Abstract
AIM: To establish if a distinct urinary metabolic profile could be identified in Bangladeshi hepatitis-B hepatocellular carcinoma (HCC) patients compared to cirrhosis patients and controls.
METHODS: Urine samples from 42 Bangladeshi patients with HCC (39 patients with hepatitis-B HCC), 47 with cirrhosis on a background of hepatitis B, 46 with chronic hepatitis B, and seven ethnically-matched healthy controls were analyzed using nuclear magnetic resonance (NMR) spectroscopy. A full dietary and medication history was recorded for each subject. The urinary NMR data were analyzed using principal component analysis (PCA) and orthogonal partial least squared discriminant analysis (OPLS-DA) techniques. Differences in relative signal levels of the most discriminatory metabolites identified by PCA and OPLS-DA were compared between subject groups using an independent samples Kruskal-Wallis one-way analysis of variance (ANOVA) test with all pairwise multiple comparisons. Within the patient subgroups, the Mann-Whitney U test was used to compare metabolite levels depending on hepatitis B e-antigen (HBeAg) status and treatment with anti-viral therapy. A Benjamini-Hochberg adjustment was applied to acquire the level of significance for multiple testing, with a declared level of statistical significance of P < 0.05.
RESULTS: There were significant differences in age (P < 0.001), weight (P < 0.001), and body mass index (P < 0.001) across the four clinical subgroups. Serum alanine aminotransferase (ALT) was significantly higher in the HCC group compared to controls (P < 0.001); serum α-fetoprotein was generally markedly elevated in HCC compared to controls; and serum creatinine levels were significantly reduced in the HCC group compared to the cirrhosis group (P = 0.004). A three-factor PCA scores plot showed clustering of the urinary NMR spectra from the four subgroups. Metabolites that contributed to the discrimination between the subgroups included acetate, creatine, creatinine, dimethyamine (DMA), formate, glycine, hippurate, and trimethylamine-N-oxide (TMAO). A comparison of relative metabolite levels confirmed that carnitine was significantly increased in HCC; and creatinine, hippurate, and TMAO were significantly reduced in HCC compared to the other subgroups. HBeAg negative patients showed a significant increase in creatinine (P = 0.001) compared to HBeAg positive patients in the chronic hepatitis B subgroup, whilst HBeAg negative patients showed a significant decrease in DMA (P = 0.004) in the cirrhosis subgroup compared to HBeAg positive patients. There were no differences in metabolite levels in HCC patients who did or did not receive antiviral treatment.
CONCLUSION: Urinary NMR changes in Bangladeshi HCC were identified, corroborating previous findings from Egypt and West Africa. These findings could form the basis for the development of a cost-effective HCC dipstick screening test.
Keywords: Urinary metabolic profiling, Hepatocellular carcinoma, Nuclear magnetic resonance spectroscopy, Hepatitis B, Bangladesh
Core tip: Previous urinary metabolic profiling studies using nuclear magnetic resonance (NMR) spectroscopy of hepatocellular carcinoma (HCC) from Egypt and West Africa suggested the reproducibility of an identifying urinary metabolic profile in HCC. Here, a Bangladeshi HCC cohort was studied to identify similar changes. Urine samples from 142 subjects with hepatitis B HCC, cirrhosis, chronic hepatitis B, or no history of liver disease were analyzed using NMR. Urinary NMR from HCC differed across a range of metabolites, including reduced hippurate and creatinine and increased carnitine levels, consistent with the diverse effects of liver cancer on metabolic pathways and the interrelationship with the gut microbiome. Previous findings were corroborated, suggesting that a panel of metabolic markers could form the basis of a cost-effective HCC dipstick screening test.
INTRODUCTION
The incidence of hepatocellular carcinoma (HCC) in Asia was estimated to be approximately 460000 in 2000, and since then, it has been increasing every year[1]. Studies have shown that hepatitis B virus (HBV) underlies 33% of the HCC cases in the People’s Republic of Bangladesh, a small, but densely-populated country in the Indian sub-continent, bordering India and Myanmar[2]. Bangladesh is considered a region of intermediate prevalence for HBV infection, with the lifetime risk of HBV infection at 20%-60%[3]. While HBV infection is mainly transmitted during infancy and childhood in Bangladesh, all age groups are affected.
It is estimated that 2 billion people, one-third of the world-wide population, have been infected by HBV[4] and that approximately 400 million people are living with a chronic form of the disease[5]. Chronic HBV results in a wide range of liver diseases, spanning asymptomatic acute hepatitis to HCC. Recent studies have concluded that serum α-fetoprotein (AFP) lacks sufficient sensitivity to be widely used as a surveillance test for HCC[6]. Ultrasound-based surveillance is generally considered more sensitive than AFP, but the quality of the images can be both equipment- and user-dependent[6]. In addition, these procedures are generally unavailable in resource-constrained countries of Asia and Africa that harbor a majority population of HBV-related cirrhosis and HCC. A simple and versatile biomarker for the early stages of HCC, when treatment would still be effective, would, therefore, be of considerable value, both in resource-poor Bangladesh and in other parts of the world with a high HBV prevalence.
There has recently been considerable interest in analyzing the chemical composition of urine to establish if one or more urinary biomarkers can be used to distinguish HCC from cirrhosis or uncomplicated chronic HBV infection. Urinary metabolic profiling using proton nuclear magnetic resonance (NMR) spectroscopy may provide objective diagnostic and prognostic assessment for a range of diseases[7]. It would be particularly valuable if subtle changes in the chemical composition of urine could be interpreted to improve early diagnosis of HCC. Diagnosis is currently often not made until such a late stage of disease that treatment measures are ineffective. Clinical cohorts from Egypt[8], Nigeria[9,10], and The Gambia[10] have been studied, and a consensus is emerging for a urinary fingerprint of HCC. The aim of this study was to establish if a similar urinary NMR fingerprint for HCC could be identified in a Bangladeshi cohort, since these patients have a different environmental and genetic background than the African populations.
MATERIALS AND METHODS
Study population
Urine samples were obtained with informed consent from patients attending the Department of Hepatology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh. Ethical approval was granted by the research ethics committee at Bangabandhu Sheikh Mujib Medical University and Imperial College London (REC 09/H0712/82), and the study conformed to the 1975 Declaration of Helsinki.
A total of 152 subjects, all of Indian ethnic origin, were recruited for the study between January 2013 and November 2014. The study cohort comprised 46 patients with HCC on a background of hepatitis B (43 patients) (HCC-HBV), hepatitis C (one patient), and cryptogenic (two patients); 50 patients with clinical or histologically confirmed hepatitis B related cirrhosis (CIR), 48 patients with non-cirrhotic chronic hepatitis B related liver disease (CHB), and eight healthy volunteers with no history of liver disease from the same Bangladeshi population (CTR). All patients with HBV-related HCC provided a 5-10 year history of their liver disease, and all were seropositive for hepatitis B surface antigen (HBsAg) and expressed antibodies to hepatitis B core antigen (anti-HBc) at the time of sampling. Forty two of these patients had hepatitis B e-antigen (HBeAg) status determined, and levels of HBV DNA were quantified in 35 subjects. The diagnosis of HCC was made from past history of HBV-related chronic liver disease, clinical presentation, ultrasound assessment of HCC nodules, and elevated AFP levels. The diagnosis was confirmed by fine needle aspiration cytology (FNAC).
Urine sample collection and preparation for NMR analysis
A full dietary and medication history was recorded for each subject. Mid-stream urine samples were collected in the morning into tubes and stored at -20 °C, 2 to 4 h after collection, in Bangladesh until transport to the Institute of Hepatology, London, United Kingdom on dry ice and by air. Samples were prepared for NMR study according to previously published standard methodology[9]. Specifically, 400 μL of urine was added to 160 μL of phosphate buffer solution (0.2 mol/L Na2HPO4/0.2 mol/L NaH2PO4, pH 7.4) and 40 μL of 3-trimethylsilyl-(2,2,3,3-d4)-1-propionate (TSP)/D2O solution. After centrifuging, 550 μL of buffered urine was pipetted into an NMR tube of 5 mm diameter (Norell 502-7 from Glass Precision Engineering Ltd, Leighton Buzzard, United Kingdom) for proton NMR spectroscopy at the Department of Chemistry, University College London. Samples were placed in a sample queue at 21 °C on the auto sampler, and some samples may have remained in the queue for up to 6 h before NMR analysis.
Proton NMR spectral acquisition
The urine samples were prepared for proton NMR study and analyzed in a random order. NMR spectra were recorded on a Bruker Avance III 600 NMR spectrometer (Billerica, MA, United States) operating at proton NMR frequency of 600.13 MHz equipped with a 5 mm DCH cryoprobe and a 60-position sample changer BACS60. Data acquisition and processing were performed using standard TopSpin (version 3.2, Bruker) software. NMR spectra were recorded at 300 K. Temperature calibration was carried out using a sample of 99.8% deuterated methanol in a 5 mm NMR tube. A standard water suppression sample of 2 mmol/L sucrose in 90%H2O + 10%D2O with 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS) was used for manual iterative optimization of high-order shims (z6 in particular) via inspection of the shape of the residual water signal after presaturation. Each sample was shimmed using a modified topshim routine, in which the z shim was incremented by +24 units at the final stage in order to achieve optimum resolution for organic species dissolved in water. The increment applied was determined using a sample of H2O:CD3CN (3:1) with a small amount of DSS added to it. This sample was shimmed using first deuterium of CD3CN and then the protons of H2O. The change of the z shim from the shimming using CD3CN to that using H2O was -24 units. The deuterium lock phase was autocorrected both before and after shimming. The presaturation frequency (o1, Hz) was determined using a single 360° pulse sequence followed by further manual iterations where the phase of the pre-saturated residual water signal was monitored and dispersive contributions were minimized. This was done for the first sample for each set of 20-25 samples, and the o1 value was then kept constant for the remaining samples. The variation in the o1 value for all samples was found to be within less than ± 0.5 Hz. Similarly, probe tuning and matching was carried out manually for the first sample in each set of 20-25 samples and then kept unchanged for the remaining samples of the set. Proton NMR spectra with water presaturation during relaxation delay were acquired using a standard pulse sequence noesygppr1d, which suppressed effectively the probe background signal, giving a flat baseline. In addition, a digital filter (known as “BASEOPT” under TopSpin) with a pre-optimized correction for filter delay (1.0 μs in our case) was used to give spectra with a flat baseline, which required no first order phase correction. Prior to the start of data acquisition for each sample, the 90° pulse was determined (typically 14.0 μs) and the power level was adjusted for a 25 Hz-wide solvent presaturation automatically. Four dummy scans were used for equilibration followed by 64 scans collected into 144 K points with a total repetition time of 8.0 s at each scan (acquisition time = 4.0 s; relaxation delay = 4.0 s). NMR spectra were processed using the Bruker AMIX data processing package and the KnowItAll Informatics System v9.0 (Bio-Rad, Philadelphia, PA, United States). The Free Induction Decays were zero-filled, and an exponential 0.3 Hz line-broadening function was applied before Fourier transformation. All NMR spectra were automatically phased, and a baseline correction was applied. The TSP peak was assigned to be at δ 0.00 ppm for an internal chemical shift reference. NMR peaks in the range δ 0.50-9.50 ppm were analyzed, although the region δ 4.50-6.40 ppm was excluded to remove the residual water signal and the signal from urea. The urinary NMR peaks were assigned to metabolites on the basis of chemical shifts and coupling patterns and with reference to the published literature[8-13].
Statistical analysis
Demographic and blood biochemistry data between subject groups were compared using an independent sample Kruskal-Wallis one-way analysis of variance (ANOVA) test with pairwise multiple comparisons (IBM® SPSS® v21), and a P value of < 0.05 was considered significant.
The NMR data were analyzed using principal component analysis (PCA) (KnowItAll Informatics System v9.0) and orthogonal partial least squared discriminant analysis (OPLS-DA) techniques [SIMCA v14 (Umetrics AB, Umeå, Sweden)]. Using the intellibucketing option in KnowItAll v9.0, the NMR spectra were subdivided into smaller regions of about 0.02 ppm. Regions corresponding to particular metabolites were additionally selected, including those assigned to hippurate (7.82-7.85 ppm, 7.61-7.66 ppm, 7.52-7.58 ppm); creatinine (3.0425-3.0550 ppm, 4.04-4.07 ppm); creatine (3.035-3.0425 ppm); citrate (2.64-2.72 ppm, 2.52-2.58 ppm), and dimethylamine (2.72-2.74 ppm). All spectral regions were integrated, normalized to the sum of the total spectral integral, and mean-centred prior to multivariate analysis. PCA was performed to highlight outliers and clustering (KnowItAll v9.0). PCA was then repeated with all outliers excluded, and the metabolites contributing to the separation of groupings were identified from the loadings plot. This final data set was also analyzed by OPLS-DA using SIMCA v14. The discriminatory power of the model was validated using leave-one-out cross validation. An R2 value was determined to give a measure of the goodness of fit by the model. A cross-validated Q2 statistic (based on a 1:7 leave one out algorithm) was calculated as a quantitative measure of the predictability of the model for the Y variable, where a positive Q2 indicated a good predictive.
The NMR spectral regions corresponding to the most important discriminatory metabolite peaks, as determined by the PCA and OPLS-DA loadings plots, were normalized to the sum of the total spectral integral, and differences in these relative metabolite signal levels were compared between the subject groups using an independent samples Kruskal-Wallis one-way ANOVA test with all pairwise multiple comparisons (IBM® SPSS® v21). Within the patient subgroups, the Mann-Whitney U test was used to compare relative metabolite levels depending on HBeAg status and treatment with anti-viral therapy. A Benjamini-Hochberg adjustment[14] was applied to the obtained P values to acquire the level of significance for multiple testing, with a declared level of statistical significance of P < 0.05.
RESULTS
Subject demographics
Nine samples from across the four cohorts were identified as outliers on subsequent NMR analysis (one CTR, two CHB, three CIR, and three HCC). In addition, one HCC subject was excluded for diagnostic uncertainty. The final study cohort, therefore, comprised 142 subjects (seven CTR, 46 CHB, 47 CIR, and 42 HCC). Subject demographics are summarized in Table 1, and the serum biochemistry results are shown in Table 2. All HCC patients had underlying cirrhosis, and the diagnosis of HCC was confirmed by FNAC in all cases. The levels of HBV DNA showed considerable variation. All HCC patients were considered to have advanced HCC, which is a typical finding in Bangladesh.
Table 1.
Subject demographics of the Bangladeshi study cohort
| Group | n | Age (yr), median (range) | M:F (%male) | Etiology, n | HBeAg positive | Height (cm), median (range) | Weight (kg), median (range) | BMI (kg/m2), median (range) |
| CTR | 7 | 37 (24-46) | 7:0 (100%) | NA | NA | 170 (152-173) | 65 (54-85) | 26.0 (18.7-29.4) |
| CHB | 46 | 27 (15-45) | 40:6 (87%) | HBV (100%) | 27 | 166 (147- 180) | 55 (35-79) | 20.6 (13.9-26.6) |
| (-59%) | ||||||||
| CIR | 47 | 42 (15-67) | 39:8 (83%) | HBV (98%) | 21 | 165 (142–175) | 58 (42-82) | 21.9 (14.9-29.3) |
| Cryptogenic HBV (2%) | (-45%) | |||||||
| HCC | 42 | 48 (27-90) | 38:3 (90%) | HBV (93%) | 15 | 162 (145-180) | 48 (38-75) | 18.3 (12.3-25.2) |
| HCV (2%) | (-36%) | |||||||
| Cryptogenic (5%) |
CTR: Healthy controls; CHB: Chronic hepatitis-B related liver disease; CIR: Cirrhosis; HCC: Hepatocellular carcinoma; NA: Unavailable; BMI: Body mass index.
Table 2.
Serum biochemistry profiles
| Group | ALT | ALP | Bil | Alb | AFP | Creatinine |
| (U/L) | (U/L) | (mg/dL) | (g/dL) | (ng/mL) | (mg/dL) | |
| CTR | 31 (18-42) [7/7] | NA | NA | NA | NA | NA |
| CHB | 40 (22-232) [32/46] | 96 (63-171) [14/46] | 1.4 (0.4-2.2) [5/46] | 3.4 (2.9-3.9) [2/46] | NA | 1.4 (1.3-1.5) [2/46] |
| CIR | 51 (10-243) [30/47] | 286 (75-558) [5/47] | 1.9 (0.3-22.4) [19/47] | 2.7 (1.5-39.0) [20/47] | 6 (2-12) [4/47] | 1.4 (0.8-2.8) [17/47] |
| HCC | 74 (28-332) [32/42] | 259 (82-648) [25/42] | 1.8 (0.3-21.5) [28/42] | 3.1 (1.3-35.0) [26/42] | 4900 (4-70000) [34/42] | 0.9 (0.4-1.3) [23/42] |
Data are represented as median (range) [number of subjects included/total number of subjects in subgroup]. CTR: Healthy controls; CHB: Chronic hepatitis-B related liver disease; CIR: Cirrhosis; HCC: Hepatocellular carcinoma; ALT: Alanine transaminase; ALP: Alkaline phosphatase; Bil: Bilirubin; Alb: Albumin; AFP: α-fetoprotein; NA: Unavailable.
There were significant differences in age (P < 0.001), weight (P < 0.001), and body mass index (BMI) (P < 0.001) across the four subgroups. For example, the CHB cohort was significantly younger than both the CIR (P = 0.000) and HCC (P < 0.001) cohorts, and BMI was significantly lower in the HCC cohort compared to the CTR (P = 0.001) and CIR (P < 0.001) cohorts. While 83% of the CIR subgroup was male, the control group was entirely male, and so the urinary NMR data were analyzed both as a complete cohort and as a subset of males only.
The majority of subjects had eaten a similar diet of rice, dhal, water with vegetables, fish or chicken, in the 6 h prior to collection of a urine sample. The patients reported a varied drug history: the majority of the CHB group were taking multivitamins, although some were also taking an oral antiviral drug (n = 10, entecavir or tenofovir) and/or an oral proton pump inhibitor (PPI) (omeprazole, pantoprazole); the CIR group reported a combination of non-absorbable sugars (lactulose), PPI (omeprazole, pantoprazole), oral antiviral (n = 7, entecavir, tenofovir or telbivudine), and/or multivitamins; and the HCC cohort reported a wider range of medication, including an oral antiviral (entecavir, tenofovir), hormonal therapy (tamoxifen), PPI (omeprazole, pantoprazole), beta blockers, non-absorbable sugars (lactulose), multivitamins, and pain killers (including tramadol).
Serum biochemical analysis
The median (range) values for the available serum alanine aminotransferase (ALT), alkaline phosphatase (ALP), bilirubin, albumin, AFP, and creatinine levels are summarized in Table 2. Serum ALT was significantly higher in the HCC group than controls (P < 0.001) and patients with CHB (P < 0.001) or CIR (P = 0.006); serum ALP was higher in the HCC group than the CHB group (P < 0.001). Serum AFP was generally markedly elevated in HCC, although two HCC patients showed AFP ≤ 20 ng/mL in the presence of large space occupying lesion(s) in the right lobe of the liver as observed on axial imaging. Serum creatinine levels were significantly lower in the HCC group than in the CIR group (P = 0.004).
Representative urinary NMR spectra
Illustrative urinary proton NMR spectra from each of the four subject groups are summarized in Figure 1. The spectral resolution was defined by a TSP linewidth of < 1 Hz in all NMR data sets. On visual inspection, a number of trends could be seen across the groups, including a reduction in hippurate and an increase in creatine, when comparing CTR, CHB, and CIR through to HCC (Figures 1A-D and 1A’-D’, respectively).
Figure 1.
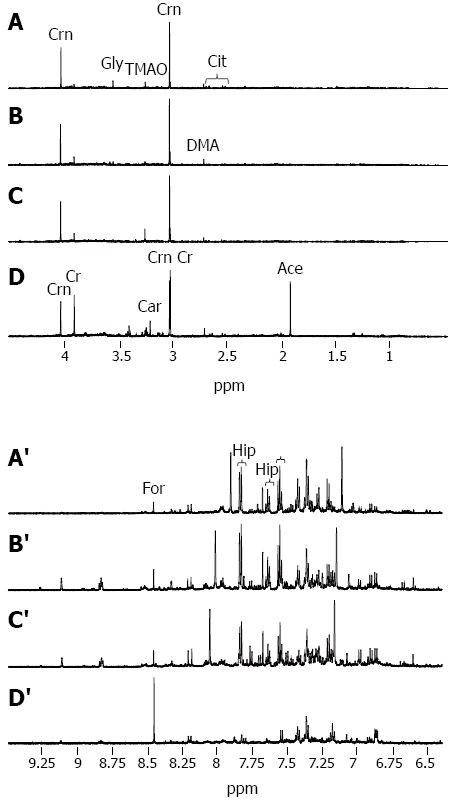
Illustrative urinary proton nuclear magnetic resonance spectra. From a 35 year old healthy control (CTR) (A, A’); a 22 year old male with chronic hepatitis-B related liver disease (CHB) (B, B’); a 36 year old male with hepatitis B virus (HBV)-cirrhosis (C, C’); and a 50 year old male with HBV-hepatocellular carcinoma (AFP > 30000 mg/dL) displaying (A-D) the aliphatic region 0.5-4.5 ppm and (A’-D’) the aromatic region 6.4-9.5 ppm (D, D’). Each NMR spectrum is scaled independently. The more prominent peaks are assigned and include acetate (Ace), carnitine (Car), citrate (Cit), creatine (Cr), creatinine (Crn), dimethylamine (DMA), formate (For), glycine (Gly), hippurate (Hip), histidine (His), and trimethylamine-N-oxide (TMAO).
Analyses of the urinary NMR spectra
Nine outliers were identified on five iterations of PCA: one CTR showing particularly high levels of hippurate; two CHB showing high levels of glucose and lactate and overlap of creatinine into the creatine peak; three CIR showing either high glucose, high glucose and lactate, or ethanol present; and three HCC showing either paracetamol resonances, absence of creatine and creatinine, or unassigned additional dominant peaks.
A three-factor PCA score plot of the final study cohort of 142 subjects is illustrated in Figure 2, showing clustering of each of the four subgroups. Metabolites that contributed to the discrimination between groups in the loadings plot included acetate, creatine, creatinine, dimethyamine (DMA), formate, glycine, hippurate, and trimethylamine-N-oxide (TMAO). An OPLS-DA plot is illustrated in Figure 3 and the R2(X), R2(Y) and Q2 values were 0.468, 0.289, and 0.195, respectively.
Figure 2.
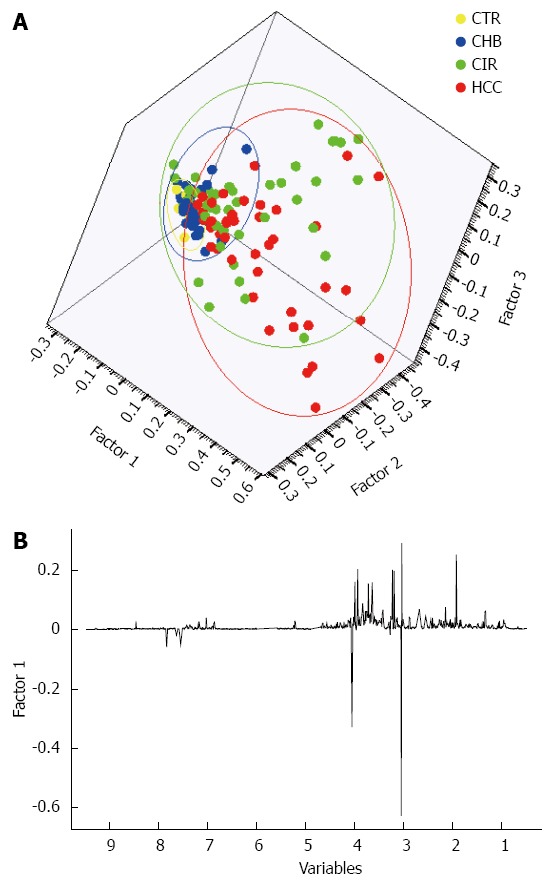
A three-factor principal components analysis scores plot of the cohort of 142 subjects and clustering of each of the four subgroups. A: Principal components analysis (PCA) scatter plot showing clustering and separation of healthy controls (CTR), chronic hepatitis-B related liver disease (CHB), cirrhosis (CIR), and hepatocellular carcinoma (HCC) subgroups; B: The associated loadings plot illustrating the metabolites contributing to the separation of the subgroups.
Figure 3.
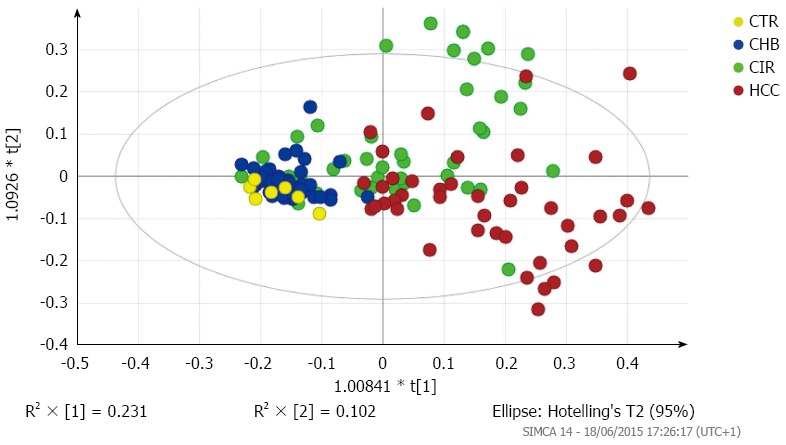
Scores plot of orthogonal partial least squares discriminant analysis (OPLS-DA) model of CTR, CHB, CIR, and HCC subgroups. R2(X), R2(Y) and Q2 values were 0.468, 0.289, and 0.195, respectively.
Acetate, carnitine, citrate, creatine, creatinine, DMA, hippurate, and TMAO metabolite levels were significantly different across the four groups, when considering all subjects (Table 3) and only the males. A comparison of relative metabolite levels between HCC vs CTR and/or HCC vs CHB in all subjects confirmed that carnitine was significantly increased in HCC, and creatinine, hippurate, and TMAO were significantly reduced in HCC (Figure 4). A significant increase in carnitine and significant reductions in creatinine and hippurate were observed in the HCC group compared to the CIR group (Figure 4).
Table 3.
Comparison of relative signal levels (mean ± SD) of discriminatory metabolites between study cohorts1
| Selected metabolites |
Study cohort (number of subjects in group) |
P value2 | ||||
| Metabolite | δ/ppm (multiplicity) of peak analysed | CTR (n = 7) | CHB (n = 46) | CIR (n = 47) | HCC (n = 42) | |
| Acetate | 1.92 (s) | 0.34 ± 0.03 | 0.57 ± 0.64 | 1.70 ± 2.14 | 1.24 ± 1.49 | 0.007 |
| Carnitine | 3.23 (s) | 0.57 ± 0.41 | 0.42 ± 0.22 | 0.44 ± 0.53 | 1.19 ± 1.04 | 0.000 |
| Citrate | 2.52 (d) | 1.49 ± 0.61 | 1.35 ± 0.61 | 1.19 ± 1.21 | 1.07 ± 0.46 | 0.005 |
| Creatine | 3.03 (s) | 1.34 ± 0.35 | 1.24 ± 0.38 | 1.07 ± 0.95 | 1.54 ± 1.82 | 0.007 |
| Creatinine | 3.04 (s) | 10.88 ± 1.30 | 10.06 ± 1.76 | 7.74 ± 3.00 | 5.85 ± 1.83 | 0.000 |
| DMA | 2.72 (s) | 2.14 ± 0.57 | 2.11 ± 0.58 | 1.95 ± 1.21 | 1.91 ± 0.45 | 0.022 |
| Formate | 8.46 (s) | 0.07 ± 0.03 | 0.07 ± 0.05 | 0.16 ± 0.16 | 0.16 ± 0.18 | 0.059 |
| Glycine | 3.56 (s) | 1.04 ± 0.39 | 0.84 ± 0.42 | 0.74 ± 0.38 | 0.79 ± 0.63 | 0.129 |
| Hippurate | 7.85 (d) | 0.99 ± 0.47 | 0.85 ± 0.47 | 0.44 ± 0.35 | 0.28 ± 0.30 | 0.000 |
| TMAO | 3.27 (s) | 1.79 ± 0.44 | 1.95 ± 0.77 | 1.62 ± 1.04 | 1.32 ± 0.75 | 0.000 |
1Percentage normalised metabolite signal level relative to total NMR signal in the region 0.50-9.50 ppm (excluding 4.50-6.40 ppm);
Significance for independent samples Kruskal-Wallis one-way ANOVA test with pairwise multiple comparisons. CTR: Healthy controls; CHB: Chronic hepatitis-B related liver disease; CIR: Cirrhosis; HCC: Hepatocellular carcinoma.
Figure 4.
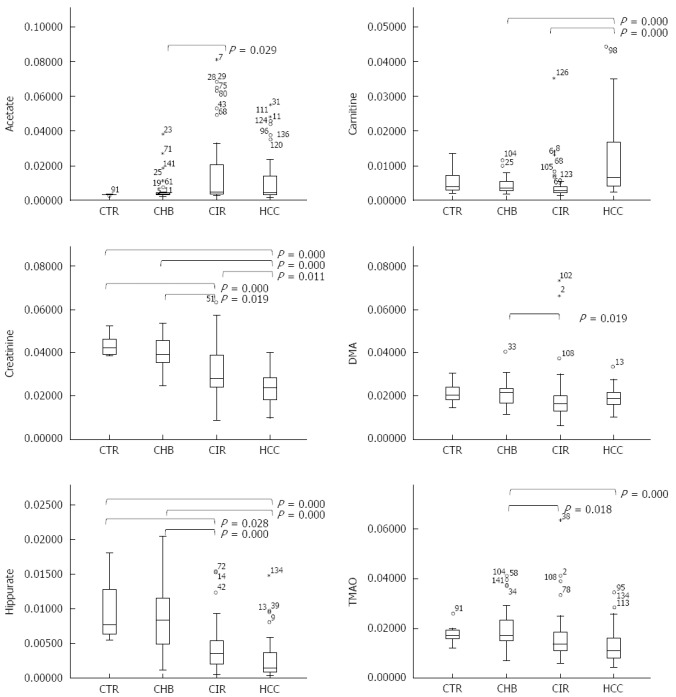
Boxplots of selected metabolites. Metabolites [acetate, carnitine, creatinine, dimethylamine (DMA), hippurate, and trimethylamine-N-oxide (TMAO)], showing the results of a comparison of signal levels (normalized to 1) using an independent-samples Kruskal-Wallis test.
HBeAg negative patients showed a significant increase in creatinine (P = 0.001) compared to HBeAg positive patients in the CHB subgroup. In the CIR group, HBeAg negative patients showed a significant decrease in DMA (P = 0.004) compared to HBeAg positive patients; there were no significant differences in the HCC subgroup according to HBeAg status.
There were no differences in metabolite levels in the HCC or CHB groups when comparing subjects receiving antiviral treatment with those not. However, creatine levels were lower in subjects receiving antiviral therapy in the CIR group (n = 7) than those who were not (n = 40) (P = 0.002).
DISCUSSION
In this study of Bangladeshi subjects, all of Indian ethnic origin, the urinary NMR metabolic profile measured in patients with HCC was distinguishable from the urinary profile of patients with CIR and CHB and also healthy control subjects. The majority of the patients (131 patients) had a background of CHB, while one patient with cirrhosis and two patients with HCC were defined as cryptogenic and one HCC subject had a background of HCV. Metabolites that contributed to the differences in urinary NMR profiles of HCC, compared to CIR, CHB and/or CTR, included acetate, carnitine, citrate, creatine, creatinine, DMA, hippurate, and TMAO. Within the CHB and CIR patient subgroups, metabolite differences were also observed according to HBeAg status and, only in CIR, to treatment with oral antiviral therapy.
Our findings extend previous studies using urinary NMR to identify metabolic changes in HCC. Previous studies showed that urinary NMR changes in HCC could be distinguished from CIR and CTR in patient cohorts from Egypt, Nigeria, and The Gambia, with HCC on a background of either chronic HCV[8,10] or chronic HBV[9,10]. An NMR pattern has emerged to separate HCC from CIR and CTR, which includes a reduction in hippurate, citrate, creatinine, and TMAO and an increase in acetate, carnitine, and creatine. In this population, neither glycine nor formate was significantly different in HCC between the other study cohorts, although both of these metabolites were identified as discriminators in the West African study. Overall, given that the urinary profile of HCC could be distinguished from CIR, CHB, and CTR, this study provides further support for the suggestion that a diagnostic marker may be feasible, as consistent urinary NMR changes are seen across differing ethnicities as well as varying disease etiologies.
The urinary metabolite changes observed in HCC subjects relate to alterations in both host and gut bacterial metabolism. For example, a reduced urinary concentration of citrate, which is a tricarboxylic acid intermediate, might be in agreement with Warburg’s hypothesis of altered mitochondrial aerobic respiration and heightened physiological stress of cancer cells[8]. The alterations observed in carnitine are interesting, and its increase in HCC is consistent with the previous urinary NMR profiling studies in HCC[8-10]. Carnitine is required for energy metabolism, enabling fatty acids to enter the mitochondria for β-oxidation[15]. Carnitine can be absorbed from the diet or synthesised in the liver, testis, and kidney and reabsorbed via the renal system[8]. Increased urinary carnitine may, therefore, correspond to excess carnitine ingestion, increased biosynthesis, or poor reabsorption and may reflect overproduction of tumor carnitine to maintain rapid growth and fuel mitochondrial activity[8]. Further evidence of heightened β-oxidation is shown by relatively higher acetate in the urine of HCC patients than in controls[10]. Advanced HCC can be complicated by cancer cachexia with associated sarcopenia, and urinary creatinine concentration has been suggested as a biomarker of sarcopenia[16]. In combination with other factors, however, urinary NMR creatinine concentration may contribute to a biomarker panel for HCC. Our findings on urinary creatine suggest that it is elevated in HCC in comparison to ethnically-matched healthy controls but is reduced in CIR patients in comparison to the same controls. Creatine has a direct function in cellular energy transport, and it may be that creatine is elevated in rapidly growing cells; and, indeed, urinary creatine levels may be increased 24 h after partial hepatectomy in rats[17].
The urinary changes in DMA, hippurate, and TMAO implicate alterations in the gut microbiome. TMAO is the oxidation product of trimethylamine (TMA), which can be produced by bacterial degradation of dietary phosphatidylcholine and choline. It is likely that a decrease in TMAO reflects dysregulation of the intestinal microbiota. DMA can also be a product of gut bacterial metabolism of dietary choline, although it can originate from the N-methylation of methylamines from the breakdown of creatine. While diet was not controlled for in the study, and such a proposition would be difficult to implement, the observation that HBeAg status has an influence on DMA levels does further suggest that gut bacterial metabolism might have pathophysiological consequences in CHB and HCC. Hippurate is formed by the conjugation of benzoate with glycine in liver and kidney mitochondria. Benzoate is formed from dietary aromatic compounds via gut microbial metabolism. It has been suggested that less efficient benzoate conjugation and lower urinary hippurate excretion levels may result from reduced hepatic function in patients with HCC[8].
The differences observed in the urinary profiles according to HBeAg status within the CHB and CIR subgroups are worth exploring in more detail in future studies. In this cohort, differences were seen in DMA and creatinine levels between HBeAg positive and negative subjects in the CHB and CIR subgroups respectively. The rate of disease progression has been shown to be influenced by HBeAg status[18], and it would be particularly interesting if these urinary metabolic differences could be attributed to differences in cell turnover, for example.
An impact of antiviral treatment on relative urinary creatine levels was observed in patients with cirrhosis. While antiviral therapy may influence renal function[19], it is not clear why urinary creatine levels might be altered. Regardless, any influence of treatment effects do need to be considered when interpreting the urinary metabolite changes.
There are a number of limitations to this present study. There was a logistical delay between sample collection and sample analysis, as the Bangladeshi samples were archived, stored, and transported to London for NMR analysis, although this would not be expected to have had any impact on the urinary NMR profile. The NMR urinary profile from nine subjects were shown to be outliers using PCA, and confounding factors included high levels of glucose and overlapping peaks from unreported over-the-counter medication. These factors need to be considered for developing an NMR analysis protocol for inclusion of all NMR data sets, without obscuring overlapping peaks in the spectral regions with outlier resonances. There was gender imbalance between the subgroups, but analyzing males only as a separate cohort did not alter the urinary NMR differences seen across the patient groups. Increased study numbers would allow inclusion of training and validation data sets and also a more detailed comparison of NMR findings with currently available diagnostic serum and clinical biomarkers. Further prospective studies looking at the urinary NMR differences in patients with early HCC, for example stage 0 using the Barcelona Clinic Liver Cancer score[20] and also following treatment, would underline the potential of implementing a urinary screening test for HCC.
In conclusion, urinary NMR changes in HCC are consistent with the diverse effects of liver cancer on human physiology and gut bacterial action and may aid the development of a cost-effective HCC urinary dipstick screening test. Such a diagnostic urine test for HCC could be a paradigm shift in liver cancer screening. It could provide a practical and cost-effective test that is easy to use in primary care and particularly applicable to countries of Central and East Asia. Clinically and economically, such a simple approach could have a major impact, not only in the developed economies of Europe and North America, but also in severely resource limited settings, such as sub-Saharan Africa. Further validation work is required, but the pattern emerging from different studies around the world is promising.
ACKNOWLEDGMENTS
We thank colleagues at the Institute of Hepatology, London (Drs M Briones, S Chokshi, J Coombes, P Manka) and Imperial College London (Ms A Ledlie, Drs N Ladep, MJW McPhail, MIF Shariff, CA Wadsworth) for advice and helpful discussions. We thank Dr. Peter Gierth (Bruker, United Kingdom) for his help with the initial setup of NMR measurements at UCL Chemistry, including optimization of shimming routines. Crossey MME, Dawood M, and Taylor-Robinson SD are grateful to the United Kingdom NIHR Biomedical Facility at Imperial College London for infrastructure support.
COMMENTS
Background
Previous studies of hepatocellular carcinoma (HCC) from Egypt and West Africa suggest reproducibility of urinary metabolic profiling in HCC using nuclear magnetic resonance (NMR) spectroscopy techniques. This study aimed to establish if similar changes were found in a Bangladeshi HCC cohort.
Research frontiers
To explore the use of metabolic profiling of urine to provide markers of hepatocellular cancer, with the aim of developing a panel of metabolic markers to form the basis of a cost-effective dipstick test for hepatocellular cancer.
Innovations and breakthroughs
Urinary NMR from patients with HCC differed across a range of metabolites, including reduced hippurate and creatinine and increased carnitine levels, when compared to the urinary NMR profile of cirrhotics, chronic hepatitis B patients, and controls, consistent with the diverse effects of liver cancer on metabolic pathways and its interrelationship with the gut microbiome. These results corroborated previous urinary NMR findings from patients with HCC from Egypt and West Africa.
Applications
Urinary NMR changes in HCC are consistent with the diverse effects of liver cancer on human physiology and gut bacterial action and may aid in the development of a cost-effective HCC urinary dipstick screening test. Further validation work is required, but the pattern emerging from different studies around the world is promising.
Terminology
NMR spectroscopy describes a state-or-the-art analytical chemistry technique that enables a non-selective snap-shot assessment of sample composition.
Peer-review
The authors should ensure as much reliability as is possible to diagnose appropriately liver disease. The authors recommend subdividing the results from patients with HCC into early and advanced disease and comparing NMR findings to other biomarker results.
Footnotes
Institutional review board statement: Ethical approval was granted by the research ethics committee at Bangabandhu Sheikh Mujib Medical University and Imperial College London (REC 09/H0712/82).
Informed consent statement: Informed consent was obtained from the study participants
Conflict-of-interest statement: To the best of our knowledge the authors declare no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 4, 2015
First decision: January 28, 2016
Article in press: March 2, 2016
P- Reviewer: Guan YS S- Editor: Yu J L- Editor: Filipodia E- Editor: Wang CH
References
- 1.Al-Mahtab M, Uddin H, Akbar SM. Epidemiology and Risk Factors of Hepatocellular Carcinoma in Asia. Journal of Gastroenterology and Hepatology Research. 2014;3:1019–1023. [Google Scholar]
- 2.Khan M, Zaki KM, Ahmed KU, Ali SM, Islam N. Clinical profile: prognostic index in hepatocellular carcinoma. Bangladesh Med Res Counc Bull. 1991;17:49–62. [PubMed] [Google Scholar]
- 3.Mahtab MA, Rahman S, Karim MF, Khan M, Foster G, Solaiman S, Afroz S. Epidemiology of hepatitis B virus in Bangladeshi general population. Hepatobiliary Pancreat Dis Int. 2008;7:595–600. [PubMed] [Google Scholar]
- 4.Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L. Hepatitis B: Epidemiology and prevention in developing countries. World J Hepatol. 2012;4:74–80. doi: 10.4254/wjh.v4.i3.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thio CL, Guo N, Xie C, Nelson KE, Ehrhardt S. Global elimination of mother-to-child transmission of hepatitis B: revisiting the current strategy. Lancet Infect Dis. 2015;15:981–985. doi: 10.1016/S1473-3099(15)00158-9. [DOI] [PubMed] [Google Scholar]
- 6.Daniele B, Bencivenga A, Megna AS, Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127:S108–S112. doi: 10.1053/j.gastro.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Dona AC, Jiménez B, Schäfer H, Humpfer E, Spraul M, Lewis MR, Pearce JT, Holmes E, Lindon JC, Nicholson JK. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal Chem. 2014;86:9887–9894. doi: 10.1021/ac5025039. [DOI] [PubMed] [Google Scholar]
- 8.Shariff MI, Gomaa AI, Cox IJ, Patel M, Williams HR, Crossey MM, Thillainayagam AV, Thomas HC, Waked I, Khan SA, et al. Urinary metabolic biomarkers of hepatocellular carcinoma in an Egyptian population: a validation study. J Proteome Res. 2011;10:1828–1836. doi: 10.1021/pr101096f. [DOI] [PubMed] [Google Scholar]
- 9.Shariff MI, Ladep NG, Cox IJ, Williams HR, Okeke E, Malu A, Thillainayagam AV, Crossey MM, Khan SA, Thomas HC, et al. Characterization of urinary biomarkers of hepatocellular carcinoma using magnetic resonance spectroscopy in a Nigerian population. J Proteome Res. 2010;9:1096–1103. doi: 10.1021/pr901058t. [DOI] [PubMed] [Google Scholar]
- 10.Ladep NG, Dona AC, Lewis MR, Crossey MM, Lemoine M, Okeke E, Shimakawa Y, Duguru M, Njai HF, Fye HK, et al. Discovery and validation of urinary metabotypes for the diagnosis of hepatocellular carcinoma in West Africans. Hepatology. 2014;60:1291–1301. doi: 10.1002/hep.27264. [DOI] [PubMed] [Google Scholar]
- 11.Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, Bjorndahl TC, Krishnamurthy R, Saleem F, Liu P, et al. The human urine metabolome. PLoS One. 2013;8:e73076. doi: 10.1371/journal.pone.0073076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, Cheng D, Jewell K, Arndt D, Sawhney S, et al. HMDB: the Human Metabolome Database. Nucleic Acids Res. 2007;35:D521–D526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzmann SS, Merrifield CA, Rezzi S, Kochhar S, Lindon JC, Holmes E, Nicholson JK. Stability and robustness of human metabolic phenotypes in response to sequential food challenges. J Proteome Res. 2012;11:643–655. doi: 10.1021/pr2005764. [DOI] [PubMed] [Google Scholar]
- 14.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 15.Vaz FM, Wanders RJ. Carnitine biosynthesis in mammals. Biochem J. 2002;361:417–429. doi: 10.1042/0264-6021:3610417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pahor M, Manini T, Cesari M. Sarcopenia: clinical evaluation, biological markers and other evaluation tools. J Nutr Health Aging. 2009;13:724–728. doi: 10.1007/s12603-009-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bollard ME, Contel NR, Ebbels TM, Smith L, Beckonert O, Cantor GH, Lehman-McKeeman L, Holmes EC, Lindon JC, Nicholson JK, et al. NMR-based metabolic profiling identifies biomarkers of liver regeneration following partial hepatectomy in the rat. J Proteome Res. 2010;9:59–69. doi: 10.1021/pr900200v. [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro RM, Germanidis G, Powers KA, Pellegrin B, Nikolaidis P, Perelson AS, Pawlotsky JM. Hepatitis B virus kinetics under antiviral therapy sheds light on differences in hepatitis B e antigen positive and negative infections. J Infect Dis. 2010;202:1309–1318. doi: 10.1086/656528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gane EJ, Deray G, Liaw YF, Lim SG, Lai CL, Rasenack J, Wang Y, Papatheodoridis G, Di Bisceglie A, Buti M, et al. Telbivudine improves renal function in patients with chronic hepatitis B. Gastroenterology. 2014;146:138–146.e5. doi: 10.1053/j.gastro.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 20.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]


