Abstract
AIM: To assess the rate of infection, appropriateness of antimicrobial-therapy and mortality on intensive care unit (ICU). Special focus was drawn on patients with liver cirrhosis.
METHODS: The study was approved by the local ethical committee. All patients admitted to the Internal Medicine-ICU between April 1, 2007 and December 31, 2009 were included. Data were extracted retrospectively from all patients using patient charts and electronic documentations on infection, microbiological laboratory reports, diagnosis and therapy. Due to the large hepatology department and liver transplantation center, special interest was on the subgroup of patients with liver cirrhosis. The primary statistical-endpoint was the evaluation of the influence of appropriate versus inappropriate antimicrobial-therapy on in-hospital-mortality.
RESULTS: Charts of 1979 patients were available. The overall infection-rate was 53%. Multiresistant-bacteria were present in 23% of patients with infection and were associated with increased mortality (P < 0.000001). Patients with infection had significantly increased in-hospital-mortality (34% vs 17%, P < 0.000001). Only 9% of patients with infection received inappropriate initial antimicrobial-therapy, no influence on mortality was observed. Independent risk-factors for in-hospital-mortality were the presence of septic-shock, prior chemotherapy for malignoma and infection with Pseudomonas spp. Infection and mortality-rate among 175 patients with liver-cirrhosis was significantly higher than in patients without liver-cirrhosis. Infection increased mortality 2.24-fold in patients with cirrhosis. Patients with liver cirrhosis were at an increased risk to receive inappropriate initial antimicrobial therapy.
CONCLUSION: The results of the present study report the successful implementation of early-goal-directed therapy. Liver cirrhosis patients are at increased risk of infection, mortality and to receive inappropriate therapy. Increasing burden are multiresistant-bacteria.
Keywords: Intensive care unit, Sepsis-bundle, Early goal-directed therapy, Liver cirrhosis, Mortality
Core tip: This is a retrospective study evaluating the association of appropriate and inappropriate antimicrobial therapy on intensive care unit-mortality with special focus on patients with liver cirrhosis. Charts of 1979 patients were available for analysis. Patients with infection had significantly increased in-hospital mortality. Only 9% of patients with infection received inappropriate initial antimicrobial therapy. Multiresistant bacteria were detected in 23% of patients with infection and were associated with increased mortality. Infection increased mortality 2.24-fold in patients with cirrhosis. Patients with liver cirrhosis were at an increased risk to receive inappropriate initial antimicrobial therapy.
INTRODUCTION
Infections are a worldwide problem concerning patients admitted to intensive care units (ICU), since they are associated with an increased mortality, morbidity and financial burden[1-4]. A 51%-prevalence of infection has been reported for ICU-patients and previously 20% of patients admitted with septic-shock to an ICU received inappropriate antimicrobial-therapy[5]. This knowledge led to increased efforts to implement early-goal-directed therapy with timely initiation of appropriate antimicrobial-therapy in clinical practice[4].
The aim of the present study was to assess the rate of infection, as well as appropriate- and inappropriate antimicrobial-therapy after implementation of early-goal-directed therapy of infection and sepsis on an Internal-Medicine-ICU and to assess the association of appropriate-and inappropriate antimicrobial-therapy on mortality. In addition, special focus was drawn on the subgroup of patients with liver cirrhosis. This special interest was derived due to the hepatologic focus of our unit with a large hepatology outpatient and inn-patient clinic including transplantation unit. A previous meta-analysis reported a 4-fold increase in mortality in patients with liver-cirrhosis that acquire an infection[6].
MATERIALS AND METHODS
All patients admitted to the Internal-Medicine-ICU of the Goethe-University-Hospital-Frankfurt between April 1, 2007 and December 31, 2009 were included in the study. Data were extracted retrospectively from all patients using patient-charts and electronic-documentations on infection, microbial-isolates, diagnosis and therapy. The study was approved by the local ethical-committee. The study protocol included the special focus on liver cirrhosis patients and was approve prior to the study recruitment.
Demographic, physiological, antimicrobial and therapeutic-data were collected from all patients. Only the first seven days after ICU-admission were evaluated concerning microbial-isolates and antibiotic-regimen. Patient characteristics are shown in Tables 1 and 2.
Table 1.
Characteristics of patients n (%)
| Characteristic | All patients (n = 1979) | Patients without infection (n = 937) | Patients with infection (n = 1042) | OR (95%CI) | P value |
| Age (yr), mean ± SD (range) | 61 ± 16 (16-96) | 61 ± 16 (16-96) | 61 ± 16 (16-95) | 1.003 (0.997-1.008) | > 0.20 |
| Male sex | 1236 (62.5) | 614 (65.5) | 622 (60) | 0.779 (0.649-0.935) | 0.0074 |
| Days on ICU, mean ± SD (range) | 5.53 ± 9.88 (1-217) | 2.89 ± 5.97 (1-76) | 8.12 ± 12 (1-217) | 0.877 (0.858-0.897) | < 0.000001 |
| Hospital-mortality | 489 (24.7) | 140 (14.9) | 349 (33.5) | 2.883 (2.312-3.595) | < 0.000001 |
| Severity score on admission day | |||||
| SAPS II, mean ± SD (range) | 35 ± 16 (5-93) | 30 ± 14 (5-80) | 39 ± 16 (5-93) | 0.958 (0.950-0.965) | < 0.000001 |
| TISS, mean ± SD (range) | 9.5 ± 8.8 (0-55) | 6.3 ± 7.4 (0-35) | 11.6 ± 9.1 (0-55) | 0.925 (0.912-0.938) | < 0.000001 |
| Highest severity score within 7 d after admission | |||||
| SAPS II, mean ± SD (range) | 39 ± 17 (6-93) | 32 ± 15 (6-91) | 44 ± 17 (6-93) | 0.954 (0.947-0.961) | < 0.000001 |
| TISS, mean ± SD (range) | 12.0 ± 9.4 (0-55) | 7.5 ± 8.0 (0-35) | 14.6 ± 9.1 (0-55) | 0.909 (0.897-0.921) | < 0.000001 |
| Type of admission | < 0.000001 | ||||
| Internal medicine ICU | 825 (41.7) | 275 (29.3) | 550 (52.8) | ||
| Cardiology ICU | 1154 (58.3) | 663 (70.8) | 491 (47.1) | ||
| Source of admission | < 0.000001 | ||||
| Emergency department | 923 (46.6) | 468 (49.9) | 455 (43.7) | Reference | |
| Other hospital | 274 (13.8) | 87 (9.3) | 187 (17.9) | 2.211 (1.662-2.941) | < 0.000001 |
| Hospital ward | 613 (31.0) | 276 (29.5) | 337 (32.2) | 1.256 (1.023-1.541) | 0.029 |
| Operating room | 132 (67.0) | 98 (10.5) | 34 (3.3) | 0.357 (0.237-0.538) | 0.000001 |
| Surgical IMC/ICU | 38 (1.9) | 9 (0.96) | 29 (2.8) | 3.314 (1.552-7.079) | 0.0020 |
| Comorbid conditions | 1410 (71.2) | 607 (64.8) | 803 (77.1) | 1.840 (1.511-2.241) | < 0.000001 |
| COPD | 233 (11.8) | 84 (9.0) | 149 (14.3) | 1.696 (1.278-2.252) | 0.00026 |
| Heart failure (EF < 30%) | 114 (5.8) | 59 (6.3) | 55 (5.3) | 0.830 (0.569-1.212) | > 0.20 |
| Coronary artery disease | 455 (23.0) | 244 (26.0) | 211 (20.2) | 0.722 (0.585-0.891) | 0.0024 |
| Chronic renal failure | 342 (34.2) | 140 (14.9) | 202 (19.4) | 1.371 (1.082-1.736) | 0.0089 |
| Diabetes mellitus | 474 (24.0) | 227 (24.2) | 247 (23.7) | 0.973 (0.791-1.197) | > 0.20 |
| Liver-cirrhosis | 175 (8.8) | 57 (6.1) | 118 (11.3) | 1.974 (1.420-2.744) | 0.000052 |
| Cancer | 229 (11.6) | 89 (9.5) | 140 (13.4) | 1.481 (1.117-1.962) | 0.0063 |
| Hematologic neoplasia | 111 (5.6) | 39 (4.2) | 72 (6.9) | 1.711 (1.147-2.553) | 0.0085 |
| HIV | 76 (3.8) | 15 (1.6) | 61 (5.9) | 3.826 (2.160-6.779) | 0.0000043 |
| Immunosuppressive Tx | 106 (5.4) | 29 (3.1) | 77 (7.4) | 2.501 (1.616-3.870) | 0.000039 |
| Chemotherapy | 108 (5.5) | 32 (3.4) | 76 (7.3) | 2.230 (1.461-3.403) | 0.00020 |
| Number of cormobid conditions | 0.000023 | ||||
| 0 | 566 (28.6) | 330 (35.2) | 236 (22.6) | ||
| 1 | 593 (30.0) | 257 (27.4) | 336 (32.2) | ||
| 2 | 415 (21.0) | 174 (18.6) | 241 (23.1) | ||
| 3 | 260 (13.1) | 109 (11.6) | 151 (14.5) | ||
| > 3 | 146 (7.4) | 68 (7.3) | 78 (7.5) | ||
| Mechanical ventilation | 942 (47.6) | 265 (28.3) | 677 (65.0) | 4.710 (3.892-5.701) | <0.000001 |
| Hemodialysis | 274 (13.8) | 61 (6.5) | 213 (20.4) | 3.694 (2.736-4.987) | <0.000001 |
SAPS II: Simplified Acute Physiology Score II; TISS: Therapeutic Intervention Scoring System; ICU: Intensive care unit; IMC: Intermediate care unit, Internal Medicine ICU includes patients from Gastroenterology, Hepatology, Pulmonology, Endocrinology, Oncology, Hematology, Infectiology, Rheumatology departments, while Cardiology-ICU include patients from Cardiology, Angiology, Nephrology department; EF: Ejection fraction.
Table 2.
Site of infection and types of microorganisms in culture-positive infected patients n (%)
| All patients with infection (n = 1042) | Appropriate therapy (n = 948) | Inappropriate therapy (n = 94) | P value | |
| Site of primary infection | ||||
| Respiratory tract | 716 (68.7) | 658 (69.4) | 58 (61.7) | 0.12 |
| Abdomen/GIT | 49 (4.7) | 46 (4.9) | 3 (3.2) | > 0.20 |
| Renal/urinary tract | 33 (3.2) | 32 (3.4) | 1 (1.1) | > 0.20 |
| Skin | 13 (1.2) | 13 (1.4) | 0 | > 0.20 |
| CNS | 12 (1.2) | 10 (1.1) | 2 (2.1) | > 0.20 |
| Endocarditis | 26 (2.5) | 24 (2.5) | 2 (2.3) | > 0.20 |
| Bloodstream | 5 (0.5) | 5 (0.5) | 0 | > 0.20 |
| Others | 35 (3.4) | 33 (3.5) | 2 (2.1) | > 0.20 |
| Sepsis | 207 (19.8) | 179 (18.9) | 28 (29.8) | 0.011 |
| Sites with detection of microorganism | ||||
| Respiratory tract | 400 (38.4) | 345 (36.4) | 55 (58.5) | 0.000026 |
| Gastrointestinal | 215 (20.6) | 184 (19.4) | 31 (33.0) | 0.0019 |
| Bloodstream | 156 (15.0) | 130 (13.7) | 26 (27.7) | 0.00030 |
| Renal/urinary tract | 185 (17.8) | 155 (16.4) | 30 (31.9) | 0.000165 |
| Skin | 174 (16.7) | 145 (15.3) | 29 (30.9) | 0.00012 |
| Catheter-related | 51 (4.9) | 39 (4.1) | 12 (12.8) | 0.00021 |
| CNS | 10 (1.0) | 10 (1.1) | 0 | > 0.20 |
| Others | 87 (8.4) | 70 (7.4) | 17 (18.1) | 0.00055 |
| Culture-positive | 697 (66.9) | 606 (63.9) | 91 (69.8) | < 0.000001 |
| Gram-positive isolates | 473 (45.4) | 400 (42.2) | 73 (77.7) | < 0.000001 |
| S. aureus | 187 (17.9) | 171 (18.0) | 16 (17.0) | > 0.20 |
| MRSA | 45 (4.3) | 31 (3.3) | 14 (14.9) | < 0.000001 |
| S. epidermidis | 17 (1.6) | 15 (1.6) | 2 (2.1) | > 0.20 |
| S. pneumoniae | 16 (1.5) | 12 (1.3) | 4 (4.3) | 0.025 |
| VSE | 93 (8.9) | 67 (7.1) | 26 (27.7) | < 0.000001 |
| VRE | 91 (8.7) | 68 (7.2) | 23 (24.5) | < 0.000001 |
| Others | 172 (16.5) | 138 (14.5) | 34 (36.2) | < 0.000001 |
| Gram-negative isolates | 368 (35.3) | 320 (33.8) | 48 (51.1) | 0.00081 |
| E. coli | 99 (9.5) | 89 (9.4) | 10 (10.6) | > 0.20 |
| Enterobacter spp. | 45 (4.3) | 39 (4.1) | 6 (6.4) | > 0.20 |
| Klebsiella spp. | 56 (5.4) | 49 (5.2) | 7 (7.4) | > 0.20 |
| Pseudomonas spp. | 93 (8.9) | 79 (8.3) | 14 (14.9) | 0.033 |
| Acinetobacter spp. | 10 (1.0) | 5 (0.5) | 5 (5.3) | 0.0000055 |
| ESBL-producing Enterobacteriaceae | 42 (4.0) | 37 (3.9) | 5 (5.3) | > 0.20 |
| Others | 148 (14.2) | 122 (12.8) | 26 (27.7) | 0.00099 |
| Other bacteria | 17 (1.6) | 11 (1.2) | 6 (6.4) | 0.00014 |
| Fungi | 484 (46,5) | 421 (40,4) | 63 (6,1) | 0.00028 |
| Candia albicans | 406 (39.0) | 359 (37.9) | 47 (50) | 0.021 |
| Other Candida spp. | 197 (18.9) | 165 (17.4) | 32 (3.4) | 0.010 |
| C. glabrata | 128 (12.3) | 108 (11.4) | 20 (21.3) | |
| C. tropicalis | 39 (3.7) | 33 (3.5) | 6 (6.4) | |
| C. krusei | 17 (1.6) | 13 (1.4) | 4 (4.3) | |
| Others | 13 (1.3) | 11 (1.2) | 2 (2.1) | |
| Aspergillus spp. | 27 (2.6) | 8 (0.8) | 19 (20.2) | < 0.000001 |
| Pneumocystis jiroveci | 14 (1.3) | 4 (0.4) | 10 (10.6) | < 0.000001 |
| Other fungi | 10 (1.0) | 10 (1.1) | 0 | > 0.20 |
| Parasites | 1 (0.1) | 1 (0.1) | 0 | 0.00072 |
| Multiresistant-bacteria | 235 (22.6) | 180 (19.0) | 55 (58.5) | < 0.000001 |
| MRSA | 45 (4.3) | 31 (3.3) | 14 (14.9) | |
| VRE | 91 (8.7) | 68 (7.2) | 23 (24.5) | |
| ESBL-producing | 42 (4.0) | 37 (3.9) | 5 (5.3) | |
| Enterobacteriaceae | ||||
| Pseudomonas spp. | 16 (1.5) | 14 (1.5) | 2 (2.2) | |
| Stenotrophomonas spp. | 19 (1.8) | 12 (1.3) | 7 (7.4) | |
| Acinetobacter spp. | 3 (0.29) | 1 (0.1) | 2 (2.2) | |
| Others | 21 (2.01) | 19 (2.0) | 2 (2.2) | |
Appropriate therapy: Patients who received appropriate antimicrobial-therapy; Inappropriate therapy: Patients who received inappropriate antimicrobial-therapy; GIT: Gastrointestinal tract; CNS: Central nervous system; VSE: Vancomycin-sensitive Enterococcus; VRE: Vancomycin-resistant Enterococcus; ESBL: Extended-spectrum β-lactamase producing bacteria; MRSA: Methicilline-resistant Staphylococcus aureus.
Infection was defined according to the definitions of the International Sepsis-Forum[7] and adjudicated by the senior-physicians. Data concerning infection were extracted from the ICU-doctor´s final-report, from imaging (Computed-Tomogaphy, Magnetic-Resonance-Imaging, X-ray, ultrasound, endoscopy) and from the microbiological laboratory-reports.
Appropriate/inappropriate antimicrobial-therapy was determined for all patients with infection. Appropriate initial antimicrobial-therapy was defined as: (1) Initiation of appropriate empirical antimicrobial-therapy at symptom begin (recurrence or persistent hypotension) with in vitro activity appropriate to isolated pathogenic organisms according to the Sanford Guide on Antimicrobial Therapy 2010 (40th edition)[8] and the national guidelines 2010 of the Paul-Ehrlich-society for chemotherapy[9] or according to the antibiotic susceptibility-testing; and (2) Appropriate empiric therapy for culture negative infections according to the underlying clinical syndrome, risk-factors (hospital-stay, previous antibiotics, immunosuppressive patient) in accordance to the Sanford Guide on Antimicrobial Therapy 2010 (40th edition)[8] and national guidelines 2010 of the Paul-Ehrlich-society for chemotherapy and the local hospital resistance-statistics[9] and initiation of appropriate antimicrobial-therapy at symptom begin.
In addition appropriate adjustment of antimicrobial-therapy within the first 7 d of ICU-stay was recorded.
Statistical analysis
The primary statistical-endpoint was the evaluation of the influence of appropriate versus inappropriate antimicrobial-therapy on in-hospital-mortality. Multivariate-logistic regression-analysis was performed. The statistical analysis was performed using SPSS-version-20 (SPSS-Inc, Chicago, IL, United States). Data were expressed as mean ± SD for normally distributed variables and median and range for others. All statistical tests were 2-sided and P < 0.05 was considered statistically significant. No correction for multiple-testing was done. Wilcoxon-Mann-Whitney-test was used to compare numeric variables between the main-groups of patients. Ordinal-variables were analyzed using Chi-square-test and via binary-logistic regression-analysis. A multivariate-logistic regression-analysis was performed by forward binary-logistic-regression including all relevant nominal-variables, in which the significant variables were selected.
RESULTS
Characteristics of infection
Between April 1, 2007 and December 31, 2009, 2148 patients were admitted to the Internal-Medicine-ICU of the Goethe-University-Hospital-Frankfurt. From 1979 patients enough data were available to include them into the analysis. Of the 1979 patients, 1042 (53%) were classified as having an infection. Infected patients had more comorbid conditions, higher SAPS-II and TISS-scores, received more often mechanical-ventilation and hemodialysis, and had a higher in-hospital-mortality-rate than patients without infection. Details are shown in Table 1.
The most frequent site of infection was the respiratory-tract, followed by gastrointestinal-infections, genitourinary-infections, endocarditis and skin-infections (Table 2).
In 697 patients (67%) from overall 1042 patients with infection underlying microorganisms were identified. Gram-positive isolates (S. aureos, S. epidermidis, S. pneumoniae, VSE, VRE, and others) were found in 45% (473/1042) of patients with infections and gram-negative isolates (E. coli, Enterobacter spp., Klebsiella spp., Pseudomonas spp., Acinetobacter spp., ESBL-producing Enterobacteriaceae, and others) in 35% (368/1042). Details are shown in Table 2. Multiresistant-bacteria were present in 23% of patients with infection; these were most frequently vancomycin-resistant Enterococcus faecium (VRE) (8.7%), followed by methicilline-resistant Staphylococcus aureus (MRSA) (4.3%) and extended-spectrum-betalactamase (ESBL)-producing enterobacteriaceae (4.0%).
Overall, the rate of survival to hospital-discharge was 66% in patients with infection as compared to 83% of patients without infection (P < 0.000001).
Appropriateness of initial antimicrobial-therapy
From all patients with infection, 91% of patients received appropriate and 9% inappropriate initial antimicrobial-therapy. From the 697 patients with infection and positive blood-culture, 87% received appropriate initial antimicrobial-therapy. The survival-rate to hospital discharge was higher in the group of patients receiving appropriate antimicrobial-therapy (67% vs 57%). However, this difference was not statistically significant (P = 0.054). The rate of inappropriate antimicrobial-therapy in septic-shock was 13.5%. In the group of patients with septic-shock (20% of patients with infection) no significant difference in survival-rate to hospital discharge was found between patients receiving appropriate (n = 179) versus inappropriate (n = 28) initial antimicrobial-therapy (44% vs 36%, P > 0.20). Details are shown in Figure 1 and Table 3.
Figure 1.
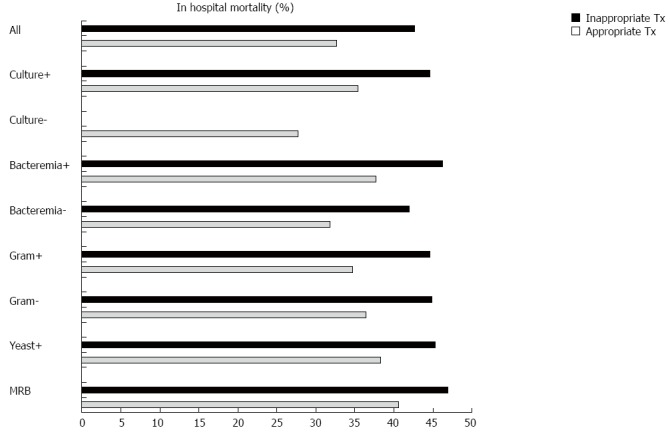
Impact of appropriate and inappropriate antimicrobial-therapy on in hospital-mortality. Culture+: Culture-positive infection; Culture-: Culture-negative infection; Bacteremia+: Patients with positive blood cultures; Bacteremia-: Patients with negative blood cultures; Gram+: Culture-positive for gram-positive bacteria; Gram-: Culture-positive for gram-negative bacteria; Yeast+: Culture-positive for yeast; MRB: Multiresistant-bacteria.
Table 3.
Characteristics of patients with infection according to appropriate or inappropriate antimicrobial-therapy n (%)
| Characteristic | All patients with infection (n = 1042) | Appropriate therapy (n = 948) | Inappropriate therapy (n = 94) | OR (95%CI) | P value |
| Age (yr), mean ± SD (range) | 61 ± 16 (16-95) | 61 ± 16 (16-95) | 61 ± 14 (21-88) | 1.003 (0.99-1.017) | > 0.20 |
| Male sex | 622 (60.0) | 376 (39.7) | 0.747 (0.488-1.143) | 0.18 | |
| Days on ICU, mean ± SD (range) | 8.12 ± 12 (1-217) | 7.68 ± 11.958 (1-217) | 10.02 ± 11.134 (1-59) | 1.011 (0.998-0.024) | 0.092 |
| ICU-mortality | 324 (31.3) | 287 (30.3) | 37 (39.4) | 0.669 (0.432-1.035) | 0.071 |
| Hospital-mortality | 350 (33.6) | 310 (32.7) | 40 (42.6) | 0.656 (0.426-1.009) | 0.055 |
| Severity score on admission day | |||||
| SAPS II, mean ± SD (range) | 38.67 ± 15.64 (5-93) | 38.54 ± 15.82 (5-93) | 39.95 ± 13.78 (8-68) | 1.006 (0.992-1.020) | > 0.20 |
| TISS, mean ± SD (range) | 11.57 ± 9.11 (0-55) | 11.74 ± 9.25 (0-55) | 9.86 ± 7.42 (0-28) | 0.976 (0.951-1.002) | 0.071 |
| Highest severity score within 7 d after admission | |||||
| SAPS II, mean ± SD (range) | 43.88 ± 16.57 (6-93) | 43.68 ± 16.71 (6-93) | 45.87 ± 15.09 (8-81) | 1.008 (0.994-1.022) | > 0.20 |
| TISS. mean ± SD (range) | 14.65 ± 9.14 (0-55) | 14.68 ± 9.27 (0-55) | 14.32 ± 7.73 (0-38) | 0.996 (0.971-1.021) | > 0.20 |
| Type of Admission | 0.0022 | ||||
| Internal Medicine ICU | 550 (52.8) | 486 (51.3) | 64 (68.1) | ||
| Cardiology ICU | 491 (47.1) | 461 (48.7) | 30 (31.9) | ||
| Source of admission | > 0.20 | ||||
| Emergency department | 455 (43.7) | 418 (44.1) | 37 (39.4) | Reference | |
| Other hospital | 187 (17.9) | 170 (17.9) | 17 (18.1) | 0.425 (0.153-1.179) | 0.10 |
| Hospital ward | 337 (32.3) | 305 (32.2) | 32 (34.0) | 0.48 (0.162-1.429) | 0.19 |
| Operating room | 34 (3.3) | 31 (3.3) | 3 (3.2) | 0.504 (0.18-1.411) | 0.19 |
| Surgical IMC/ICU | 29 (2.7) | 24 (2.6) | 5 (5.3) | 0.465 (0.101-2.14) | > 0.20 |
| Comorbid conditions | 806 (77.4) | 729 (76.9) | 77 (81.9) | 0.842 (0.498-1.424) | > 0.20 |
| COPD | 149 (14.3) | 137 (14.5) | 12 (12.8) | 1.154 (0.613-2.172) | > 0.20 |
| Heart failure (EF < 30%) | 55 (5.3) | 50 (5.3) | 5 (5.3) | 0.991 (0.385-2.549) | > 0.20 |
| Coronary artery disease | 211 (20.2) | 195 (20.6) | 16 (17.0) | 1.262 (0.721-2.211) | > 0.20 |
| Chronic renal failure | 202 (19.4) | 182 (19.2) | 20 (21.3) | 0.879 (0.523-1.478) | > 0.20 |
| Diabetes mellitus | 247 (23.7) | 220 (23.2) | 27 (28.7) | 0.750 (0.468-1.202) | > 0.20 |
| Liver-cirrhosis | 118 (11.3) | 100 (10.5) | 18 (19.1) | 0.498 (0.286-0.866) | 0.014 |
| Cancer | 140 (13.4) | 122 (12.9) | 18 (19.1) | 0.624 (0.361-1.079) | 0.091 |
| Hematologic neoplasia | 72 (6.9) | 68 (7.2) | 4 (4.3) | 1.739 (0.620-4.877) | > 0.20 |
| HIV | 61 (5.9) | 51 (5.4) | 10 (10.6) | 0.478 (0.234-0.975) | 0.042 |
| Immunosuppressive Tx. | 77 (7.4) | 69 (7.3) | 8 (8.5) | 0.844 (0.393-1.813) | > 0.20 |
| Chemotherapy | 53 (5.1) | 47 (5.0) | 6 (6.4) | 2.634 (1.691-4.102) | 0.000018 |
| Number of cormobid conditions | > 0.20 | ||||
| 0 | 236 | 219 (23.1) | 17 (18.1) | ||
| 1 | 336 | 304 (32.1) | 32 (34.0) | ||
| 2 | 241 | 219 (23.1) | 22 (23.4) | ||
| 3 | 151 | 138 (14.6) | 13 (13.8) | ||
| > 3 | 78 | 68 (7.2) | 10 (10.6) | ||
| Mechanical ventilation | 677 (65.0) | 621 (65.5) | 56 (59.6) | 1.289 (0.836-1.987) | > 0.20 |
| Hemodialysis | 213 (20.4) | 194 (20.5) | 19 (20.2) | 1.016 (0.599-1.721) | > 0.20 |
Appropriate therapy: Patients who received appropriate antimicrobial-therapy; Inappropriate therapy: Patients who received inappropriate antimicrobial-therapy; SAPS II: Simplified Acute Physiology Score II; TISS: Therapeutic Intervention Scoring System; ICU: Intensive care unit; IMC: Internal Medicine ICU includes patients from Gastroenterology, Hepatology, Pulmonology, Endocrinology, Oncology, Hematology, Infectiology, Rheumatology departments, while Cardiology-ICU include patients from Cardiology, Angiology, Nephrology department; EF: Ejection fraction.
Patients who received inappropriate initial antimicrobial-therapy had a longer ICU stay and suffered from higher rates of liver-cirrhosis, HIV, and prior chemotherapy. In addition, patients with multiresistant-bacterial infection received significantly more often inappropriate initial antimicrobial-therapy as compared to patients with other bacterial-infection [58% vs 19%, P < 0.000001; OR = 6.25 (2.59-15.15), P = 0.000046]. Details are shown in Table 3.
Escalation of antimicrobial-therapy was documented within the first 7 d of ICU stay in 20% of patients with infection, and de-escalation in 31%, respectively. From the 94 patients with infection who received inappropriate initial antimicrobial-therapy, antimicrobial-therapy was adjusted appropriately within the first seven days in 38 (40%) of patients according to the AST.
Mortality
In hospital-mortality-rate was 25% in the overall ICU population and 34% in patients with infection. Details are shown in Table 4.
Table 4.
Risk factors for in hospital mortality n (%)
| Characteristic | Patients who died in hospital | Patients surviving | Odds ratio | P value |
| Age (yr) | 63 | 60 | 0.0011 | |
| SAPS II score (mean) | 47 | 37 | < 0.000001 | |
| TISS score (mean) | 12.8 | 8.5 | < 0.000001 | |
| Number of comorbid conditions | 1.851 [1.446-2.369] | < 0.000001 | ||
| Chemotherapy for malignoma within 3 mo prior to admission | 2.385 [1.605-3.543] | 0.000017 | ||
| Clinical infection on admission | 2.883 [2.312-3.595] | < 0.000001 | ||
| Septic-shock on admission | 4.993 [3.707-6.727] | < 0.000001 | ||
| Bacteriemia | 2.119 [1.515-2.962] | < 0.000001 | ||
| Multiresistant-bacterial infections | 2.189 [1.664-2.879] | < 0.000001 | ||
| mechanical ventilation during ICU stay | 5.018 [3.981-6.324] | < 0.000001 | ||
| C-reactive-protein at admission (mg/dL) | 8.76 | 6.23 | < 0.000001 | |
| Creatinine-values at admission (mg/dL) | 1.93 | 1.644 | < 0.000001 | |
| Bilirubin-values at admission (mg/dL) | 3.175 | 1.818 | 0.0014 | |
| INR | 1.81 | 1.46 | < 0.000001 |
SAPS II: Simplified Acute Physiology Score II; TISS: Therapeutic Intervention Scoring System; ICU: Intensive care unit.
Multivariate-logistic regression-analysis including all significant variables revealed as independent risk-factors for in hospital-mortality the presence of septic-shock at admission [OR = 2.928 (1.246-6.787), P = 0.0057], chemotherapy for malignoma received within 3 mo prior to ICU admission [OR = 4.274 (1.37-13.33), P = 0.012] and infection with Pseudomonas spp.[OR = 4.187 (1.665-12.579), P = 0.0030].
Risk factors for in-hospital mortality in patients with infection are shown in Table 5.
Table 5.
Risk factors for in hospital mortality of patients with infection
| Characteristic | Patients who died in hospital | Patients surviving | Odds ratio | P value |
| SAPS II score (mean) | 42 | 37 | 0.00021 | |
| TISS score (mean) | 12.7 | 11.03 | 0.0084 | |
| Number of comorbid conditions | 1.824 [1.311-2.539] | 0.00036 | ||
| mechanical ventilation during ICU stay | 2.363 [1.765-3.164] | < 0.000001 | ||
| pulmonary-infections | 1.741 [1.301-2.33] | 0.00020 | ||
| catheter-related infection | 2.578 [1.51-4.399] | 0.00052 | ||
| bilirubin-values at admission (mg/dL) | 3.39 | 2.458 | 0.0300 | |
| INR | 1.83 | 1.55 | 0.0000082 |
ICU: Intensive care unit.
Multivariate-logistic regression-analysis for patients with infection only revealed as independent risk-factors for in hospital-mortality the presence of septic-shock at admission [OR = 3.576 (2.347-5.448), P < 0.000001], SAPS-II-score at admission [OR = 1.014 (1.002-1.027), P = 0.017], presence of comorbid-disease [OR = 2.228 (1.443-3.627), P = 0.00043], mechanical-ventilation [OR = 1.712 (0.993-2.959), P = 0.034], pulmonary focus of infection [OR = 1.885 (1.200-2.963), P = 0.0061], liver-cirrhosis [OR = 1.816 (1.061-3.106), P = 0.029] and INR [OR = 1.248 (1.031-1.508), P = 0.022].
Liver disease
Chronic liver-disease was known in 272 patients (14%) with liver-cirrhosis present in 175 of these patients (64%). From these 272 patients with chronic liver disease, chronic viral hepatitis infection was present in 150 patients (55%), of whom 101 were infected with chronic hepatitis C and 49 with chronic hepatitis B. Only one patient had chronic alcoholic and one patient autoimmune liver disease. Patients with spontaneous bacterial infection as primary infection site were not included in the analysis.
Patients with chronic liver-disease had a higher infection-rate (69% vs 50%, P < 0.000001) and presented more often with septic-shock (15% vs 10%, P = 0.014) as compared to patients without chronic liver-disease. From all patients with chronic liver-disease, only patients infected with chronic hepatitis C had significantly higher risk of in hospital-mortality [OR = 1.660 (1.087-2.534), P = 0.019].
The presence of liver-cirrhosis significantly increased the risk of infection (OR = 1.974 (1.420-2.744), P = 0.000052], the risk of receiving inappropriate initial antimicrobial-therapy [OR = 2.008 (1.155-3.497), P = 0.014] and in hospital-mortality-rate [OR = 2.320 (1.684-3.195), P < 0.000001]. Infection-rate and sepsis-rate was significantly higher in patients with liver-cirrhosis as compared to patients without liver-cirrhosis (67% vs 51%, P = 0.000040 and 15% vs 10%, P = 0.046). In patients with liver-cirrhosis the presence of infection significantly increased in hospital-mortality (49% vs 25%, P = 0.0019). Liver-cirrhosis was an independent risk-factor for in hospital-mortality in patients with infection [OR = 1.816 (1.061-3.106), P = 0.029] (Figure 2).
Figure 2.
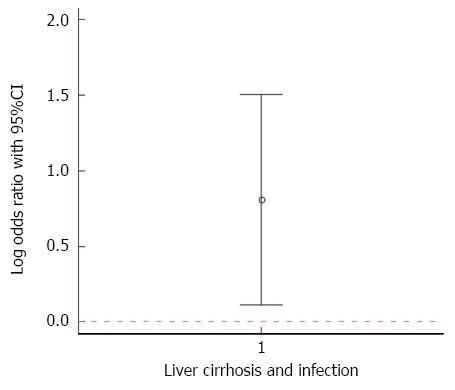
49% of patients with liver-cirrhosis and infection died in hospital, compared to 25% with liver-cirrhosis and without infection. The plot shows the log odds ratio (0.8063, marked by the dot) and its 95%CI [(0.1086-1.5039), marked by the whiskers], i.e. patients with liver-cirrhosis and infection had a 2.24 greater in-hospital-mortality than patients with liver-cirrhosis without infection.
MELD-score was significantly higher in patients with liver-cirrhosis who died in hospital as compared to survivors (MELD-score 28 vs 23, P = 0.012) and significantly more patients with liver-cirrhosis received hemodialysis (15% vs 8%, P = 0.043). In patients with liver-cirrhosis infections with multiresistant-bacteria were found in 32% (38/118) of patients. From these 38 patients, 18% were infected with ESBL-producing bacteria enterobacteriaceae, 18% with MRSA and 55% with VRE.
No significant difference was found for diagnostic-accuracy of MELD-score (AUROC 73%, 95%CI: 65%-82%) and SAPS-II-score (AUROC 72%, 95%CI: 63%-81%) at admission in identifying risk of in-hospital-mortality (P > 0.20).
DISCUSSION
In the present study the infection-rate of patients on the Internal Intensive Care Unit was 53%. Patients with infection had more often comorbid conditions and higher SAPS II scores on admission and during the first seven days of ICU treatment. The results of the present study are in accordance with a 1-d point international multicenter prevalence study by Vincent et al[5] reporting infections in 51% of ICU patients and similar associations. While the present study included Internal Medicine ICU only, the study by Vincent et al. included also surgery and trauma patients. The most common infection focus in the present study was the lung, followed by abdomen, and genitourinary tract infection. This again is in accordance with epidemiological studies concerning infections on ICU[10-12]. Positive microbiological cultures were obtained in 65% of infected patients with more gram-positive than gram-negative microorganisms. This is in accordance with previous studies that reported an increasing incidence of gram-positive microorganisms on ICUs[2]. In hospital-mortality was significantly higher in patients with infections and patients with sepsis as compared to patients without. This again supports results of previous studies[2,5].
Studies have reported higher hospital-mortality in patients with blood stream infections that receive inappropriate initial antimicrobial-therapy[13-15]. In 2006 Kumar et al[16] reported, that effective antimicrobial administration within the first hour of documented hypotension was associated with increased survival to hospital-discharge in patients with septic-shock. However, only 50% of septic-shock patients received effective antimicrobial-therapy within 6 h of documented hypotension[16]. In 2004, the Surviving-Sepsis-Campaign developed guidelines for the management of severe sepsis and septic-shock[17]. This included besides others, early cultures for microbial evaluation and “early-goal-directed therapy” with initiation of antimicrobial-therapy as soon as possible[17,18]. After implementation of the “sepsis-bundle” the Surviving-Sepsis-Campaign study group published a study performed at 165 sites with 15022 subjects and reported an increase in compliance with the sepsis bundle from 11 to 31% two years after implementation and a reduction in mortality[19]. Other studies have reported a similar association[20]. The present study also started after implementation of the sepsis-bundle with early-goal-directed antimicrobial-therapy with empiric calculated therapy on the ICU-ward. Only 9% of patients with infection and 13.5% of patients with septic-shock received inappropriate antimicrobial-therapy. This was lower than reported in a study of Kumar et al[16] published in 2009 including 5715 patients with septic-shock with 20% of inappropriate therapy of patients with septic-shock and significant reduction of survival in those receiving inappropriate therapy (10% vs 52%)[16]. Although these two studies cannot be directly compared, it shows a trend towards improvement of initial antimicrobial-therapy. While in the study of Kumar[16] a significant reduction of survival (42%) was found in patients receiving inappropriate antimicrobial-therapy as compared to patients receiving appropriate antimicrobial-therapy, no significant reduction was found in the present study (10%). Antimicrobial-therapy is only one aspect of the sepsis bundle, therefore other factors might have also played a role in the present study.
Multiresistant-microorganisms are an increasing burden on ICUs worldwide[21]. Also in the present study multiresistant-bacteria were detected in 23% of patients with infection. This is higher than the overall prevalence in German hospitals[22,23]. A reason might be the high proportion of patients colonized with such bacteria which arrive from countries with high prevalence of multiresistant-bacteria to be treated at Frankfurt-University-hospital due to its close location to Frankfurt international-airport. Therefore, successful infection control measurements are strictly implemented inhibiting transmission of such pathogens from patient to patient. In addition, Frankfurt-University-Hospital is a referral-hospital and therefore many severely ill patients, who received multiple antibiotic-regimens prior to referral, receive further treatment here.
Nevertheless, inappropriate therapy was significantly associated with the presence of multiresistant-bacteria in the present study, suggesting that empirical therapy has to cover multiresistant-bacteria more regularly. Studies have shown that inappropriate antimicrobial-therapy for gram negative ESBL producers was associated with significant increase in mortality (59.5% vs 18.5%, P < 0.001)[24]. Common risk factors for infection with multiresistant-bacteria are: residence in nursery home, recent hospital stay, mechanical ventilation, age, prior antibiotic therapy and foreign citizenship[9,25]. Also in the present study prior antimicrobial-therapy was an independent risk-factor for infection.
Since the University-Hospital is a liver transplantation center, a high number of patients with liver-disease are treated here. Chronic liver-disease was present in 14% of patients admitted to the ICU with liver-cirrhosis in 64% of these patients. Patients with liver-cirrhosis had a significantly higher infection-rate than patients without liver-cirrhosis. This is in accordance with previous studies reporting infection-rates of 32%-34% in liver-cirrhosis as compared to 5%-7% in the general hospitalized population[26,27]. A meta-analysis reported a 4-fold increase in mortality in patients with liver-cirrhosis that acquire an infection[6]. In addition, patients with sepsis and liver-cirrhosis have a significant higher mortality-rate as compared to patients with sepsis in the general hospitalized population[27], this was supported by the results of the present study. The presence of liver-cirrhosis was an independent risk factor for in hospital-mortality in patients with infection. In addition, also in patients with liver-cirrhosis infection with multiresistant-bacteria is increasing. A recent study resported an incidence of 39% multiresistant-bacteria in patients with liver-cirrhosis and nosocomial infection[28]. In the present study an infection with multiresistant-bacteria could be found in 32% of patients with liver-cirrhosis. Inappropriate antimicrobial-therapy was significantly more often in patients with liver-cirrhosis. The high rate of multiresistant-bacteria could be an explanation herefore. A recent study revealed a significantly higher rate of septic-shock and in-hospital-mortality in patients with liver-cirrhosis infected with multiresistant-bacteria (26% vs 10% and 25% vs 12%)[29].
MELD-score and SAPS-II-score at admission performed equally good concerning the prediction of in-hospital-mortality with diagnostic accuracies of 73% and 72%. A previous systematic review including 21 studies reported diagnostic accuracies of 81% for MELD-score and 83% for SOFA (sequential-organ-failure-assessment)-score[29]. Therefore both studies report comparable results for general ICU prognostic-markers and liver-specific prognostic-markers for patients with liver-cirrhosis on ICU and therefore might supplement each other.
A limitation of the present study is its monocentric retrospective study-design. Nevertheless, the distribution of infections was comparable to previous multicenter-studies[5].
The inclusion of patients with culture-negative infection might be criticized and lead to bias. However, no difference in percentage of appropriate to inappropriate antimicrobial-therapy was found in a subanalysis of patients with culture-positive infections only.
In conclusion, the results of the present study report the successful implementation of early-goal-directed therapy. Increasing burden are multiresistant-bacteria, which are associated with increased mortality.
COMMENTS
Background
Infections are a worldwide problem concerning patients admitted to intensive care units, since they are associated with an increased mortality, morbidity and financial burden. A 51%-prevalence of infection has been reported for intensive care unit (ICU)-patients and previously 20% of patients admitted with septic-shock to an ICU received inappropriate antimicrobial-therapy.
Research frontiers
Infection rate and management on ICU with special focus on liver cirrhosis patients.
Innovations and breakthroughs
In the present study the infection-rate of patients on the Internal Intensive Care Unit was 53%. Infection increased mortality 2.24-fold in patients with cirrhosis.
Applications
Retrospective analysis reporting the high prevalence of infections and the importance of early goal-directed therapy.
Peer-review
The paper presents the influence of antibiotic-regimens on ICU-mortality. It underlines the successful implementation of early-goal-directed therapy in patients treated in ICU. The topic is interesting, especially considering the rate of mortality in these patients and financial burden.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of the University Clinic Frankfurt.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained retrospectively.
Conflict-of-interest statement: We have no financial relationships to disclose.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 16, 2016
First decision: January 28, 2016
Article in press: March 14, 2016
P- Reviewer: Ampuero J, Cichoz-Lach H, He JY, Kayashima H S- Editor: Gong ZM L- Editor: A E- Editor: Zhang DN
References
- 1.Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Crit Care. 2006;10:R42. doi: 10.1186/cc4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, Dodek P, Wood G, Kumar A, Simon D, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136:1237–1248. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 5.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 6.Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256, 1256e1-e5. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Calandra T, Cohen J. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33:1538–1548. doi: 10.1097/01.ccm.0000168253.91200.83. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert DN, Moellering RC, Eliopoulos GM. Clinical approach to intitial choice of antimicrobial therapy. The Sanford Guide To Antimicrobial Therapy 2010, Antimicrobial Therapy Inc., 2010. Available from: http://www.sanfordguide.com/
- 9.Bodmann KF, Grabein B, Expertenkommission der Paul-Ehrlich-Gesellschaft fur Chemotherapie e.V. [Empfehlungen zur kalkulierten parenteralen Initialtherapie bakterieller Erkrankungen bei Erwachsenen-Update 2010. Available from: http://www.p-e-g.org/aktuelles/435/
- 10.Martin CM, Priestap F, Fisher H, Fowler RA, Heyland DK, Keenan SP, Longo CJ, Morrison T, Bentley D, Antman N. A prospective, observational registry of patients with severe sepsis: the Canadian Sepsis Treatment and Response Registry. Crit Care Med. 2009;37:81–88. doi: 10.1097/CCM.0b013e31819285f0. [DOI] [PubMed] [Google Scholar]
- 11.Brun-Buisson C, Meshaka P, Pinton P, Vallet B. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med. 2004;30:580–588. doi: 10.1007/s00134-003-2121-4. [DOI] [PubMed] [Google Scholar]
- 12.Engel C, Brunkhorst FM, Bone HG, Brunkhorst R, Gerlach H, Grond S, Gruendling M, Huhle G, Jaschinski U, John S, et al. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med. 2007;33:606–618. doi: 10.1007/s00134-006-0517-7. [DOI] [PubMed] [Google Scholar]
- 13.Prowle JR, Echeverri JE, Ligabo EV, Sherry N, Taori GC, Crozier TM, Hart GK, Korman TM, Mayall BC, Johnson PD, et al. Acquired bloodstream infection in the intensive care unit: incidence and attributable mortality. Crit Care. 2011;15:R100. doi: 10.1186/cc10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462–474. doi: 10.1378/chest.115.2.462. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 17.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536–555. doi: 10.1007/s00134-004-2210-z. [DOI] [PubMed] [Google Scholar]
- 18.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 19.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36:222–231. doi: 10.1007/s00134-009-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiramizo SC, Marra AR, Durão MS, Paes ÂT, Edmond MB, Pavão dos Santos OF. Decreasing mortality in severe sepsis and septic shock patients by implementing a sepsis bundle in a hospital setting. PLoS One. 2011;6:e26790. doi: 10.1371/journal.pone.0026790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgmann H, Hiesmayr JM, Savey A, Bauer P, Metnitz B, Metnitz PG. Impact of nosocomial infections on clinical outcome and resource consumption in critically ill patients. Intensive Care Med. 2010;36:1597–1601. doi: 10.1007/s00134-010-1941-2. [DOI] [PubMed] [Google Scholar]
- 22.Kresken M, Hafner D, Schmitz FJ, Wichelhaus TA. PEG-Resistenzstudie 2004. Paul-Ehrlich-Gesellschaft für Chemotherapie e.V. Available from: http://www.p-e-g.org.
- 23.Geffers C, Gastmeier P. Nosocomial infections and multidrug-resistant organisms in Germany: epidemiological data from KISS (the Hospital Infection Surveillance System) Dtsch Arztebl Int. 2011;108:87–93. doi: 10.3238/arztebl.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumbarello M, Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, Fiori B, Citton R, D’Inzeo T, Fadda G, Cauda R, et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother. 2007;51:1987–1994. doi: 10.1128/AAC.01509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaye KS, Fraimow HS, Abrutyn E. Pathogens resistant to antimicrobial agents. Epidemiology, molecular mechanisms, and clinical management. Infect Dis Clin North Am. 2000;14:293–319. doi: 10.1016/s0891-5520(05)70249-x. [DOI] [PubMed] [Google Scholar]
- 26.Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology. 2009;50:2022–2033. doi: 10.1002/hep.23264. [DOI] [PubMed] [Google Scholar]
- 27.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 28.Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551–1561. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 29.Cholongitas E, Senzolo M, Patch D, Kwong K, Nikolopoulou V, Leandro G, Shaw S, Burroughs AK. Risk factors, sequential organ failure assessment and model for end-stage liver disease scores for predicting short term mortality in cirrhotic patients admitted to intensive care unit. Aliment Pharmacol Ther. 2006;23:883–893. doi: 10.1111/j.1365-2036.2006.02842.x. [DOI] [PubMed] [Google Scholar]


