Abstract
The available oocyte pool is determined before birth, with the majority of oocytes lost before puberty. We hypothesised that events occurring before birth, in childhood or in adolescence (‘early-life risk factors’) could influence the size of the oocyte pool and thus the timing of menopause. We included cross-sectional data from 273,474 women from the UK Biobank, recruited in 2006–2010 from across the UK. We analysed the association of early menopause with events occurring before adulthood in 11,781 cases (menopause aged under 45) and 173,641 controls (menopause/pre-menopausal at ≥45 years), in models controlling for potential confounding variables. Being part of a multiple birth was strongly associated with early menopause (odds ratio = 1.42, confidence interval: 1.11, 1.82, P = 8.0 × 10−9, fully-adjusted model). Earlier age at menarche (odds ratio = 1.03, confidence interval: 1.01, 1.06, P = 2.5 × 10−6) and earlier year of birth were also associated with EM (odds ratio = 1.02, confidence interval: 1.00, 1.04, P = 8.0 × 10−6). We also confirmed previously reported associations with smoking, drinking alcohol, educational level and number of births. We identified an association between multiple births and early menopause, which connects events pre-birth, when the oocyte pool is formed, with reproductive ageing in later life.
Age at menopause influences health in later life with earlier menopause associated with increased osteoporosis and cardiovascular disease, and poorer cognitive function, but lower risks of several reproductive cancers1,2. Over half of the population variation in menopause age is estimated to be non-genetic, however only smoking and nulliparity have been reproducibly linked to earlier menopause3.
Natural menopause occurs on average at 51 years of age in Caucasian populations when the number of oocytes in the ovary are reduced below about 1000, however the factors that determine the timing of this event are poorly understood4. The number of ovarian follicles is determined before birth: approximately 7 million oocytes are produced by 6 months post conception, though this number declines rapidly before birth, continuing after birth so that by puberty only about 400,000 primary oocytes remain. Over 99% of ovarian follicle loss is due to atresia5, hence the rate of loss will influence age at menopause3. Menopause under the age of 45 affects approximately 5% of women and is of clinical relevance since it is associated with increased morbidity and mortality and affected individuals may benefit from treatment with hormone therapy6.
Early life events (defined in this study as occurring before birth, in childhood or in adolescence) have shown inconsistent relationships with age at menopause3. Previously reported positive associations with menopause include birth weight7,8,9, birth year10, whether breast fed as a baby11,12, food deprivation13,14, age at menarche15 childhood socio-economic status16, maternal smoking during pregnancy17, and weight in early childhood7,11,12. However, several studies have also reported null associations with these same traits, creating substantial uncertainty in the epidemiological literature10,18,19,20.
We hypothesised that pre-birth and early-life events could influence the oocyte pool and thus influence the timing of menopause. In this, one of the largest epidemiological analyses of age at menopause, we investigated whether early life variables are associated with the clinically-relevant outcome of menopause below the age of 45 years (early menopause (EM)). We investigated the early-life risk factors birth year, maternal smoking around birth, birth weight, being part of a multiple birth, whether breastfed as a baby, whether adopted as a child, handedness, comparative height and body size at age 10, age of menarche and illnesses occurring at under 20 years-of-age.
Methods
Source of data
We analysed data from 273,474 women from the UK Biobank, which includes 503,325 people aged 40–69 years recruited in 2006–2010 from across the UK21 (further details in Supplementary Methods).
Identification of early menopause cases and controls
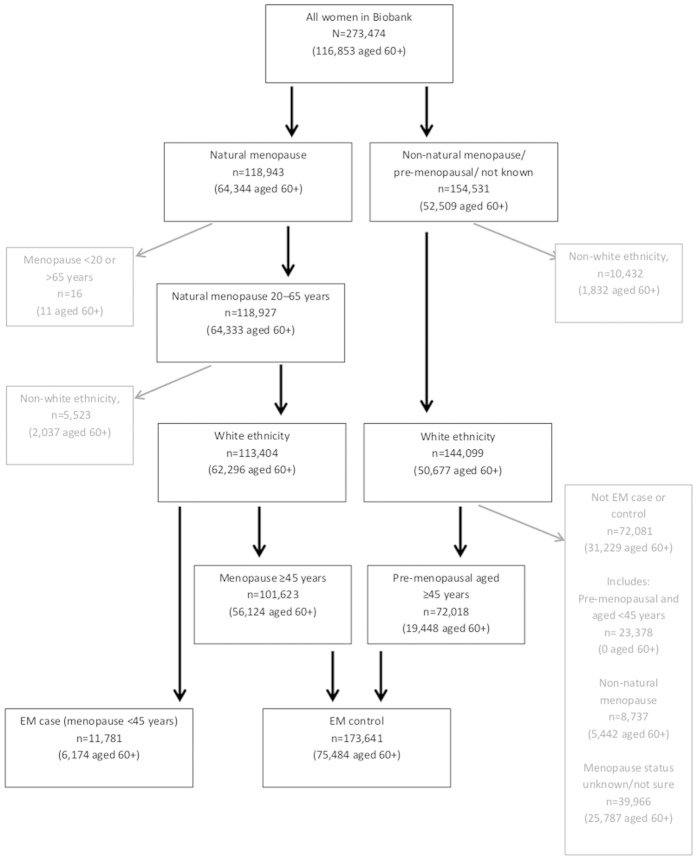
We considered the outcome EM in our analyses, since this is clinically relevant and captures the lower extreme of normal reproductive ageing. EM cases were women with menopause under 45 years while controls had menopause or were known to be pre-menopausal at age 45 or over. Age at natural menopause was defined as age at last menstrual period excluding those with surgical menopause or taking hormone replacement therapy (further details in Supplementary Methods). Of the 257,503 white women, there were 11,781 EM cases (4.6%) and 173,641 controls (67.4%) (Fig. 1).
Figure 1. Selection of data used in the analysis.
Early life variables
We hypothesised that early-life risk factors influence the timing of menopause. In this study, we defined an early-life risk factor as a risk-factor occurring before adulthood, i.e. before birth, in childhood or in adolescence. Early-life risk factors have a clear temporal relationship with menopause and are suitable for analysis in cross-sectional data since they are unlikely to be biased by, or cause bias in, age at menopause. Sixteen early-life risk factors were present in the data: birth year, maternal smoking around birth, birth weight, part of a multiple birth, breastfed as a baby, adopted as a child, handedness, comparative height at age 10, comparative body size at age 10, age of menarche and six illnesses occurring at under 20 years-of-age. Illnesses diagnosed under the age of 20 were analysed in broad categories and were selected on the basis of frequency and relevance to reproductive phenotypes: ‘allergy’, ‘diabetes’, ‘infections’, ‘headaches’, ‘gynaecological issues’ and ‘cancer’ (more details are provided in Supplementary Methods). Women providing no answer to any of the relevant questions, answering ‘Prefer not to answer’ or answering ‘Not sure’ were coded as missing.
Statistical methods
We used logistic regression to conduct analyses of early-life variables and odds of EM (n included from 98,273 to 181,778). All analyses were performed in Stata/SE v13.1. We restricted our analyses to women of white ethnicity (95% of cohort) and removed extreme outliers as appropriate (see Supplementary Methods). Potential confounding variables were controlled for in two ways: Firstly, the ‘partially-adjusted’ model included variables associated with age at menopause in published studies that were significant in exploratory univariate analyses of our UK Biobank data (approximately 1000 variables tested), and included Townsend deprivation index, BMI and smoking status; Secondly, the ‘fully-adjusted’ model, included all variables significantly associated with menopause in exploratory univariate analyses, and included smoking pack-years, frequency of alcohol intake, number of live births, educational level and whether the participant ate meat.
We included all available individuals including those with some missing data in each analysis. We also conducted sensitivity analyses in women with only complete data for all variables tested (n = 78,042): both sets of results were consistent. Due to the number of variables present in our UK Biobank data (approximately 1000), we used a conservative significance threshold of P < 5 × 10−5 for all tests and calculated 99.995% confidence intervals (CIs) around the odds ratio (OR).
Consistency of relationships and sensitivity analyses
We performed parallel analysis using Cox proportional hazards regression models to check for consistency of relationships with menopause as a quantitative trait (Supplementary Methods) (n included from 126,001 to 228,221). To investigate the sensitivity of our results to age at recruitment which was different in cases and controls (Supplementary Fig. 1), we performed the same analyses in a subset of women aged 60 and over. Of 112,887 women aged 60 and over, there were 6,174 EM cases (5.5%) and 75,484 controls (66.9%) (Fig. 1). We investigated whether results that were significant for EM were also significant for primary ovarian insufficiency (POI) by conducting logistic regression in 2,549 POI cases (menopause under 40 years) and 218,269 controls (known to be pre-menopausal at age 40 or over).
Results
Distribution of age at menopause is skewed
We identified 113,417 women with natural menopause, with a mean age at menopause of 50 years (Table 1). The distribution of age at menopause was skewed even when only women aged 60 and over were considered, and had peaks at values ending in zero, two and five (Supplementary Fig. 2). Descriptive statistics for the cohort are presented in Table 2 and Supplementary Table 1.
Table 1. Age at natural menopause of women with self-reported white ethnicity in UK Biobank.
| n | N | % | Mean age at menopause in years (standard deviation) | Median age at menopause in years (range) | |
|---|---|---|---|---|---|
| Natural menopause | 113,417 | 257,516 | 44.0 | 50.0 (4.5) | 50 (18,65) |
| Early menopause (<45 years) | 11,794 | 233,394 | 5.1 | 40.6 (3.5) | 42 (18,44) |
n is number of women with natural menopause or early menopause (includes women with menopause at <20 years or >65 years) and women with early menopause aged <45 years at recruitment). N is the total number of women for whom whom menopause (all women) or early menopause status (women aged 45 and over at recruitment) could be determined
Table 2. Descriptive statistics for early life variables.
| All cohort | Natural menopause | Early menopause (<45 years) | |||||
|---|---|---|---|---|---|---|---|
| Age of menarche | n | 250,143 | 111,034 | 11,565 | |||
| mean | 13.0 | 13.0 | 12.9 | ||||
| s.d. | 1.6 | 1.6 | 1.7 | ||||
| median (range) | 13 (5,25) | 13 (5,25) | 13 (5,21) | ||||
| Birth weight (kg) | n | 164,448 | 69,367 | 7,210 | |||
| mean | 3.2 | 3.2 | 3.2 | ||||
| s.d. | 0.6 | 0.6 | 0.7 | ||||
| median (range) | 3.2 (0.4,9) | 3.2 (0.4,9) | 3.2 (0.7,6.3) | ||||
| Year of birth | n | 257,516 | 113,417 | 11,794 | |||
| mean | 1951.5 | 1948.4 | 1949.6 | ||||
| s.d. | 8.0 | 5.6 | 7.1 | ||||
| median (range) | 1950 (1936,1970) | 1948 (1936,1969) | 1948 (1936,1969) | ||||
| n | % | n | % | n | % | ||
| Breastfed as a baby | No | 64,388 | 31.0 | 23,563 | 26.1 | 2,807 | 30.1 |
| Yes | 143,195 | 69.0 | 66,689 | 73.9 | 6,531 | 69.9 | |
| Total | 207,583 | 90,252 | 9,338 | ||||
| Comparative height at age 10 | Shorter | 53,619 | 21.2 | 23,549 | 21.1 | 2,502 | 21.6 |
| Taller | 64,628 | 25.5 | 28,113 | 25.2 | 2,987 | 25.8 | |
| Average | 134,773 | 53.3 | 59,943 | 53.7 | 6,105 | 52.7 | |
| Total | 253,020 | 111,605 | 11,594 | ||||
| Comparative body size at age 10 | Thinner | 79,326 | 31.3 | 34,402 | 30.7 | 3,890 | 33.5 |
| Plumper | 45,203 | 17.8 | 19,358 | 17.3 | 2,050 | 17.6 | |
| Average | 129,271 | 50.9 | 58,170 | 52.0 | 5,685 | 48.9 | |
| Total | 253,800 | 111,930 | 11,625 | ||||
| Maternal smoking around birth | No | 156,728 | 70.3 | 70,228 | 71.6 | 6,977 | 68.2 |
| Yes | 66,291 | 29.7 | 27,904 | 28.4 | 3,256 | 31.8 | |
| Total | 223,019 | 98,132 | 10,233 | ||||
| Part of a multiple birth | No | 247,777 | 97.7 | 109,292 | 97.6 | 11,242 | 96.9 |
| Yes | 5,928 | 2.3 | 2,637 | 2.4 | 365 | 3.1 | |
| Total | 253,705 | 111,929 | 11,607 | ||||
Based on women with self-reported white ethnicity in UK Biobank. Women providing no answer, answering ‘Prefer not to answer’ or answering ‘Not sure’ were excluded from the analysis.
Associations of potential confounding variables
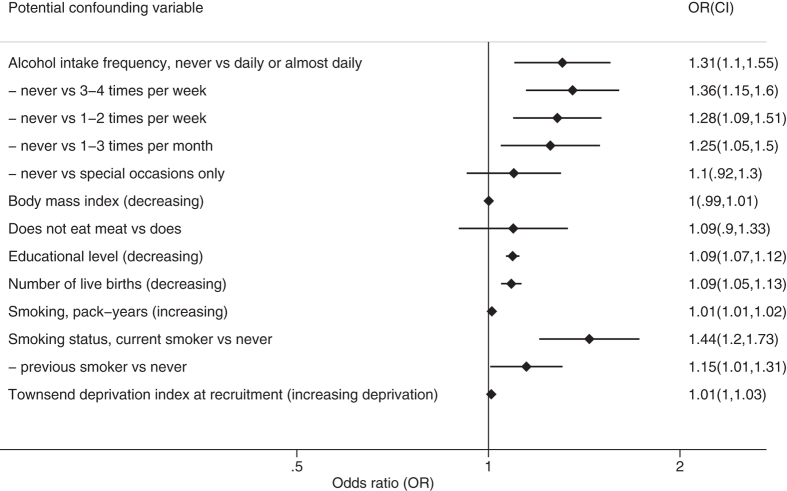
Being a current smoker had the largest effect on EM (for current smokers compared with never smokers, OR = 1.44, CI: 1.20, 1.73, P < 1 × 10−15) (Fig. 2) (Supplementary Tables 2 and 3). Other non-early life factors associated with earlier menopause were never drinking alcohol (e.g. OR = 1.28, CI: 1.09, 1.51, P = 2.5 × 10−10 for never drinking compared with drinking 1–2 times per week), decreasing educational level (OR = 1.09, CI: 1.07, 1.12, P < 1 × 10−15 per level), and fewer live births (OR = 1.09, CI: 1.05, 1.13, P < 1 × 10−15 per birth).
Figure 2. Associations of potential confounding variables with early menopause.
Results shown are for the fully-adjusted logistic regression model including the potential confounding variables Townsend deprivation index, BMI, smoking status, smoking pack-years, frequency of alcohol intake, number of live births, educational level and whether the participant ate meat (n = 152,701). Confidence intervals are 99.995%.
Events before birth are associated with EM
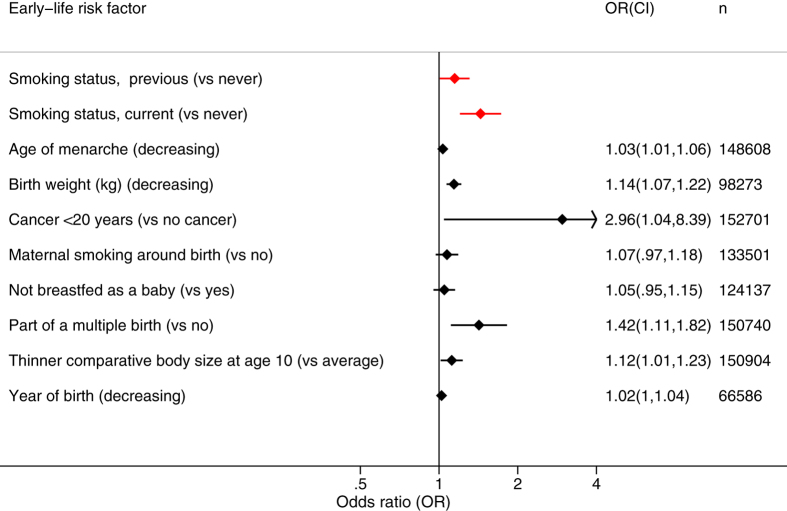
Earlier year of birth was associated with EM (OR = 1.02, CI: 1.00, 1.04, P = 8 × 10−6) in the partially- and fully-adjusted models, as was decreasing birth weight and being part of a multiple birth (Fig. 3) (Supplementary Tables 2 and 3). For the effect of year of birth on EM to be as large as being a current smoker, a women would need to be born 16.9 years earlier (2.1 standard deviations).
Figure 3. Associations between early-life risk factors and early menopause.
Results are for the logistic regression model including one early-life risk factor at a time, adjusted for the potential confounding variables Townsend deprivation index, BMI, smoking status, smoking pack-years, frequency of alcohol intake, number of live births, educational level and whether the participant ate meat. Analyses are in all ages, except for year of birth which was analysed in women aged 60 and over at recruitment. Smoking status is included for reference. Confidence intervals are 99.995%. To achieve the same effect size as being a current smoker (the binary variable with nominally the largest effect size), for the continuous variables the required change in risk factor would be: age at menarche, 11.1 years (7.0 s.d.); birth weight 2.8 kg (4.7 s.d.); year of birth, 16.9 years (2.1 s.d.).
Maternal smoking was significantly associated with EM only in the partially-adjusted model. The effect size for being part of a multiple birth (OR = 1.42, CI: 1.11, 1.82, P = 8 × 10−9, fully-adjusted model) was similar to that of being a current smoker (OR = 1.44, CI: 1.20, 1.73, P < 1 × 10−15, fully-adjusted model). These associations remained when multiple birth, decreasing birth weight and maternal smoking were considered in the same logistic regression model (Supplementary Table 4).
Since the association of EM with birth weight is adjusted for BMI, it could be considered that this is actually an association between EM and change in size22. The association with birth weight persisted regardless of adjustment for BMI (fully-adjusted model including BMI, OR = 1.14 per kg, P = 2.5 × 10−10; fully-adjusted model without BMI, OR = 1.14 per kg, P = 2.3 × 10−10), indicating that the association was with birth weight and not change in size.
Events in childhood and adolescence are associated with earlier menopause
Earlier age at menarche was associated with EM in the fully-adjusted logistic model (OR = 1.03, CI: 1.01, 1.06, P = 2.5 × 10−6), but not the partially-adjusted model (Fig. 3, Supplementary Table 2). The perception of being ‘thinner at age 10’ was significantly associated with EM in the partially- and fully-adjusted models (OR = 1.12, CI: 1.01, 1.23, P = 3.5 × 10−6 for fully-adjusted) (Fig. 3). When comparative body size at age 10 was included with age at menarche in the model (Supplementary Table 5), both remained significantly associated with EM. The description of being ‘plumper at age 10’ was not associated with age at menopause in any of the models. Handedness, whether adopted as a child and being breastfed were not associated with EM (Fig. 3) (Supplementary Tables 2 and 3).
EM is associated with cancer but not with other illnesses
Considering illnesses under the age of 20, only cancer was associated with EM (Fig. 3, Supplementary Tables 2 and 3), but only in the fully-adjusted model (OR = 2.96, CI: 1.04, 8.39, P = 2.4 × 10−5). There were no significant associations with allergy, gynaecological problems, infections and headache/migraine.
Consistency of relationships in Cox proportional hazards model of menopause as a quantitative trait and sensitivity analysis
Using Cox proportional hazards regression, we were able to explore whether associations with EM also held for menopause as a quantitative trait, where the outcome is hazard ratio (HR) of menopause. The potential confounding variables that were significantly associated with EM – drinking alcohol, educational level, number of live births, and smoking – also had consistent directions of effect in the Cox model. Increasing social deprivation, lower BMI and not eating meat were associated with increased HR of menopause, though these risk factors were not associated with EM (Supplementary Tables 2 and 3) (Supplementary Fig. 3). The directions of effect for the early-life risk factors were consistent in the EM and Cox models (Supplementary Tables 2 and 3). In the analysis based on women aged 60 and over, the direction of effects was consistent with the analysis in all age groups (Supplementary Table 3). In the analysis of the outcome POI, none of the early-life variables reached our significance threshold though directions of effect were consistent (Supplementary Table 6).
Multiple birth and menarche remain strongly associated in models including all early-life risk factors
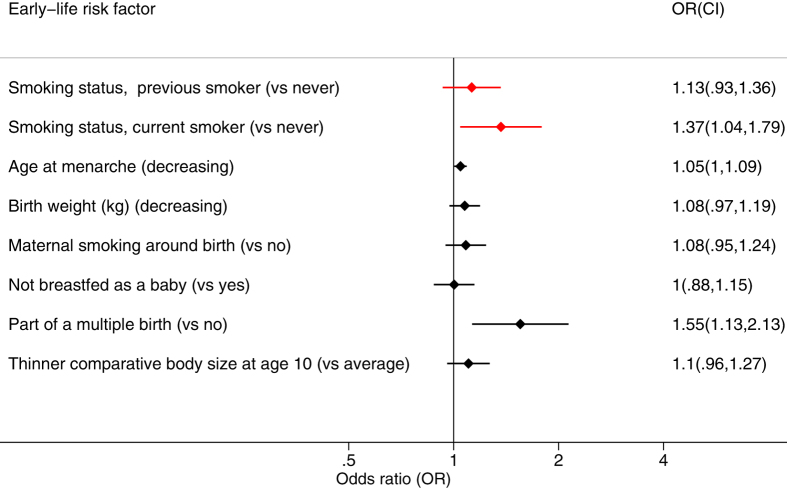
In a fully-adjusted model including all early-life risk factors that were associated with EM (age at menarche, birth weight, maternal smoking, breast fed as a baby, part of multiple birth and comparative body size at age 10), only the risk factors younger age at menarche (OR = 1.05, CI: 1.0, 1.09, P = 7.27 × 10−6) and being part of a multiple birth (OR = 1.55, CI: 1.13, 2.13, P = 2.11 × 10−8) remained significantly associated with EM (Fig. 4, Supplementary Table 7). Only age at menarche remained significantly associated in the Cox model including all early-life risk factors (Supplementary Table 7). Cancer at under 20 years and year of birth were not included in the model including all early-life risk factors, due to the small number of cases in the former, and bias due to age at recruitment in the latter.
Figure 4. Associations in the multiple early-life risk factor model.
Results shown are for the logistic regression model in all age groups including all early-life risk factors at the same time (n = 78,603), adjusted for the potential confounding variables Townsend deprivation index, BMI, smoking status, smoking pack-years, frequency of alcohol intake, number of live births, educational level and whether the participant ate meat. The early life risk-factors included are age at menarche, birth weight, maternal smoking, breast fed as a baby, part of multiple birth and comparative body size at age 10. Cancer at under 20 years and year of birth were not included due to the small number of cases in the former, and confounding with age at recruitment in the latter. Confidence intervals are 99.995%.
Discussion
Our study is the largest to date on the effect of non-genetic risk factors on age at menopause, compared with the previous largest studies in 95,704 US women and 50,678 UK women15,23. In this study, we have shown that pre-birth and early-life risk factors are associated with EM, an event in later life. Being part of a multiple birth demonstrated the most robust association, with an effect size comparable to being a smoker in this study, the single biggest predictor previously identified10,15,18,19,23,24,25. The association with multiple births persisted when we included all significant early-life variables and adjusted for all potential confounders. Non-early life risk factors are better characterised in the current literature than early-life variables and our results were consistent with previous studies. We found that odds of EM were increased by being a current smoker10,15,18,19,23,24,25, having decreased levels of education18,19,25,26 and being nulliparous15,23. However, we did not find consistent associations between age at menopause and BMI15,23, socio-economic status and not eating meat16,23,27, as suggested by previous studies.
Being part of a multiple birth and birth weight were the pre-birth risk factors with the strongest associations with EM. An association of EM with multiple births has not previously been reported, though the prevalence of POI has been found to be higher in monozygotic and dizygotic twins20,28. This suggests that the shared intra-uterine or early postnatal environment may play a role, or that there could be overlap in the genetics of twinning and age at menopause. The rate of multiple births in our study (2.4%) was slightly lower than recent estimates (3%), since the rate has increased in recent years due to assisted reproduction9,29,30. The association of multiple births with EM in this study should be independent of any potential effects of assisted reproduction since women in the study were born prior to its introduction. Babies from a multiple pregnancy are more likely to suffer complications including intrauterine growth restriction and are smaller at birth. However, intra-uterine growth restriction has not been found to restrict ovary growth and development31. Multiple births are more common as women age30 and therefore the size of the oocyte pool could be reduced in offspring of older mothers. This could be mediated by genetic abnormalities, hypertension or birth complications, which are known to be more common in older women. However, we tested for effects of increased maternal age in our data and found no increased odds of EM, in fact there was a modest effect in the opposite direction, though we were unable to adjust for potential confounding factors such as maternal socio-economic status and age at menopause and survivor bias. In our study there was an association of EM with birth weight, however the effect was less strong than that of multiple births but this may be because we were unable to adjust our analysis by gestational age. Evidence from other studies of the effects of birth weight has been contradictory7,9,20,32.
The association between earlier age at menarche and increased odds of EM agrees with another analysis using the UK Biobank data33, and is supported by findings from a study on over 90,000 women15 and a smaller study on several thousand women34 which both found associations between earlier menarche and earlier menopause. In addition, a published study has reported a correlation (using LD score regression) between genetic variants associated with age at menarche and age at menopause (rg = 0.14, P = 0.003) and five loci associated with age at menopause contain genes associated with hypogonadotropic hypogonadism50. We found that being thinner at age 10 was associated with increased odds of EM. It is unlikely that this association is driven by an effect on menarche timing as being thinner in childhood is associated with delayed menarche35,36 and in our data early menarche was a risk factor for EM. The role of childhood nutrition has been highlighted by previous studies that found associations between not being breast fed and earlier age at menopause11,12 (though this was not significantly associated with EM in our study) and earlier menopause in women who are lighter at 1–2 years7,11,12 or who have been exposed to famine in early childhood37.
In our cohort of women born in 1936–1970, there was an increased odds of EM in women born in earlier birth years. We estimate an increase in age at menopause of about 1.3–2.5 months per year of later birth (data not presented), which agrees with two smaller studies on cohorts born between 1908–1930 in Sweden (n = 1,017) and 1912–1969 in the USA (n = 22,851)34,38. The US study found a 17-month increase in age at menopause during 1915–193938, while the Swedish study found an increase of 1.2 months per later birth year.
In our analysis of illnesses occurring before the age of 20, only cancer showed an association with EM. It is likely that cancer treatment will have had an effect on age at menopause – such treatment is known to affect fertility39 and has late side-effects such as cardiovascular disease and cancers in later life40,41. The illness data used were self-reported and may possibly be affected by recall bias42,43. By restricting these analyses to cases under 20 we have minimised uncertainty about cause and effect of the illness and menopause. However, compared to a prospective study of incident cases, our cohort will be biased since the women included will have had to have survived until the study
A limitation of this study is that the UK Biobank data is based on volunteers and so there may be selection bias. The data used in our analysis was collected retrospectively and so may be subject to recall bias. Clear rounding of menopause age was evident, as has been seen in previous studies44. Previous studies have indicated that menopause is reasonably well recalled, though error increases with time after menopause45,46. Ethnicity has been shown to be associated with age at menopause15,18,26 and we restricted our analysis to white women as we had insufficient numbers of women of other ethnicities to conduct sub-analyses. Age at menopause is limited by age at recruitment, however this should not confound the effect of variables other than year of birth, which was analysed in a post-menopausal cohort to address this. In addition there could be additional confounders that were not captured by our data. There was variation in the number of responses for each variable, with only about 40% of the case–control cohort included in the full analysis model with all early-life risk factors, and we cannot rule out that such missing-ness could be informative.
Due to the number of women included in this study, we provide powerful evidence for the influence of early-life risk factors on EM, and hence women’s health. More specifically, that being a multiple birth, having menarche at a younger age, and being born in an earlier birth cohort are associated with EM. It is plausible that early-life events could influence the oocyte pool since the number of ovarian follicles is determined before birth and the number of primary oocytes decline dramatically prior to puberty – from about 7 million oocytes at 6 months post conception to about 400,000 oocytes by puberty. Early life events identified in our study could affect the oocyte reserve in utero or in early development and could be mediated through increased stress to oocytes, perhaps caused by reduced nutritional or oxygen availability. In rats, suboptimal nutrition during pregnancy is associated with transgenerational accelerated female reproductive ageing47. Factors affecting the number of follicles and their quality, and the rate of follicle decline both in utero and following birth could plausibly affect age at menopause, which itself is a consequence of a reduction in the follicle pool. At the cellular level, DNA repair has been identified as an important mechanism influencing age at menopause and may result in maintenance of a larger, better quality follicle pool48,49. Since up to 50% of population variation in age at menopause is thought to be due to genetics, risk factors affecting the size of the follicle pool are likely to have different effects on age at menopause according to the genetic variants present. We anticipate that these findings should provide direction for further work into the biological processes driving menopause, and in particular how genetics and the environment interact.
Additional Information
How to cite this article: Ruth, K. S. et al. Events in Early Life are Associated with Female Reproductive Ageing: A UK Biobank Study. Sci. Rep. 6, 24710; doi: 10.1038/srep24710 (2016).
Supplementary Material
Acknowledgments
This research has been conducted using the UK Biobank Resource. This work was generously supported by a Wellcome Trust Institutional Strategic Support Award [WT097835MF to University of Exeter].
Footnotes
Author Contributions A.M. designed and directed the study, reviewed results of analysis and revised the manuscript. K.S.R. performed the statistical analysis and prepared the manuscript. W.E.H., D.M., M.N.W. and J.R.B.P advised on the analytic strategy, reviewed results of statistical analysis and commented on the manuscript.
References
- Epperson C. N., Sammel M. D. & Freeman E. W. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocrinol Metab 98, 3829–3838, doi: 10.1210/jc.2013-1808 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C. & Murabito J. M. Genome-wide association studies of age at menarche and age at natural menopause. Mol Cell Endocrinol, doi: 10.1016/j.mce.2012.05.003 (2012). [DOI] [PubMed] [Google Scholar]
- Mishra G. D., Cooper R., Tom S. E. & Kuh D. Early life circumstances and their impact on menarche and menopause. Womens Health (Lond Engl) 5, 175–190, doi: 10.2217/17455057.5.2.175 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde E. R. & Pearson P. L. The variability of female reproductive ageing. Hum Reprod Update 8, 141–154 (2002). [DOI] [PubMed] [Google Scholar]
- Matsuda F., Inoue N., Manabe N. & Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. The Journal of reproduction and development 58, 44–50 (2012). [DOI] [PubMed] [Google Scholar]
- Faubion S. S., Kuhle C. L., Shuster L. T. & Rocca W. A. Long-term health consequences of premature or early menopause and considerations for management. Climacteric: the journal of the International Menopause Society 18, 483–491, doi: 10.3109/13697137.2015.1020484 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell J. L. et al. Is the age of menopause determined in-utero? Early Human Development 49, 143–148 (1997). [DOI] [PubMed] [Google Scholar]
- Tom S. E. et al. Fetal environment and early age at natural menopause in a British birth cohort study. Human Reproduction 25, 791–798 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar S. A., Sadrzadeh S., Do K. A., Martin N. G. & Lambalk C. B. Birth weight and age at menopause in Australian female twin pairs: exploration of the fetal origin hypothesis. Hum Reprod 15, 55–59 (2000). [DOI] [PubMed] [Google Scholar]
- van Noord P. A., Dubas J. S., Dorland M., Boersma H. & te Velde E. Age at natural menopause in a population-based screening cohort: the role of menarche, fecundity, and lifestyle factors. Fertil Steril 68, 95–102 (1997). [DOI] [PubMed] [Google Scholar]
- Hardy R. & Kuh D. Does early growth influence timing of the menopause? Evidence from a British birth cohort. Hum Reprod 17, 2474–2479 (2002). [DOI] [PubMed] [Google Scholar]
- Mishra G., Hardy R. & Kuh D. Are the effects of risk factors for timing of menopause modified by age? Results from a British birth cohort study. Menopause 14, 717–724, doi: 10.1097/GME.0b013e31802f3156 (2007). [DOI] [PubMed] [Google Scholar]
- Elias S. G., van Noord P. A., Peeters P. H., den Tonkelaar I. & Grobbee D. E. Childhood exposure to the 1944–1945 Dutch famine and subsequent female reproductive function. Hum Reprod 20, 2483–2488, doi: 10.1093/humrep/dei090 (2005). [DOI] [PubMed] [Google Scholar]
- Lumey L. H. & Stein A. D. In utero exposure to famine and subsequent fertility: The Dutch Famine Birth Cohort Study. American journal of public health 87, 1962–1966 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson K. D., Bernstein L., Henderson B., Kolonel L. & Pike M. C. Predictors of the timing of natural menopause in the Multiethnic Cohort Study. Am J Epidemiol 167, 1287–1294, doi: 10.1093/aje/kwn046 (2008). [DOI] [PubMed] [Google Scholar]
- Lawlor D. A., Ebrahim S. & Smith G. D. The association of socio-economic position across the life course and age at menopause: the British Women’s Heart and Health Study. BJOG: an international journal of obstetrics and gynaecology 110, 1078–1087 (2003). [PubMed] [Google Scholar]
- Strohsnitter W. C. et al. The association between in utero cigarette smoke exposure and age at menopause. Am J Epidemiol 167, 727–733, doi: 10.1093/aje/kwm351 (2008). [DOI] [PubMed] [Google Scholar]
- Cooper G. S. & Sandler D. P. Age at natural menopause and mortality. Ann Epidemiol 8, 229–235 (1998). [DOI] [PubMed] [Google Scholar]
- Mikkelsen T., Graff-Iversen S., Sundby J. & Bjertness E. Early menopause, association with tobacco smoking, coffee consumption and other lifestyle factors: a cross-sectional study. BMC Public Health 7, 149 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. Z., D’Aloisio A. A., Deroo L. A., Sandler D. P. & Baird D. D. Association of intrauterine and early-life exposures with age at menopause in the sister study. American Journal of Epidemiology 172, 140–148 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen N. E., Sudlow C., Peakman T., Collins R. & Biobank o. b. o. U. UK Biobank Data: Come and Get It. Science Translational Medicine 6, 224ed224, doi: 10.1126/scitranslmed.3008601 (2014). [DOI] [PubMed] [Google Scholar]
- Lucas A., Fewtrell M. S. & Cole T. J. Fetal origins of adult disease-the hypothesis revisited. BMJ (Clinical research ed.) 319, 245–249 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. H. et al. Body mass index, exercise, and other lifestyle factors in relation to age at natural menopause: analyses from the breakthrough generations study. Am J Epidemiol 175, 998–1005, doi: 10.1093/aje/kwr447 (2012). [DOI] [PubMed] [Google Scholar]
- Kinney, A. K. Alcohol, caffeine and smoking in relation to age at menopause. Maturitas 54, 27–38 (2006). [DOI] [PubMed] [Google Scholar]
- Luoto R., Kaprio J. & Uutela A. Age at natural menopause and sociodemographic status in Finland. Am J Epidemiol 139, 64–76 (1994). [DOI] [PubMed] [Google Scholar]
- Kinney A., Kline J. & Levin B. Alcohol, caffeine and smoking in relation to age at menopause. Maturitas 54, 27–38, doi: 10.1016/j.maturitas.2005.10.001 (2006). [DOI] [PubMed] [Google Scholar]
- Hardy R. & Kuh D. Social and environmental conditions across the life course and age at menopause in a British birth cohort study. BJOG: an international journal of obstetrics and gynaecology 112, 346–354, doi: 10.1111/j.1471-0528.2004.00348.x (2005). [DOI] [PubMed] [Google Scholar]
- Gosden R. G. et al. Prevalence of premature ovarian failure in monozygotic and dizygotic twins. Hum Reprod 22, 610–615, doi: 10.1093/humrep/del382 (2007). [DOI] [PubMed] [Google Scholar]
- Platt M. J., Marshall A. & Pharoah P. O. The effects of assisted reproduction on the trends and zygosity of multiple births in England and Wales 1974–99. Twin research: the official journal of the International Society for Twin Studies 4, 417–421 (2001). [DOI] [PubMed] [Google Scholar]
- Smith L. K. et al. Trends in the incidence and mortality of multiple births by socioeconomic deprivation and maternal age in England: population-based cohort study. BMJ open 4, e004514, doi: 10.1136/bmjopen-2013-004514 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin J. P. et al. Morphometry of human ovaries in normal and growth-restricted fetuses. Early Human Development 60, 179–192, doi: http://dx.doi.org/10.1016/S0378-3782(00)00118-3 (2001). [DOI] [PubMed] [Google Scholar]
- Tom S. E. et al. Fetal environment and early age at natural menopause in a British birth cohort study. Hum Reprod 25, 791–798, doi: 10.1093/humrep/dep451 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day F. R., Elks C. E., Murray A., Ong K. K. & Perry J. R. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Scientific reports 5, 11208, doi: 10.1038/srep11208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodstrom K. et al. Evidence for a secular trend in menopausal age: a population study of women in Gothenburg. Menopause 10, 538–543, doi: 10.1097/01.gme.0000094395.59028.0f (2003). [DOI] [PubMed] [Google Scholar]
- Anderson S. E., Dallal G. E. & Must A. Relative weight and race influence average age at menarche: results from two nationally representative surveys of US girls studied 25 years apart. Pediatrics 111, 844–850 (2003). [DOI] [PubMed] [Google Scholar]
- Garn S. M., LaVelle M., Rosenberg K. R. & Hawthorne V. M. Maturational timing as a factor in female fatness and obesity. The American journal of clinical nutrition 43, 879–883 (1986). [DOI] [PubMed] [Google Scholar]
- Elias S. G., van Noord P. A., Peeters P. H., den Tonkelaar I. & Grobbee D. E. Caloric restriction reduces age at menopause: the effect of the 1944–1945 Dutch famine. Menopause 10, 399–405, doi: 10.1097/01.gme.0000059862.93639.c1 (2003). [DOI] [PubMed] [Google Scholar]
- Nichols H. B. et al. From menarche to menopause: trends among US Women born from 1912 to 1969. Am J Epidemiol 164, 1003–1011, doi: 10.1093/aje/kwj282 (2006). [DOI] [PubMed] [Google Scholar]
- Bath L. E., Wallace W. H. B. & Critchley H. O. D. Late effects of the treatment of childhood cancer on the female reproductive system and the potential for fertility preservation. BJOG: An International Journal of Obstetrics & Gynaecology 109, 107–114, doi: 10.1111/j.1471-0528.2002.t01-1-01007.x (2002). [DOI] [PubMed] [Google Scholar]
- Geenen M. M., Cardous-Ubbink M. C., Kremer L. M. et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 297, 2705–2715, doi: 10.1001/jama.297.24.2705 (2007). [DOI] [PubMed] [Google Scholar]
- Oeffinger K. C. et al. Chronic Health Conditions in Adult Survivors of Childhood Cancer. New England Journal of Medicine 355, 1572–1582, doi: doi: 10.1056/NEJMsa060185 (2006). [DOI] [PubMed] [Google Scholar]
- Krall E. A., Valadian I., Dwyer J. T. & Gardner J. Recall of childhood illnesses. J Clin Epidemiol 41, 1059–1064 (1988). [DOI] [PubMed] [Google Scholar]
- Moberg C., Meding B., Stenberg B., Svensson A. & Lindberg M. Remembering childhood atopic dermatitis as an adult: factors that influence recollection. The British journal of dermatology 155, 557–560, doi: 10.1111/j.1365-2133.2006.07372.x (2006). [DOI] [PubMed] [Google Scholar]
- Hahn R. A., Eaker E. & Rolka H. Reliability of reported age at menopause. Am J Epidemiol 146, 771–775 (1997). [DOI] [PubMed] [Google Scholar]
- den Tonkelaar I. Validity and reproducibility of self-reported age at menopause in women participating in the DOM-project. Maturitas 27, 117–123 (1997). [DOI] [PubMed] [Google Scholar]
- Hahn R., Eaker E. & Rolka H. Reliability of reported age at menopause. [erratum appears in Am J Epidemiol 1999 Jan 15;149(2):201]. American Journal of Epidemiology 146, 771–775 (1997). [DOI] [PubMed] [Google Scholar]
- Aiken C. E., Tarry-Adkins J. L. & Ozanne S. E. Transgenerational Developmental Programming of Ovarian Reserve. Scientific reports 5, 16175, doi: 10.1038/srep16175 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk L. et al. Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat Genet 44, 260–268, doi: 10.1038/ng.1051 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day F. R. et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet 47, 1294–1303, doi: 10.1038/ng.3412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.