Abstract
AIM: To evaluate transient elastography (TE) as a noninvasive tool in staging liver fibrosis compared with liver biopsy and morphometry in children with different chronic liver diseases.
METHODS: A total of 90 children [50 with chronic hepatitis C virus (HCV), 20 with autoimmune hepatitis (AIH) and 20 with Wilson disease] were included in the study and underwent liver stiffness measurement (LSM) using TE. Liver biopsies were evaluated for fibrosis, qualitatively, by Ishak score and quantitatively by fibrosis area fraction (FAF) using digital image analysis (morphometry). LSM was correlated with fibrosis and other studied variables using spearman correlation. A stepwise multiple regression analysis was also performed to examine independent factors associated with LSM. Different cut-off values of LSM were calculated for predicting individual fibrosis stages using receiver-operating characteristic curve. Cut-off values with optimal clinical performance (optimal sensitivity and specificity simultaneously) were selected.
RESULTS: The majority of HCV group had minimal activity (80%) and no/mild fibrosis (72%). On the other hand, the majority of AIH group had mild to moderate activity (70%) and moderate to severe fibrosis (95%) and all Wilson disease group had mild to moderate activity (100%) and moderate to severe fibrosis (100%). LSM correlated significantly with both FAF and Ishak scores and the correlation appeared better with the latter (r = 0.839 vs 0.879, P < 0.0001 for both). LSM discriminated individual stages of fibrosis with high performance. Sensitivity ranged from 81.4% to 100% and specificity ranged from 75.0% to 97.2%. When we compared LSM values for the same stage of fibrosis, they varied according to the different etiologies. Higher values were in AIH (16.15 ± 7.23 kPa) compared to Wilson disease (8.30 ± 0.84 kPa) and HCV groups (7.43 ± 1.73 kPa). Multiple regression analysis revealed that Ishak fibrosis stage was the only independent variable associated with higher LSM (P < 0.0001).
CONCLUSION: TE appears reliable in distinguishing different stages of liver fibrosis in children. However, its values vary according to the disease type. For that, a disease-specific estimation of cut-off values for fibrosis staging is worthy.
Keywords: Autoimmune hepatitis, Chronic hepatitis C, Liver fibrosis, Liver stiffness, Morphometry, Pediatrics, Transient elastography, Wilson disease
Core tip: Noninvasive prediction of liver fibrosis is a challenging issue especially in pediatric population. Liver stiffness, as assessed by transient elastography, was reported to be associated with liver fibrosis and therefore proposed as a candidate for fibrosis prediction. The accuracy of transient elastography has been shown to be excellent in a large number of adult studies, most of which are concerned with viral hepatitis. A few studies have also been performed in children. In addition to viral etiology, the current study is concerned with noninvasive assessment of fibrosis in other etiologies of pediatric chronic liver diseases. We compared the liver stiffness measurements with both the Ishak fibrosis score and the quantitative assessment of fibrosis using morphometry.
INTRODUCTION
Severity of liver fibrosis and its progression are the most important factors for the treatment policy and its outcome in children with chronic liver disease. Those with more advanced stages of fibrosis are more likely to develop cirrhosis[1]. Evidence of significant fibrosis is an indication to commence treatment in certain conditions, such as chronic hepatitis C[2]. Evaluation of treatment efficacy and fibrosis regression in autoimmune hepatitis (AIH)[3] and Wilson disease[4] is also important. In those with cirrhosis, surveillance for gastroesophageal varices is of utmost importance[5].
Liver biopsy has been considered the gold standard for evaluating fibrosis. However, it is limited by the need for hospital admission and sedation in children. The accuracy of liver histology assessment has been challenged because of sampling error (1/50000 of the liver is sampled with needle biopsy) and up to 30% of cirrhotic cases might be missed, especially when the liver specimens are small or fragmented[6], in addition to serious risks including bleeding[7,8].
Development of reliable and accurate noninvasive methods for determining fibrosis stage is important across all chronic liver diseases of childhood. Several noninvasive methods have achieved acceptance in adults during the past decade. Biochemical, hematological and serological markers were derived, including the aspartate transaminase-to-platelet ratio index (APRI)[9], the Forns index[10], the fibrotest[11], the hepascore[12], and the Egy-Score[13]. However, most of these fibrosis markers have been evaluated only in chronic hepatitis C virus (HCV) infected patients.
Serum markers often discriminate higher stages of fibrosis, but do not discriminate adequately between earlier stages of fibrosis[14-16]. Identifying early stages of liver fibrosis is essential to initiate therapy and to prevent disease progression. An efficient tool allowing the detection of a slight variation in liver fibrosis would be essential for antifibrotic trials[17].
During the past few years, liver stiffness measurement (LSM) by the use of transient elastography (TE) has become increasingly accepted as a noninvasive marker of liver disease[18]. The accuracy of TE has been shown to be excellent in a large number of adult studies[18-21]. A few studies have also been performed in children[22-26].
The narrow step changes between categorical Ishak or Metavir fibrosis stages are defined by qualitative criteria, which may not closely reflect quantitative fibrosis changes. Digital image analysis (morphometry) allows quantitative assessment of fibrosis area fraction (FAF) on picrosirius red stained liver tissue sections. It provides more objective data and detecting smaller changes between biopsies particularly in patients with early stages of fibrosis[27-29].
We aimed to study the value of transient elastography as a noninvasive tool in staging liver fibrosis compared with liver biopsy and morphometry in children with different chronic liver diseases.
MATERIALS AND METHODS
Study population
This prospective cross-sectional study included a total of 90 children with three different etiologies of chronic liver disease (50 with chronic HCV infection, 20 with AIH, and 20 with Wilson disease). Patients were recruited consecutively from the Pediatric Hepatology department, National Liver Institute, Menofiya University and Pediatric Hepatology and Gastroenterology Unit, Mansoura University Children Hospital, Egypt in the period between 2012 and 2014. Those with systemic diseases such as cardiac, pulmonary or renal diseases were excluded from the study. Patients with ascites or decompensated liver disease or those with body mass index more than 28 were also excluded. A signed informed consent was obtained from the parents of the patients before enrollment in the study. The study was approved by the Research Ethics Committee of National Liver Institute, Menofiya University, Egypt.
Etiological diagnosis and group allocation
After complete history taking, thorough clinical examination and routine investigations, diagnosis of chronic hepatitis C was based on the presence of serum anti-HCV antibody (Ab) and persistently positive HCV-RNA for six months or more[30,31], negative hepatitis B viral markers and absence of any associated liver disease; supported by the histopathological feature of HCV infection in liver biopsy. AIH was defined according to the simplified AIH score[32]. Wilson disease was diagnosed by clinical and laboratory evidence of chronic hepatitis, the presence of low plasma ceruloplasmin level in some patients, significant urinary copper excretion with penicillamine challenge test, positive Kayser-Flischer ring by slit lamp examination, positive staining for copper in liver biopsy with absent evidence for any other associated liver disease. Severity of liver disease was assessed by the pediatric end-stage liver disease (PELD) score or the model for end-stage liver disease (MELD) score according to the age of the patient[33].
Serum autoantibodies, protein electrophoresis and urinary copper excretion
All patients were tested for serum autoantibodies and gammaglobulins at presentation. Antinuclear antibody (ANA), anti-smooth muscle antibody (ASMA), liver kidney microsomal antibody- 1 (LKM1), and antimitochondrial antibody (AMA) were tested by indirect immunofluorescence technique using a Fluoro-KitTM Combo Pak (All from DiaSorin, Stillwater, Minnesota, United States). Protein electrophoresis was performed using Titan III Cellulose Acetate Plate and scanned using Helena QuickScan 2000 (both from Helena laboratories, Beaumont, Texas, United States). Twenty-four hours urinary copper was determined by spectrophotometry (Biosystems, BTS-310)[4].
Serum viral markers and ultrasonography
Hepatitis B surface antigen (HBsAg), anti-hepatitis B core immunoglobulin (Ig)M and IgG types were tested by enzyme linked immunosorbent assay (ELISA) kit (All from Sorin Biomedica Co, Spain). Anti-HCV Ab was tested by 4th generation ELISA (Innogenetics, Ghent-Belgium). Real-time polymerase chain reaction for HCV-RNA was performed using COBAS® Ampliprep/COBAS® TaqMan®, Roche Molecular Systems, Inc., Branchburg, NJ, 08876 United States. Ultrasonography was performed by using 2-5-MHz curved linear and 4-8-MHz linear transducers (Xario XG; Toshiba, Tokyo, Japan).
Transient elastography
TE was performed by a single operator who was unaware of the fibrosis stage or blood biomarkers results. TE was performed using the standard M probe using the right lobe of the liver through the intercostal space. Liver stiffness was measured through a device called FibroScan which is composed of an ultrasound transducer probe mounted on the axis of a vibrator. Vibrations of mild amplitude and low frequency are transmitted by the transducer, inducing an elastic shear wave that propagates through the underlying tissues. Pulse echo ultrasound acquisition is used to follow the propagation of the shear wave and to measure its velocity in kilopascals (kPa), which is directly related to tissue stiffness: the stiffer the tissue, the faster the shear wave propagates[34]. Ten successful acquisitions were performed on each patient. The success rate was calculated as the ratio of the number of successful acquisitions over the total number of acquisitions. The median value was kept as representative of the liver elastic modulus. The LSM was considered reliable only if 10 successful acquisitions were obtained, with interquartile range ≤ 30% of liver stiffness and success rate > 60%. A 10-mm diameter core of tissue was measured in depth between 25 mm and 65 mm. The liver stiffness was expressed in kPa. A higher kPa reflects a stiffer liver and more severe liver fibrosis.
Liver biopsy and histopathological evaluation
Liver biopsy was performed by percutaneous ultrasonography-guided Tru-Cut needle 14 G using local anesthesia and sedation. A core of at least 1.5 cm length or encompassing five portal areas was considered suitable for interpretation. Specimens were fixed in formalin, embedded in paraffin and stained with hematoxylin and eosin, Masson’s trichrome, reticulin, Perl’s stains, Prussian blue and picrosirius red (for collagen). Hepatic necroinflammatory activity and liver fibrosis were evaluated according to Ishak score. Necroinflammatory activity was classified into minimal (score A1-A3), mild (score A4-A8), moderate (score A9-A12), and severe (score A13-A18)[35]. Fibrosis was classified into mild (F1), moderate (F2-F3), and severe fibrosis or cirrhosis (F4-F6)[36].
FAF by morphometery
The morphometric assessment of liver fibrosis was performed as previously described[3] using the fully automated X-Y motorized stage Leica microscope (Leica, Cambridge, England) and Leica image processor with Leica Qwin software 2004. Liver biopsy assessment by Ishak score and morphometry was performed by a highly experienced hepatopathologist (KRZ) who was unaware of the clinical data or the final diagnosis of the patients. Assessment of liver fibrosis was performed within a week from serological tests.
Statistical analysis
Values were expressed as mean ± SD or number (percentage) of individuals with a condition. For quantitative data, statistical significance was tested by either independent samples t-test or by the non-parametric Mann-Whitney U test according to the nature of the data. For qualitative data, significance was tested by χ2 test or Fisher’s exact test. Correlation was tested by Spearman test. A stepwise multiple regression analysis was also performed to examine independent factors associated with LSM. The final model was determined using Pin < 0.05 and Pout < 0.10. Standardized coefficient (β), R squared (R2) and P values of the independent variables are presented. The diagnostic value of liver stiffness was assessed by calculating the area under the receiver-operating characteristic (AUROC) curves. The diagnostic performance was measured as sensitivity, specificity, positive predictive value and negative predictive value. The cut-off values for optimal clinical performance (optimal sensitivity and specificity simultaneously) were determined from the ROC curves (The upper most left point). Results were considered significant if P-value was < 0.05. Statistical analysis was performed using SPSS, version 13 (SPSS Inc, Chicago, IL, United States).
RESULTS
Study population characteristics
A total of 90 children were recruited to the study between 2012 and 2014; 50 children with chronic HCV, 20 children with AIH (18 patients were type-1 AIH and 2 patients were seronegative AIH), and 20 children with Wilson disease. None of the HCV group had jaundice, hepatomegaly or splenomegaly and their liver enzymes were significantly lower compared to the other studied groups (P < 0.0001 for all) (Table 1). The majority of HCV group had minimal activity (80%) and no/mild fibrosis (72%). On the other hand, the majority of AIH group had mild to moderate activity (70%) and moderate to severe fibrosis (95%) and all Wilson disease group had mild to moderate activity (100%) and moderate to severe fibrosis (100%) (Table 1).
Table 1.
Basic clinical characteristics of the studied groups
| Parameter | HCV | AIH | Wilson disease | P value |
| (n = 50) | (n = 20) | (n = 20) | ||
| Age (yr) | 8.50 ± 3.51 | 8.98 ± 2.98 | 12.05 ± 4.25 | 0.003 |
| Sex [male, n (%)] | 39 (78) | 7 (35) | 13 (65) | 0.003 |
| Jaundice, n (%) | 0 (0) | 18 (90) | 5 (25) | < 0.0001 |
| Abdominal enlargement, n (%) | 0 (0) | 5 (25) | 15 (75) | < 0.0001 |
| Liver US, n (%) | < 0.0001 | |||
| Normal | 50 (100) | 0 (0) | 1 (5) | |
| Enlarged | 0 (0) | 20 (100) | 13 (65) | |
| Shrunken | 0 (0) | 0 (0) | 6 (30) | |
| Splenomegaly US, n (%) | 0 (0) | 16 (80) | 20 (100) | < 0.0001 |
| Total bilirubin (mg/dL) | 0.97 ± 0.24 | 7.70 ± 3.72 | 3.37 ± 2.85 | < 0.0001 |
| Direct bilirubin (mg/dL) | 0.24 ± 0.17 | 5.58 ± 3.24 | 1.8 ± 1.30 | < 0.0001 |
| Albumin (g/dL) | 4.40 ± 0.61 | 3.71 ± 1.71 | 3.55 ± 0.71 | < 0.0001 |
| Aspartate transaminase (U/L) | 27.21 ± 14.75 | 225.10 ± 21.7 | 76.59 ± 68.43 | < 0.0001 |
| Alanine transaminase (U/L) | 29.52 ± 14.93 | 218.65 ± 14.2 | 87.05 ± 56.26 | < 0.0001 |
| Gamma-glutamyl transpeptidase (U/L) | 28.66 ± 13.80 | 52.50 ± 19.22 | 51.25 ± 37.89 | < 0.0001 |
| Alkaline phosphatase (U/L) | 127.82 ± 74.13 | 122.25 ± 45.03 | 222.30 ± 134.23 | < 0.0001 |
| Prothrombin time (s) | 13.2 ± 1.1 | 14.87 ± 3.13 | 16.18 ± 5.31 | 0.003 |
| Activity grade, n (%) | < 0.0001 | |||
| Minimal (A1-A3) | 40 (80) | 6 (30) | 0 (0) | |
| Mild (A4-A8) | 8 (16) | 8 (40) | 18 (90) | |
| Moderate (A9-A12) | 2 (4) | 6 (30) | 2 (10) | |
| Fibrosis category, n (%) | < 0.0001 | |||
| Absent (F0) | 4 (8) | 0 (0) | 0 (0) | |
| Mild (F1) | 32 (64) | 1 (5) | 0 (0) | |
| Moderate (F2-F3) | 14 (28) | 7 (35) | 6 (30) | |
| Severe (F4-F6) | 0 (0) | 12 (60) | 14 (70) | |
| Fibrosis area fraction (%) | 2.72 ± 1.66 | 17.41 ± 9.24 | 12.68 ± 4.59 | < 0.0001 |
| Liver stiffness (kPa) | 5.75 ±1.81 | 22.13 ± 14.23 | 20.05 ± 10.57 | < 0.0001 |
AIH: Autoimmune hepatitis; HCV: Hepatitis C virus; US: Ultrasonography.
Correlation of LSM with liver fibrosis in liver biopsy
LSM correlated significantly with both FAF and Ishak fibrosis scores and the correlation appeared better with the latter (Figure 1). The mean value of LSM increases successively with higher stages of fibrosis. Comparing the individual fibrosis stages in all the patients, a significant increase in LSM was found except between F0 and F1 and between F5 and F6 (Figure 2A). Comparing LSM of each fibrosis stage within different etiological groups revealed higher LSM with higher fibrosis stages (Figure 2B-D). Comparing FAF in different Ishak fibrosis stages revealed a significant difference between individual stages except between F4 and F5 (P = 0.376) (Figure 3).
Figure 1.
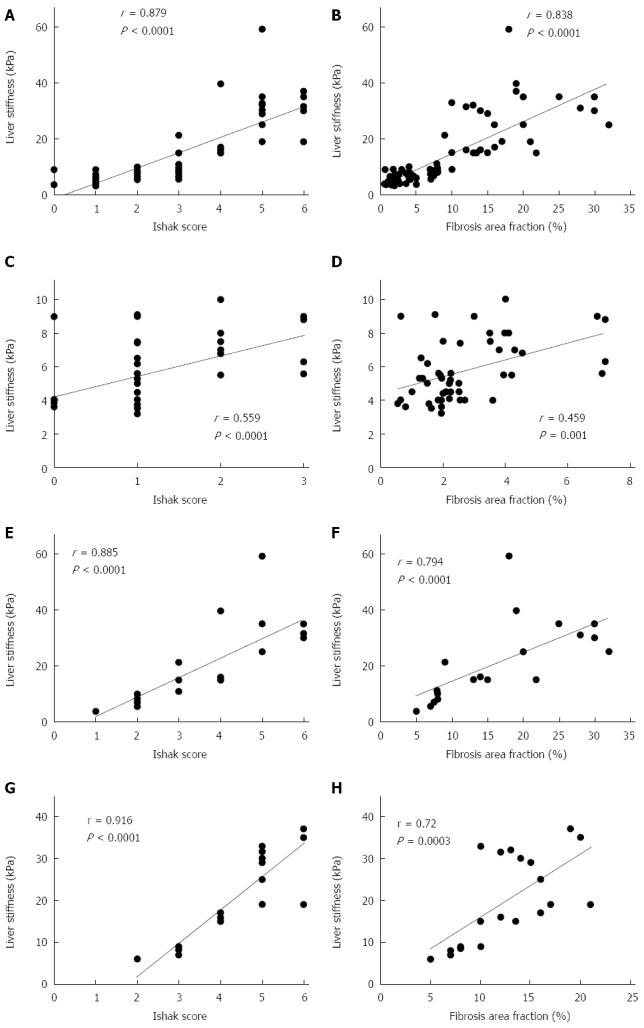
Correlation of LSM with fibrosis category by Ishak scores (left panel) and fibrosis area fraction (right panel). A and B: All patients; C and D: HCV patients; E and F: AIH patients; G and H: Wilson disease patients.
Figure 2.
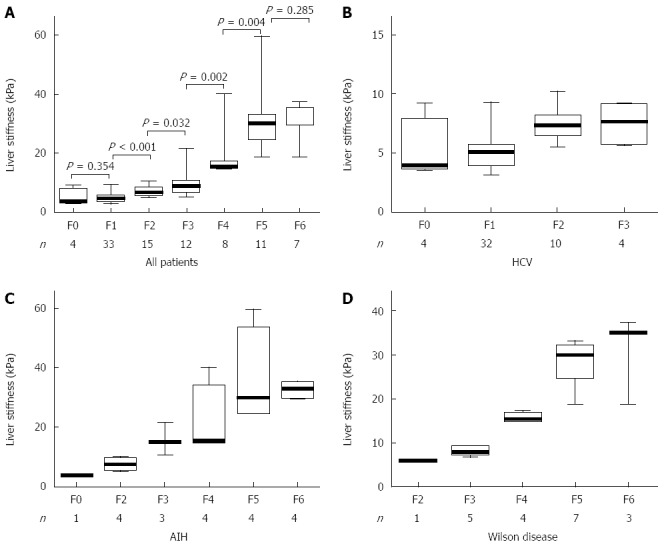
Distribution of liver stiffness measurement according to categorical fibrosis scores. A: All patients; B: HCV group; C: AIH group; D: Wilson disease group. Box-and-whiskers plot for liver stiffness measurement. The top and bottom of each box are the 75th and 25th percentiles. The line through the box is the median and the error bars are the maximum and minimum. The horizontal bar represents the significance between the designated groups.
Figure 3.
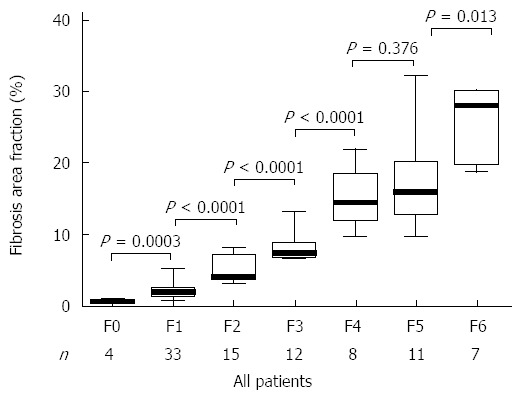
Distribution of fibrosis area fraction according to the categorical fibrosis scores. Box-and-whiskers plot for liver stiffness measurement. The top and bottom of each box are the 75th and 25th percentiles. The line through the box is the median and the error bars are the maximum and minimum. The horizontal bar represents the significance between the designated groups.
Performance of transient elastography in predicting individual fibrosis stages
LSM was a good discriminator of any fibrosis (≥ F1) from absent fibrosis (F0) (P = 0.039); of fibrosis ≥ F2 from < F2 (P < 0.0001); of fibrosis ≥ F3 from < F3 (P < 0.0001); of fibrosis ≥ F4 from < F4 (P < 0.0001), of fibrosis ≥ F5 from < F5 (P < 0.0001) and cirrhosis (F6) from < F6 (P < 0.0001) (Table 2). The performance of LSM in the etiological subgroups was calculated as the AUROC and its P-value, but the number of patients in each fibrosis category was too small to calculate sensitivity and specificity (Table 3). When we compared LSM values for the same stage of fibrosis, they varied according to the different etiologies with higher values in AIH (16.15 ± 7.23 kPa) compared to Wilson disease (8.30 ± 0.84 kPa) and HCV groups (7.43 ± 1.73 kPa) (Table 4).
Table 2.
Clinical performance of liver stiffness in predicting individual Ishak fibrosis stages in all the patients
| Ishak fibrosis stage | Cut-off (kPa) | AUROC | P value | Sensitivity | Specificity | PPV | NPV |
| ≥ F1 | 4.75 | 0.807 | 0.039 | 81.4 | 75.0 | 98.6 | 15.7 |
| ≥ F2 | 6.65 | 0.947 | < 0.0001 | 88.7 | 86.5 | 90.4 | 84.2 |
| ≥ F3 | 8.25 | 0.960 | < 0.0001 | 89.5 | 90.4 | 87.2 | 92.2 |
| ≥ F4 | 13.0 | 0.993 | < 0.0001 | 100 | 96.9 | 92.9 | 100 |
| ≥ F5 | 18.0 | 0.985 | < 0.0001 | 100 | 97.2 | 90.0 | 100 |
| F6 | 29.5 | 0.941 | < 0.0001 | 85.7 | 91.6 | 46.2 | 98.7 |
AUROC: Area under receiver operating characteristic curve; PPV: Positive predictive value; NPV: Negative predictive value.
Table 3.
Area under receiver operating characteristic curve for liver stiffness in predicting individual Ishak fibrosis stages in the different etiological groups
| Ishak fibrosis stage |
HCV |
AIH |
Wilson disease |
|||
| AUROC | P value | AUROC | P value | AUROC | P value | |
| ≥ F1 | 0.698 | 0.192 | NA | -- | NA | -- |
| ≥ F2 | 0.870 | < 0.0001 | 1.000 | < 0.0001 | NA | -- |
| ≥ F3 | 0.799 | 0.049 | 1.000 | < 0.0001 | 1.000 | < 0.0001 |
| ≥ F4 | NA | -- | 0.958 | 0.001 | 1.000 | < 0.0001 |
| ≥ F5 | NA | -- | 0.927 | 0.002 | 1.000 | < 0.0001 |
| F6 | NA | -- | 0.828 | 0.047 | 0.837 | 0.044 |
AIH: Autoimmune hepatitis; AUROC: Area under receiver operating characteristic; HCV: Hepatitis C virus.
Table 4.
Liver stiffness measurement in every fibrosis stage by the disease group
| Ishak fibrosis stage | All patients | HCV | AIH | Wilson disease |
| (n = 90) | (n = 50) | (n = 20) | (n = 20) | |
| kPa | kPa | kPa | kPa | |
| F0 | 5.10 ± 2.61 | 5.10 ± 2.61 | -- | -- |
| F1 | 5.10 ± 1.45 | 5.13 ± 1.45 | 3.70 | -- |
| F2 | 7.32 ± 1.4 | 7.33 ± 1.32 | 7.50 ± 0.71 | 6.00 |
| F3 | 9.88 ± 4.33 | 7.43 ± 1.73 | 16.15 ± 7.23 | 8.30 ± 0.84 |
| F4 | 18.58 ± 8.57 | -- | 21.43 ± 12.19 | 15.75 ± 0.96 |
| F5 | 31.25 ± 10.38 | -- | 36.08 ± 16.19 | 28.49 ± 4.93 |
| F6 | 31.71 ± 6.13 | -- | 32.75 ± 3.63 | 30.33 ± 9.87 |
AIH: Autoimmune hepatitis; HCV: Hepatitis C virus.
Correlation of LSM with the studied variables
LSM correlated significantly with age, serum bilirubin, hepatocellular enzymes, prothrombin time, activity grade and fibrosis stage in liver biopsy; P = 0.011 for age, P = 0.013 for alkaline phosphatase, P = 0.002 for prothrombin time and P < 0.0001 for the remaining studied parameters (Table 5). Subsequently, stepwise multiple regression analysis was performed. As a result, Ishak fibrosis stage was the only independent variable associated with higher LSM (β = 0.855; R2 = 0.731). Other variables were excluded from the model.
Table 5.
Correlation of liver stiffness with age, laboratory, and histopathological parameters of the studied patients
| Parameter |
Correlation |
|
| r | P value | |
| Age (yr) | 0.267 | 0.011 |
| Total bilirubin (mg/dL) | 0.433 | < 0.0001 |
| Direct bilirubin (mg/dL) | 0.506 | < 0.0001 |
| Albumin (g/dL) | -0.419 | < 0.0001 |
| Alanine transaminase (U/L) | 0.622 | < 0.0001 |
| Aspartate transaminase (U/L) | 0.559 | < 0.0001 |
| Alkaline phosphatase (U/L) | 0.261 | 0.013 |
| gamma-glutamyl transpeptidase (U/L) | 0.559 | < 0.0001 |
| Prothrombin time (s) | 0.322 | 0.002 |
| Ishak activity grade | 0.760 | < 0.0001 |
| Ishak fibrosis stage | 0.879 | < 0.0001 |
FAF according to Ishak scores and correlation of both with disease severity
FAF varied significantly with increasing stage of Ishak score. There was overlap in the values of FAF in F5 and F6 with no significant statistical difference (P = 0.376) (Figure 3). PELD/MELD scores correlated significantly with both FAF and Ishak score, nonetheless, the correlation with Ishak score appeared better than with FAF (P = 0.001 and 0.022 respectively) (Figure 4).
Figure 4.
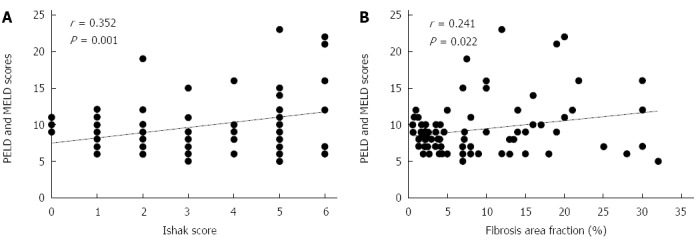
Correlation of PELD/MELD scores of all studied patients with categorical Ishak fibrosis scores (A) and fibrosis area fraction (B). PELD: Pediatric end-stage liver disease; MELD: Model for end-stage liver disease.
DISCUSSION
The current study showed a significant correlation between LSM and both the Ishak fibrosis stage and FAF with better correlation with Ishak fibrosis stage. The close correlation of TE readings with the extent of fibrosis suggests that TE is an ideal tool to follow liver fibrosis over years. In previous studies, among patients with viral hepatitis, the diagnostic performance of LSM was best for histologic cirrhosis and was less satisfactory for earlier stages of liver fibrosis[19,37-40].
Our results demonstrated that LSM could significantly discriminate individual stages of fibrosis even the earlier stages (≥ F1) from absent fibrosis (F0). Different LSM cut-off values have been proposed by different investigators for various stages of liver fibrosis. The optimal LSM cut-off for cohorts with predominantly chronic hepatitis C patients was between 10.4 and 17.6 kPa, and the AUROC for liver cirrhosis was 0.93-0.96[19,37-39].
de Lédinghen et al[22] reported a significant correlation of LSM with Metavir score in children with chronic liver disease but the performance was studied only for discriminating cirrhosis bacause F2 and F3 values were comparable. The better results in our study may be due to the wider range of Ishak score (7 stages) compared to Metavir score (5 stages).
The performance of LSM in discriminating individual stages of fibrosis was highly significant (P < 0.0001) for stages ≥ F2 with AUROC more than 0.94, and for ≥ F1 (i.e., discriminating F0) the AUROC was 0.807 and P = 0.039. The significance for discriminating F0 was abolished when comparing LSM according to the etiology (P = 0.192). F0 was found only in patients with chronic HCV in our study, but in none of the other studied groups. This may be due to the milder course of the HCV disease which induces mild changes in the liver with a low level of fibrosis and a low rate of progression[41].
The higher LSM values for different Ishak stages in AIH patients compared to the other studied groups in our study was in agreement with that of Fitzpatrick et al[26] and others[42]. In our study, AIH patients had higher stages of fibrosis and higher grades of inflammatory activity. Increased histologic necroinflammation, which is a logical cause of increased hepatic fibrosis, may explain the higher results of LSM in AIH patients compared with HCV and Wilson disease groups. One possible explanation for the higher LSM was a stiffer liver during active inflammation[1].
When we compared LSM for the same stage of fibrosis, it varied according to the etiology. This carries the possibility that, patients only having mild fibrosis would be misdiagnosed as moderate to severe fibrosis and even cirrhosis if they had increase histological activity. That is why it is better to estimate disease-specific cut-off values for predicting different stages of fibrosis due to the variable nature of liver diseases. For that, we checked the performance of LSM in the etiological subgroups which showed wide variability among the calculated AUROC and their P-values, but the number of patients in each fibrosis category was too small to calculate sensitivity and specificity. Similar to our results, other reports found that LSM was associated with the Ishak score in AIH[3,43], in HCV[44,45] and in Wilson disease patients[46].
The better correlation of the LSM in our study with Ishak scores compared to FAF suggests that LSM reflect the pattern (or quality) of fibrosis better than the quantity of hepatic collagen deposition. Liver diseases involve several types of cell injury, each of which leads to wound healing by fibrosis of distinct types and location. In chronic hepatitis (seen most often in chronic viral hepatitis, as well as in AIH, drug reactions, and some metabolic disorders as Wilson disease), fibrosis mainly develop from portal myofibroblasts and give rise to a dense, stellate, and regularly-distributed portal and periportal fibrosis that may form septa. On the other hand, fibrosis is mainly located in the perisinusoidal space of the centrilobular area, and in the wall of the centrilobular vein in alcoholic and nonalcoholic steatohepatitis[17,47,48].
Sandrini et al[49] investigated what type of fibrosis influences LSM. They found that the area of portal-bridging fibrosis better correlated with the liver stiffness than did the area of whole fibrosis or the area of perisinusoidal fibrosis. In the early fibrosis stages, there was a significant increase of perisinusoidal fibrosis from F0 to F2 Metavir, more than that of portal-bridging fibrosis. In subsequent stages F3 and F4, the area of perisinusoidal fibrosis stabilized[49]. Ishak scoring system is based on the pattern and extension of portal fibrosis, while FAF evaluates the whole amount of collagen whether portal or perisinusoidal. Taken together, this may explain the better correlation of LSM with Ishak scores in our study.
In contrast to semiquantitative fibrosis scores that rely on the pattern of architectural distortion caused by fibrosis, morphometry determines the proportion of collagenous tissue in a specimen, regardless of the pattern, and provides precise measurements on a continuous scale. Within any given semiquantitative stage, a wide range of collagen may be present, and considerable overlap exists within Metavir and Ishak stages[45]. In our study, FAF was largely overlapping between F4 and F5 with no significant difference (P = 0.376).
Biopsy sampling error will always be present to some extent, so it must be emphasized that morphometry is not appropriate for evaluation of changes in individual patients but should be reserved for statistical analysis and comparison of changes in large cohorts[45].
The question whether the severity and deterioration of liver disease is related to the pattern (quality) or to the quantity of fibrosis remains to be answered. The PELD/MELD scores were developed to create an integrated system of deceased donor liver allocation based on the severity of the chronic liver disease[50]. In our study, PELD/MELD scores were significantly correlated with both Ishak score and FAF but the correlation appeared better with Ishak scores (P = 0.001 vs 0.022). This may suggest that the information about the pattern (or quality) of fibrosis by semiquantitative Ishak score is more important than the overall amount of fibrosis by FAF in predicting the outcome of liver disease.
In the current study, LSM at cut-off values of 4.75, 6.65, 8.25, 13.0, 18.0, and 29.5 kPa was able to discriminate ≥ F1, ≥ F2, ≥ F3, ≥ F4, ≥ F5, and F6 respectively. Fitzpatrick et al[26] using Metavir score in pediatric patients with chronic liver diseases reported an LSM cut-off values of 6.1, 6.9, 7.5 and 14.1 kPa in discriminating ≥ F1, ≥ F2, ≥ F3, and F4. Other studies reported only the discrimination of cirrhosis. de Lédinghen et al[22] studied LSM performance in children with chronic liver disease. Using Metavir score, they reported that LSM was able to discriminate cirrhosis with AUROC of 0.88.
Wong et al[1] reported that a cut-off value of 8.4 kPa was associated with cirrhosis in adults with chronic liver disease at a very high sensitivity (93%) and a specificity of 79%. On the other hand, a cut-off value of 13.4 kPa was associated with high specificity (95%) but with low sensitivity (41%). In addition, Wang et al[51] reported that values below 6 kPa are considered as normal and exclude ongoing liver disease, while cut-off values of 8 and 12.5 kPa represent generally accepted values for discriminating F3 and F4 fibrosis in adults with chronic liver disease.
Staging of fibrosis with biopsy will always carry a risk, albeit low, of misclassification thus making the term ‘‘best” standard more appropriate than ‘‘gold” standard for liver biopsy[52]. As liver biopsy with its limitations[53] is used as a reference, a perfect surrogate will never reach maximal value[52].
Because LSM represents a volume of liver tissue at least 100 times bigger than a biopsy sample, a possibility that the performance of LSM has been underestimated can not be excluded. Yet it represents an efficient, noninvasive rapid painless and reproducible tool for fibrosis evaluation[54].
The limitation of the present study was the relatively small numbers in each of the etiological groups. In addition, the paucity of children with no fibrosis (F0) made it difficult to differentiate between no and minimal fibrosis. Future studies on larger population number are advisable.
In conclusion, TE appears reliable in distinguishing different stages of liver fibrosis in children with different chronic liver diseases. Routine use of this technique may significantly decrease the number of biopsies performed and provide a reliable method of noninvasive monitoring of liver disease progression or regression in children. Patients with increased necroinflammatory activity tend to have higher LSM values. For that, a disease-specific estimation of cut-off values for fibrosis staging is worthy.
ACKNOWLEDGMENTS
The authors would like to thank the doctors and nursing staff of Pediatric Hepatology and Gastroenterology Unit, Mansoura University Children Hospital, for their help and contribution.
COMMENTS
Background
The need for repetition of liver biopsy in patients with chronic liver disease, especially in assessing the degree of fibrosis and follow-up of treatment protocols, justifies an intensive search for non-invasive alternatives. Of these alternatives, liver stiffness measurement, as assessed by transient elastography, has been proposed. The accuracy of transient elastography has been shown to be excellent in a large number of adult studies. A few studies have also been performed in children.
Research frontiers
The natural course of hepatitis C viral infection in children differs from that in adults since the infection is relatively benign and induces mild changes in the liver with a low level of fibrosis. Progression of liver fibrosis in other liver diseases such as autoimmune hepatitis and Wilson disease is somehow faster. In patients with no or minimal fibrosis at presentation, treatment could possibly be delayed or withheld. On the other hand, patients with significant fibrosis progress almost invariably to cirrhosis more rapidly and treatment should be strongly considered. The decision to stop treatment in some cases, such as autoimmune hepatitis, may necessitate a second liver biopsy.
Innovations and breakthroughs
Most of liver stiffness studies have been conducted only in chronic hepatitis C virus infected patients. The current study includes different etiologies of pediatric chronic liver diseases. When the authors compared liver stiffness values for the same stage of fibrosis, they varied according to the different etiologies. Patients with increased necroinflammatory activity tend to have higher liver stiffness values. For that, a disease-specific estimation of cut-off values is worthy.
Terminology
Hepatic fibrosis is the final common path of liver injury in most chronic liver diseases and can lead to cirrhosis, which is responsible for the majority of clinical complications. Fibrosis is characterized by excess deposition of extracellular matrix components including different collagens and non-collagenous proteins. The more deposition of extracellular matrix in the liver the stiffer it gets. Liver stiffness is directly correlated with the amount of liver fibrosis. Digital image analysis (morphometry) allows quantitative assessment of fibrosis area fraction in liver tissue sections regardless of the pattern (or quality) of fibrosis. It detects smaller changes between biopsies particularly in patients with early stage fibrosis.
Peer-review
The authors demonstrated that Ishak fibrosis stage was the only independent variable associated with liver stiffness measurement assessed by TE. They concluded that TE may be useful for distinguishing different stages of liver fibrosis in pediatric patients with chronic liver diseases. Overall impression: The authors well demonstrated usefulness of TE for assessing liver fibrosis in pediatric patients with different chronic liver diseases.
Footnotes
Supported by the National Liver Institute, Menofiya University, Egypt, No. 2011.MDT013.
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of National Liver Institute, Menofiya University (Egypt).
Informed consent statement: All the legal guardians of the study participants provided informed written consent prior to study enrollment.
Conflict-of-interest statement: There are no conflicts of interest to report.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 8, 2016
First decision: February 18, 2016
Article in press: March 18, 2016
P- Reviewer: Konno T, Wang R S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
References
- 1.Wong GL, Wong VW, Choi PC, Chan AW, Chum RH, Chan HK, Lau KK, Chim AM, Yiu KK, Chan FK, et al. Assessment of fibrosis by transient elastography compared with liver biopsy and morphometry in chronic liver diseases. Clin Gastroenterol Hepatol. 2008;6:1027–1035. doi: 10.1016/j.cgh.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 2.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 3.Abdalla AF, Zalata KR, Ismail AF, Shiha G, Attiya M, Abo-Alyazeed A. Regression of fibrosis in paediatric autoimmune hepatitis: morphometric assessment of fibrosis versus semiquantiatative methods. Fibrogenesis Tissue Repair. 2009;2:2. doi: 10.1186/1755-1536-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47:2089–2111. doi: 10.1002/hep.22261. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 6.Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 8.Terjung B, Lemnitzer I, Dumoulin FL, Effenberger W, Brackmann HH, Sauerbruch T, Spengler U. Bleeding complications after percutaneous liver biopsy. An analysis of risk factors. Digestion. 2003;67:138–145. doi: 10.1159/000071293. [DOI] [PubMed] [Google Scholar]
- 9.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 10.Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 11.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 12.Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, Kench J, Farrell G, McCaughan GW, Jeffrey GP. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867–1873. doi: 10.1373/clinchem.2005.048389. [DOI] [PubMed] [Google Scholar]
- 13.Alboraie M, Khairy M, Elsharkawy A, Elsharkawy M, Asem N, Abo El-Seoud AR, Elghamry FG, Esmat G. Egy-score as a noninvasive score for the assessment of hepatic fibrosis in chronic hepatitis C: a preliminary approach. Saudi J Gastroenterol. 2014;20:170–174. doi: 10.4103/1319-3767.133003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel-Ghaffar TY, Behairy EB, Abd El-Shaheed A, Mahdy K, El-Batanony M, Hussein MH, Sira MM. Clinical Benefits of Biochemical Markers of Fibrosis in Egyptian Children With Chronic Liver Diseases. Gastroenterol Res. 2010;3:262–271. doi: 10.4021/gr246w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sira MM, Behairy BE, Abd-Elaziz AM, Abd Elnaby SA, Eltahan EE. Serum Inter-Alpha-Trypsin Inhibitor Heavy Chain 4 (ITIH4) in Children with Chronic Hepatitis C: Relation to Liver Fibrosis and Viremia. Hepat Res Treat. 2014;2014:307942. doi: 10.1155/2014/307942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behairy BE, El-Mashad GM, Abd-Elghany RS, Ghoneim EM, Sira MM. Serum complement C4a and its relation to liver fibrosis in children with chronic hepatitis C. World J Hepatol. 2013;5:445–451. doi: 10.4254/wjh.v5.i8.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziol M, Kettaneh A, Ganne-Carrié N, Barget N, Tengher-Barna I, Beaugrand M. Relationships between fibrosis amounts assessed by morphometry and liver stiffness measurements in chronic hepatitis or steatohepatitis. Eur J Gastroenterol Hepatol. 2009;21:1261–1268. doi: 10.1097/MEG.0b013e32832a20f5. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 19.Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968–973. doi: 10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arena U, Vizzutti F, Abraldes JG, Corti G, Stasi C, Moscarella S, Milani S, Lorefice E, Petrarca A, Romanelli RG, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008;57:1288–1293. doi: 10.1136/gut.2008.149708. [DOI] [PubMed] [Google Scholar]
- 21.Chon YE, Choi EH, Song KJ, Park JY, Kim do Y, Han KH, Chon CY, Ahn SH, Kim SU. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One. 2012;7:e44930. doi: 10.1371/journal.pone.0044930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Lédinghen V, Le Bail B, Rebouissoux L, Fournier C, Foucher J, Miette V, Castéra L, Sandrin L, Merrouche W, Lavrand F, et al. Liver stiffness measurement in children using FibroScan: feasibility study and comparison with Fibrotest, aspartate transaminase to platelets ratio index, and liver biopsy. J Pediatr Gastroenterol Nutr. 2007;45:443–450. doi: 10.1097/MPG.0b013e31812e56ff. [DOI] [PubMed] [Google Scholar]
- 23.Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, Fruhwirth R, Marcellini M, Pinzani M. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008;48:442–448. doi: 10.1002/hep.22376. [DOI] [PubMed] [Google Scholar]
- 24.Alkhouri N, Sedki E, Alisi A, Lopez R, Pinzani M, Feldstein AE, Nobili V. Combined paediatric NAFLD fibrosis index and transient elastography to predict clinically significant fibrosis in children with fatty liver disease. Liver Int. 2013;33:79–85. doi: 10.1111/liv.12024. [DOI] [PubMed] [Google Scholar]
- 25.Breton E, Bridoux-Henno L, Guyader D, Daniélou H, Jouan H, Beuchée A, Nousbaum JB, Dabadie A. [Value of transient elastography in noninvasive assessment in children’s hepatic fibrosis] Arch Pediatr. 2009;16:1005–1010. doi: 10.1016/j.arcped.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Fitzpatrick E, Quaglia A, Vimalesvaran S, Basso MS, Dhawan A. Transient elastography is a useful noninvasive tool for the evaluation of fibrosis in paediatric chronic liver disease. J Pediatr Gastroenterol Nutr. 2013;56:72–76. doi: 10.1097/MPG.0b013e31826f2760. [DOI] [PubMed] [Google Scholar]
- 27.Caballero T, Pérez-Milena A, Masseroli M, O’Valle F, Salmerón FJ, Del Moral RM, Sánchez-Salgado G. Liver fibrosis assessment with semiquantitative indexes and image analysis quantification in sustained-responder and non-responder interferon-treated patients with chronic hepatitis C. J Hepatol. 2001;34:740–747. doi: 10.1016/s0168-8278(01)00006-x. [DOI] [PubMed] [Google Scholar]
- 28.Lazzarini AL, Levine RA, Ploutz-Snyder RJ, Sanderson SO. Advances in digital quantification technique enhance discrimination between mild and advanced liver fibrosis in chronic hepatitis C. Liver Int. 2005;25:1142–1149. doi: 10.1111/j.1478-3231.2005.01155.x. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien MJ, Keating NM, Elderiny S, Cerda S, Keaveny AP, Afdhal NH, Nunes DP. An assessment of digital image analysis to measure fibrosis in liver biopsy specimens of patients with chronic hepatitis C. Am J Clin Pathol. 2000;114:712–718. doi: 10.1309/D7AU-EYW7-4B6C-K08Y. [DOI] [PubMed] [Google Scholar]
- 30.Alisi A, Comparcola D, Nobili V. Treatment of chronic hepatitis C in children: is it necessary and, if so, in whom? J Hepatol. 2010;52:472–474. doi: 10.1016/j.jhep.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47–52. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 33.Freeman RB, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, Merion R, Wolfe R, Turcotte J, Teperman L. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851–858. doi: 10.1053/jlts.2002.35927. [DOI] [PubMed] [Google Scholar]
- 34.Ji D, Shao Q, Han P, Li F, Li B, Zang H, Niu X, Li Z, Xin S, Chen G. The frequency and determinants of liver stiffness measurement failure: a retrospective study of “real-life” 38,464 examinations. PLoS One. 2014;9:e105183. doi: 10.1371/journal.pone.0105183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 36.Esmat G, Metwally M, Zalata KR, Gadalla S, Abdel-Hamid M, Abouzied A, Shaheen AA, El-Raziky M, Khatab H, El-Kafrawy S, et al. Evaluation of serum biomarkers of fibrosis and injury in Egyptian patients with chronic hepatitis C. J Hepatol. 2007;46:620–627. doi: 10.1016/j.jhep.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganne-Carrié N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Castera L, Dhumeaux D, Trinchet JC, Beaugrand M. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44:1511–1517. doi: 10.1002/hep.21420. [DOI] [PubMed] [Google Scholar]
- 40.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 41.Camarero C, Ramos N, Moreno A, Asensio A, Mateos ML, Roldan B. Hepatitis C virus infection acquired in childhood. Eur J Pediatr. 2008;167:219–224. doi: 10.1007/s00431-007-0472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilder J, Patel K. The clinical utility of FibroScan(®) as a noninvasive diagnostic test for liver disease. Med Devices (Auckl) 2014;7:107–114. doi: 10.2147/MDER.S46943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang QX, Shen L, Qiu DK, Bao H, Chen XY, Zeng MD, Mao YM, Ma X. [Validation of transient elastography (Fibroscan) in assessment of hepatic fibrosis in autoimmune hepatitis] Zhonghua Ganzangbing Zazhi. 2011;19:782–784. doi: 10.3760/cma.j.issn.1007-3418.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 44.Muñoz R, Ramírez E, Fernandez I, Martin A, Romero M, Romero E, Dominguez-Gil B, Hernandez A, Morales E, Andres A, et al. Correlation between fibroscan, liver biopsy, and clinical liver function in patients with hepatitis C virus infection after renal transplantation. Transplant Proc. 2009;41:2425–2426. doi: 10.1016/j.transproceed.2009.06.103. [DOI] [PubMed] [Google Scholar]
- 45.Goodman ZD, Stoddard AM, Bonkovsky HL, Fontana RJ, Ghany MG, Morgan TR, Wright EC, Brunt EM, Kleiner DE, Shiffman ML, et al. Fibrosis progression in chronic hepatitis C: morphometric image analysis in the HALT-C trial. Hepatology. 2009;50:1738–1749. doi: 10.1002/hep.23211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karlas T, Hempel M, Tröltzsch M, Huster D, Günther P, Tenckhoff H, Mössner J, Berg T, Keim V, Wiegand J. Non-invasive evaluation of hepatic manifestation in Wilson disease with transient elastography, ARFI, and different fibrosis scores. Scand J Gastroenterol. 2012;47:1353–1361. doi: 10.3109/00365521.2012.719924. [DOI] [PubMed] [Google Scholar]
- 47.Sokol RJ. Copper metabolism and copper storage disorders. In: Suchy FJ, Sokol RJ, Balistreri WF, editors. Liver Disease in Children. 4th ed. New York: Cambridge University Press; 2014. pp. 465–492. [Google Scholar]
- 48.Goodman ZD, Becker RL, Pockros PJ, Afdhal NH. Progression of fibrosis in advanced chronic hepatitis C: evaluation by morphometric image analysis. Hepatology. 2007;45:886–894. doi: 10.1002/hep.21595. [DOI] [PubMed] [Google Scholar]
- 49.Sandrini J, Boursier J, Chaigneau J, Sturm N, Zarski JP, Le Bail B, de Ledinghen V, Calès P, Rousselet MC. Quantification of portal-bridging fibrosis area more accurately reflects fibrosis stage and liver stiffness than whole fibrosis or perisinusoidal fibrosis areas in chronic hepatitis C. Mod Pathol. 2014;27:1035–1045. doi: 10.1038/modpathol.2013.225. [DOI] [PubMed] [Google Scholar]
- 50.McDiarmid SV, Merion RM, Dykstra DM, Harper AM. Selection of pediatric candidates under the PELD system. Liver Transpl. 2004;10:S23–S30. doi: 10.1002/lt.20272. [DOI] [PubMed] [Google Scholar]
- 51.Wang JH, Changchien CS, Hung CH, Eng HL, Tung WC, Kee KM, Chen CH, Hu TH, Lee CM, Lu SN. FibroScan and ultrasonography in the prediction of hepatic fibrosis in patients with chronic viral hepatitis. J Gastroenterol. 2009;44:439–446. doi: 10.1007/s00535-009-0017-y. [DOI] [PubMed] [Google Scholar]
- 52.Bedossa P, Carrat F. Liver biopsy: the best, not the gold standard. J Hepatol. 2009;50:1–3. doi: 10.1016/j.jhep.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 53.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 54.Mendoza J, Trapero-Marugán M, González-Moreno L, Jones EA, Gómez-Domínguez E, Moreno-Otero R. Hepatic fibrosis in patients with chronic hepatitis C assessed by transient elastography: implications for determining the efficacy of antiviral therapy. Rev Esp Enferm Dig. 2010;102:426–434. doi: 10.4321/s1130-01082010000700005. [DOI] [PubMed] [Google Scholar]


