Abstract
Atypical amygdala function and connectivity have reliably been associated with psychopathy. However, the amygdala is not a unitary structure. To examine how psychopathic traits in a nonforensic sample are linked to amygdala response to violence, this study used probabilistic tractography to classify amygdala subnuclei based on anatomical projections to and from amygdala subnuclei in a group of 43 male participants. The segmentation identified the basolateral complex (BLA; lateral, basal, and accessory basal subnuclei) and the central subnucleus (CE), which were used as seeds in a functional connectivity analysis to identify differences in neuronal coupling specific to observed violence. While a full amygdala seed showed significant connectivity only to right middle occipital gyrus, subnuclei seeds revealed unique connectivity patterns. BLA showed enhanced coupling with anterior cingulate and prefrontal regions, while CE showed increased connectivity with the brainstem, but reduced connectivity with superior parietal and precentral gyrus. Further, psychopathic personality factors were related to specific patterns of connectivity. Fearless Dominance scores on the psychopathic personality inventory predicted increased coupling between the BLA seed and sensory integration cortices, and increased connectivity between the CE seed and posterior insula. Conversely, Self‐Centered Impulsivity scores were negatively correlated with coupling between BLA and ventrolateral prefrontal cortex, and Coldheartedness scores predicted increased functional connectivity between BLA and dorsal anterior cingulate cortex. Taken together, these findings demonstrate how subnuclei segmentations reveal important functional connectivity differences that are otherwise inaccessible. Such an approach yields a better understanding of amygdala dysfunction in psychopathy. Hum Brain Mapp 36:1417–1428, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: amygdala, cognition, fMRI, diffusion weighted MRI, neuroimaging, diffusion tensor imaging, psychopathy, violence
INTRODUCTION
Psychopathy is a personality disorder exemplified by high levels of callousness, grandiosity, manipulation, impulsivity, criminal versatility, and other antisocial and affective characteristics. Psychopathy can be assessed with different measures. For institutionalized and forensic populations, the most widely measure is Hare's Psychopathy Checklist‐Revised (PCL‐R), a well‐validated psychological assessment tool [Hare, 2003]. While the PCL‐R is often considered as the gold standard, the Psychopathy Personality Inventory‐Revised (PPI‐R) [Lilienfeld and Widows, 2005] is a comprehensive index of personality traits which does not assume particular links to criminal or antisocial behavior. This latter measure is, therefore, more appropriate for assessing variation in the personality traits associated with psychopathy, which are distributed along a continuum that extends into the general population [Patrick et al., 2009; Skeem et al., 2011]. These traits cluster into three high‐level factors [Benning et al., 2005], each of which can differentially influence behavior, cognition, and neural processing [Almeida et al., 2014; Sadeh and Verona, 2008].
In particular, amygdala dysfunction and its connectivity have been argued to underlie many of these effects [Anderson and Kiehl, 2012; Birbaumer et al., 2005; Blair, 2007], yet there are conflicting accounts of the exact nature of the relationship between psychopathy and amygdala functioning. While some studies report reduced functional connectivity between the amygdala and orbitofrontal (OFC) or ventromedial prefrontal (vmPFC) regions [Decety et al., 2013a; Motzkin et al., 2011], or reduced activity elicited by emotionally salient stimuli [Decety et al., 2014; Kiehl et al., 2001], others report enhanced amygdala activity, but only for specific classes of stimuli [Carré et al., 2013; Decety et al., 2013b].
One possible explanation for these divergent findings is that most previous studies have examined the amygdala as a unitary structure. However, the amygdaloid complex is comprised of multiple nuclei that have unique cytoarchitectonics, histochemistry, and hodology [Sah et al., 2003]. These nuclei are commonly grouped into the basolateral amygdala (BLA; lateral, basal, and accessory basal nuclei) and central amygdala (CE; central and medial nuclei). CE is the primary amygdala output nucleus [Price and Amaral, 1981] and projects to many cortical and subcortical regions that are important for the stress response, including the brainstem and hypothalamus. One of the primary functions of CE is to coordinate physiology and behavior in response to threatening cues. BLA is the primary initiator and recipient of neocortical projections [Freese and Amaral, 2009], and is reciprocally connected to frontal regions such as OFC, vmPFC, and anterior cingulate cortex (ACC) [Ongür and Price, 2000; St Onge et al., 2012]. In addition to fear conditioning [Fanselow and LeDoux, 1999], BLA has also been implicated in decision‐making tasks that require updating representations of subjective stimulus‐value associations [Balleine and Killcross, 2006; Baxter and Murray, 2002].
These functional distinctions are relevant to psychopathy personality research because a recently proposed model argues that the various attentional and affective components of psychopathy can be explained by focal amygdala dysfunction. Specifically, the differential amygdala activation model (DAAM) [Moul et al., 2012] argues that psychopathy is characterized by chronic BLA hypoactivation and normal or intact CE functioning. Partial anatomical support for this model comes from a recent study showing that psychopathy (scores of 21–40 on the PCL‐R) is associated with increased CE volume and decreased BLA volume [Boccardi et al., 2011]. Moreover, selective disruption of BLA in nonhuman animals produces behavioral changes that are similar to those observed in individuals with increased psychopathic personality traits. For instance, individuals with high scores on psychopathic traits show more impulsive response styles across a range of tasks, and exhibit reduced error‐related negativity (ERN) amplitude, a component associated with error monitoring [Heritage and Benning, 2013]. In rodents, impulsivity is often measured with a delay discounting task, where the animal can choose between a small immediate reward and a larger reward for which the animal must wait. When BLA is experimentally lesioned, rats exhibit more impulsive response styles, preferring the smaller reward option after shorter delay latencies [Winstanley et al., 2004]. Similarly, just as criminal psychopaths perseverate on a gambling task, even in the face of increased punishment [Newman et al., 1987], disrupting medial prefrontal‐BLA circuitry decreases rats' sensitivity to negative feedback [St Onge et al., 2012]. Finally, the “low‐fear” model of psychopathy posits that psychopathy is characterized as a specific deficit in the experience and comprehension of fear, likely from abnormal processing in OFC, amygdala, and paralimbic areas [Birbaumer et al., 2005; Kiehl et al., 2001]. In rats, BLA lesions disrupt fear‐potentiated startle (FPS) for both auditory and visual conditioned stimuli [Campeau and Davis, 1995], and deficient FPS has been repeatedly related to psychopathy [Baskin‐Sommers et al., 2011; Larson et al., 2013].
Importantly, as discussed above, psychopathic personality traits can be clustered into three high‐level factors [Benning et al., 2005]. These factors independently relate to neural and behavioral differences seen in psychopathy. For instance, higher scores on Fearless Dominance are associated with increased attention to motivationally salient stimuli, at the expense of attention to less relevant information [Sadeh and Verona, 2008]. Conversely, impulsive responding and decreased sensitivity to negative feedback are specifically related to Self‐Centered Impulsivity. Moreover, scores on Self‐Centered Impulsivity, but not Fearless Dominance, predict reduced amplitudes in the ERN [Heritage and Benning, 2013]. However, only scores on Fearless Dominance correlate with reduced amplitudes in the feedback‐related negativity [Schulreich et al., 2013]. These event‐related potential (ERP) components are closely related, both in terms of their function and proposed neural generators, so the differential impact of Fearless Dominance and Self‐Centered Impulsivity scores suggests that these factors are capable of characterizing fine‐grained distinctions.
Coldheartedness, the third high‐level factor, has not been studied as extensively. One functional neuroimaging study found Coldheartedness scores negatively correlated with amygdala response to social stimuli [Han et al., 2012]. A recent ERP study used different spatial frequencies to distinguish between cortical and subcortical visual pathways in healthy adults [Almeida et al., 2014]. Fearless Dominance scores predicted reduced N170 amplitudes to faces at low spatial frequencies which are dependent on the tectopulvinar pathway (which includes the amygdala). However, Coldheartedness scores were related to the geniculostriate pathway (which does not include the amygdala). Coldheartedness scores have also been related to increased suppression of motor evoked potentials when viewing physical pain [Fecteau et al., 2008], as well as decreased preferred interpersonal distance [Vieira and Marsh, 2014].
Thus, one important question that has been largely unaddressed is how dysfunction in a single neural structure (BLA), as proposed by DAAM, could underlie both fearlessness and impulsivity in psychopathy. One appealing hypothesis, and the one under investigation in this article, is that different factors of psychopathy are associated with unique patterns of activity within cortical and subcortical networks which overlap in the amygdala. To that end, this study sought to examine how variation in specific psychopathic personality traits in a nonforensic sample is associated with variation in neural coupling based in either BLA or CE in the right amygdala. These regions were identified on an individual‐participant basis using tractography‐based segmentation [Saygin et al., 2011], and these masks were used in a functional connectivity analysis. The right amygdala was selected for two reasons. First, previous work suggests that the right as compared to left BLA and CE are more widely connected to cortical targets [Roy et al., 2009]. Second, two meta‐analyses of functional neuroimaging studies have reported differences in temporal dynamics and habituation rates, specifically a shorter duration responses in the right amygdala to emotional‐laden stimuli [Sergerie et al., 2008].
Rather than a resting‐state scan, this study used video clips of interpersonal interactions with the presence or absence of violence. Interpersonal violence/harm was chosen because violence is a universally salient stimulus, and viewing other individuals being hurt is reliably associated with recruitment of several well‐defined cortical regions, including dorsal ACC (dACC) and anterior insula cortex (AIC) [Decety et al., 2013a, b; Lamm et al., 2011]. However, in addition to perception of harm and physical pain, the dACC has also been implicated in nociception, conflict‐monitoring [Botvinick et al., 2004], and is the putative neural generator of the ERN [Allman et al., 2001]. Thus, a more recent conception, and parsimonious interpretation, argues that rather than serving as a dedicated pain perception network, these regions are part of a more general salience network that integrates information to coordinate widespread cortical and subcortical activity to support the rapid deployment of attentional resources toward motivationally relevant stimuli [Decety, 2011; Decety et al., 2013a, b; Fox et al., 2013; Harsay et al., 2012; Shackman et al., 2011]. Finally, and especially relevant for this study, dACC (as well as AIC) shows extensive reciprocal connections with BLA [Shackman et al., 2011].
Fearless Dominance, because it has been linked to enhanced attention to salient stimuli [Sadeh and Verona, 2008], was expected to relate to increased coupling between BLA and regions involved in sensory processing and selective attention. Because Self‐Centered Impulsivity indexes impulsivity, it was expected to predict connectivity with ventrolateral prefrontal (e.g., vlPFC) areas that work with the amygdala to regulate emotion [Wager et al., 2008]. Finally, Coldheartedness scores (i.e., decreased concern for others) was expected to be related to decreased coupling between CE and regions of the salience network.
MATERIALS AND METHODS
Participants
Forty‐three healthy adult males (mean age = 25 years, range = 18–35 years) from the Chicago metropolitan area participated in the study, which was approved by the Institutional Review Board at the University of Chicago. Participants responded to flyers designed to attract a range of individuals, ranging from those who liked to those who disliked Mixed Martial Arts (MMA). All individuals who responded were included, provided they were male and between the ages of 18 and 35 years of age. All participants had normal or corrected to normal vision.
The sample was restricted to males because the majority of consumers of violent sports are males, and also to avoid the influence of hormonal fluctuations. Prior to participation, participants answered a survey about MMA. These questions assessed how often participants watched (1‐Never; 5‐More than 4 h/week) or participated in (1‐Never; 5‐More than 2 times/week) MMA, as well as how pleasurable (1‐Extremely unpleasurable; 5‐Most pleasurable) they found watching MMA. Pleasurability ratings were similar to those obtained from another study of 51 healthy adults who were not recruited based on MMA viewing preference [Smith et al., 2014].
Personality Assessment
Participants completed the Psychopathic Personality Inventory‐Revised (PPI‐R). This instrument was developed to assess psychopathy in nonpsychiatric and nonforensic samples [Lilienfeld and Widows, 2005]. Subscores from the PPI‐R cluster into three high‐level factors [Benning et al., 2005; Patrick et al., 2009; Skeem et al., 2011], which have been shown to differentially influence behavior, attention, and neural processing [Almeida et al., 2014; Carré et al., 2013; Sadeh and Verona, 2008; Schulreich et al., 2013]. Fearless Dominance includes social influence, fearlessness, and stress immunity. Self‐Centered Impulsivity is comprised of Machiavellian egocentricity, rebellious nonconformity, blame externalization, and carefree nonplanfulness. Finally, Coldheartedness (i.e., callousness, unsentimentality, and lack of sympathy) stands alone as a third factor because it does not load on either of the first two factors [Benning et al., 2003]. Scores for Fearless Dominance, Self‐Centered Impulsivity, and Coldheartedness were calculated and used as covariates at the whole‐brain level and in the effective connectivity analysis.
Stimuli
Twenty video clips (10 s each) of MMA and Capoeira were used. MMA is a full contact combat sport that incorporates aspects of boxing, kick boxing, wrestling, and other martial arts. Athletes win “fights” by knocking their opponent unconscious, forcing their opponent to “give up,” or being awarded the most points by a panel of judges. Clips for MMA were extracted from a five round fight that was previously used in a study of autonomic arousal in response to perceiving harm in others [Smith et al., 2014]. The Brazilian dance form Capoeira was included as a control condition because it involves similar movements (e.g., kicking and hand strikes) between pairs of individuals, but without the intent or execution of injury or contact.
Task
To avoid contamination of neural activity with motor planning or anticipation, participants passively viewed stimuli. Before scanning, participants were shown samples of each stimulus type. Stimuli were presented using E‐Prime 2.0 (Psychology Software Tools, Inc., Pittsburgh, PA) on a back‐projection Avotec projection system (Avotec Inc., Stuart, FL). During scanning, a 1 s cue slide appeared with text informing participants of which clip they would see next. Clips were interspersed with a 10 s interblock interval, and stimulus order was pseudorandom, with no stimulus type repeating more than twice in a row. Structural scans were conducted last.
Scanning Parameters
Functional volumes were collected using a Philips Achieva 3T scanner with a T2*‐weighted EPI sequence (TR/TE: 2000/25ms; FOV 240 × 240 × 127.5 mm; matrix: 80 × 80 × 32 mm; flip angle 80°; in plane resolution: 3 × 3 mm). Whole brain coverage was achieved using 32 3.5‐mm‐thick slices with a 0.5 mm gap. A Philips provided Z‐Shim sequence was used to recovered signal in orbitofrontal regions. Next, T1‐weighted anatomical scans were collected (FOV: 250 × 250 × 180 mm; matrix: 240 × 240 × 300). Finally, diffusion‐weighted images were collected with single shot echo planar imaging (70 slices, no skip gap, voxel size 0.88 × 0.88 × 2 mm, matrix size 112 × 112, b‐value 1,000 along 32 directions) on the same scanner using an 8‐channel SENSE head coil.
Image Processing
First, FreeSurfer's automated routines [Fischl, 2004; Fischl et al., 2002] were used to parcellate and segment cortical and subcortical regions in individual subjects' native space. These resulting classifications were visually inspected and errors were corrected if necessary. Concurrently, diffusion‐weighted images were skull‐stripped [Smith, 2002], and the FMRIB Diffusion Toolbox [Behrens et al., 2003, 2007] was used to correct for eddy currents, fit diffusion tensors to each voxel, and estimate diffusion directions within each voxel (BEDPOSTX). Structural segmentations were then coregistered to diffusion images. Preprocessing of functional magnetic resonance imaging (fMRI) data was performed using FMRIB Software Library FSL's FMRI Expert Analysis Tool with default settings (highpass cutoff filter = 100s; motion correction; smoothing with FWHM = 8mm). MMA and CAPO blocks were modeled separately in a general linear model and convolved with a gamma function that mimics the hemodynamic response.
Subnuclei Classification
Each voxel within the right amygdala was taken as a seed for probabilistic tractography and connectivity was calculated for each target mask using 25,000 streamline samples avoiding a mask of the combined ventricles (PROBTRACKX). Path estimates were then normalized by their maximum, thresholded at 0.1, and binarized, rendering each amygdala voxel either 0 or 1 for each of the anatomical targets. Subnuclei were then classified using Boolean expressions as belonging to lateral (LA), basal and accessory basal (BA), or central (CE) subnuclei complexes. After this initial classification, three‐dimensional smoothing was applied to each subnuclei, and only voxels that with six contiguous neighbors belonging to the same nuclei, we included in the mask. Finally, LA and BA were combined into a single mask for the basolateral complex (BLA). Segmentations from a representative single subject are shown in Figure 1.
Figure 1.
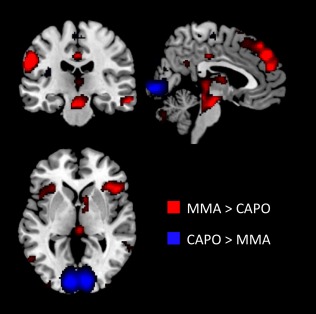
Regions show sensitivity to violence. Rendered whole‐brain results (P < 0.001) for MMA compared to Capoiera (CAPO). Video clips of MMA were associated with greater hemodynamic response in anterior insula, caudate, brainstem, and dorsal attention network. Posterior occipital cortex showed greater activity for the CAPO videos. For more details, see Porges and Decety [2013]. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Functional Connectivity
For each participant, the MMA>CAPO contrast was used to isolate violence‐specific neural activity and constituted the psychological regressor in a psychophysiological interaction. Detailed results of this contrast are present elsewhere [Porges and Decety, 2013]. Separate physiological regressors were constructed by extracting the mean time‐course from within 3 mm radius spheres centered at the peak voxels within each participant's BLA and CE masks. For comparison, another connectivity analysis was also conducted using each participant's anatomically defined right amygdala as a seed. The resulting first‐level psychophysiological interactions were then averaged to create group‐level maps of regions showing significant functional connectivity seeded in BLA, CE, or full right amygdala.
Finally, PPI‐R factor scores were entered as covariates of interest at the second level to determine how individual differences in psychopathic personality traits predicted changes in functional connectivity between individuals. Images were thresholded at P < 0.001 with a cluster extend threshold of 50 contiguous voxels.
RESULTS
Descriptive statistics and Pearson correlations for PPI‐R factor scores and MMA questions are shown in Table 1. PPI‐R total scores (M = 307.82, SD = 35.03, range 240–408) were unimodally distributed and comparable to other community samples [Almeida et al., 2014; Vieira and Marsh, 2014]. No participants had inconsistent responding scores over 40 so all were used. Self‐Centered Impulsivity scores were positively correlated with watching MMA (r = 0.37, P = 0.014). Watching MMA was also positively correlated with liking MMA (r = 0.53, P < 0.001) and participating in MMA (r = 0.41, P = 0.006). There was also a trend toward a positive relationship between Fearless Dominance scores and participation in MMA (r = 0.30, P = 0.051). There were no other significant correlations between these independent variables (all Ps > 0.3).
Table 1.
Means, standard deviations, and bivariate correlations amongst dispositions and MMA familiarity ratings
| Fearless Dominance | Self‐Centered Impulsivity | Coldheartedness | Watch | Like | Do | |
|---|---|---|---|---|---|---|
| Fearless dominance | — | |||||
| Self‐centered antisociality | 0.11 | — | ||||
| Coldheartedness | 0.02 | 0.19 | — | |||
| Watch | −0.07 | 0.37* | 0.11 | — | ||
| Like | 0.04 | −0.12 | 0.21 | 0.53** | — | |
| Do | 0.30 | 0.15 | 0.21 | 0.41** | 0.28 | — |
| Mean | 130.07 | 143.23 | 33.58 | 2.14 | 3.44 | 1.88 |
| SD | 16.42 | 23.76 | 9.66 | 1.23 | 1.14 | 1.35 |
| Min‐Max | 97–208 | 92–164 | 18–48 | 1–5 | 1–5 | 1–5 |
P < 0.05; ** P < 0.01
The results of the MMA>CAPO contrast are reported in depth elsewhere [Porges and Decety, 2013] and are shown in Figure 1 for reference. PPI‐R factor scores significantly predicted activity in several cortical and subcortical regions (Table 2). Fearless Dominance scores were related to reduced activity in the right superior temporal cortex, overlapping with the right temporoparietal junction (rTPJ). Similarly, Coldheartedness was negatively related to activity in bilateral occipital cortex and cerebellum, as well as left middle frontal gyrus. Conversely, scores on Self‐Centered Impulsivity were related to significantly greater activity bilateral caudate.
Table 2.
Significant whole‐brain clusters for each factor score from the Psychopathic Personality Inventory‐Revised
| Covariate | Brain region | MNI coordinates | Cluster size | T | Cohen's d | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Fearless Dominance | |||||||
| R Superior Temporal | 66 | −46 | 22 | 178 | −4.33 | −1.35 | |
| Brainstem | 0 | −40 | −35 | 130 | 5.04 | 1.57 | |
| Self‐Centered Impulsivity | |||||||
| L Caudate | −12 | 22 | 0 | 93 | 3.00 | 0.94 | |
| R Caudate | 20 | 26 | 0 | 64 | 3.87 | 1.21 | |
| Coldheartedness | |||||||
| L Middle Occipital | −38 | −94 | 2 | 271 | −4.37 | −1.36 | |
| R Middle Occipital | 24 | −94 | 6 | 206 | −3.94 | −1.23 | |
| Cerebellum | 10 | −70 | −40 | 195 | −4.32 | −1.35 | |
| L Middle Frontal | −32 | 18 | 38 | 60 | −4.25 | −1.33 | |
P < 0.001.
R, Right; L, left.
Significant whole‐brain clusters from the functional connectivity analysis are listed in Table 3. In response to viewing MMA, the right BLA showed enhanced coupling with the dorsal anterior cingulate cortex, vmPFC, and to left prefrontal regions, including dorsolateral prefrontal cortex (Fig. 2E). Right CE showed increased coupling with the brainstem and reduced connectivity bilaterally with postcentral gyri and left superior parietal lobule (Fig. 2E). The whole right amygdala seed showed enhanced connectivity to left middle occipital (x = −46, y = −77, z = 37).
Table 3.
Whole‐brain results for psychophysiological interaction in MMA>CAPO seeded in right BLA and right CE with and without factor scores on the Psychopathic Personality Inventory‐Revised as a covariate.
| Seed | Brain region | MNI coordinates | Cluster size | T | Cohen's d | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| BLA | |||||||
| R ventromedial Prefrontal | 13 | 53 | 3 | 921 | 4.11 | 1.27 | |
| L Dorsolateral Prefrontal | −34 | 52 | 17 | 162 | 4.35 | 1.34 | |
| L Superior Frontal | −22 | 38 | 26 | 1366 | 4.41 | 1.36 | |
| R Dorsal Anterior Cingulate | 10 | 20 | 45 | 61 | 3.93 | 1.21 | |
| BLA‐Fearless Dominance | |||||||
| Paracentral Lobule | −2 | −30 | 75 | 508 | 4.55 | 1.42 | |
| R Lingual | 22 | −80 | 0 | 444 | 3.98 | 1.24 | |
| L Middle Occipital | −36 | −83 | 1 | 302 | 3.80 | 1.19 | |
| R Middle Occipital | 37 | −72 | 33 | 212 | 3.76 | 1.17 | |
| R Fusiform | 34 | −49 | −13 | 183 | 3.66 | 1.14 | |
| L Middle Temporal | −48 | −30 | −13 | 148 | 3.79 | 1.18 | |
| Supplementary Motor Area | 1 | −2 | 75 | 135 | 4.16 | 1.30 | |
| R Temporal Pole | 50 | 19 | −24 | 130 | 3.90 | 1.22 | |
| R Superior Parietal Lobule | 17 | −56 | 58 | 109 | 3.64 | 1.14 | |
| R Precentral | 44 | −7 | 39 | 103 | 3.54 | 1.11 | |
| BLA‐Self‐Centered Impulsivity | |||||||
| L Inferior Frontal Gyrus | −41 | 23 | −11 | 205 | −4.00 | −1.25 | |
| BLA‐Coldheartedness | |||||||
| n.s. | |||||||
| CE | |||||||
| R Postcentral | 57 | −18 | 38 | 297 | −3.92 | −1.21 | |
| L Postcentral | −37 | −30 | 41 | 980 | −4.29 | −1.32 | |
| Brainstem | −11 | −10 | −24 | 51 | 3.85 | 1.19 | |
| L Superior Parietal Lobule | −42 | −34 | 60 | 71 | −3.98 | −1.23 | |
| CE‐Fearless Dominance | |||||||
| L Posterior Insula | −34 | −25 | 21 | 157 | 4.01 | 1.25 | |
| L Cerebellum | −44 | −70 | −36 | 103 | 3.64 | 1.14 | |
| CE‐Self‐Centered Impulsivity | |||||||
| n.s. | |||||||
| CE‐Coldheartedness | |||||||
| L Dorsal Anterior Cingulate | −12 | 32 | 27 | 429 | −3.85 | −1.20 | |
P < 0.001.
BLA, basolateral; CE, central; R, Right; L, left.
Figure 2.
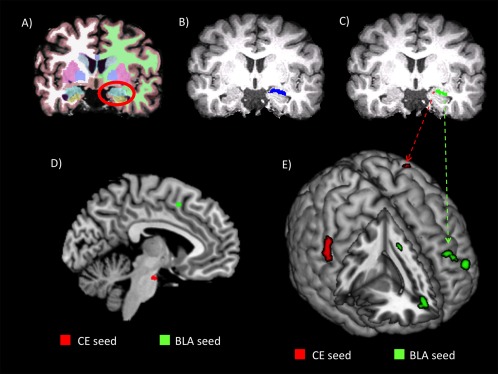
Subnuclei segmentation and connectivity results. A representative single subject's structural MRI is shown with semiautomatic parcellations and segmentations (A), right amygdala mask (B), and central (red; CE) and basolateral complex (green; BLA) masks (C). D: Connectivity results (P < 0.001) on a midline sagittal slice (MNI x = −5) for regions showing increased connectivity with CE (red) and BLA (green). E: Rendered connectivity results (P < 0.001) for regions showing increased connectivity with BLA (green) and decreased connectivity with CE (red) for MMA > CAPO. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Fearless Dominance scores from the PPI‐R were positively related to increased functional connectivity between BLA and regions in bilateral occipital, fusiform, temporal, and parietal cortex (Fig. 3). Fearless Dominance scores also predicted increased connectivity between CE and left posterior insula and cerebellum. Fearless Dominance was not significantly related to connectivity seeded with the full right amygdala. Higher scores on Self‐Center Antisociality predicted decreased coupling between BLA and left inferior frontal gyrus, while high Coldheartedness scores were related to reduce connectivity between CE and the left dorsal anterior cingulate cortex.
Figure 3.
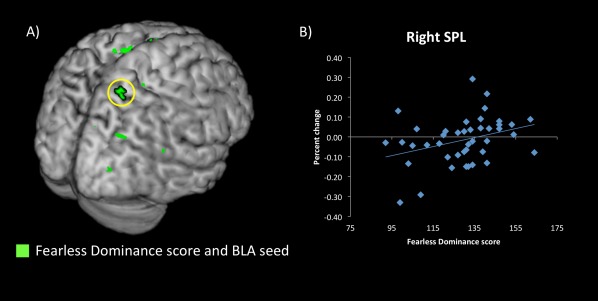
Effects of psychopathic personality traits. A: Whole‐brain regions showing significant positive relationships (P < 0.001) between fearless dominance score on psychopathic personality inventory‐revised and functional connectivity seeded in BLA (green). B: Fearless dominance scores and percent change in connectivity within right superior parietal lobule from whole‐brain cluster (circled in yellow in A). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
This study was designed to investigate the influence of psychopathic personality traits on the neuronal coupling of BLA and CE, respectively. While observing video clips depicting violence, as compared to the nonviolent control condition, effective coupling increased between BLA and frontal regions involved in salience processing, socioemotional reasoning, and attentional control. Conversely, functional connectivity decreased between CE areas important for somatosensory representations and visual processing. Importantly, there was strong convergence between the observed functional connectivity and previously established anatomical connectivity based in BLA and CE, yet these connectivity patterns were absent when the full amygdala was used as a seed, revealing the important insights offered by subnuclei‐specific investigations.
These findings suggest that observing interpersonal violence is associated not only with increased recruitment of prefrontal and midline structures involved in salience processing and decision‐making [Harsay et al., 2012; Porges and Decety, 2013; Yoder and Decety, 2014], but also with enhanced effective connectivity between these regions and specific subnuclei of the amygdala. The dorsal anterior cingulate (dACC) is one of the core nodes of the salience network [Harsay et al., 2012]. One of the only other studies examining BLA and CE functional connectivity in humans found that dACC showed increased coupling with CE and decreased coupling with BLA [Roy et al., 2009]. This is opposite to the pattern of connectivity found here (Table 3; Fig. 2D,E). However, Roy and colleagues assessed functional connectivity during resting state, whereas the current study used video clip stimuli selected to be inherently salient. As dACC and the salience network have been shown to modulate activity in the default network [Chiong et al., 2013], this reversal in neuronal coupling is taken as evidence that participants were engaged by the task.
The critical role of the amygdala in assigning emotional significance or value to sensory information was proposed long ago, starting with lesion studies in nonhuman primates [Kluver and Bucy, 1939]. Moreover, substantial converging evidence from lesion studies in nonhuman models and neurological patients as well as functional neuroimaging studies clearly indicate that specific nuclei are important for a wide range of functions, including but not limited to fear conditioning [Ledoux et al., 1990], social and emotional processing [Adolphs et al., 2002; Phelps and LeDoux, 2005; Sander et al., 2003], processing stimulus relevance for the goals and motivations of the perceiver [Cunningham and Brosch, 2012] and the development of moral reasoning [Anderson et al., 2006; Decety et al., 2012; Taber‐Thomas et al., 2014].
However, data about subnuclei functioning in humans has so far been scarce. Some preliminary insights come from Urbach–Wiethe disease, a genetic disorder that causes the calcification of the medial temporal lobes. One study conducted with a group of patients with focal bilateral BLA damage showed that these individuals were hypervigilant to fearful faces [Terburg et al., 2012]. They were also worse than controls at ignoring task‐irrelevant threatening body postures during an emotional facial expression identification task [De Gelder et al., 2014], further supporting the importance of BLA for filtering out potentially threatening, but task‐irrelevant, information. This is similar to the “attentional bottleneck” hypothesis of psychopathy [Baskin‐Sommers et al., 2011], especially with regards to Fearless Dominance. In this study, we find specific influences of Fearless Dominance scores on connectivity between BLA and regions involved in sensory processing (occipital, fusiform, and temporal pole), as well as selective attention (superior parietal cortex and SMA; Table 3; Fig. 3). The increased neuronal coupling with superior parietal cortex is interesting, since Fearless Dominance scores were negatively related to signal in rTPJ (Table 2). Given the role of the pSTS/rTPJ in processing mental state information and moral judgment [Decety and Cacioppo, 2012; Yoder and Decety, 2014; Young and Dungan, 2012; Young and Saxe, 2009], these results suggest that individuals with high trait Fearless Dominance are less concerned about the moral implications of violence, but may still find interpersonal violence especially threatening, salient, or less stressful.
Because Self‐Centered Impulsivity scores are related to impulsivity, it was expected that individuals with high Self‐Centered Impulsivity scores would show reduced connectivity with prefrontal regions involved in emotion regulation. In support of this hypothesis, Self‐Centered Impulsivity scores were negatively related to neuronal coupling between BLA and ventrolateral prefrontal cortex (Table 3). A previous study used structural equation modeling and found that vlPFC was related to two competing circuits for emotional reappraisal [Wager et al., 2008]. Connectivity from vlPFC to amygdala was important for decreasing negative affect, while connectivity to ventral striatum was responsible for generating positive reappraisals [Wager et al., 2008]. Since high Self‐Centered Impulsivity scores were related to decreased BLA‐vlPFC connectivity, and Self‐Centered Impulsivity predicted increased recruitment of bilateral caudate for the whole‐brain MMA>CAPO contrast (Table 2), these results suggest that Self‐Centered Impulsivity may be related to a network imbalance that produces affective appraisals that are positively biased. Given that a recent study showed that it is specifically descending projections from prefrontal regions to BLA that produce behavioral disruption [St Onge et al., 2012], it is likely that this disruption is specifically related to decreased inhibitory cortical input to BLA.
Finally, scores on Coldheartedness were related to decreased hemodynamic response in bilateral occipital and left middle frontal cortex (Table 2). Moreover, Coldheartedness predicted decreased connectivity between CE and left dorsal anterior cingulate cortex (Table 3), consistent with previous work showing Coldheartedness predicted reduced amygdala response to social stimuli [Han et al., 2012]. As an important node in the salience network, dACC is important for orchestrating cortical networks in response to motivationally relevant stimuli. Thus, reduced CE‐dACC connectivity, along with decreased recruitment of early sensory areas, could indicate that the reason individuals with high trait Coldheartedness show disregard for the well‐being of others is because they do not encode violence as inherently salient.
Taken together, the results of our study largely support and extend DAAM by linking Fearless Dominance and Self‐Centered Impulsivity to specific patterns of functional connectivity with right BLA. For instance, specific BLA dysfunction and Fearless Dominance scores have separately been linked to abnormal selective attention [De Gelder et al., 2014; Sadeh and Verona, 2008]. Here, Fearless Dominance scores predicted increased functional coupling between BLA and regions important for selective attention and sensory processing, including bilateral occipital areas and right temporal pole and superior parietal lobule (Table 3). Conversely, BLA dysfunction and Self‐Centered Impulsivity have independently been related to reduced sensitivity to negative feedback [Heritage and Benning, 2013; St Onge et al., 2012]. This study found negative relationships between Self‐Centered Impulsivity scores and connectivity between BLA and vlPFC (Table 3), a region known to be important for updating the incentive salience value of stimuli [St Onge et al., 2012]. Thus, these data are consistent with the hypothesis that abnormal BLA functioning underlies both Fearless Dominance and Self‐Centered Impulsivity, because it is a nexus of separable subcortical‐cortical networks, and provide a potential neurobiological basis for specific contribution of psychopathic personality traits to amygdala subnuclei functional connectivity.
Importantly, to assess psychopathic traits, we used the PPI‐R rather than PCL‐R, and while total scores on these metrics are often highly correlated, their respective factor scores appear to measure related, but not identical aspects of personality [Malterer et al., 2010]. Moreover, the PCL‐R is conventionally divided (and largely used) into two factors, rather than three. Thus, we tentatively suggest that studies utilizing the PCL‐R may find that the neural activity in response to violence is predicted by scores on PCL‐R Factor 1 (interpersonal‐affective) is similar to both Fearless Dominance and Coldheartedness, with increased connectivity between BLA and occipital and parietal areas, as well as decreased connectivity between CE and dACC. Conversely, PCL‐R Factor 2 (antisociality) might predict reduced connectivity between BLA and vlPFC. However, there is some recent evidence that factors scores on PPI‐R and PCL‐R are not easily related to each other [Copestake et al., 2011]. Therefore, the observed patterns of functional connectivity specifically related to Fearless Dominance and Self‐Centered Impulsivity may not be directly related to Factor 1 and Factor 2 of the PCL‐R. Additionally, the current results are expected to be most applicable to the general population, and may not necessarily hold in forensic populations.
One limitation of this study is that it cannot distinguish between the specific contributions of violence‐specific processing and general affective arousal. In fact, previous work indicates that viewing MMA can elicit autonomic arousal, as measured by electrodermal activity [Smith et al., 2014], suggesting that the perception of harm, even in the context of a sporting event, is inherently physiologically arousing. While the CAPO condition is an appropriate control for many aspects of nonviolent information present in the MMA condition (e.g., two males engaged in dynamic movements filmed in front of an audience), future studies could use other nonviolent but arousing images to further tease apart neuronal responses specific to violence processing. Similarly, the observed patterns of connectivity may not generalize to less salient stimuli.
This study is the first to examine the differential functional connectivity between input and output amygdala subnuclei using tractography‐based segmentation [Saygin et al., 2011], which classifies amygdala subnuclei based on the anatomical projections between specific subnuclei and distinct cortical and subcortical regions [Freese and Amaral, 2009] in an individual's native space. Previous work has labeled nuclei using probabilistic atlases on normalized images, and shown that resting state fMRI connectivity is largely in concert with nonhuman animal studies [Roy et al., 2009]. However, the tractography‐based segmentation approach allows for greater specificity than defining lateral and medial divisions based on two primary diffusion directions [Solano‐Castiella et al., 2010] and is less time consuming than approaches using ultrahigh resolution structural MRI [Entis et al., 2012; Solano‐Castiella et al., 2011]. Future studies could likely improve on this method using smaller, isotropic voxels for the DTI acquisition. Also, it is important to note that the psychophysiological interaction methodology highlights regions that show significant coactivity, but cannot indicate the directionality of information transfer, or the degree of anatomical connectivity between coactive regions. This is especially true for the BLA seed, since the neocortex, lateral nucleus, and basal nucleus, in general, form a circuit [Pitkänen and Amaral, 1991, 1998]. Thus, this study does not offer information about whether specific psychopathic traits are linked with disruption of ascending sensory processing, descending cortical regulation, or some combination of both. However, tractography‐based segmentation provides a powerful tool to use anatomically defined subnuclei masks to identify unique functional connectivity patterns that would otherwise not be found. Ideally, this method of subdividing the amygdala and examining its functional connectivity in response to specific socially relevant stimuli will be used in future neuroimaging studies with both forensic and community samples, rather than treating the amygdala as a whole.
CONCLUSION
Overall, this study, by leveraging anatomical amygdala connectivity, assessed the relationships between specific psychopathic personality traits and effective amygdala connectivity, and casts new light on the unique response of this region to highly salient affective stimuli. These findings indicate how socioemotional processing is impacted by psychopathy personality, and provide evidence for partially distinct neural networks which overlap in the amygdala and are associated with different aspects of psychopathic personality. Moreover, the data provide further evidence that psychopathic personality traits extend into the general population and differentially influence patterns of neural connectivity.
Conflict of interest: None of the authors have any conflict of interest.
REFERENCES
- Adolphs R, Baron‐Cohen S, Tranel D (2002): Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci 14:1264–1274. [DOI] [PubMed] [Google Scholar]
- Allman JM, Hakeem AY, Erwin JM, Nimchinsky E, Hof PR (2001): The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci 935:107–117. [PubMed] [Google Scholar]
- Almeida PR, Ferreira‐Santos F, Vieira JB, Moreira PS, Barbosa F, Marques‐Teixeira J (2014): Dissociable effects of psychopathic traits on cortical and subcortical visual pathways during facial emotion processing: An ERP study on the N170. Psychophysiology 51:645–657. [DOI] [PubMed] [Google Scholar]
- Anderson NE, Kiehl KA (2012): The psychopath magnetized: insights from brain imaging. Trends Cogn Sci 16:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SW, Barrash J, Bechara A, Tranel D (2006): Impairments of emotion and real‐world complex behavior following childhood‐ or adult‐onset damage to ventromedial prefrontal cortex. J Int Neuropsychol Soc 12:224–235. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross S (2006): Parallel incentive processing: An integrated view of amygdala function. Trends Neurosci 29:272–279. [DOI] [PubMed] [Google Scholar]
- Baskin‐Sommers AR, Curtin JJ, Newman JP (2011): Specifying the attentional selection that moderates the fearlessness of psychopathic offenders. Psychol Sci 22:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA (2002): The amygdala and reward. Nat Rev Neurosci 3:563–573. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen‐Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM (2003): Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magn Reson Med 50:1077–1088. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW (2007): Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Hicks BM, Blonigen DM, Krueger RF (2003): Factor structure of the psychopathic personality inventory: Validity and implications for clinical assessment. Psychol Assess 15:340–350. [DOI] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Blonigen DM, Hicks BM, Iacono WG (2005): Estimating facets of psychopathy from normal personality traits: A step toward community epidemiological investigations. Assessment 12:3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H (2005): Deficient fear conditioning in psychopathy. Arch Gen Psychiatry 62:799–805. [DOI] [PubMed] [Google Scholar]
- Blair RJR (2007): The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn Sci 11:387–392. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Frisoni GB, Hare RD, Cavedo E, Najt P, Pievani M, Rasser PE, Laakso MP, Aronen HJ, Repo‐Tiihonen E, Vaurio O, Thompson PM, Tiihonen J (2011): Cortex and amygdala morphology in psychopathy. Psychiatry Res 193:85–92. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS (2004): Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci 8:539–546. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M (1995): Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear‐potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci 15:2301–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré JM, Hyde LW, Neumann CS, Viding E, Hariri AR (2013): The neural signatures of distinct psychopathic traits. Soc Neurosci 8:122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiong W, Wilson SM, D'Esposito M, Kayser AS, Grossman SN, Poorzand P, Seeley WW, Miller BL, Rankin KP (2013): The salience network causally influences default mode network activity during moral reasoning. Brain 136:1929–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copestake S, Gray NS, Snowden RJ (2011): A comparison of a self‐report measure of psychopathy with the psychopathy checklist‐revised in a UK sample of offenders. J Forens Psychiatry Psychol 22:169–182. [Google Scholar]
- Cunningham WA, Brosch T (2012): Motivational salience: Amygdala tuning from traits, needs, values, and goals. Curr Dir Psychol Sci 21:54–59. [Google Scholar]
- Decety J (2011): Dissecting the neural mechanisms mediating empathy. Emot Rev 3:92–108. [Google Scholar]
- Decety J, Cacioppo S (2012): The speed of morality: A high‐density electrical neuroimaging study. J Neurophysiol 108:3068–3072. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Kinzler KD (2012): The contribution of emotion and cognition to moral sensitivity: A neurodevelopmental study. Cereb Cortex 22:209–220. [DOI] [PubMed] [Google Scholar]
- Decety J, Chen C, Harenski C, Kiehl KA (2013a): An fMRI study of affective perspective taking in individuals with psychopathy: Imagining another in pain does not evoke empathy. Front Hum Neurosci 7:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Skelly LR, Kiehl KA (2013b): Brain response to empathy‐eliciting scenarios involving pain in incarcerated individuals with psychopathy. JAMA Psychiatry 70:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Skelly L, Yoder KJ, Kiehl KA (2014): Neural processing of dynamic emotional facial expressions in psychopaths. Soc Neurosci 9:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gelder B, Terburg D, Morgan B, Hortensius R, Stein DJ, van Honk J (2014): The role of human basolateral amygdala in ambiguous social threat perception. Cortex 52:28–34. [DOI] [PubMed] [Google Scholar]
- Entis JJ, Doerga P, Barrett LF, Dickerson BC (2012): A reliable protocol for the manual segmentation of the human amygdala and its subregions using ultra‐high resolution MRI. Neuroimage 60:1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow M, LeDoux J (1999): Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23:229–232. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Pascual‐Leone A, Théoret H (2008): Psychopathy and the mirror neuron system: Preliminary findings from a non‐psychiatric sample. Psychiatry Res 160:137–144. [DOI] [PubMed] [Google Scholar]
- Fischl B (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002): Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–55. [DOI] [PubMed] [Google Scholar]
- Fox GR, Sobhani M, Aziz‐Zadeh L (2013): Witnessing hateful people in pain modulates brain activity in regions associated with physical pain and reward. Front Psychol 4:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese J, Amaral DG (2009): Neuroanatomy of the primate amygdala In: Whalen PJ, Phelps EA, editors. The Human Amygdala. New York, NY: The Guilford Press; pp 3–42. [Google Scholar]
- Han T, Alders GL, Greening SG, Neufeld RWJ, Mitchell DGV (2012): Do fearful eyes activate empathy‐related brain regions in individuals with callous traits? Soc Cogn Affect Neurosci 7:958–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD (2003): The Hare Psychopathy Checklist‐Revised, 2nd ed Toronto, Ontario, Canada: Multi‐Health Systems. [Google Scholar]
- Harsay HA, Spaan M, Wijnen JG, Ridderinkhof KR (2012): Error awareness and salience processing in the oddball task: Shared neural mechanisms. Front Hum Neurosci 6:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heritage AJ, Benning SD (2013): Impulsivity and response modulation deficits in psychopathy: Evidence from the ERN and N1. J Abnorm Psychol 122:215–222. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, Liddle PF (2001): Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol Psychiatry 50:677–684. [DOI] [PubMed] [Google Scholar]
- Kluver H, Bucy P (1939): Preliminary analysis of functions of the temporal lobes in monkeys. Arch Neurol Psychiatry 42:979–1000. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T (2011): Meta‐analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54:2492–2502. [DOI] [PubMed] [Google Scholar]
- Larson CL, Baskin‐Sommers AR, Stout DM, Balderston NL, Curtin JJ, Schultz DH, Kiehl KA, Newman JP (2013): The interplay of attention and emotion: top‐down attention modulates amygdala activation in psychopathy. Cogn Affect Behav Neurosci 13:757–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux J, Cicchetti P, Xagoraris A, Romanski L (1990): The lateral amygdaloid in fear conditioning nucleus: sensory interface amygdala. J Neurosci 10:1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld SO, Widows MR (2005): Psychopathic Personality Inventory‐Revised: Professional Manual. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Malterer MB, Lilienfeld SO, Neumann CS, Newman JP (2010): Concurrent validity of the psychopathic personality inventory with offender and community samples. Assessment 17:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M (2011): Reduced prefrontal connectivity in psychopathy. J Neurosci 31:17348–17357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moul C, Killcross S, Dadds MR (2012): A model of differential amygdala activation in psychopathy. Psychol Rev 119:789–806. [DOI] [PubMed] [Google Scholar]
- Newman JP, Patterson CM, Kosson DS (1987): Response perseveration in psychopaths. J Abnorm Psychol 96:145–148. [DOI] [PubMed] [Google Scholar]
- Ongür D, Price JL (2000): The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10:206–219. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Fowles DC, Krueger RF (2009): Triarchic conceptualization of psychopathy: Developmental origins of disinhibition, boldness, and meanness. Dev Psychopathol 21:913–938. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE (2005): Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 48:175–187. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Amaral DG (1991): Demonstration of projections from the lateral nucleus to the basal nucleus of the amygdala: A PHA‐L study in the monkey. Exp brain Res 83:465–470. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Amaral DG (1998): Organization of the intrinsic connections of the monkey amygdaloid complex: Projections originating in the lateral nucleus. J Comp Neurol 398:431–458. [DOI] [PubMed] [Google Scholar]
- Porges EC, Decety J (2013): Violence as a source of pleasure or displeasure is associated with specific functional connectivity with the nucleus accumbens. Front Hum Neurosci 7:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Amaral DG (1981): An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci 1:1242–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP (2009): Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45:614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N, Verona E (2008): Psychopathic personality traits associated with abnormal selective attention and impaired cognitive control. Neuropsychology 22:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ESL, Lopez De Armentia M, Power J (2003): The amygdaloid complex: Anatomy and physiology. Physiol Rev 83:803–834. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T (2003): The human amygdala: An evolved system for relevance detection. Rev Neurosci 14:303–316. [DOI] [PubMed] [Google Scholar]
- Saygin ZM, Osher DE, Augustinack J, Fischl B, Gabrieli JDE (2011): Connectivity‐based segmentation of human amygdala nuclei using probabilistic tractography. Neuroimage 56:1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulreich S, Pfabigan DM, Derntl B, Sailer U (2013): Fearless dominance and reduced feedback‐related negativity amplitudes in a time‐estimation task—Further neuroscientific evidence for dual‐process models of psychopathy. Biol Psychol 93:352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL (2008): The role of the amygdala in emotional processing: A quantitative meta‐analysis of functional neuroimaging studies. Neurosci Biobehav Rev 32:811–830. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ (2011): The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12:154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeem JL, Polaschek DLL, Patrick CJ, Lilienfeld SO (2011): Psychopathic personality: Bridging the gap between scientific evidence and public policy. Psychol Sci Public Interest 12:95–162. [DOI] [PubMed] [Google Scholar]
- Smith KE, Porges EC, Norman GJ, Connelly JJ, Decety J (2014): Oxytocin receptor gene variation predicts empathic concern and autonomic arousal while perceiving harm to others. Soc Neurosci 9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano‐Castiella E, Anwander A, Lohmann G, Weiss M, Docherty C, Geyer S, Reimer E, Friederici AD, Turner R (2010): Diffusion tensor imaging segments the human amygdala in vivo. Neuroimage 49:2958–2965. [DOI] [PubMed] [Google Scholar]
- Solano‐Castiella E, Schäfer A, Reimer E, Türke E, Pröger T, Lohmann G, Trampel R, Turner R (2011): Parcellation of human amygdala in vivo using ultra high field structural MRI. Neuroimage 58:741–748. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Stopper CM, Zahm DS, Floresco SB (2012): Separate prefrontal‐subcortical circuits mediate different components of risk‐based decision making. J Neurosci 32:2886–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber‐Thomas BC, Asp EW, Koenigs M, Sutterer M, Anderson SW, Tranel D (2014): Arrested development: Early prefrontal lesions impair the maturation of moral judgement. Brain 137:1254–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terburg D, Morgan BE, Montoya ER, Hooge IT, Thornton HB, Hariri AR, Panksepp J, Stein DJ, van Honk J (2012): Hypervigilance for fear after basolateral amygdala damage in humans. Transl Psychiatry 2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira JB, Marsh AA (2014): Don't stand so close to me: Psychopathy and the regulation of interpersonal distance. Front Hum Neurosci 7:907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN (2008): Prefrontal‐subcortical pathways mediating successful emotion regulation. Neuron 59:1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Cardinal RN, Robbins TW (2004): Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci 24:4718–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KJ, Decety J (2014): The good, the bad, and the just: Justice sensitivity predicts neural response during moral evaluation of actions performed by others. J Neurosci 34:4161–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Dungan J (2012): Where in the brain is morality? Everywhere and maybe nowhere. Soc Neurosci 7:1–10. [DOI] [PubMed] [Google Scholar]
- Young L, Saxe R (2009): An FMRI investigation of spontaneous mental state inference for moral judgment. J Cogn Neurosci 21:1396–405. [DOI] [PubMed] [Google Scholar]


