Abstract
Background
Premature ejaculation (PE) is a highly prevalent sexual dysfunction among patients with diabetes mellitus (DM). Despite this, the underlying mechanism of this association is poorly understood. In this study, we aimed to investigate the prevalence of PE in a group of patients with DM and explore possible associations linking both conditions together.
Methods
This was a prospective study of subjects recruited with advertisement pamphlets and whose sexual function was assessed using the international index of erectile function-5 (IIEF-5) and the Arabic index of premature ejaculation (AIPE) questionnaires together with stopwatch measured intravaginal ejaculatory latency time (ELT). Participants were divided into two groups; group A subjects had DM and group B were healthy adult males.
Results
A total of 488 subjects were recruited. Group A included 199 (40.8%) subjects, while group B included 289 (59.2%). The prevalence of PE and ED was significantly higher in group A subjects (P<0.001). Mean ELT ± standard deviation (SD) was 3.6±2.7 in group A versus 4.3±2.8 in group B (P<0.014). Diabetic patients with erectile dysfunction (ED) showed a significantly higher incidence of PE with significantly shorter ELT.
Conclusions
PE is more prevalent in diabetic patients. DM is a multi-systemic disorder with complications that could help explain the pathophysiology of PE.
Keywords: Diabetes mellitus type 2, erectile dysfunction (ED), glycosylated hemoglobin (HbA1c), premature ejaculation (PE)
Introduction
Premature ejaculation (PE) is the most common sexual dysfunction affecting around 15–30% of males above 18 years of age (1). The diagnosis of PE has long been subject to scrutiny as it was mainly based on self evaluation, resulting in exaggeration in the prevalence of this condition (2). For this reason, efforts have been made to reach universally agreed-on definition and valid screening tools that can reduce false patient misperception and over-diagnosis of PE (3). As such, in addition to patient-perceived deviation from the norm, intravaginal ejaculation latency time (ELT) is taken into account to help in establishing a diagnosis (4). A recent update by the international society of sexual medicine has classified PE into acquired PE (when symptoms appear after a period of normal ejaculatory function) and lifelong PE (5).
For years, PE was thought to be a psychological ailment, mainly resulting from anxiety or conditioning toward rapid ejaculation based on rushed early sexual experiences (6). However, recently organic etiologies for early ejaculation have been hypothesized. Factors such as hypersensitivity of the glans penis (7), abnormalities of serotonergic neurotransmission (8), erectile dysfunction (ED) (9) and comorbid conditions such as thyroid disease (10) and metabolic syndrome (11) have been proposed.
As of 2014, an estimated 387 million people have diabetes mellitus (DM) worldwide; this is equal to about 9% of the adult population (12,13). Diabetes is a chronic progressive disease usually diagnosed late due to its silent nature. Its association with sexual dysfunction is well investigated, with the latter occurring as a consequence of either vascular or neurological complications of DM or of its psychological impact on patients (14). Few studies have reported an association between DM and PE, indicating that PE is more common in diabetic patients (15,16). The underlying mechanism of this association is still poorly understood. Although some have advocated it to be secondary to psychogenic factors such as performance anxiety and depression (16), the search for organic causes to this observation should be considered. Microvascular complications of diabetes such as diabetic neuropathy is common and can justify the presence of PE due to the fact that ejaculation depend on the integrity of the autonomic nervous system and its central and peripheral neurotransmitters (17).
DM is a very common condition in our community with prevalence much higher than what is internationally reported (18). The aim of the present study is to investigate the association of PE and DM in our population. To our knowledge, this is the first study to inspect such an association utilizing validated questionnaires.
Material and methods
This is a cross-sectional study held at the out patient department (OPD) of our institute. Between November 2013 and April 2015, male subjects presenting to the OPD were recruited using advertisement pamphlets to participate in this study. Responders included patients of various OPD clinics, companions, clerks, nurses and doctors. They were seen in a private clinic and were screened for inclusion and exclusion criteria and informed fully about the purpose of this study. The inclusion criteria were male subjects between 18 and 65 years of age, while the exclusion criteria were patients with type 1 DM, consumption of medical treatment for ED or PE, presence of co-morbid illness besides DM (such as endocrinopathies, Peyronie’s disease, urethritis, persistent urinary tract infection, spinal cord injuries, etc.), and presence of psychiatric illnesses or intake of any psychiatric medications. All subjects signed a written informed consent before participating in this study. The study protocol was approved by the internal review board (IRB) in our institute.
A detailed medical and sexual history was taken from all participants. Patients found to have DM were asked about the duration of their illness, type of medication and their compliance. Additionally, their medical records were checked for the presence of glycosylated hemoglobin (HbA1c) test result. Those who did not do an HbA1c test in the past 6 months from their presentation were asked to provide a new sample. HbA1c is performed using the enzymatic assay method (Abbott Diagnostics, Illinois, USA). The participant’s sexual history was assessed using the international index of erectile function-5 (IIEF-5) questionnaire and the Arabic index of premature ejaculation questionnaire (AIPE). Both questionnaires have been validated in Arabic language and were proven to be reliable tools for diagnosis of ED and PE, respectively (19-21). ED is considered when the IIEF-5 score is ≤21 (20); while PE is considered when the AIPE is ≤30 (19). AIPE is a seven-question questionnaire that deals with various areas of sexual activity, namely, libido (Q1), erection (Q2), intravaginal ejaculatory latency time (ELT) (Q3), control over ejaculation (Q4), couple satisfaction (Q5–6) and psychological impact of PE (Q7). Results of both questionnaires were sub-classified according to severity. For AIPE, severe [7–13], moderate [14–19], mild-moderate [20–25], mild [26–30], and no PE [31–35]. While for IIEF, severe [0–7], moderate ED [8–11], mild-moderate ED [12–16], mild ED [17–21], and no ED [14, 22–24]. All participants were provided with validated stopwatches and were instructed on how to utilize them. They were asked to let their partner use it to record the ELT. An arithmetic mean ELT is used for data analysis.
Subjects are then divided into two groups. Group A participants were those found to have DM, while group B participants were healthy adult males. Results of the diagnostic tools utilized in this study were compared between both groups. Participants in each group were also reclassified according to the type of PE, acquired or lifelong. A chi-square test was used to analyze categorical variables, while a student-t-test was used to analyze non-categorical variables. Data was presented as mean ± standard deviation (SD) or as numbers and percentages. In all statistical tests, a value of P<0.05 was considered significant. Statistical analysis of collected data was performed using SPSS version 20 (IBM, Armonk, NY, USA).
Results
A total of 807 participants responded to the advertisement. A total of 233 were excluded, while 96 participants refused to continue in the study. Out of the excluded participants, 40 had type 1 DM, 114 had comorbidities and 79 were receiving medical treatment for ED and/or PE. Four hundred and eighty-eight continued the study and met the inclusion and exclusion criteria. Group A included 199 (40.8%) subjects, while group B included 289 (59.2%).
Characteristics of the study population are shown in Table 1. The mean age ± SD of participants was 38.6±9.5 years. Overall, 294 (60.2%) participants reported PE and 115 (23.6%) reported ED. The prevalence of PE and ED was significantly higher in group A subjects in comparison to group B subjects (P<0.001). All grades of PE severity (according to AIPE classification) were also significantly higher in group A than group B. Similarly, acquired PE was significantly higher in group A participants (P=0.017). Mean intravaginal ELT ± SD was significantly lower in group A than group B, 3.6±2.7 and 4.3±2.8, respectively (P=0.014). Results of the IIEF-5 and AIPE questionnaires and reported ELT in both groups are presented in Table 2.
Table 1. Study population (n=488).
| Variable | Value |
|---|---|
| Age, years (mean ± SD) | 38.6±9.5 |
| DM (No., %) | 199 (40.8%) |
| HbA1c (mean ± SD) | 8.3±2.1 |
| PE (No., %) (AIPE ≤30) | 294 (60.2%) |
| ED (No., %) (IIEF-5≤21) | 115 (23.6%) |
| ELT, minutes (mean ± SD) | 4.0±2.8 |
SD, standard deviation; DM, diabetes mellitus; HbA1c, glycosylated hemoglobin; PE, premature ejaculation; ED, erectile dysfunction; ELT, ejaculatory latency time.
Table 2. Comparison between diabetics and non-diabetics.
| Variable | Group A (n=199) | Group B (n=289) | P value |
|---|---|---|---|
| Age, years (mean ± SD) | 43.8±9.9 | 35.3±7.6 | <0.001* |
| PE types, No. (%) | <0.017* | ||
| Acquired | 33 (16.5) | 14 (4.8) | |
| Lifelong | 124 (62.3) | 123 (42.5) | |
| AIPE, No. (%) | <0.001* | ||
| No PE | 42 (21.1) | 152 (52.6) | |
| Mild PE | 39 (19.6) | 24 (8.3) | |
| Mild-moderate PE | 68 (34.2) | 86 (29.8) | |
| Moderate PE | 42 (21.1) | 25 (8.7) | |
| Severe PE | 8 (4.0) | 2 (0.7) | |
| Total PE | 157 (78.9) | 137 (47.4) | |
| ELT | |||
| Total, minutes (mean ± SD) | 3.6±2.7 | 4.3±2.8 | 0.014* |
| IIEF-5, No. (%) | <0.001* | ||
| No ED | 120 (60.3) | 253 (87.5) | |
| Mild ED | 45 (22.6) | 16 (5.6) | |
| Mild-moderate ED | 19 (9.5) | 13 (4.4) | |
| Moderate ED | 10 (5.0) | 5 (1.7) | |
| Severe ED | 5 (2.5) | 2 (0.8) | |
| Total ED | 79 (39.7) | 36 (12.5) |
PE, premature ejaculation; ED, erectile dysfunction; AIPE, Arabic index for premature ejaculation; no PE, AIPE=31–35; mild PE, AIPE=26–30; mild-moderate, AIPE=20–25; moderate, AIPE=14–19; severe, AIPE=7–13; IIEF-5, International index for erectile function-5; no ED, IIEF=22–25; mild ED, IIEF=17–21; mild-moderate ED, IIEF=12–16; moderate ED, IIEF=8–11; severe ED, IIEF=0–7; *, significant P value.
Figure 1 demonstrates reported answers for all areas of the AIPE questionnaire. Questions 1 through 7 were significantly lower in group A than group B participants.
Figure 1.
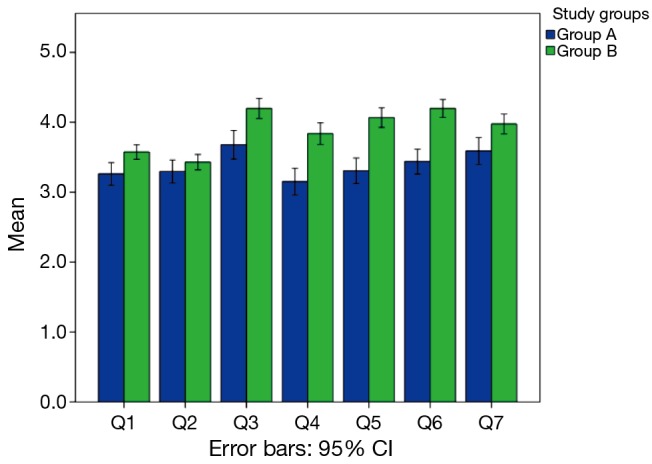
Demonstration of different areas of Arabic index of premature ejaculation (AIPE) questionnaire among group A and group B participants.
Diabetic patients were investigated for the duration of their illness and HbA1c level. There was no statistically significant difference in the duration of DM or HbA1c level between patients with and without PE, P=0.13 and P=0.09, respectively (Table 3).
Table 3. Comparison between PE and non PE in 199 diabetic patients.
| Variable | PE (n=151) | Non PE (n=38) | P value |
|---|---|---|---|
| Age, years (mean ± SD) | 44.7±9.8 | 39.6±9.4 | 0.040* |
| HbA1c (mean ± SD) | 8.1±1.6 | 8.8±1.8 | 0.090 |
| Duration of diabetes, years (mean ± SD) | 5.7±2.4 | 4.8±1.5 | 0.130 |
| ELT, minutes (mean ± SD) | 3.1±2.5 | 5.6±2.8 | 0.001* |
PE, premature ejaculation; SD, standard deviation; HbA1c, glycosylated hemoglobin; ELT, ejaculatory latency time. *, significant P value.
Diabetic patients with ED showed a significantly higher incidence of PE with significantly shorter ELT (Table 4).
Table 4. Comparison between ED and non ED in 199 diabetic patients.
| Variable | ED (n=146) | Non ED (n=43) | P value |
|---|---|---|---|
| Age, years (mean ± SD) | 46.5±9.9 | 41.7±9.5 | 0.001* |
| PE (No., %) | 71 (89.9) | 86 (71.7) | 0.001* |
| ELT, minutes (mean ± SD) | 2.9±2.5 | 3.9±2.8 | 0.017* |
ED, erectile dysfunction; SD, standard deviation; PE, premature ejaculation; ELT, ejaculatory latency time. *, significant P value.
Discussion
The international society of sexual medicine’s latest definition of PE considered the length of time from penetration to ejaculation, the inability to delay ejaculation and the negative personal consequences resulting from PE (1). ELT has been used in clinical trials to assess men with PE. Prospectively, stopwatch assessment of ELTs has superior accuracy compared with spontaneous reported latency (22). The mean ELT ± SD reported by our study population was 4.0±2.8 min, which was comparable to internationally documented results (22). Since the perception of “normal” ejaculatory latency varies by country and differs when assessed by patients from multiple diversities (23), the search for diagnostic tools unique to the area of research is advisable. This should address linguistic and cultural differences that may lead to misinterpretation of the problem. The AIPE, developed for this particular reason, was proven to be a reliable aid in diagnosis of PE (19) with a cutoff value of 30 having the highest sensitivity and specificity. Utilizing this tool, 79.4% of our study population reported PE; this result coincides with described PE prevalence in our region (24).
Patients with DM often tend to unveil the presence of various sexual dysfunctions (14) that are directly related to the deleterious complications of their disease. Previous reports have documented that diabetic men are prone to developing sexual dysfunctions, even at an earlier age (25), with an incidence reaching up to 85% (25). Of these sexual dysfunctions, PE has also been quite commonly reported (16). In a population of 300 diabetic men, Owiredu et al. found a prevalence of PE of 56.6% (26). Contrary to his results, we detected a higher rate of PE among type 2 DM patients (78.9%) when compared to non-diabetic controls (47.4%). Such variation in reported rates may be secondary to differences in the studied population or to the tools used in PE assessment. In our study, we investigated type 2 DM only in participants from Middle East and North Africa regions utilizing the AIPE questionnaire, whereas Owiredu et al. used the Golombok Rust Inventory of Sexual Satisfaction (GRISS) questionnaire on a cohort from sub-Saharan Africa with an unspecified type of DM. It is worth mentioning here that differences in lifestyles among varied populations may have a direct influence on sexual function. This study has been conducted in a community known to have very high rates of diabetes and obesity (18), possibly due to a sedentary lifestyle. It is difficult to interpret whether this may have a direct effect on the prevalence of PE. Despite the fact that diabetic patients reported a significantly lower ELT than non-diabetics in this study population, their mean measured ELT (3.6 min) falls within the norm, representing an exaggerated impact for patient perceived sexual dysfunction.
The connection between PE and DM is still not clearly understood. The incidence of PE was found to be proportionate with the duration of DM and its severity. El-Sakka et al., determined that PE was more likely reported by patients who have been diabetic for more than 10 years. Additionally, those with poor glycemic control (HbA1c >7%) were 10 times as likely to report PE than patients with HbA1c <7% (16). In the present study such a correlation could not be reached. This may be due to the relatively young recruited population, which may have affected the duration and control of DM.
The search for reasons linking PE and DM entails a great deal of effort due to poor understanding of PE etiology from one hand, and due to a multi-systemic and poly-etiologic nature of DM on the other hand. Possible apparent associations between PE and DM include neurologic, neurotransmitter and psychologic dysfunctions.
Neurologic derangement is one of many pathophysiologic theories explaining the development of PE. In the spinal cord, a spinal generator of ejaculation has been discovered that triggers ejaculation aided by inputs from central and peripheral somatic nerves and by autonomic nerves (27). It is still vague how this center controls ejaculation though it can malfunction in diabetic patients. Diabetic neuropathy is a well-recognized microvascular complication of DM. It may manifest in different forms, including sensory, focal/multifocal, and autonomic neuropathies (28). Diabetic neuropathy is also known to be associated with impaired nitric oxide (NO) metabolism as a consequence of insulin resistance (29). NO is a key regulatory molecule with extensive metabolic, vascular, and cellular effects (30). Its effects on the cardiovascular system are well known making it the molecule of choice for all forms of phosphodiesterase inhibitors treating ED. Animal studies have shown that NO helps with PE, mainly inhibiting seminal emission, probably by decreasing sympathetic nervous system activity (31).
The ejaculatory reflex depends on the orchestrated action of many neurotransmitters such as central serotonergic and dopaminergic neurons, acetylcholine, adrenaline, neuropeptides, oxytocin, and γ-aminobutyric acid (GABA).
Serotonin
Waldinger et al., hypothesized that PE in humans may be explained by hyposensitivity of serotonin neurotransmitter receptor 5-HT2C (32). This serotonin dysregulation is also encountered in DM. Recent animal studies have demonstrated a distinct role for the 5-HT2C receptors in glucose homeostasis (33). 5-HT2C receptor agonists were investigated in obese and diabetic mice and initiated an improvement in glucose tolerance and insulin sensitivity (33).
Dopamine
Dopamine is another neurotransmitter that is thought to play major role in sexual arousal and ejaculation. Dopamine levels in the medial preoptic area of the hypothalamus increase step by step during sexual activity until reaching orgasm. In recent years, dopamine receptors were also discovered in rat and human seminal vesicles, indicating a possible role of dopamine in seminal emission (34). Animal experiments with dopamine receptor antagonists were successful in inhibition of ejaculation (35). Moreover, a glycemic effect to dopamine exists. Mammalian species living in the wild have an incredible ability to alter their metabolism from the insulin-sensitive/glucose-tolerant state to the insulin-resistant/glucose-intolerant state at exactly the right time of the year to survive long periods when food is sparse (36). Such animals are able to do so through rhythmic changes in dopamine levels in hypothalamic nuclei (36). Dopamine levels are low during the insulin-resistant state and increase to normal following return of the insulin-sensitive state (37). Moreover, selective destruction of the dopaminergic nuclei in the hypothalamus results in insulin resistance and diabetes (38). Although this data indicate a potential protective effect of dopamine on both conditions, future studies are still warranted to further elucidate the dopaminergic system influence on different levels.
Oxytocin
The so-called “love hormone” has been shown in various studies to play an important role in ejaculation in both rats and humans. Oxytocin levels in men have been shown to increase during sexual activity and decrease shortly after ejaculation. The idea of inhibiting oxytocin as a method to delay PE has been discussed for more than a decade. Clément et al. was able to document inhibition of ejaculation using oxytocin receptor antagonists (35). Accumulating evidence indicates a role for oxytocin in the regulation of food balance and stimulation of glucose uptake in rat skeletal muscles (39). Interestingly, recent research demonstrated that oxytocin can lead to reversal of obesity as well as related glucose and insulin disorders in mouse models (40). However in another study, although oxytocin was found to reduce the fat mass in obese diabetic mice, it was accompanied by a worsening of basal glycemia and glucose tolerance (41).
Psychological theories of rapid ejaculation postulate that the lowered ejaculatory threshold stems from anxiety. This aspect is certainly shared by patients who suffer from DM, ED and/or PE. Diabetes is a well-known risk factor for ED (42). Anxiety and depression are often experienced by men with DM and are main reasons for non-compliance to treatment (43). Diabetes complications and treatment strategies were also found to reduce patient perceived quality of life (44). On the other hand, a close relationship between ED and PE exists. The longer the erectile problem, the worse the anxiety and the more marked is the PE (45). As a consequence, diabetic men develop performance anxiety regarding their erectile reliability and accordingly rush through intercourse thinking that they have limited time to complete the task. With this mindset, an additional dysfunction appears as men become more anxious about their sexual life (46). Results of IIEF-5 questionnaire used in this study coincide with a higher prevalence of PE and lower ELT in patients with DM.
This study has few limitations. Patients with DM had a significantly older age, a factor that may have influenced the study results. Furthermore, participants were recruited from the hospital’s OPD, who may be presenting with an unforeseen medical condition that can also affect the prevalence of PE.
Conclusions
PE is found to be significantly prevalent in diabetic patients compared to non-diabetics. Diabetic patients reported higher incidence of PE with increased severity of ED. The HbA1c level is not a predictive factor for occurrence of PE in this cohort. Although few proposed etiologic factors can explain this relationship, further clinical and bio-cellular research is needed to help unveil this observation.
Acknowledgements
Funding: This research has been funded by the medical research center at Hamad Medical Corporation.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Serefoglu EC, McMahon CG, Waldinger MD, et al. An evidence-based unified definition of lifelong and acquired premature ejaculation: report of the second international society for sexual medicine ad hoc committee for the definition of premature ejaculation. Sex Med 2014;2:41-59. 10.1002/sm2.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segraves RT. Considerations for an evidence-based definition of premature ejaculation in the DSM-V. J Sex Med 2010;7:672-9. 10.1111/j.1743-6109.2009.01682.x [DOI] [PubMed] [Google Scholar]
- 3.Althof SE, Abdo CH, Dean J, et al. International Society for Sexual Medicine's guidelines for the diagnosis and treatment of premature ejaculation. J Sex Med 2010;7:2947-69. 10.1111/j.1743-6109.2010.01975.x [DOI] [PubMed] [Google Scholar]
- 4.Waldinger MD, Schweitzer DH. Changing paradigms from a historical DSM-III and DSM-IV view toward an evidence-based definition of premature ejaculation. Part II--proposals for DSM-V and ICD-11. J Sex Med 2006;3:693-705. 10.1111/j.1743-6109.2006.00276.x [DOI] [PubMed] [Google Scholar]
- 5.Althof SE, McMahon CG, Waldinger MD, et al. An update of the International Society of Sexual Medicine's guidelines for the diagnosis and treatment of premature ejaculation (PE). Sex Med 2014;2:60-90. 10.1002/sm2.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schapiro B. Potency disorders in the male; a review of 1960 cases of premature ejaculation. Harefuah 1953;45:39-41. [PubMed] [Google Scholar]
- 7.Xin ZC, Chung WS, Choi YD, et al. Penile sensitivity in patients with primary premature ejaculation. J Urol 1996;156:979-81. 10.1016/S0022-5347(01)65677-5 [DOI] [PubMed] [Google Scholar]
- 8.Waldinger MD, Berendsen HH, Blok BF, et al. Premature ejaculation and serotonergic antidepressants-induced delayed ejaculation: the involvement of the serotonergic system. Behav Brain Res 1998;92:111-8. 10.1016/S0166-4328(97)00183-6 [DOI] [PubMed] [Google Scholar]
- 9.Jannini EA, Lombardo F, Lenzi A. Correlation between ejaculatory and erectile dysfunction. Int J Androl 2005;28 Suppl 2:40-5. 10.1111/j.1365-2605.2005.00593.x [DOI] [PubMed] [Google Scholar]
- 10.Carani C, Isidori AM, Granata A, et al. Multicenter study on the prevalence of sexual symptoms in male hypo- and hyperthyroid patients. J Clin Endocrinol Metab 2005;90:6472-9. 10.1210/jc.2005-1135 [DOI] [PubMed] [Google Scholar]
- 11.Mosli HA, Mosli HH, Bokhari AA. The effect of obesity and components of metabolic syndrome on urinary and sexual functions in Saudi men. Res Rep Urol 2013;5:91-7. 10.2147/RRU.S43925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels, Belgium, 2013. [Google Scholar]
- 13.International Diabetes Federation. IDF Diabetes Atlas, 6th edition, update poster 2014. Available online: http://www.idf.org/diabetesatlas/
- 14.Thomas AM, LoPiccolo J. Sexual functioning in persons with diabetes: Issues in research, treatment, and education. Clinical Psychology Review 1994;14:61-86. 10.1016/0272-7358(94)90048-5 [DOI] [Google Scholar]
- 15.Hakim LS, Goldstein I. Diabetic sexual dysfunction. Endocrinol Metab Clin North Am 1996;25:379-400. 10.1016/S0889-8529(05)70329-7 [DOI] [PubMed] [Google Scholar]
- 16.El-Sakka AI. Premature ejaculation in non-insulin-dependent diabetic patients. Int J Androl 2003;26:329-34. 10.1111/j.1365-2605.2003.00433.x [DOI] [PubMed] [Google Scholar]
- 17.Saenz de Tejada I, Goldstein I. Diabetic penile neuropathy. Urol Clin North Am 1988;15:17-22. [PubMed] [Google Scholar]
- 18.Bener A, Zirie M, Janahi IM, et al. Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar. Diabetes Res Clin Pract 2009;84:99-106. 10.1016/j.diabres.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 19.Arafa M, Shamloul R. Development and evaluation of the Arabic Index of Premature Ejaculation (AIPE). J Sex Med 2007;4:1750-6. 10.1111/j.1743-6109.2006.00213.x [DOI] [PubMed] [Google Scholar]
- 20.Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822-30. 10.1016/S0090-4295(97)00238-0 [DOI] [PubMed] [Google Scholar]
- 21.Shamloul R, Ghanem H, Abou-zeid A. Validity of the Arabic version of the sexual health inventory for men among Egyptians. Int J Impot Res 2004;16:452-5. 10.1038/sj.ijir.3901248 [DOI] [PubMed] [Google Scholar]
- 22.Waldinger MD, Quinn P, Dilleen M, et al. A multinational population survey of intravaginal ejaculation latency time. J Sex Med 2005;2:492-7. 10.1111/j.1743-6109.2005.00070.x [DOI] [PubMed] [Google Scholar]
- 23.Montorsi F. Prevalence of premature ejaculation: a global and regional perspective. J Sex Med 2005;2 Suppl 2:96-102. 10.1111/j.1743-6109.2005.20369.x [DOI] [PubMed] [Google Scholar]
- 24.Shaeer O, Shaeer K. The Global Online Sexuality Survey (GOSS): ejaculatory function, penile anatomy, and contraceptive usage among Arabic-speaking Internet users in the Middle East. J Sex Med 2012;9:425-33. 10.1111/j.1743-6109.2011.02338.x [DOI] [PubMed] [Google Scholar]
- 25.Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54-61. [DOI] [PubMed] [Google Scholar]
- 26.Owiredu WK, Amidu N, Alidu H, et al. Determinants of sexual dysfunction among clinically diagnosed diabetic patients. Reprod Biol Endocrinol 2011;9:70. 10.1186/1477-7827-9-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staudt MD, Truitt WA, McKenna KE, et al. A pivotal role of lumbar spinothalamic cells in the regulation of ejaculation via intraspinal connections. J Sex Med 2012;9:2256-65. 10.1111/j.1743-6109.2011.02574.x [DOI] [PubMed] [Google Scholar]
- 28.Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956-62. 10.2337/diacare.28.4.956 [DOI] [PubMed] [Google Scholar]
- 29.Goligorsky MS, Chen J, Brodsky S. Workshop: endothelial cell dysfunction leading to diabetic nephropathy: focus on nitric oxide. Hypertension 2001;37:744-8. 10.1161/01.HYP.37.2.744 [DOI] [PubMed] [Google Scholar]
- 30.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 1993;329:2002-12. 10.1056/NEJM199312303292706 [DOI] [PubMed] [Google Scholar]
- 31.Hull EM, Lumley LA, Matuszewich L, et al. The roles of nitric oxide in sexual function of male rats. Neuropharmacology 1994;33:1499-504. 10.1016/0028-3908(94)90054-X [DOI] [PubMed] [Google Scholar]
- 32.Waldinger MD. The neurobiological approach to premature ejaculation. J Urol 2002;168:2359-67. 10.1016/S0022-5347(05)64146-8 [DOI] [PubMed] [Google Scholar]
- 33.Zhou L, Sutton GM, Rochford JJ, et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab 2007;6:398-405. 10.1016/j.cmet.2007.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyun JS, Baig MR, Yang DY, et al. Localization of peripheral dopamine D1 and D2 receptors in rat and human seminal vesicles. J Androl 2002;23:114-20. [DOI] [PubMed] [Google Scholar]
- 35.Clément P, Bernabé J, Compagnie S, et al. Inhibition of ejaculation by the non-peptide oxytocin receptor antagonist GSK557296: a multi-level site of action. Br J Pharmacol 2013;169:1477-85. 10.1111/bph.12198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cincotta AH. Hypothalamic Role in the Insulin Resistance Syndrome. In: Hansen B, Shaffrir E, editors. Reistance and Insulin Resistance Syndrome. London: Taylor and Francis, 2002. [Google Scholar]
- 37.Luo S, Meier AH, Cincotta AH. Bromocriptine reduces obesity, glucose intolerance and extracellular monoamine metabolite levels in the ventromedial hypothalamus of Syrian hamsters. Neuroendocrinology 1998;68:1-10. 10.1159/000054344 [DOI] [PubMed] [Google Scholar]
- 38.Luo S, Luo J, Meier AH, et al. Dopaminergic neurotoxin administration to the area of the suprachiasmatic nuclei induces insulin resistance. Neuroreport 1997;8:3495-9. 10.1097/00001756-199711100-00016 [DOI] [PubMed] [Google Scholar]
- 39.Gutkowska J, Broderick TL, Bogdan D, et al. Downregulation of oxytocin and natriuretic peptides in diabetes: possible implications in cardiomyopathy. J Physiol 2009;587:4725-36. 10.1113/jphysiol.2009.176461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang G, Bai H, Zhang H, et al. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron 2011;69:523-35. 10.1016/j.neuron.2010.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altirriba J, Poher AL, Caillon A, et al. Divergent effects of oxytocin treatment of obese diabetic mice on adiposity and diabetes. Endocrinology 2014;155:4189-201. 10.1210/en.2014-1466 [DOI] [PubMed] [Google Scholar]
- 42.Al Naimi A, Majzoub AA, Talib RA, et al. Erectile dysfunction in qatar: prevalence and risk factors in 1,052 participants-a pilot study. Sex Med 2014;2:91-5. 10.1002/sm2.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eriksson AK, Ekbom A, Granath F, et al. Psychological distress and risk of pre-diabetes and Type 2 diabetes in a prospective study of Swedish middle-aged men and women. Diabet Med 2008;25:834-42. 10.1111/j.1464-5491.2008.02463.x [DOI] [PubMed] [Google Scholar]
- 44.Huang ES, Brown SE, Ewigman BG, et al. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care 2007;30:2478-83. 10.2337/dc07-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oztürk B, Cetinkaya M, Saglam H, et al. Erectile dysfunction in premature ejaculation. Arch Ital Urol Androl 1997;69:133-6. [PubMed] [Google Scholar]
- 46.Althof SE. Pharmacological treatment for rapid ejaculation: Preliminary strategies, concerns and questions. Sex Marital Ther 1995;10:247-51. [Google Scholar]


