Abstract
We aimed to systematically investigate the potential association of matrix metalloproteinase (MMP)-9, -3, -2, and -8 gene polymorphisms with susceptibility to periodontitis using meta-analysis. A literature search in PubMed, Embase, and Web of Sciencewas conducted to obtain relevant publications. Finally a total of 16 articles with 24 case-control studies (nine on MMP-9-1562 C/T, seven on MMP-3-1171 A5/A6, four on MMP-2-753C/T, and four on MMP-8-799 C/T) were considered in this meta-analysis. The results based on 2,724 periodontitis patients and 3,438 controls showed that MMP-9-1562C/T, MMP-3-1171 A5/A6, and MMP-8-799C/T polymorphisms were associated with periodontitis susceptibility. No significant association was found between MMP-2-753 C/T and periodontitis susceptibility. Subgroup analyses suggested that the MMP-9-1562 C/T polymorphism reduced chronic periodontitis susceptibility and MMP-3-1171 A5/A6polymorphism increased chronic periodontitis susceptibility. In summary, current evidence demonstrated that MMP-9-753 C/Tpolymorphism reduced the risk of periodontitis, MMP-3-1171 5A/6A and MMP-8-799 C/Tpolymorphisms increased the risk of periodontitis, and MMP-2-753 C/T was not associated with risk of periodontitis.
Periodontitis is considered as an inflammatory disease caused by bacterial infection and characterized by loss of connective tissue and alveolar bone1,2. It is classified into two major types, namely chronic periodontitis (CP) and aggressive periodontitis (AgP)3,4, and is generally regarded as one of the most common diseases around the world with a prevalence of 15–20%5. Of greater importance, periodontitis has been suggested to be associated with other disorders such as head and neck cancer, coronary heart disease, and chronic obstructive pulmonary disease6,7,8,9. Therefore, to elucidate the etiology, identify risk factors, and prevent its onset are very important. In the past few years, great efforts had been taken to elucidate potential background leading to the etiology of periodontitis. In addition to bacterial infection, individuals’ susceptibility to periodontitis is likely to be of major importance in determining the manifestation and progression of the disease10. Genetic factors, such as cyclooxygenase-2 gene polymorphisms and interleukin gene polymorphisms had been demonstrated by meta-analyses that whether they were associated with periodontitis susceptibility11,12,13,14,15,16.
Periodontal health needs a balance between tissue destruction enzymes, e.g. matrix metalloproteinase (MMP), and its inhibitors. The MMP is a family of proteolytic enzymes involved in matrix remodeling and basement membranes in the begging and developing course of a wide range of diseases17. In addition, it has been confirmed to be involved in the pathogenesis of periodontitis18. Many meta-analyses showed that MMP-1 1607 G1/G2 gene polymorphism was associated with CP susceptibility, as well with the severity of disease condition19,20. Regarding MMP-9-1562 C/T polymorphism, a meta-analysis indicated that it had no association with periodontitis19, but another meta-analysis suggested that it might be involved in the development of periodontitis21. Therefore, we determined to perform a meta-analysis with improved quality and to investigate the interaction association between gene polymorphism and environmental factors such as smoking. Moreover, many molecular epidemiological studies has been conducted to investigate the association between MMP-3-1171 A5/A6, MMP-8-799 C/T, and MMP-2-753 C/T polymorphisms and periodontitis susceptibility. Nevertheless, the results still remain inconsistency and individual studies based on small sample sizes have low statistical power to investigate the real association22. These facts warranted us to perform a meta-analysis to further investigate the role of these polymorphisms in the pathogenesis of periodontitis.
Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statementin reporting this meta-analysis23.
Eligible criteria
The following criteria were used for literature selection: 1) articles that explored the association between MMP gene polymorphisms and periodontitis (CP and/or AgP) susceptibility; 2) the study design was cohort or case-control study; 3) there are adequate data to estimate odds ratios (ORs) and corresponding 95% confidence intervals (CIs). Two investigators independently screened all papers by title or abstract and then by a full content evaluation. Any discrepancy between the two authors was solved by discussion with a third investigator.
Search strategy
A comprehensive electronic database search was conducted for studies that examined associations between MMP polymorphisms and periodontitis. We utilized the PubMed, Embase, and Web of Science citation index to identify papers in which MMP polymorphisms were examined in patients with periodontitis and controls (up to 20 September, 2015). In addition, bibliographies of all potentially relevant studies and recently reviews were reviewed to identify additional articles not indexed by the aforementioned databases. The following text words and MeSH terms were searched: “matrix metalloproteinase” (MeSH term and text word), “MMP” (text word), “genetic, polymorphism” (MeSH term), “polymorphism” (text word), “periodontal disease” (MeSH term and text word), and “periodontitis” (MeSH and text word). We limited the search to studies that were carried out on humans and were written in English.
Data extraction and quality assessment
Two authors independently extracted the data and assessed the methodological quality. The following data were extracted from each study: first author’s surname, publication year, country, ethnicity, type of disease, source of control, sample size, number of genotype distribution, Hardy-Weinberg equilibrium (HWE) for controls, site of polymorphism, genotyping method, smoking percentile, and other factors for environment. Quality assessment of included studies was evaluated using the Newcastle-Ottawa scale (NOS) by two authors (P = 0.90 for κ test) independently22.
Data Analysis
A χ2test was used to assess the deviation of genotype distribution from HWE among controls. We performed meta-analyses using allelic contrast, homozygote contrast, heterozygote contrast, recessive, and dominant models. The associations between the polymorphisms and periodontitis risk were assessed by ORs and corresponding 95% CIs. Cochran’s Q metric was used to assess between tric was used to assess between-study heterogeneity. When the Q metric (P < 0.1) indicated significant heterogeneity among studies, the random-effects model (the Der-Simonian and Laird method) was applied to perform the meta-analysis. If the between study heterogeneity was not significant (P ≥ 0.1), the fixed-effects model (Mantel-Haenszel method) was used. We quantified the degree of heterogeneity using I2 statistic24. I2 ranges between 0 and 100%, and stands for the proportion of inter-study variability attributable to heterogeneity rather than random error. I2 values of 75%, 50%, and 25% were defined as high, moderate, and low estimates, respectively. If the number of included studies was applicable, we conducted stratified analyses on the bias of type of disease, ethnicity, agreement with HWE for controls, source of control, severity of CP, smoking status, and genotyping method. Funnel plots are used to detect publication bias. In addition, we performed Egger’s linear regression test to measure the asymmetry of funnel plot (P < 0.05)25. The meta-analysis was conducted using the Stata 12.0 software (Stata Corp LP, College Station, TX, USA), and the P value was two-sided.
Results
Study identification and characteristics
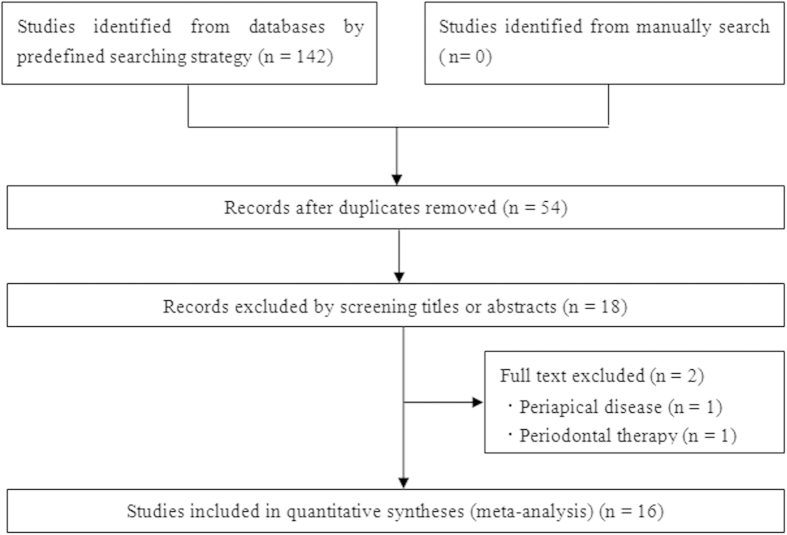
As shown in Fig. 1, we initially identified 142 articles. Finally we included 16 articles26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41 with 24 case-control studies involving 2,724 cases and 3,438 controls. Among them, there were four polymorphisms of MMP gene included in our meta-analysis: nine on MMP-9-1562 C/T, seven on MMP-3-1171 A5/A6, four on MMP-2-753C/T, and four on MMP-8-799 C/T. Two of these studies28,37 contained data on two different groups (CP and AgP), which were considered independently. One article contained two independent case-control studies in different countries and in this study only allele number was available39. Three articles27,40,41 were focused on two polymorphisms, and two articles31,32 were focused on three polymorphisms, which were treated as independent case-control studies as well. Four articles with six case-control studies did not satisfy the HWE for control group26,39,40,41. Main characteristics of included studies were summarized in Table 1.
Figure 1. Flow chart for this meta-analysis.
Table 1. Characteristics of the studies included in the meta-analysis.
| Polymorphism | Reference | Year | Country | Ethnicity | Disease type | Control source | Case | Control | Smoking (%) | Genotyping method | P# value | NOS score | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | CC | CT | TT | Case | Control | ||||||||||
| MMP-9-1562 C/T | de Souza | 2005 | Brazil | Mixed | CP | HB | 42 | 20 | 0 | 24 | 13 | 1 | 0 | 0 | PCR-RFLP | 0.62 | 7 |
| Holla | 2006 | Czech | Caucasian | CP | HB | 122 | 43 | 4 | 93 | 37 | 5 | 26.0 | 29.6 | PCR | 0.59 | 8 | |
| Keles | 2006 | Turkish | Caucasian | CP | HB | 57 | 13 | 0 | 42 | 24 | 4 | NA | NA | PCR | 0.82 | 7 | |
| Chen | 2007 | China | Asian | AgP | PB | 62 | 15 | 2 | 101 | 26 | 1 | NA | NA | PCR-RFLP | 0.63 | 7 | |
| Gurkan | 2007 | Turkish | Caucasian | AgP | HB | 58 | 53 | 1 | 78 | 72 | 7 | 32.6 | 5.1 | PCR | 0.06 | 7 | |
| Gurkan | 2008 | Turkish | Caucasian | CP | PB | 54 | 32 | 1 | 52 | 52 | 3 | 47.1 | 3.7 | PCR | 0.02 | 6 | |
| Loo | 2011 | China | Asian | CP | PB | 143 | 73 | 64 | 43 | 72 | 135 | NA | NA | PCR-RFLP | <0.01 | 6 | |
| Isaza-Guzman | 2011 | Colombia | Mixed | CP | HB | 58 | 11 | 0 | 47 | 6 | 1 | 14.6 | 4.9 | PCR-RFLP | 0.16 | 7 | |
| Li | 2012 | China | Asian | CP | PB | 68 | 26 | 28 | 99 | 156 | 277 | 93.4 | 97.7 | PCR-RFLP | <0.01 | 6 | |
| MMP-3-1171 A5/A6 | A5/A5 | A5/A6 | A6/A6 | A5/A5 | A5/A6 | A6/A6 | |||||||||||
| Itagaki | 2004 | Japan | Asian | AgP | HB | 0 | 17 | 20 | 4 | 38 | 100 | NA | NA | Taqman | 0.87 | 8 | |
| Itagaki | 2004 | Japan | Asian | CP | HB | 5 | 58 | 142 | 4 | 38 | 100 | NA | NA | Taqman | 0.87 | 8 | |
| Astolfi | 2006 | Brazil | Mixed | CP | PB | 19 | 52 | 19 | 8 | 70 | 25 | NA | NA | PCR-RFLP | <0.01 | 6 | |
| Loo | 2011 | China | Asian | CP | PB | 154 | 115 | 11 | 100 | 135 | 15 | NA | NA | PCR-RFLP | <0.01 | 6 | |
| Li | 2012 | China | Asian | CP | PB | 75 | 44 | 3 | 213 | 283 | 36 | 93.4 | 97.7 | PCR-RFLP | <0.01 | 6 | |
| Letra* | 2012 | Brazil | Mixed | CP | HB | NA | NA | NA | 121 | 114 | 64 | NA | NA | Taqman | <0.01 | 6 | |
| Letra* | 2012 | US | Caucasian | CP | HB | NA | NA | NA | 51 | 91 | 62 | NA | NA | Taqman | 0.13 | 7 | |
| MMP-8-799C/T | CC | CT | TT | CC | CT | TT | |||||||||||
| Chou | 2011 | China | Asian | CP | PB | 122 | 191 | 48 | 53 | 40 | 13 | 25.5 | 15.1 | PCR-RFLP | 0.22 | 8 | |
| Chou | 2011 | China | Asian | AgP | PB | 34 | 50 | 12 | 53 | 40 | 13 | 25 | 15.1 | PCR-RFLP | 0.22 | 7 | |
| Holla | 2012 | Czech | Caucasian | CP | HB | 88 | 163 | 90 | 84 | 134 | 60 | 30 | 28 | PCR-RFLP | 0.63 | 8 | |
| Emingil | 2014 | Turkey | Caucasian | AgP | PB | 11 | 57 | 32 | 76 | 66 | 25 | 29 | 3.6 | TaqMan | 0.10 | 8 | |
| MMP-2-753C/T | CC | CT | TT | CC | CT | TT | |||||||||||
| Holla | 2005 | Czech | Caucasian | CP | PB | 107 | 38 | 4 | 93 | 30 | 4 | NA | NA | PCR-RFLP | 0.42 | 8 | |
| Chen | 2007 | China | Asian | AgP | PB | 63 | 15 | 1 | 98 | 28 | 2 | NA | NA | PCR-RFLP | 1.00 | 7 | |
| Gurkan | 2007 | Turkish | Caucasian | AgP | HB | 49 | 39 | 4 | 98 | 54 | 5 | 32.6 | 5.1 | PCR | 0.06 | 7 | |
| Gurkan | 2008 | Turkish | Caucasian | CP | PB | 51 | 32 | 4 | 67 | 37 | 3 | 47.1 | 3.7 | PCR | 0.43 | 6 | |
CP = chronic periodontitis, AgP = aggressive periodontitis, NA = not available, HB = hospital-based, PB = population-based.
P# for Hardy-Weinberg equilibrium, *Only allele number available in controls.
MMP-9-1562 C/T polymorphism and periodontitis susceptibility
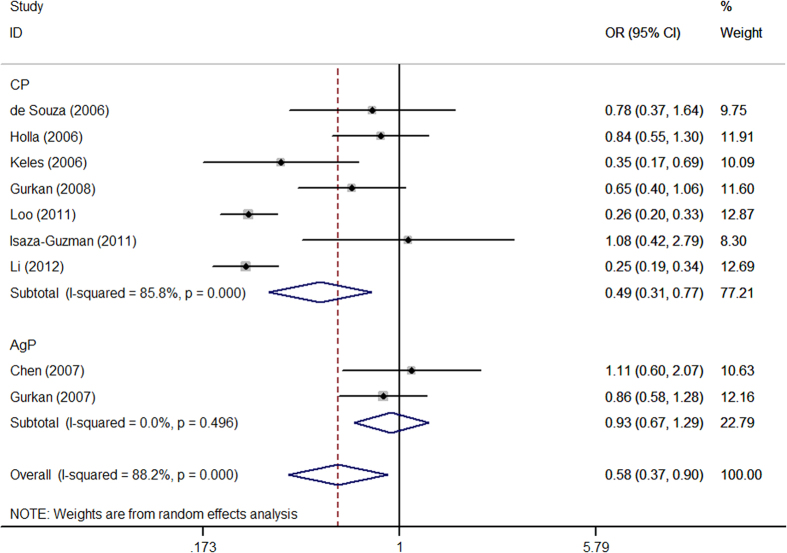
In all study subjects, meta-analysis showed a reduced risk between the MMP-9-1562 C/T polymorphism and periodontitis susceptibility in all tested genetic model (T vs. C: OR = 0.58, 95% CI = 0.37-0.90; TT vs. CC: OR = 0.17, 95% CI = 0.13–0.23; CT vs. CC: OR = 0.61, 95% CI = 0.41–0.93; TT + CT vs. CC: OR = 0.54, 95% CI = 0.32–0.93; TT vs. CC + CT: OR = 0.28, 95% CI = 0.21–0.36) with some evidence of between-study heterogeneity (Table 2, Fig. 2). Sensitivity analysisby excluding studies with control inconsistent with HWE showed that the decreased risk was only observed in recessive model (OR = 0.41, 95% CI = 0.18–0.93). Stratification analysis by type of disease indicated that individuals were more susceptible to CP than AgP (Table 2).
Table 2. Meta-analysis of the association between the MMP-9-1562 C/Tpolymorphism and periodontitis.
| Genetic Model and Subgroup | Subgroups | N | Test of Association | Test of Heterogeneity | P* value | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | Model | P value | I2(%) | ||||
| T vs. C | Overall | 9 | 0.58 | 0.37–0.90 | 0.015 | R | <0.001 | 88.2 | 0.039 |
| HWE (yes) | 6 | 0.8 | 0.64–1.00 | 0.052 | F | 0.198 | 31.6 | ||
| AgP | 2 | 0.93 | 0.67–1.29 | 0.656 | F | 0.496 | 0 | ||
| CP | 7 | 0.49 | 0.31–0.77 | 0.002 | R | <0.001 | 85.8 | ||
| Caucasian | 4 | 0.72 | 0.57–0.90 | 0.005 | F | 0.122 | 48.2 | ||
| Asian | 3 | 0.39 | 0.20–0.73 | 0.004 | R | <0.001 | 89.9 | ||
| Mixed | 2 | 0.89 | 0.49–1.59 | 0.684 | F | 0.596 | 0 | ||
| HB | 5 | 0.76 | 0.60–0.97 | 0.027 | F | 0.19 | 34.7 | ||
| PB | 4 | 0.44 | 0.25–0.79 | 0.006 | R | <0.001 | 89.7 | ||
| PCR | 4 | 0.72 | 0.57–0.90 | 0.005 | F | 0.122 | 48.2 | ||
| PCR-RFLP | 5 | 0.52 | 0.29–0.93 | 0.029 | R | <0.001 | 87.8 | ||
| Moderate CP | 2 | 0.65 | 0.40–1.07 | 0.09 | F | 0.358 | 0 | ||
| Sever CP | 2 | 0.98 | 0.64–1.50 | 0.926 | F | 0.343 | 0 | ||
| Non-smokers | 2 | 0.67 | 0.46–1.00 | 0.048 | F | 0.86 | 0 | ||
| TT vs. CC | Overall | 9 | 0.17 | 0.13–0.23 | <0.001 | F | 0.217 | 25.6 | 0.121 |
| HWE (yes) | 6 | 0.4 | 0.18–0.89 | 0.026 | F | 0.405 | 1.7 | ||
| AgP | 2 | 0.74 | 0.05–12.16 | 0.836 | R | 0.082 | 66.8 | ||
| CP | 7 | 0.16 | 0.12–0.22 | <0.001 | F | 0.564 | 0 | ||
| Caucasian | 4 | 0.3 | 0.12–0.76 | 0.011 | F | 0.578 | 0 | ||
| Asian | 3 | 0.18 | 0.09–0.38 | <0.001 | R | 0.043 | 68.1 | ||
| Mixed | 2 | 0.23 | 0.02–2.25 | 0.206 | F | 0.883 | 0 | ||
| HB | 5 | 0.29 | 0.12–0.72 | 0.008 | F | 0.718 | 0 | ||
| PB | 4 | 0.19 | 0.10–0.36 | <0.001 | R | 0.083 | 55 | ||
| PCR | 4 | 0.3 | 0.12–0.76 | 0.011 | F | 0.578 | 0 | ||
| PCR-RFLP | 5 | 0.16 | 0.11–0.22 | <0.001 | F | 0.171 | 37.6 | ||
| Moderate CP | 2 | 0.34 | 0.06–2.08 | 0.245 | F | 0.831 | 0 | ||
| Sever CP | 2 | 0.75 | 0.20–2.81 | 0.667 | F | 0.563 | 0 | ||
| Non-smokers | 2 | 1.38 | 0.38–4.92 | 0.623 | F | 0.579 | 0 | ||
| CT vs. CC | Overall | 9 | 0.61 | 0.41–0.93 | 0.02 | R | <0.001 | 74.8 | 0.322 |
| HWE (yes) | 6 | 0.87 | 0.66–1.14 | 0.293 | F | 0.406 | 1.6 | ||
| AgP | 2 | 0.97 | 0.65–1.46 | 0.896 | F | 0.906 | 0 | ||
| CP | 7 | 0.53 | 0.33–0.85 | 0.008 | R | 0.001 | 73.4 | ||
| Caucasian | 4 | 0.75 | 0.57–0.99 | 0.046 | F | 0.192 | 36.8 | ||
| Asian | 3 | 0.39 | 0.19–0.80 | 0.01 | R | 0.008 | 79.4 | ||
| Mixed | 2 | 1.09 | 0.56–2.11 | 0.808 | F | 0.453 | 0 | ||
| HB | 5 | 0.85 | 0.64–1.14 | 0.286 | F | 0.285 | 20.4 | ||
| PB | 4 | 0.43 | 0.25–0.76 | 0.004 | R | 0.007 | 75.3 | ||
| PCR | 4 | 0.75 | 0.57–0.99 | 0.046 | F | 0.192 | 36.8 | ||
| PCR-RFLP | 5 | 0.56 | 0.29–1.09 | 0.09 | R | 0.001 | 79.2 | ||
| Moderate CP | 2 | 0.7 | 0.39–1.24 | 0.218 | F | 0.319 | 0 | ||
| Sever CP | 2 | 1.06 | 0.64–1.74 | 0.667 | F | 0.414 | 0 | ||
| Non-smokers | 2 | 1 | 0.63–1.57 | 0.988 | F | 0.51 | 0 | ||
| TT + CT vs. CC | Overall | 9 | 0.54 | 0.32–0.93 | 0.025 | R | <0.001 | 86.9 | 0.104 |
| HWE (yes) | 6 | 0.82 | 0.63–1.06 | 0.133 | F | 0.276 | 20.9 | ||
| AgP | 2 | 0.95 | 0.64–1.42 | 0.814 | F | 0.798 | 0 | ||
| CP | 7 | 0.46 | 0.26–0.82 | 0.009 | R | <0.001 | 85.8 | ||
| Caucasian | 4 | 0.71 | 0.54–0.93 | 0.013 | F | 0.134 | 46.2 | ||
| Asian | 3 | 0.32 | 0.13–0.77 | 0.011 | R | <0.001 | 89.9 | ||
| Mixed | 2 | 0.98 | 0.51–1.88 | 0.953 | F | 0.512 | 0 | ||
| HB | 5 | 0.79 | 0.59–1.04 | 0.098 | F | 0.21 | 31.7 | ||
| PB | 4 | 0.37 | 0.17–0.78 | 0.009 | R | <0.001 | 88.8 | ||
| PCR | 4 | 0.71 | 0.54–0.93 | 0.013 | F | 0.134 | 46.2 | ||
| PCR-RFLP | 5 | 0.48 | 0.22–1.07 | 0.072 | R | <0.001 | 88.6 | ||
| Moderate CP | 2 | 0.65 | 0.37–1.14 | 0.132 | F | 0.323 | 0 | ||
| Sever CP | 2 | 1.02 | 0.63–1.66 | 0.939 | F | 0.363 | 0 | ||
| Non-smokers | 2 | 1.02 | 0.66–1.58 | 0.935 | F | 0.439 | 0 | ||
| TT vs. CC + CT | Overall | 9 | 0.28 | 0.21–0.36 | <0.001 | F | 0.601 | 0 | 0.379 |
| HWE (yes) | 6 | 0.41 | 0.18–0.93 | 0.033 | F | 0.437 | 0 | ||
| AgP | 2 | 0.75 | 0.05–12.36 | 0.372 | R | 0.08 | 67.3 | ||
| CP | 7 | 0.27 | 0.20–0.35 | <0.001 | F | 0.897 | 0 | ||
| Caucasian | 4 | 0.33 | 0.13–0.83 | 0.018 | F | 0.624 | 0 | ||
| Asian | 3 | 0.27 | 0.21–0.36 | <0.001 | F | 0.119 | 53 | ||
| Mixed | 2 | 0.23 | 0.02–2.22 | 0.202 | F | 0.915 | 0 | ||
| HB | 5 | 0.3 | 0.12–0.75 | 0.01 | F | 0.753 | 0 | ||
| PB | 4 | 0.28 | 0.21–0.37 | <0.001 | F | 0.224 | 31.3 | ||
| PCR | 4 | 0.33 | 0.13–0.83 | 0.018 | F | 0.624 | 0 | ||
| PCR-RFLP | 5 | 0.27 | 0.21–0.36 | <0.001 | F | 0.368 | 6.7 | ||
| Moderate CP | 2 | 0.37 | 0.06–2.26 | 0.284 | F | 0.891 | 0 | ||
| Sever CP | 2 | 0.73 | 0.20–2.72 | 0.64 | F | 0.618 | 0 | ||
| Non-smokers | 2 | 1.35 | 0.38–4.80 | 0.641 | F | 0.618 | 0 | ||
N = number of studies, OR = odds ratio, CI = confidence interval, F = fixed model, R = random model, CP = chronic periodontitis, AgP = aggressive periodontitis, HWE = Hardy-Weinberg equilibrium, HB = hospital-based, PB = population-based.
P* value for publication bias (Egger’s test).
Figure 2. Forest plot for MMP-9-1562 C/T polymorphism associated with periodontitis susceptibility in C versus T allele comparison based on random-effects model.
MMP-3-1171 A5/A6 polymorphism and periodontitis susceptibility
Meta-analysis of the MMP-3-1171 A5/A6 polymorphism showed an elevated risk between the polymorphism and periodontitis susceptibility in three tested genetic model (A5 vs. A6: OR = 1.45, 95% CI = 1.26–1.66; A5/A5 vs. A6/A6: OR = 2.32, 95% CI = 1.42–3.81; A5/A5 vs. A6/A5 + A6/A6: OR = 2.03, 95% CI = 1.59–2.59) with low between-study heterogeneity (Table 3. Stratification by disease type indicated that individuals were more susceptible to CP rather than AgP (Table 3).
Table 3. Meta-analysis of the association between the MMP-3-1171 5A/6A polymorphism and periodontitis.
| Genetic Model and Subgroup | Subgroups | N | Test of Association | Test of Heterogeneity | P* value | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | Model | P value | I2(%) | ||||
| A5 vs. A6 | Overall | 7 | 1.45 | 1.26–1.66 | <0.001 | F | 0.263 | 21.8 | 0.721 |
| HWE (yes) | 3 | 1.34 | 1.01–1.70 | 0.04 | F | 0.315 | 13.4 | ||
| AgP | 1 | 1.54 | 0.82–2.89 | 0.175 | NA | NA | NA | ||
| CP | 6 | 1.44 | 1.25–1.66 | <0.001 | F | 0.178 | 34.4 | ||
| Caucasian | 1 | 1.55 | 1.05–2.30 | 0.029 | NA | NA | NA | ||
| Asian | 4 | 1.53 | 1.28–1.83 | <0.001 | F | 0.135 | 46 | ||
| Mixed# | 2 | 1.25 | 0.96–1.62 | 0.095 | F | 0.477 | 0 | ||
| HB | 4 | 1.25 | 1.02–1.54 | 0.033 | F | 0.444 | 0 | ||
| PB | 3 | 1.62 | 1.35–1.95 | <0.001 | F | 0.394 | 0 | ||
| Taqman | 4 | 1.25 | 1.02–1.54 | 0.033 | F | 0.444 | 0 | ||
| PCR-RFLP | 3 | 1.62 | 1.35–1.95 | <0.001 | F | 0.394 | 0 | ||
| A5/A5 vs. A6/A6 | Overall | 5 | 2.32 | 1.42–3.81 | 0.001 | F | 0.371 | 6.2 | 0.412 |
| HWE (yes) | 2 | 0.8 | 0.24–2.65 | 0.713 | F | 0.771 | 0 | ||
| AgP | 1 | 0.54 | 0.03–10.51 | 0.688 | NA | NA | NA | ||
| CP | 4 | 2.45 | 1.47–4.08 | 0.001 | F | 0.336 | 11.3 | ||
| Asian | 4 | 2.13 | 1.21–3.75 | 0.009 | F | 0.293 | 19.5 | ||
| Mixed# | 1 | 3.13 | 1.13–8.66 | 0.028 | NA | NA | NA | ||
| A6/ A 5 vs. A6/A6 | Overall | 5 | 1.26 | 0.92–1.71 | 0.148 | F | 0.458 | 0 | 0.367 |
| HWE (yes) | 2 | 1.32 | 0.88–1.99 | 0.176 | F | 0.106 | 61.7 | ||
| AgP | 1 | 2.24 | 1.06–4.72 | 0.035 | NA | NA | NA | ||
| CP | 4 | 1.12 | 0.80–1.58 | 0.504 | F | 0.836 | 0 | ||
| Asian | 4 | 1.34 | 0.85–1.89 | 0.101 | F | 0.39 | 0.4 | ||
| Mixed# | 1 | 0.98 | 0.49–1.96 | 0.949 | NA | NA | NA | ||
| (A5/A5 + A6/A5) vs. A6/A6 | Overall | 5 | 1.94 | 0.92–4.13 | 0.083 | R | <0.001 | 81.3 | 0.021 |
| HWE (yes) | 2 | 1.65 | 0.30–8.98 | 0.565 | R | <0.001 | 92.8 | ||
| AgP | 1 | 4.05 | 1.82–9.00 | 0.001 | NA | NA | NA | ||
| CP | 4 | 1.59 | 0.74–3.38 | 0.232 | R | 0.004 | 77.2 | ||
| Asian | 4 | 2.12 | 0.78–5.79 | 0.141 | R | <0.001 | 85.9 | ||
| Mixed# | 1 | 1.56 | 0.78–3.10 | 0.207 | NA | NA | NA | ||
| A5/A5vs. (A6/A5 + A6/A6) | Overall | 5 | 2.03 | 1.59–2.59 | <0.001 | F | 0.323 | 14.4 | 0.458 |
| HWE (yes) | 2 | 0.73 | 0.22–2.39 | 0.606 | F | 0.649 | 0 | ||
| AgP | 1 | 0.41 | 0.02–7.79 | 0.553 | NA | NA | NA | ||
| CP | 4 | 2.06 | 1.61–2.64 | <0.001 | F | 0.317 | 14.9 | ||
| Asian | 4 | 1.95 | 1.51–2.52 | <0.001 | F | 0.306 | 17 | ||
| Mixed# | 1 | 3.18 | 1.32–7.67 | 0.01 | NA | NA | NA | ||
N = number of studies, OR = odds ratio, CI = confidence interval, F = fixed model, R = random model, CP = chronic periodontitis, AgP = aggressive periodontitis, HWE = Hardy-Weinberg equilibrium, HB = hospital-based, PB = population-based.
P* value for publication bias (Egger’s test), mixed# for ethnicity.
MMP-2-753C/T and MMP-8-799 C/T polymorphisms and periodontitis susceptibility
Meta-analysis of the MMP-2-753 C/T showed no association between thepolymorphism and periodontitis susceptibility (T vs. C: OR = 1.13, 95% CI = 0.88–1.44; TT vs. CC: OR = 1.25, 95% CI = 0.58–2.73; CT vs. CC: OR = 1.14, 95% CI = 1.14, 95% CI = 0.85–1.53; CT + TT vs. CC: OR = 1.15, 95% CI = 0.87–1.53; TT vs. CC + CT: OR = 1.18, 95% CI = 0.55–2.56) with no between-study heterogeneity (I2 = 0% for all genetic models) (Table 4). The results of stratification analyses according to disease type, ethnicity, and smoking statuswere similar to the overall results (Table 4).
Table 4. Meta-analysis of the association between the MMP-2-753C/T and MMP-8-799C/T polymorphisms and periodontitis.
| Genetic Model and Subgroup | N | T vs. C | TT vs. CC | CT vs. CC | (CT + TT) vs. CC | TT vs. (CC + CT) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | I2 (%) | OR (95% CI) | I2 (%) | OR (95% CI) | I2 (%) | OR (95% CI) | I2 (%) | OR (95% CI) | I2 (%) | ||
| MMP-2-753 C/T | |||||||||||
| Total | 4 | 1.13 (0.88–1.44) | 0 | 1.25 (0.58–2.73) | 0 | 1.14 (0.85–1.53) | 0 | 1.15 (0.87–1.53) | 0 | 1.18 (0.55–2.56) | 0 |
| CP | 2 | 1.11 (0.79–1.55) | 0 | 1.20 (0.43–3.37) | 0 | 1.12 (0.74–1.68) | 0 | 1.12 (0.76–1.66) | 0 | 1.16 (0.42–3.24) | 0 |
| AgP | 2 | 1.15 (0.81–1.64) | 29.9 | 1.33 (0.41–4.31) | 0 | 1.18 (0.77–1.79) | 32.8 | 1.18 (0.78–1.78) | 39.3 | 1.21 (0.38–3.88) | 0 |
| Caucasian | 3 | 1.19 (0.91–1.55) | 0 | 1.33 (0.58–3.03) | 0 | 1.23 (0.89–1.69) | 0 | 1.23 (0.90–1.69) | 0 | 1.24 (0.55–2.80) | 0 |
| Asian | 1 | 0.84 (0.45–1.58) | NA | 0.78 (0.07–8.76) | NA | 0.83 (0.41–1.68) | NA | 0.83 (0.42–1.64) | NA | 0.81 (0.07–9.06) | NA |
| Non-smokers | 3 | 1.27 (0.92–1.75) | 0 | 1.38 (0.52–3.62) | 0 | 1.35 (0.91–2.00) | 0 | 1.35 (0.92–1.97) | 0 | 1.24 (0.48–3.22) | 0 |
| Smokers | 1 | 0.85 (0.36–1.99) | NA | 1.10 (0.09–13.00) | NA | 0.73 (0.26–2.08) | NA | 0.77 (0.28–2.10) | NA | 1.21 (0.10–14.00) | NA |
| MMP-8-799 C/T | |||||||||||
| Total | 4 | 1.61 (1.11–2.35) | 81.6 | 2.26 (1.03–4.97) | 80.8 | 2.18 (1.19–4.00) | 81.5 | 2.22 (1.21–4.08) | 94.5 | 1.44 (0.90–2.16) | 47.3 |
| CP | 2 | 1.28 (1.07–1.54) | 0 | 1.48 (1.02–2.15) | 0 | 1.52 (0.86–2.69) | 72 | 1.48 (1.13–1.95) | 59.9 | 1.25 (0.90–1.73) | 0 |
| AgP | 2 | 2.01 (0.98–4.11) | 85.3 | 3.60 (0.61–21.33) | 88.4 | 3.34 (1.11–10.04) | 81.7 | 3.45 (0.95–12.55) | 87.9 | 1.73 (0.68–4.42) | 70.2 |
| Caucasian | 2 | 1.84 (0.78–4.32) | 93.8 | 3.44 (0.58–20.47) | 93.2 | 2.55 (0.51–12.76) | 93.6 | 2.82 (0.53–14.96) | 94.5 | 1.79 (0.89–3.61) | 75 |
| Asian | 2 | 1.43 (1.11–1.85) | 0 | 1.54 (0.89–2.67) | 0 | 2.03 (1.40–2.93) | 0 | 1.91 (1.35–2.70) | 0 | 1.07 (0.64–1.79) | 0 |
| Non-smokers | 4 | 1.79 (1.19–2.69) | 79.4 | 2.88 (1.21–6.84) | 78.3 | 2.37 (1.24–4.55) | 77.2 | 2.52 (1.30–4.88) | 80.5 | 1.64 (1.19–2.25) | 38.7 |
| Smokers | 1 | 0.91 (0.58–1.42) | NA | 0.81 (0.33–2.00) | NA | 0.96 (0.46–1.99) | NA | 0.91 (0.46–1.82) | NA | 0.84 (0.39–1.81) | NA |
OR = odds ratio, CI = confidence interval, CP = chronic periodontitis, AgP = aggressive periodontitis, NA = not available.
Meta-analysis of the MMP-8-799 C/T showed an increased risk between the polymorphism and periodontitis susceptibility in four genetic model (T vs. C: OR = 1.61, 95% CI = 1.11–2.35; TT vs. CC: OR = 2.26, 95% CI = 1.03–4.97; CT vs. CC: OR = 2.18, 95% CI = 1.19–4.00; CT + TT vs. CC: OR = 2.22, 95% CI = 1.21–4.08) with moderate to highbetween-study heterogeneity (Table 4). Subgroup analyses according to disease type, ethnicity, and smoking status showed that the increased risk was predominant in CP, Asians, and non-smokers (Table 4).
Publication bias
Due to limitations of the quantity of included studies, we just test the publication bias for MMP-9-1562 C/T and MMP-3-1171 A5/A6 polymorphisms. The funnel plots based on allele model for MMP-9-1562 C/T and MMP-3-1171 A5/A6 polymorphism were asymmetry and indicated that publication bias probably existed in the present study. The Egger’s test showed there was some publication bias existed in the MMP-9-1562 C/T allele model (Table 2).
Discussion
In the present meta-analysis, we aggregated data from published studies to estimate genetic associations between MMP gene, namely MMP-2-753 C/T, MMP-3-1171 A5/A6, MMP-8-799 C/T, and MMP-9-1562 C/T polymorphisms, and periodontitis susceptibility. Our results provided some evidence to support an elevated risk between periodontitis susceptibility and MMP-3-1171 A5 allele and MMP-8-799 T allele, and a reduced risk between periodontitis susceptibility and MMP-9-1562 T allele. But our study provided no evidence to support an association between MMP-2-753 C/T polymorphism and periodontitis. Appreciable differences were identified in the etiology characteristic between CP and AgP42, indicating that there might be different genetic mechanism between them. In stratified analysis by disease type, their association with susceptibility of CP rather than AgP was observed. For MMP-9-1562 C/T polymorphism, we did not observe any meaningful associations in stratified analysis by ethnicity, severity of CP, and smoking status. The results were similar to MMP-3-1171 A5/A6 and MMP-2-753 C/T polymorphisms for periodontitis susceptibility. However, in stratified analysis by ethnicity and smoking status for MMP-8-799 C/T polymorphism indicated an elevated risk in Asian populations rather than Caucasian populations, and non-smokers rather than smokers.
To our knowledge, this is the first quantitative analysis that assessed the association between MMP-2-753 C/T, MMP-3-1171 A5/A6, and MMP-8-799 C/T polymorphisms and periodontitis susceptibility. With regard to MMP-9-1562 C/T polymorphism, two meta-analyses waspublished in 2013. Pan et al.21 indicated that MMP-9-1562 C/T polymorphism might be involved in the development of periodontitis. However, Song et al.19 suggested no association between MMP-9-1562 C/T polymorphism and periodontitis susceptibility. Compared with the previous meta-analyses, we had a lager sample size than them, which increased the statistical power, and we found a reduced risk of MMP-9-1562 T allele with periodontitis susceptibility. Genetic association researches designed to investigate relations between gene polymorphisms and complex outcomes must be interpreted with caution, because many factors could potentially affect the results. Therefore, we assessed the association with severity of CP and smoking status even though we did not observe any significant difference among the moderate and severe CP and no association among non-smokers. This might be caused by small sample size that only two studies29,33 presented the association between polymorphism and disease severity, two studies31,32 investigate the interaction between polymorphism and smoking status.
However, the present meta-analysis also has certain limitations that affect the interpretation of the results. First, the heterogeneity for MMP-9-1562 C/T polymorphism was high. Subgroup analyses suggested that the heterogeneity might come from the deviation of HWE, ethnicity, and genotyping method. Certainly, other clinical heterogeneity also might contribute to it, for instance different classification and diagnosis of periodontitis and differences on the oral examination by different clinicians. In addition, it is widely acknowledged that meta-analysis is a secondary analysis and we could not handle the problem of clinical heterogeneity in a meta-analysis. Therefore, we recommend that further studies should be designed as multi-center studies and utilizea unified criterion of disease. Second, although a comprehensive literature search was performed, it was likely that some publications were overlooked because of our language restriction for the literature search. In addition, the number of included studies for each polymorphism was limited. Therefore, the statistic power of present meta-analysis might be affected and the present results might be led to false positive or false negative rate. Third, we used genotype distributions and crude estimates of effect rather than adjusted estimates of association between polymorphism and periodontitis. Even though we investigated the interaction between polymorphism and disease severity and smoking status, the statistic power was limited due to most studies did not present the relevant data. Moreover, age, sex, and gene-gene interactions could not be assessed in our study due to insufficient data. Finally, the publication bias was of concern that small studies with negative results tend not to be published.
In conclusion, this meta-analysis with published data suggested that the MMP-2-753 C/T, MMP-3-1171 A5/A6, and MMP-9-1562 C/T polymorphisms were associated with periodontitis susceptibility and there is lack of association between the MMP-8-799 C/T polymorphism and periodontitis. Further studies with large sample size, gene-gene, and gene-environment detailed information are needed to validate the present results.
Additional Information
How to cite this article: Weng, H. et al. Matrix metalloproteinase gene polymorphisms and periodontitis susceptibility: a meta-analysis involving 6,162 individuals. Sci. Rep. 6, 24812; doi: 10.1038/srep24812 (2016).
Footnotes
Author Contributions H.W. and X.-T.Z. designed this study; Y.Y. and Y.-H.J. searched databases and collected full-text papers; X.-Y.M. and D.W.H. extracted and analyzed data; Y.-Y.M. and H.W. wrote the manuscript, X.-T.Z. reviewed the manuscript.
References
- Pihlstrom B. L., Michalowicz B. S. & Johnson N. W. Periodontal diseases. Lancet 366, 1809–1820 (2005). [DOI] [PubMed] [Google Scholar]
- Mullally B. H., Coulter W. A., Hutchinson J. D. & Clarke H. A. Current oral contraceptive status and periodontitis in young adults. J Periodontol 78, 1031–1036 (2007). [DOI] [PubMed] [Google Scholar]
- Armitage G. C. Development of a classification system for periodontal diseases and conditions. Northwest Dent 79, 31–35 (2000). [PubMed] [Google Scholar]
- Wei X. M. et al. Tumor necrosis factor-alpha G-308A (rs1800629) polymorphism and aggressive periodontitis susceptibility: a meta-analysis of 16 case-control studies. Sci Rep 6, 19099 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassebaum N. J. et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res 93, 1045–1053 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X. T. et al. Periodontal disease and carotid atherosclerosis: A meta-analysis of 17,330 participants. Int J Cardiol 203, 1044–1051 (2016). [DOI] [PubMed] [Google Scholar]
- Leng W. D., Zeng X. T., Kwong J. S. & Hua X. P. Periodontal disease and risk of coronary heart disease: An updated meta-analysis of prospective cohort studies. Int J Cardiol 201, 469–472 (2015). [DOI] [PubMed] [Google Scholar]
- Zeng X. T. et al. Periodontal disease and risk of head and neck cancer: a meta-analysis of observational studies. PLos One 8, e79017 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X. T. et al. Periodontal disease and risk of chronic obstructive pulmonary disease: a meta-analysis of observational studies. PLos One 7, e46508 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi L., Amar S., Polins A. S. & Van Dyke T. E. Host mechanisms in the pathogenesis of periodontal disease. Curr Opin Periodontol 4, 3–10 (1997). [PubMed] [Google Scholar]
- Jiang L., Weng H., Chen M. Y., Zhang C. & Zeng X. T. Association between cyclooxygenase-2 gene polymorphisms and risk of periodontitis: a meta-analysis involving 5653 individuals. Mol Biol Rep (2014). [DOI] [PubMed] [Google Scholar]
- Wang W. F., Shi J., Chen S. J., Niu Y. M. & Zeng X. T. Interleukin-1alpha -899 (+4845) C– > T polymorphism is not associated with aggressive periodontitis susceptibility: A meta-analysis based on 19 case-control studies. Biomed Rep 2, 378–383 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao M. et al. Interleukin-1alpha -899 (+4845) C– > T polymorphism increases the risk of chronic periodontitis: evidence from a meta-analysis of 23 case-control studies. Gene 532, 114–119 (2013). [DOI] [PubMed] [Google Scholar]
- Chen Y. J. et al. Interleukin-1beta rs1143634 polymorphism and aggressive periodontitis susceptibility: a meta-analysis. Int J Clin Exp Med 8, 2308–2316 (2015). [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Weng H., Shen Z. H., Wu L. & Zeng X. T. Association between interleukin-4 gene -590 c/t, -33 c/t, and 70-base-pair polymorphisms and periodontitis susceptibility: a meta-analysis. J Periodontol 85, e354–362 (2014). [DOI] [PubMed] [Google Scholar]
- Zeng X. T. et al. Meta-Analysis of Association Between Interleukin-1beta C-511T Polymorphism and Chronic Periodontitis Susceptibility. J Periodontol 86, 812–819 (2015). [DOI] [PubMed] [Google Scholar]
- Ravanti L. & Kahari V. M. Matrix metalloproteinases in wound repair (review). Int J Mol Med 6, 391–407 (2000). [PubMed] [Google Scholar]
- Sorsa T., Tjaderhane L. & Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis 10, 311–318 (2004). [DOI] [PubMed] [Google Scholar]
- Song G. G., Kim J. H. & Lee Y. H. Toll-like receptor (TLR) and matrix metalloproteinase (MMP) polymorphisms and periodontitis susceptibility: a meta-analysis. Mol Biol Rep 40, 5129–5141 (2013). [DOI] [PubMed] [Google Scholar]
- Li D. et al. Association between MMP-1 g.-1607dupG polymorphism and periodontitis susceptibility: a meta-analysis. PLos One 8, e59513 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. et al. MMP-9-1562C > T contributes to periodontitis susceptibility. J Clin Periodontol 40, 125–130 (2013). [DOI] [PubMed] [Google Scholar]
- Zeng X. et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 8, 2–10 (2015). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astolfi C. M. et al. Genetic polymorphisms in the MMP-1 and MMP-3 gene may contribute to chronic periodontitis in a Brazilian population. J Clin Periodontol 33, 699–703 (2006). [DOI] [PubMed] [Google Scholar]
- Chen D. et al. MMP-2, MMP-9 and TIMP-2 gene polymorphisms in Chinese patients with generalized aggressive periodontitis. J Clin Periodontol 34, 384–389 (2007). [DOI] [PubMed] [Google Scholar]
- Chou Y. H. et al. MMP-8-799 C > T genetic polymorphism is associated with the susceptibility to chronic and aggressive periodontitis in Taiwanese. J Clin Periodontol 38, 1078–1084 (2011). [DOI] [PubMed] [Google Scholar]
- de Souza A. P. et al. Analysis of the MMP-9 (C-1562 T) and TIMP-2 (G-418C) gene promoter polymorphisms in patients with chronic periodontitis. J Clin Periodontol 32, 207–211 (2005). [DOI] [PubMed] [Google Scholar]
- Emingil G. et al. Matrix metalloproteinase (MMP)-8 and tissue inhibitor of MMP-1 (TIMP-1) gene polymorphisms in generalized aggressive periodontitis: gingival crevicular fluid MMP-8 and TIMP-1 levels and outcome of periodontal therapy. J Periodontol 85, 1070–1080 (2014). [DOI] [PubMed] [Google Scholar]
- Gurkan A. et al. Matrix metalloproteinase-2, -9, and -12 gene polymorphisms in generalized aggressive periodontitis. J Periodontol 78, 2338–2347 (2007). [DOI] [PubMed] [Google Scholar]
- Gurkan A. et al. Gene polymorphisms of matrix metalloproteinase-2, -9 and -12 in periodontal health and severe chronic periodontitis. Arch Oral Biol 53, 337–345 (2008). [DOI] [PubMed] [Google Scholar]
- Holla L. I., Fassmann A., Muzik J., Vanek J. & Vasku A. Functional polymorphisms in the matrix metalloproteinase-9 gene in relation to severity of chronic periodontitis. J Periodontol 77, 1850–1855 (2006). [DOI] [PubMed] [Google Scholar]
- Holla L. I. et al. Genetic variations in the human gelatinase A (matrix metalloproteinase-2) promoter are not associated with susceptibility to, and severity of, chronic periodontitis. J Periodontol 76, 1056–1060 (2005). [DOI] [PubMed] [Google Scholar]
- Holla L. I., Hrdlickova B., Vokurka J. & Fassmann A. Matrix metalloproteinase 8 (MMP8) gene polymorphisms in chronic periodontitis. Arch Oral Biol 57, 188–196 (2012). [DOI] [PubMed] [Google Scholar]
- Isaza-Guzman D. M., Arias-Osorio C., Martinez-Pabon M. C. & Tobon-Arroyave S. I. Salivary levels of matrix metalloproteinase (MMP)-9 and tissue inhibitor of matrix metalloproteinase (TIMP)-1: a pilot study about the relationship with periodontal status and MMP-9(-1562C/T) gene promoter polymorphism. Arch Oral Biol 56, 401–411 (2011). [DOI] [PubMed] [Google Scholar]
- Itagaki M. et al. Matrix metalloproteinase-1 and -3 gene promoter polymorphisms in Japanese patients with periodontitis. J Clin Periodontol 31, 764–769 (2004). [DOI] [PubMed] [Google Scholar]
- Keles G. C. et al. Association of matrix metalloproteinase-9 promoter gene polymorphism with chronic periodontitis. J Periodontol 77, 1510–1514 (2006). [DOI] [PubMed] [Google Scholar]
- Letra A. et al. MMP3 and TIMP1 variants contribute to chronic periodontitis and may be implicated in disease progression. J Clin Periodontol 39, 707–716 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. et al. Association of matrix metalloproteinase (MMP)-1, 3, 9, interleukin (IL)-2, 8 and cyclooxygenase (COX)-2 gene polymorphisms with chronic periodontitis in a Chinese population. Cytokine 60, 552–560 (2012). [DOI] [PubMed] [Google Scholar]
- Loo W. T., Wang M., Jin L. J., Cheung M. N. & Li G. R. Association of matrix metalloproteinase (MMP-1, MMP-3 and MMP-9) and cyclooxygenase-2 gene polymorphisms and their proteins with chronic periodontitis. Arch Oral Biol 56, 1081–1090 (2011). [DOI] [PubMed] [Google Scholar]
- Benoist H. M. et al. Profile of chronic and aggressive periodontitis among Senegalese. J Periodontal Implant Sci 41, 279–284 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]