other, similar medications have. The goal of this review is to highlight an anabolic steroid, 19-nortestosterone (i.e., nandrolone, deca-durabolin) and illustrate prospective therapeutic applications for male health. Containing a chemical structure similar to testosterone, nandrolone has a higher myotrophic: androgenic ratio resulting in improved effects on muscle mass. Animal models have suggested application in the improvement of joint healing following rotator cuff repair. Minimal literature exists regarding the use of nandrolone and, as such, further human studies are required.
Keywords: Nandrolone, erectile dysfunction (ED), testosterone supplementation therapy (TST), alopecia, joint healing, pharmacology, muscle growth
Introduction
Male hypogonadism is a clinical entity characterized by symptoms such as fatigue, erectile dysfunction (ED) and mood changes associated with decreased serum levels of testosterone (1). Treatment of hypogonadism involves the exogenous supplementation of testosterone and may be complemented with other medications such as human chorionic gonadotropin (hCG) and clomiphene citrate (clomid) (2-4). With the increasing rise in incidence of male hypogonadism, and the growing knowledge of the roles for testosterone in male health, other pharmacological steroid compounds should be evaluated to either supplement, and/or replace testosterone in the treatment of male health.
Unfortunately, numerous well-published media reports dealing with anabolic steroids in professional and Olympic athletes have brought a cloud of speculation and doubt regarding use of these “illicit” medications. The fact that these anabolic steroid medications are banned by numerous athletic associations such as the International Olympic Committee as well as the National Football and Basketball Associations and Major League Baseball, further re-enforces the public perception that these medications should not be used under any circumstances. Indeed, a recent study (5) evaluated healthcare-provider attitudes towards anabolic androgenic steroids (AAS) and found that AAS users were viewed less favorably that cocaine abusers or healthy adults. These perceptions were enhanced by the passage of the Anabolic Steroid Control Act of 2004 that listed anabolic steroids as schedule III controlled substances—similar to ketamine, opiates and morphine (6). This policy mandated that a physician prescription was necessary to obtain the medication; further challenging the opinion of the general public. As such, it is tempting to speculate that investigations into alternatives to testosterone therapy have been slowed by societal stigma and perception.
An anabolic steroid that has been investigated to some degree in the treatment of male health is 19-nortestosterone (or nandrolone, deca-durabolin). This synthetic anabolic steroid has been available for decades and has been studied in multiple clinical pathologies. Early investigations of nandrolone focused on its potential uses in the treatment of osteoporosis. In that setting, it increased gastrointestinal and renal tubular absorption of calcium and decreased bone reabsorption (7). Nandrolone also had the beneficial effects of stimulating the formation of extra-osseous collagen and soft tissue (7). However, as would be expected, ~50% of women who were prescribed nandrolone reported some component of virilization (7). Due, in part, to these adverse effects, and the development of newer and more effective therapies to treat osteoperosis (i.e., bisphosphonates), nandrolone never gained traction as an effective clinical adjunct for this indication. Despite this, nandrolone’s stimulatory effects on bone and soft tissues became the basis upon which some researchers suggested its use in anemia and muscle wasting secondary to hemodialysis and/or HIV (8,9). Furthermore, recent studies have investigated the effects of nandrolone in the treatment of chronic pulmonary obstructive disease (10,11). In the context of the current review that examines the potential uses for anabolic steroids in male health, nandrolone was chosen as a case study. Details regarding its pharmacology as well as its potential uses in male health were explored.
Materials and methods
A PubMed/MEDLINE literature search was conducted for the periods of 1960–2015 in January, 2015. There were an insignificant number of published quality data available for meta-analysis, so a systematic review was performed. Key search terms included combinations of “Nandrolone”, “testosterone”, “hypogonadism”, “deca-durabolin”, “19-nortestosterone”, “pharmacology”, “alopecia”, “joint(s)”, “rotator cuff”, and “erectile dysfunction”. In excess of 1,000 manuscript abstracts were screened by the authors using title search and abstract summaries. Applicable studies were read in-depth and included in this current review. Additionally, an internet search strategy examining blogs and discussion sites, as previously described (12) was used. These techniques have been previously validated through other reports that employed similar methods of Internet data mining to report consistent findings (13,14).
Nandrolone pharmacology
Nandrolone is a synthetic anabolic steroid that bears similarity in chemical appearance to testosterone. The only major difference between the two molecules is a single methyl group (see Figure 1). Similar to testosterone, nandrolone is administered via intramuscular (IM) injection and has a plasma half-life of approximately 8 days (7). Nandrolone binds to androgen receptors with a greater binding affinity than testosterone and with an increased anabolic, or myotrophic, activity rate (versus androgenic activity) (15). For example and to assist in comparison, the myotrophic:androgenic ratio can be used to compare testosterone (~1:1) to nandrolone (~11:1) (15) with regards to the ability to stimulate muscle growth compared to virilization.
Figure 1.
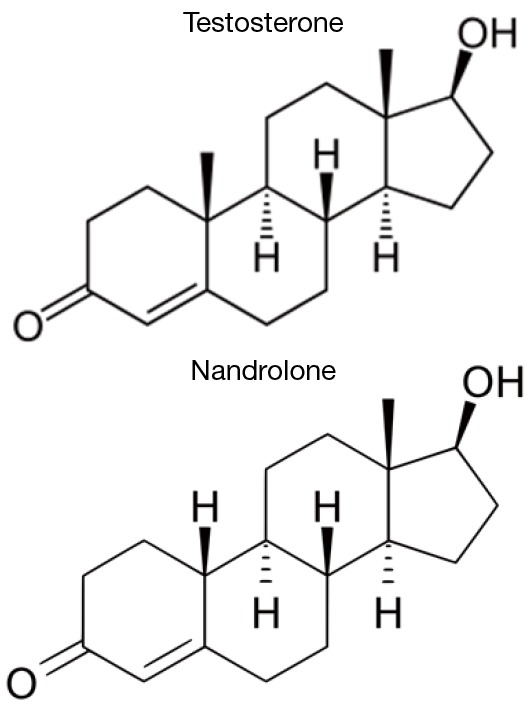
Comparison of molecular structures of testosterone and 19-nortestosterone (nandrolone).
With regards to the mechanisms of nandrolone inside the human body, understanding the pathway of testosterone action is important. In certain tissues, such as the prostate or in the hair follicles, testosterone is converted to dihydrotestosterone (DHT) by 5 alpha-reductase (5AR). High levels of 5AR activity are present in androgenic tissues from the prostate but are undetectable in skeletal muscle (15). As such, in skeletal muscle, testosterone directly binds androgen receptors contributing to muscle growth. However, in prostatic tissues and hair follicles, testosterone is converted to DHT by 5AR and is thus responsible for the known side effects of testosterone supplementation therapy (TST) on prostate growth and alopecia (15).
5AR can also act on nandrolone (19-nortestosterone) to produce 5α-dihydro-19-nortestosterone (15). This reduced form of nandrolone has a significantly decreased binding affinity for the androgen receptor compared to its parent steroid, testosterone (15). As such, the 5AR conversion of nandrolone to 5α-dihydro-19-nortestosterone in prostatic tissues results in a significantly decreased ability of nandrolone to bind androgen receptors. Theoretically, the end result could be a decrease in prostatic growth with a possible and theoretical effect on lower urinary tract symptoms such as those developed as a result of benign prostatic hyperplasia (BPH). A potential decrease in the rates of alopecia could also be observed . Furthermore, the lack of 5AR in skeletal muscle allows nandrolone to bind strongly to androgen receptors in the muscle and stimulate growth, contributing to its high myotrophic:androgenic ratio (15).
The metabolism of nandrolone is incompletely understood. Metabolites of nandrolone include 19-norandrosterone and 19-noretiocholanolone glucuronides that are detectable in urine (16). Animal studies suggest that nandrolone can also be converted to various estrogens via the actions of aromatase (17,18); however, human studies are lacking.
Nandralone and alopecia
TST in hypogonadal men results in elevated levels of free serum testosterone and, in turn, DHT. This occurs due to conversion of testosterone to DHT via 5AR inhibitors (5ARi) (19,20). Androgenic alopecia, or male pattern hair loss, typically occurs in 20% of 20-year-old men and then increases by approximately 10% every 10 years (21). A critical role for DHT in hair growth can be seen in the case study of men with Imperato-McGinley syndrome. This condition results from a mutation in the gene for type II 5AR that prevents the expression of the enzyme. As a consequence, men with this condition never become bald (22).
Androgen dependent miniaturization of scalp hair follicles by DHT is implicated as the primary cause of alopecia (23). Controlled clinical trials demonstrated that use of finasteride resulted in decreased accumulation of DHT and improvements in both subjective and objective assessments of hair growth and density (23).
Indeed, the most established management paradigm for the treatment of alopecia in the male is finasteride (24). First approved in 1992 in the treatment of benign prostatic hypertrophy, finasteride was approved for the treatment of alopecia in 1997 at a dose of 1 mg daily (propecia®) (24). In men on TST, finasteride at 1 mg is used as a means of preventing the excess exogenously administered testosterone from converting to DHT. By decreasing the amount of DHT, less is available to act on the hair follicles stabilizing the amount of hair loss.
The theory underlying the possible use of nandrolone in the context of alopecia results from the fact that it does not convert to DHT but instead gets transformed into 5α-dihydro-19-nortestosterone, a molecularly distinct compound (15,25). It is thus possible that in hypogonadal males nandrolone, in addition to or in replacement for testosterone, could alleviate concerns for the development of androgenic alopecia.
There are several lines of evidence that make this hypothesis particularly appealing. In experimental animal models, nandrolone is synthesized endogenously through a mechanism distinct from DHT (25). Furthermore, the metabolism of nandrolone in animal models yields compounds completely unrelated to DHT (15,26). It has also been proven that the actions of 5AR on nandrolone produce a compound that has decreased affinity and activity at the androgen receptor (15). Given that nandrolone is not converted to DHT it seems logical to assume that it would have less effect on hair loss than exogenous testosterone (with its subsequent conversion to DHT). Thus, nandrolone may be beneficial in treating hypogonadal men concerned about alopecia in the setting of TST.
Another aspect of this discussion also involves consideration for the possible existence of persistent sexual side effects and anxiety/depressive symptoms reported with the use of 5ARis like finasteride (27,28). Some symptoms of which have been shown to last for months to years (29). Therefore, by utilizing an agent with less direct effects on hair loss, nandrolone may represent a viable option for men in the treatment of hypogonadism. Altered levels of neuroactive steroids related to depressive symptoms have been identified in men with a history of finasteride use, even when the drug has been discontinued (30). The use of nandrolone would obviate these concerns.
Nandrolone and joint healing
Recent studies in animal models have identified a potential role for nandrolone in joint pain, particularly post rotator cuff tears (31,32). In one such study by Gerber et al. (31), 20 New Zealand white rabbits had their supraspinatus tendon released with musculotendinous retraction and observed over 6 weeks. Rabbits were organized into groups treated with placebo as well as local and systemic administration of nandrolone (31). Nandrolone, given in the phase after tendon release, was found to inhibit fatty infiltration of the supraspinatus muscle and reduced functional impairment of the rotator cuff (31).
An earlier 2010 study by Papaspiliopoulos et al. (32) examined 48 male rabbits that underwent rotator cuff incision and reconstruction after stratification into groups based on local nandrolone administration and immobilization. In this study, local administration of nandrolone proved detrimental to wound healing however, systemic administration was not studied (32). Other limitations include the fact that anabolic steroids affect the tensile strength of tendons that may then cause failure with less elongation (33). Local administration of nandrolone may impair the healing of acute tendon injuries and the perceived benefits to retracted muscle may be outweighed by its effects on tendon healing (34).
Interestingly, Internet and discussion group anecdotal data suggests that nandrolone is effective in decreasing joint pain in bodybuilders. These athletes lift large amounts of weights putting extreme pressure on their joints while reporting improvement and lowered pain with the use of nandrolone. While limited data is available, and dosages are unknown, further investigations are needed to determine the effects of nandrolone on joints in general, and the rotator cuff in particular.
Nandrolone and muscle growth
Nandrolone has long been known to have significant stimulatory effects on muscle growth. As described above, nandrolone displays a greater myotrophic:androgenic ratio compared to testosterone (15). When compared to testosterone, the high levels of 5AR in androgenic tissues (i.e., the prostate) convert nandrolone to a less active metabolite (versus DHT that is highly active). Furthermore, the lack of 5AR in skeletal muscle allows nandrolone to produce a primarily anabolic/myotrophic (i.e., muscle growing) effect (15). The ability of nandrolone to preferentially stimulate muscle growth formed the basis of its use in the treatment of anorexia and cachexia in patients with chronic medical disorders such as chronic renal failure and HIV (8,35). In these patients, administration of nandrolone has been shown to increase lean body mass as well as muscle mass and strength (8,35).
Hypogonadism has been shown to be associated with dyslipidemia, atherosclerosis, cardiovascular disease, metabolic syndrome, and diabetes (36). Testosterone supplementation in hypogonadal men improves these risk factors leading, in some patients, to complete resolution of their metabolic syndrome (36-38). Indeed, an increase in lean body mass and muscle mass with the systemic administration of nandrolone could improve body composition and augment testosterone’s effects in preventing (39) and reversing metabolic syndrome and the risk of type 2 diabetes in hypogonadal men.
Nandralone and ED
In spite of its potential beneficial uses described above, one major limitation to the use of nandrolone in hypogonadal males stems from the fact that a relationship may exist between the use of nandrolone and ED. Although the World Health Organization INCHEM database compiled by the International Program on Chemical Safety (http://www.who.int/ipcs/en) lists impotence under adverse effects of nandrolone; no consistent reports of ED associated with the use of nandrolone have been reported in the literature.
Anecdotal evidence from patients, as well as those men who have previously used nandrolone from “alternative” sources suggests a relationship with the use of nandrolone (alone, not in combination with testosterone) and ED. Indeed, nandrolone may contribute to the development of ED through two mechanisms: the suppression of testosterone/DHT via negative feedback and the buildup of estrogens.
Numerous studies have shown that DHT is the active androgen involved in maintenance of nitric oxide-mediated penile erections. Castrated rats treated with exogenous testosterone recovered erectile function but, when co-administered with a 5ARi to block DHT production, this recovery was lost (40,41). Moreover, administration of transdermal DHT in aging men resulted in improvement in early morning erections and the ability to maintain erections (42). In fact, one of the most common sexual side-effects of 5ARi’s (described above) is ED (43).
Nandrolone has also been shown to decrease LH, FSH, and endogenous testosterone levels in animal models, indicating a negative feedback loop to inhibit the hypothalamic-pituitary-gonadal (HPG) axis (44). In this context, nandrolone acts as an androgen receptor agonist that is not converted endogenously to DHT (15). As such, it provides negative feedback to the HPG axis to suppresses testosterone levels, further decreasing the available testosterone and DHT, compounding its negative effects on erectile function.
Imbalance in the testosterone to estrogen ratio has been associated with ED (45). Numerous subsets of patients with ED have elevated estradiol levels, indicating a relationship between estrogens and erectile function (45). Nandrolone and other members of the 19-nor-androgen families have been shown to undergo aromatase-mediated conversion to estrogens in animal models (17,18,46). Nandrolone itself shows significant binding affinity and full agonist activity with the alpha-estrogen receptor (47). Indeed, increased serum estrogen levels in men have been associated with development of gynecomastia, increased body fat mass, and unfavorable lipid profiles—all contributing factors to ED (48-51). As such, it can be theorized that nandrolone should be administered with testosterone to prevent ED with an eye towards regulation of a patients estradiol levels. However, specific in vivo studies examining the effects of nandrolone administration in humans has not been described.
Nandralone and male infertility
No studies currently exist.
Discussion
Nandrolone is a synthetic anabolic steroid that possesses unique qualities and is potentially beneficial in the treatment of male health alone, or as an adjunct to TST for hypogonadal men. Nandrolone has a relatively long half-life in the plasma and a strong binding affinity for androgen receptors. The lack of conversion to DHT could mean decreased hair loss in men undergoing TST suggesting a novel use for this medication in a subpopulation of hypogonadal men. Furthermore, in men with voiding dysfunction due to benign prostatic hypertrophy, the reduction of nandrolone by 5AR to generate a weaker androgen (compared to DHT) that does not stimulate the growth of androgenic tissues such as the prostate could serve as another indicator for its use. Nandrolone preferentially stimulates growth of skeletal muscle and lean body mass that may provide benefit in reducing components of metabolic syndrome. Moreover, preliminary work on nandrolone has suggested a potential role in the treatment of joint healing, particularly in rotator cuff injuries.
Limitations to the use of nandrolone include the potential for ED via suppression of the HPG axis. The lack of conversion to DHT and the concurrent increase in serum estrogens may mediate this effect. Administration of low doses of testosterone, along with nandrolone, would alleviate these effects. Further research is needed to evaluate nandrolone’s potential role in the management of male health.
Conclusions
Characterized by low serum testosterone and a multitude of debilitating symptoms, male hypogonadism is a common condition. Primarily treated with exogenous testosterone replacement, novel adjuncts to improve responses and decrease side effects are being studied. Nandrolone is an anabolic steroid compound with a high myotrophic:anabolic ratio. In this manuscript, we have explored the potential uses for nandrolone in male health. Specifically, we have reviewed the pharmacology of nandrolone and detailed a potential role for nandrolone in joint healing and muscle growth. Finally, a consideration was given to the potential adverse effects of nandrolone on ED. Further research in human subjects is required.
Acknowledgements
None.
Footnotes
Conflicts of Interest: JR Kovac is a paid speaker for AbbVie. The other author has no conflicts of interest to declare.
References
- 1.Basaria S. Male hypogonadism. Lancet 2014;383:1250-63. 10.1016/S0140-6736(13)61126-5 [DOI] [PubMed] [Google Scholar]
- 2.Moskovic DJ, Katz DJ, Akhavan A, et al. Clomiphene citrate is safe and effective for long-term management of hypogonadism. BJU Int 2012;110:1524-8. 10.1111/j.1464-410X.2012.10968.x [DOI] [PubMed] [Google Scholar]
- 3.Kim ED, Crosnoe L, Bar-Chama N, et al. The treatment of hypogonadism in men of reproductive age. Fertil Steril 2013;99:718-24. 10.1016/j.fertnstert.2012.10.052 [DOI] [PubMed] [Google Scholar]
- 4.Layton JB, Li D, Meier CR, Sharpless JL, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab 2014;99:835-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, Hildebrandt T, Lanzieri N. Healthcare professionals' stigmatization of men with anabolic androgenic steroid use and eating disorders. Body Image 2015;15:49-53. 10.1016/j.bodyim.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 6.Kovac JR, Pan M, Arent S, et al. Dietary Adjuncts for Improving Testosterone Levels in Hypogonadal Males. Am J Mens Health 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Geusens P. Nandrolone decanoate: pharmacological properties and therapeutic use in osteoporosis. Clin Rheumatol 1995;14 Suppl 3:32-9. 10.1007/BF02210686 [DOI] [PubMed] [Google Scholar]
- 8.Mulligan K, Schambelan M. Anabolic treatment with GH, IGF-I, or anabolic steroids in patients with HIV-associated wasting. Int J Cardiol 2002;85:151-9. 10.1016/S0167-5273(02)00247-4 [DOI] [PubMed] [Google Scholar]
- 9.Chen CT, Lin SH, Chen JS, et al. Muscle wasting in hemodialysis patients: new therapeutic strategies for resolving an old problem. ScientificWorldJournal 2013;2013:643954. [DOI] [PMC free article] [PubMed]
- 10.Sharma S, Arneja A, McLean L, et al. Anabolic steroids in COPD: a review and preliminary results of a randomized trial. Chron Respir Dis 2008;5:169-76. 10.1177/1479972308092350 [DOI] [PubMed] [Google Scholar]
- 11.Velema MS, Kwa BH, de Ronde W. Should androgenic anabolic steroids be considered in the treatment regime of selected chronic obstructive pulmonary disease patients? Curr Opin Pulm Med 2012;18:118-24. 10.1097/MCP.0b013e32834e9001 [DOI] [PubMed] [Google Scholar]
- 12.Rahnema CD, Lipshultz LI, Crosnoe LE, et al. Anabolic steroid-induced hypogonadism: diagnosis and treatment. Fertil Steril 2014;101:1271-9. 10.1016/j.fertnstert.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 13.Parkinson AB, Evans NA. Anabolic androgenic steroids: a survey of 500 users. Med Sci Sports Exerc 2006;38:644-51. 10.1249/01.mss.0000210194.56834.5d [DOI] [PubMed] [Google Scholar]
- 14.McCabe SE. Comparison of web and mail surveys in collecting illicit drug use data: a randomized experiment. J Drug Educ 2004;34:61-72. 10.2190/4HEY-VWXL-DVR3-HAKV [DOI] [PubMed] [Google Scholar]
- 15.Kicman AT. Pharmacology of anabolic steroids. Br J Pharmacol 2008;154:502-21. 10.1038/bjp.2008.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reznik Y, Dehennin L, Coffin C, et al. Urinary nandrolone metabolites of endogenous origin in man: a confirmation by output regulation under human chorionic gonadotropin stimulation. J Clin Endocrinol Metab 2001;86:146-50. 10.1210/jcem.86.1.7100 [DOI] [PubMed] [Google Scholar]
- 17.Sundaram K, Kumar N, Monder C, et al. Different patterns of metabolism determine the relative anabolic activity of 19-norandrogens. J Steroid Biochem Mol Biol 1995;53:253-7. 10.1016/0960-0760(95)00056-6 [DOI] [PubMed] [Google Scholar]
- 18.Dintinger T, Gaillard JL, Zwain I, et al. Synthesis and aromatization of 19-norandrogens in the stallion testis. J Steroid Biochem 1989;32:537-44. 10.1016/0022-4731(89)90387-7 [DOI] [PubMed] [Google Scholar]
- 19.Piraccini BM, Alessandrini A. Androgenetic alopecia. G Ital Dermatol Venereol 2014;149:15-24. [PubMed] [Google Scholar]
- 20.Abadilla KA, Dobs AS. Topical testosterone supplementation for the treatment of male hypogonadism. Drugs 2012;72:1591-603. 10.2165/11635620-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 21.Dušková M, Pospíšilová H. The role of non-aromatizable testosterone metabolite in metabolic pathways. Physiol Res 2011;60:253-61. [DOI] [PubMed] [Google Scholar]
- 22.Imperato-McGinley J, Guerrero L, Gautier T, et al. Steroid 5alpha-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science 1974;186:1213-5. 10.1126/science.186.4170.1213 [DOI] [PubMed] [Google Scholar]
- 23.Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol 1998;39:578-89. 10.1016/S0190-9622(98)70007-6 [DOI] [PubMed] [Google Scholar]
- 24.Libecco JF, Bergfeld WF. Finasteride in the treatment of alopecia. Expert Opin Pharmacother 2004;5:933-40. 10.1517/14656566.5.4.933 [DOI] [PubMed] [Google Scholar]
- 25.Robic A, Faraut T, Prunier A. Pathways and genes involved in steroid hormone metabolism in male pigs: a review and update. J Steroid Biochem Mol Biol 2014;140:44-55. 10.1016/j.jsbmb.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 26.Houghton E. Studies related to the metabolism of anabolic steroids in the horse: 19-nortestosterone. Xenobiotica 1977;7:683-93. 10.3109/00498257709038698 [DOI] [PubMed] [Google Scholar]
- 27.Mysore V. Finasteride and sexual side effects. Indian Dermatol Online J 2012;3:62-5. 10.4103/2229-5178.93496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganzer CA, Jacobs AR, Iqbal F. Persistent sexual, emotional, and cognitive impairment post-finasteride: a survey of men reporting symptoms. Am J Mens Health 2015;9:222-8. 10.1177/1557988314538445 [DOI] [PubMed] [Google Scholar]
- 29.Irwig MS. Persistent sexual side effects of finasteride: could they be permanent? J Sex Med 2012;9:2927-32. 10.1111/j.1743-6109.2012.02846.x [DOI] [PubMed] [Google Scholar]
- 30.Caruso D, Abbiati F, Giatti S, et al. Patients treated for male pattern hair with finasteride show, after discontinuation of the drug, altered levels of neuroactive steroids in cerebrospinal fluid and plasma. J Steroid Biochem Mol Biol 2015;146:74-9. 10.1016/j.jsbmb.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 31.Gerber C, Meyer DC, Nuss KM, et al. Anabolic steroids reduce muscle damage caused by rotator cuff tendon release in an experimental study in rabbits. J Bone Joint Surg Am 2011;93:2189-95. 10.2106/JBJS.J.01589 [DOI] [PubMed] [Google Scholar]
- 32.Papaspiliopoulos A, Papaparaskeva K, Papadopoulou E, et al. The effect of local use of nandrolone decanoate on rotator cuff repair in rabbits. J Invest Surg 2010;23:204-7. 10.3109/08941939.2010.481007 [DOI] [PubMed] [Google Scholar]
- 33.Miles JW, Grana WA, Egle D, et al. The effect of anabolic steroids on the biomechanical and histological properties of rat tendon. J Bone Joint Surg Am 1992;74:411-22. [PubMed] [Google Scholar]
- 34.Wang VM. Important preliminary findings on the potential role for nandrolone decanoate in the treatment of chronic rotator cuff tears. J Bone Joint Surg Am 2011;93:e1441-2. 10.2106/JBJS.K.01213 [DOI] [PubMed] [Google Scholar]
- 35.Macdonald JH, Marcora SM, Jibani MM, et al. Nandrolone decanoate as anabolic therapy in chronic kidney disease: a randomized phase II dose-finding study. Nephron Clin Pract 2007;106:c125-35. 10.1159/000103000 [DOI] [PubMed] [Google Scholar]
- 36.De Maddalena C, Vodo S, Petroni A, et al. Impact of testosterone on body fat composition. J Cell Physiol 2012;227:3744-8. 10.1002/jcp.24096 [DOI] [PubMed] [Google Scholar]
- 37.Kovac JR, Pastuszak AW, Lamb DJ, et al. Testosterone supplementation therapy in the treatment of patients with metabolic syndrome. Postgrad Med 2014;126:149-56. 10.3810/pgm.2014.11.2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalinchenko SY, Tishova YA, Mskhalaya GJ, et al. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf) 2010;73:602-12. 10.1111/j.1365-2265.2010.03845.x [DOI] [PubMed] [Google Scholar]
- 39.Kovac JR, Scovell J, Kim ED, et al. A positive role for anabolic androgenic steroids: preventing metabolic syndrome and type 2 diabetes mellitus. Fertil Steril 2014;102:e5. 10.1016/j.fertnstert.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 40.Lugg JA, Rajfer J, González-Cadavid NF. Dihydrotestosterone is the active androgen in the maintenance of nitric oxide-mediated penile erection in the rat. Endocrinology 1995;136:1495-501. [DOI] [PubMed] [Google Scholar]
- 41.Seo SI, Kim SW, Paick JS. The effects of androgen on penile reflex, erectile response to electrical stimulation and penile NOS activity in the rat. Asian J Androl 1999;1:169-74. [PubMed] [Google Scholar]
- 42.Kunelius P, Lukkarinen O, Hannuksela ML, et al. The effects of transdermal dihydrotestosterone in the aging male: a prospective, randomized, double blind study. J Clin Endocrinol Metab 2002;87:1467-72. 10.1210/jcem.87.4.8138 [DOI] [PubMed] [Google Scholar]
- 43.Erdemir F, Harbin A, Hellstrom WJ. 5-alpha reductase inhibitors and erectile dysfunction: the connection. J Sex Med 2008;5:2917-24. 10.1111/j.1743-6109.2008.01001.x [DOI] [PubMed] [Google Scholar]
- 44.Purkayastha S, Mahanta R. Effect of Nandrolone Decanoate on Serum FSH, LH and Testosterone Concentration in Male Albino Mice. World J Life Sci Med Res 2012;2:123. [Google Scholar]
- 45.Srilatha B, Adaikan PG. Endocrine milieu and erectile dysfunction: is oestradiol-testosterone imbalance, a risk factor in the elderly? Asian J Androl 2011;13:569-73. 10.1038/aja.2010.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obasanjo IO, Clarkson TB, Weaver DS. Effects of the anabolic steroid nandrolone decanoate on plasma lipids and coronary arteries of female cynomolgus macaques. Metabolism 1996;45:463-8. 10.1016/S0026-0495(96)90220-6 [DOI] [PubMed] [Google Scholar]
- 47.Bovee TF, Helsdingen RJ, Rietjens IM, et al. Rapid yeast estrogen bioassays stably expressing human estrogen receptors alpha and beta, and green fluorescent protein: a comparison of different compounds with both receptor types. J Steroid Biochem Mol Biol 2004;91:99-109. 10.1016/j.jsbmb.2004.03.118 [DOI] [PubMed] [Google Scholar]
- 48.Ismail AA, Barth JH. Endocrinology of gynaecomastia. Ann Clin Biochem 2001;38:596-607. 10.1258/0004563011900993 [DOI] [PubMed] [Google Scholar]
- 49.Vermeulen A, Kaufman JM, Goemaere S, et al. Estradiol in elderly men. Aging Male 2002;5:98-102. 10.1080/tam.5.2.98.102 [DOI] [PubMed] [Google Scholar]
- 50.Tomaszewski M, Charchar FJ, Maric C, et al. Association between lipid profile and circulating concentrations of estrogens in young men. Atherosclerosis 2009;203:257-62. 10.1016/j.atherosclerosis.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Treas J, Tyagi T, Singh KP. Chronic exposure to arsenic, estrogen, and their combination causes increased growth and transformation in human prostate epithelial cells potentially by hypermethylation-mediated silencing of MLH1. Prostate 2013;73:1660-72. [DOI] [PubMed] [Google Scholar]


