Abstract
LIN28B has been identified as an oncogene in various tumor entities, including neuroblastoma, a childhood cancer that originates from neural crest-derived cells, and is characterized by amplification of the MYCN oncogene. Recently, elevated LIN28B expression levels were shown to contribute to neuroblastoma tumorigenesis via let-7 dependent de-repression of MYCN. However, additional insight in the regulation of LIN28B in neuroblastoma is lacking. Therefore, we have performed a comprehensive analysis of the regulation of LIN28B in neuroblastoma, with a specific focus on the contribution of miRNAs.
We show that MYCN regulates LIN28B expression in neuroblastoma tumors via two distinct parallel mechanisms. First, through an unbiased LIN28B-3′UTR reporter screen, we found that miR-26a-5p and miR-26b-5p regulate LIN28B expression. Next, we demonstrated that MYCN indirectly affects the expression of miR-26a-5p, and hence regulates LIN28B, therefor establishing a MYCN-miR-26a-5p-LIN28B regulatory axis. Second, we provide evidence that MYCN regulates LIN28B expression via interaction with the LIN28B promotor, establishing a direct MYCN-LIN28B regulatory axis. We believe that these findings mark LIN28B as an important effector of the MYCN oncogenic phenotype and underlines the importance of MYCN-regulated miRNAs in establishing the MYCN-driven oncogenic process.
Keywords: microRNA, integrative analysis, cross-species
1. Introduction
The highly conserved RNA-binding proteins LIN28A and LIN28B play important roles in glucose metabolism [1] and during development and stem cell reprogramming by regulating the self-renewal of stem cells [2]. They mediate these pleiotropic functions by modulating the translation of target messenger RNAs (mRNAs) and by inhibiting let-7 microRNA (miRNA) biogenesis – their main mode of action studied. The let-7 family of miRNAs comprises a group of 12 sequence-related miRNAs, encoded by 8 distinct genomic loci. Because these miRNAs regulate multiple stemness regulators and bona fide proto-oncogenes, such as RAS, HMGA2 and MYC, they play an essential role in stem cell differentiation and tumor-suppression. As a consequence, deregulated LIN28A or LIN28B expression contributes to abnormal development and tumorigenesis.
Recently, elevated LIN28B expression levels were shown to contribute to neuroblastoma tumorigenesis via let-7 dependent de-repression of MYCN [3]. Neuroblastoma is a pediatric tumor of the sympathetic nervous system and is characterized by high MYCN activity through genomic amplification, which occurs in about half of high-stage neuroblastoma tumors and marks poor survival. LIN28B is frequently overexpressed in high-risk neuroblastoma, and high-level amplifications of the 6q21 region including the LIN28B gene occur at a low frequency. In addition, increased expression of LIN28B is associated with poor outcome of neuroblastoma patients [3]. This prominent LIN28B expression results in a strong increase in the amount of MYCN protein via repression of the MYCN-targeting let-7 miRNAs, as shown by expression analysis of murine Lin28b-driven tumors and a series of in vitro LIN28B model systems [3].
Increased LIN28B expression in neuroblastoma tumors could be attributed to genomic amplification in only a few samples. Therefore, additional mechanisms are likely to be involved, such as alterations in upstream regulatory pathways as well as transcriptional and post-transcriptional control. Indeed, several miRNAs, including the let-7 family miRNAs, were reported to target the LIN28B 3′ untranslated region (3′UTR) [4–8]. Interestingly, also MYCN has been proposed to induce LIN28B transcription [9,10] but other studies could not confirm these findings [3]. Taken together, the present data point at an intimate regulatory interconnection between MYCN and LIN28B, let-7 and possibly also other miRNAs.
In this study, we set about to elucidate the regulation of LIN28B expression in neuroblastoma tumors, with particular focus on the contribution of MYCN and miRNAs. To this end, we performed a comprehensive, genome-wide exploration of the miRNA-LIN28B interactome in neuroblastoma. We combined results from an unbiased and genome-wide high-throughput miRNA target reporter screen with miRNA and mRNA expression data from 200 neuroblastoma patients and identified twelve LIN28B-targeting miRNAs in neuroblastoma. Subsequent integration of the screen results and clinical data from neuroblastoma patients prioritized miR-26a-5p and miR-26b-5p for further analysis. Using different MYCN-driven in vitro and in vivo model systems, we show that MYCN induces the expression of LIN28B via indirect repression of miR-26a-5p and miR-26b-5p. In addition, we provide evidence that MYCN further enhances the expression of LIN28B via direct binding to the LIN28B promoter region. Hence, our findings clarify the role of MYCN and miRNAs in the upstream regulation of LIN28B in neuroblastoma and describe a double feed-forward loop between MYCN and LIN28B.
2. Materials and methods
2.1. LIN28B 3′UTR-miRNA library screen
Since the wild-type LIN28B (ENST00000345080, Ensemble release 78) 3′ UTR is longer than 3.5kb, GeneCopoeia (Rockville, MD, U.S.A) provided the UTR into two constructs with overlapping sequence; further referred to as construct A (1 – 3084bp, catalog number: HmiT010363a-MT01) and construct B (2905 – 4548bp, catalog number: HmiT010363b-MT01). HEK293T cells were seeded at a density of 1×104 cells/well in 96-well plates. Twenty-four hours after seeding, cells were co-transfected with 100 ng of one of the two LIN28B reporter vectors and 20 ng of pRL-TK control vector containing the Renilla luciferase gene (Promega, Madison, WI, USA) together with a library of 470 miRNA mimics (2.5 pmol) (Ambion’s Pre-miR miRNA Precursor Library - Human V3, design based on miRBase v9.2 with exclusion of hsa-miR-122a; Life Technologies, Carlsbad, CA, USA). Lipid based transfections were performed using 0.4 μl Dharmafect Duo reagent (GE Dharmacon, Lafayette, CO, USA). Forty-eight hours post-transfection, luciferase reporter gene activities were assayed using the Dual-Luciferase Reporter assay system (Promega) according to the manufacturer’s protocol with minor changes (LARII and Stop & Glo buffer volumes were reduced to 50 μl). Firefly reporter gene activities were normalized to Renilla values and then log-transformed. Subsequently, robust z-scores were calculated; the robust z-score is a variation of the outlier-sensitive z-score that substitutes the outlier-insensitive median and median absolute deviation (MAD) for mean and standard deviation in the z-score calculation. In order to determine the robust z-score cutoff that separates interactions from non-interactions, the scores for a set of validated miRNA interactions, probed in the LIN28B screen and in 36 analogous screens (of 17 cancer and disease associated genes), were used together with the scores for a set of negative control interactions from an empty-3′UTR vector miRNA library screen to perform ROC-curve analysis and determine the point of highest accuracy (z-score = −3.05, specificity = 97%, sensitivity = 31%, accuracy = 86%). LIN28B 3′UTR-miRNA library screen results were replicated in two independent experiments for both reporter constructs. To allow for correct integration of miRNA data, we used miRBase Tracker, an in-house developed web tool for miRNA reannotation [11], to re-annotate the miRNAs in the miRNA mimic library to the most up-to-date annotation at time of publication (miRBase release 21) (Supplementary Table 2).
2.2. miRNA target analysis
Potential miRNA target sites in the LIN28B 3′UTR were identified as reported previously [12]. In addition, miRNAs targeting LIN28B were predicted using the MirTarget2 algorithm [13].
2.3. miRNA and mRNA expression in patient cohort
200 primary tumor samples of neuroblastoma patients were collected prior to therapy at the Ghent University Hospital (Ghent, Belgium), the University Children’s Hospital Essen (Essen, Germany), the Hospital Clínico Universitario (Valencia, Spain), the Academic Medical Center (University of Amsterdam, Netherlands) and the National Children’s Research Centre (Dublin, Ireland). Informed consent was obtained from the patients’ relatives. mRNA data from 75 primary neuroblastoma tumors is available at the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo; Accession Number: GSE32664). Correlation of miRNA expression levels and LIN28B mRNA levels was evaluated with Spearman’s rank correlation coefficient.
2.4. Tissue culture
Human neuroblastoma cell lines were cultured in RPMI 1640 medium (Invitrogen, Waltham, MA, USA) supplemented with fetal bovine serum (10%), kanamycin (1%), penicillin/streptomycin (1%), L-glutamine (1%) and HEPES (25mM) (Life Technologies). Cells were kept at 37°C in a 5% C02/95 %02 humidified environment. Cell line authenticity was validated by Short Tandem Repeat (STR) genotyping prior to performing the described experiments. Experiments with the IMR5-75-shMYCN, SHEP-MYCN-ER and MYCN3 cell lines were performed as previously published [14–16]. In all cases, three replicate experiments were performed and analyzed.
2.5. RT-qPCR
Total RNA was isolated using the miRNeasy Mini Kit (Qiagen, Venlo, Netherlands) according to manufacturer’s instructions, including on-column DNase treatment. RT-qPCR reactions were performed and reported according to the MIQE guidelines [17]. For quantification of gene expression, cDNA was synthetized from 500 ng total RNA with the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions. qPCR reactions were performed with Sybr green detection chemistry, using the LC480 real-time PCR detection system (Bio-Rad). qPCR reactions were performed in a total volume of 5 μl consisting of 2.5 μl of SsoAdvance Universal SYB Green Supermix (Bio-Rad), 0.5 μl forward and reverse primer (5 μM; primer sequences are listed in Supplementary Table 3) and 2 μl of 2.5 ng/μl cDNA (total RNA equivalents). Cycling conditions were as follows: 2 minutes at 95 °C followed by 44 cycles of 5 seconds at 95 °C, 30 seconds at 60 °C and 1 second at 72 °C. Expression levels of LIN28B were normalized to reference genes. Expression analysis as well as error propagation was done using qbase+ software version 3.0 (http://www.biogazelle.com/qbaseplus) (Biogazelle, Zwijnaarde, Belgium) [18]. Measuring the primary miRNAs transcripts was done using stem-loop TaqMan assays. qPCR was performed according to the manufacturer’s protocol using 10 ng of the RT product, TaqMan universal master mix (2×) and TaqMan Pri-miRNA assays (20×) (Applied Biosystems) in 8 μl reaction volume. The following cycling conditions were applied: 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Expression levels were normalized to reference genes. Expression analysis as well as error propagation was done using qbase+ software version 3.0. For quantification of individual miRNA expressions, cDNA was synthesized from 500 ng total RNA with 4 μl of HiSpec Buffer, 2 μl of Nucleics Mix and 2 μl miScript RT Mix (miScript II RT Kit, Qiagen) in a final volume of 20 μl. This reaction mix was incubated for 60′ at 37°C and 5′ at 95°C using an iCycer instrument (Bio-Rad). qPCR reactions contained 3 ng of cDNA, 2.5 μl QuantiTeckt Mastermix, 0.5 μl miScript Universal Primer and 0.5 μl miRNA-specific miScript Primer Assay (Qiagen, miScript Primer Assays used are listed in Supplementary Table 4) in a total volume of 5 μl. Expression levels were normalized against two stably expressed reference miRNAs (hsa-miR-423-5p and hsa-miR-92) validated with GeNorm [19] and analyzed using qbase+ software version 3.0.
2.6. Protein isolation, antibodies and Western blotting
Total protein lysates were harvested after washing with ice-cold PBS and total protein isolation was carried out using RIPA lysis buffer, containing cOmplete Protease Inhibitor Cocktail Tablet (Roche Diagnostics, Basel, Switzerland) and PhosSTOP Phosphatase Inhibitor Cocktail Tablets (Roche Diagnostics). 30 μg of protein lysate was loaded. The antibody directed against LIN28B (4196), secondary anti-rabbit and anti-mouse antibodies were obtained from Cell Signaling Technologies (Danvers, MA, USA). The antibody directed against VCL (V9131) was obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.7. anti-MYCN ChIP-qPCR in MYCN3 cells
MYCN ChIP was performed on 1×107 MYCN3 cells either treated or untreated with doxycycline for 24 h using ChIP-IT Express Chromatin Immunoprecipitation Kit (53008; Active Motif, Carlsbad, CA, USA) according to manufacturer’s instructions. Samples were sonicated for 20 cycles of 30 sec intervals in a Bioruptor UCD-200 sonicator (Diagenode). ChIP-grade Anti-MYCN anti-body (ab16898, abcam), IgG control anti-body (12–370; EMD-Millipore, Billerica, MA, USA) antibodies were used for ChIP and input was generated by purifying DNA from the sonicated lysates of each sample. ChIP-qPCR primers were designed by analyzing putative MYCN binding sites on LIN28B promoter from previous MYCN ChIP-seq data [16] and are listed in Supplementary Table 5. Control primers for a non-specific genomic region and a distal site on LIN28B promoter was also designed and used to validate the specificity of ChIP DNA. Real-time qPCR reactions were performed in triplicates. The amount of genomic DNA co-precipitated with specific antibody was calculated by normalizing and comparing Cq values of MYCN CHIP with input and control IgG.
2.8. Statistical methods
All statistical analyses were performed using R Bioconductor software (version 3.0.2). If not further specified in the results section, statistical significance was defined as p-value < 0.05 for all statistical tests.
3. Results
3.1. An unbiased LIN28B 3′UTR-miRNA library screen identifies 28 miRNAs targeting LIN28B
Potential interactions of 470 miRNAs with the 3′UTR of LIN28B were assayed in a high-throughput luciferase reporter screen. In brief, human embryonic kidney cells (HEK293T) were co-transfected with a reporter construct, containing the LIN28B 3′UTR downstream of a luciferase reporter gene, and each of the individual miRNA mimics from a 470 miRNA mimic library. Based on the relative luciferase activities in two independent screens (Supplementary Fig. 1), a robust z-score was calculated for each miRNA-LIN28B combination (see Material and Methods). Applying this strategy, we identified 28 miRNAs with a high probability of targeting LIN28B (average robust z-score score < −3.05; see Material and Methods; Fig. 1a and Supplementary Table S1). According to miRTarBase 4.0, an experimentally validated microRNA-target interactions database [20], scientific literature holds weak (n = 8) [4,5,21] or strong (n = 2) [6,7,22] evidence for 10 miRNAs as regulator of LIN28B. Both miRNAs with strong evidence to target LIN28B, let-7b-5p and miR-125b-5p, were validated in our screen (Fig. 1a, red), whereas only 3 out of 8 miRNAs with weak evidence to target LIN28B, let-7d-5p, miR-125a-5p and miR-484, could be confirmed (Fig. 1a, blue). Additionally, 23 new LIN28B targeting miRNAs were identified, of which 14 are predicted to target LIN28B by MirTarget2 [23] (Supplementary Table S1), underscoring the value of our screening method to detect novel, predicted as well as non-predicted, miRNA-target gene interactions. Among the strongest hits in the screens, a significant enrichment was observed for miRNAs with seed-matched sites present in the LIN28B 3′UTR (Fig. 1b), thus further underscoring the sensitivity and robustness of the 3′UTR-miRNA library screen.
Figure 1. An unbiased LIN28B 3′UTR-miRNA library screen identifies 28 miRNAs targeting LIN28B.
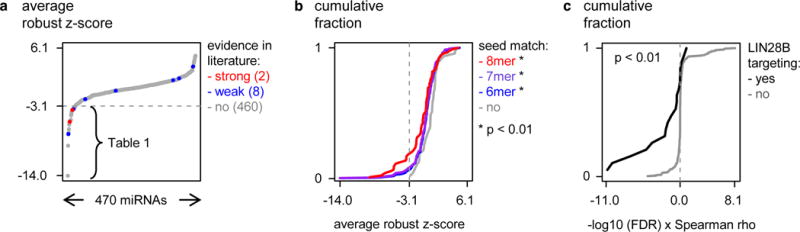
(a) Average robust z-scores are plotted (Y-axis) for 470 tested miRNAs (X-axis). The 28 miRNAs with a z-core of < −3.05 are listed in Table 1. The 10 interactions that have been reported in literature according to miRtarBase, are indicated in red (strong evidence) and blue (weak evidence). (b) Cumulative distribution (Y-axis) of the robust z-scores (X-axis) of miRNAs that respectively have 6-, 7- or 8-mer seed-matches in the LIN28B 3′UTR. P-value represents Kolmogorov-Smirnov test for the distribution of the average robust z-score for miRNAs that have a 6-, 7- or 8-mer seed-match versus miRNAs without seed-match. (c) The cumulative distribution (Y-axis) of the π-value for the correlation between miRNA and LIN28B expression (X-axis) of miRNAs that were identified as either LIN28B-targeting (black) or not LIN28B-targeting (gray). Kolmogorov-Smirnov test, p = 1.4 × 10−3.
3.2. miR-26a-5p and miR-26b-5p are top candidate LIN28B-targeting miRNAs in neuroblastoma
miRNAs are known to regulate their target genes in a highly tissue- and cell type specific manner [24]. As the LIN28B 3′UTR-miRNA library screens were performed in HEK293T cells, we next aimed to identify miRNAs specifically targeting LIN28B in neuroblastoma cells by integrating the obtained screen results with miRNA expression data from a large cohort primary neuroblastoma tumors We reasoned that the expression of a LIN28B-targeting miRNA is inversely correlated to LIN28B mRNA expression levels in primary tumor samples. Of the 19 LIN28B-targeting miRNAs that were included on the human miRNA expression platform, 12 miRNAs showed significant inverse correlation to LIN28B mRNA expression (Table 1), suggesting that they can indeed downregulate LIN28B expression in primary neuroblastoma tumors. In addition, three of the 19 LIN28B-targeting miRNAs that were included on the human miRNA expression platform were positively correlated to MYCN expression (Table 1). Overall, LIN28B targeting miRNAs identified in our screen showed stronger inverse correlation to LIN28B mRNA levels compared to non-targeting miRNAs (Fig. 1c), as indicated by a shift of the cumulative distribution of π-values (−log10 p-value × Pearson correlation coefficient) to more negative values.
Table 1. Overview of 28 LIN28B-targeting miRNAs, categorized according to LIN28B-targeting potential in primary neuroblastoma tumors.
LIN28B-targeting miRNAs are filtered for their relevance in neuroblastoma according to significant negative correlation to LIN28B expression in a neuroblastoma patient cohort. In total, 12 out of 28 miRNAs show negative correlation to LIN28B expression and are considered to be relevant for neuroblastoma, while 3 out of 28 miRNAs show positive correlation to LIN28B expression. Four miRNAs lack correlation to LIN28B expression (n.s.: not significant, Spearman correlation, Benjamini & Hochberg multiple testing corrected p-value > 0.05), and for 9 miRNAs no expression data was available (n.d.: no data available). In addition, of the 15 miRNAs showing correlation to LIN28B expression, the expression of 3 miRNAs is associated to poor outcome of stage 4 neuroblastoma patients (n = 92). n.s.: not significant (log-rank test, p-value > 0.05)
| miRNA | mirBase Accession | Reference in miRTarBase | Spearman rho | log-rank odds ratio |
|---|---|---|---|---|
| miRNA’s with negative correlation to LIN28B expression | ||||
|
| ||||
| hsa-let-7a-5p | MIMAT0000062 | −0.35 | n.s. | |
| hsa-let-7b-5p | MIMAT0000063 | [6,8] | −0.36 | n.s. |
| hsa-let-7c-5p | MIMAT0000064 | −0.23 | n.s. | |
| hsa-let-7d-5p | MIMAT0000065 | [4] | −0.59 | n.s. |
| hsa-let-7f-5p | MIMAT0000067 | −0.45 | n.s. | |
| hsa-let-7g-5p | MIMAT0000414 | −0.54 | n.s. | |
| hsa-let-7i-5p | MIMAT0000415 | −0.60 | n.s. | |
| hsa-miR-125b-5p | MIMAT0000423 | [7] | −0.25 | 14.43 |
| hsa-miR-26a-5p | MIMAT0000082 | −0.37 | 12.48 | |
| hsa-miR-26b-5p | MIMAT0000083 | −0.31 | 13.00 | |
| hsa-miR-363-3p | MIMAT0000707 | −0.23 | n.s. | |
| hsa-miR-98-5p | MIMAT0000096 | −0.49 | n.s. | |
|
| ||||
| miRNA’s with positive correlation to LIN28B expression | ||||
|
| ||||
| hsa-miR-516b-3p | MIMAT0002860 | 0.17 | n.s. | |
| hsa-miR-550a-3p | MIMAT0003257 | 0.28 | n.s. | |
| hsa-miR-641 | MIMAT0003311 | 0.29 | n.s. | |
|
| ||||
| miRNA’s without correlation to LIN28B expression | ||||
|
| ||||
| hsa-let-7e-5p | MIMAT0000066 | n.s. | – | |
| hsa-miR-125a-5p | MIMAT0000443 | [5] | n.s. | – |
| hsa-miR-367-3p | MIMAT0000719 | n.s. | – | |
| hsa-miR-383-5p | MIMAT0000738 | n.s. | – | |
|
| ||||
| miRNA’s with missing data | ||||
|
| ||||
| hsa-miR-182-3p | MIMAT0000260 | n.d. | – | |
| hsa-miR-208a-3p | MIMAT0000241 | n.d. | – | |
| hsa-miR-484 | MIMAT0002174 | [5] | n.d. | – |
| hsa-miR-499a-5p | MIMAT0002870 | n.d. | – | |
| hsa-miR-552-3p | MIMAT0003215 | n.d. | – | |
| hsa-miR-561-3p | MIMAT0003225 | n.d. | – | |
| hsa-miR-569 | MIMAT0003234 | n.d. | – | |
| hsa-miR-605-5p | MIMAT0003273 | n.d. | – | |
| hsa-miR-92b-3p | MIMAT0003218 | n.d. | – | |
Next, we reasoned that miRNAs with a putative tumor suppressive role in neuroblastoma are lower expressed in more aggressive neuroblastoma tumors. Therefore, we assessed the relationship between expression of the LIN28B-targeting miRNAs and the outcome of high-risk neuroblastoma patients (International Neuroblastoma Staging System stage 4, n = 92). For three LIN28B-targeting miRNAs, miR-26a-5p, miR-26b-5p and miR-125b-5p, low expression is associated with poor outcome (Fig. 2a–b and Supplementary Fig. 2). In addition, two miRNAs, miR-26a-5p and miR-26b-5p, were previously reported to be lower expressed in neuroblastoma tumors with MYCN amplification, a marker of aggressive neuroblastoma (Fig. 2c–d) [25]. These observations highlight miR-26a-5p and miR-26b-5p as interesting candidate regulators of LIN28B in neuroblastoma.
Figure 2. miR-26a-5p and miR-26b-5p are top candidate LIN28B-targeting miRNAs in neuroblastoma.
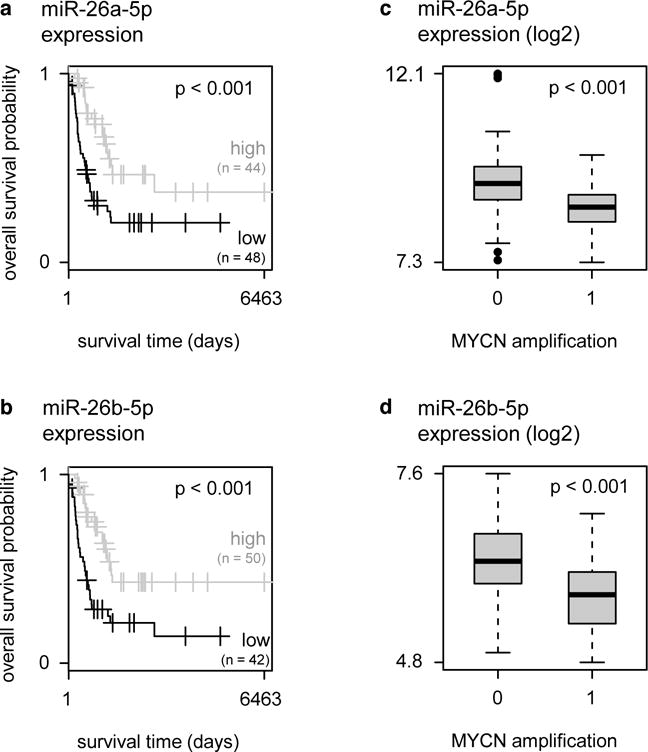
(a) Kaplan-Meier curves for miR-26a-5p in a cohort of primary neuroblastoma tumors. Patients are split in two groups with either miR-26a-5p expression above (= high) or below (= low) the average miR-26a-5p expression. Log-rank test, OR = 12.48, p = 2.4 × 10−3. (b) Kaplan-Meier curves for miR-26b-5p in a cohort of primary neuroblastoma tumors. Patients are split in two groups with either miR-26b-5p expression above (= high) or below (= low) the average miR-26b-5p expression. Log-rank test, OR = 13.00, p = 1.4 × 10−3. (c) Expression (log2) of miR-26a-5p in primary neuroblastoma tumors with MYCN amplification (= 1) versus tumors without MYCN amplification (= 0). T-test, p = 4.3 × 10−7. (d) Expression (log2) of miR-26b-5p in primary neuroblastoma tumors with MYCN amplification (= 1) versus tumors without MYCN amplification (= 0). T-test, p = 1.9 × 10−6.
3.3. Overexpression of miR-26a-5p or miR-26b-5p reduces LIN28B expression
Following the selection of miR-26a-5p and miR-26b-5p as candidate miRNAs in the context of neuroblastoma, we assessed whether these miRNAs regulate LIN28B expression levels in the neuroblastoma cell line NGP. As expected from the luciferase reporter screen, overexpression of either miR-26a-5p or miR-26b-5p induced downregulation of LIN28B expression levels compared with a non-targeting miRNA control (Fig. 3a). Finally, we investigated whether the functions of these miRNAs in neuroblastoma are related to the functions of LIN28B using a Gene Set Enrichment Approach (GSEA). To this end, we first constructed a gene set of genes upregulated by LIN28B in the LSL-Lin28b mouse model [3]. This gene set contains 256 genes that are significantly upregulated in LSL-Lin28B tumors compared to normal adrenal glands. Next, we calculated the Spearman correlation coefficient between the expression of miR-26a-5p or miR-26b-5p and all mRNAs expressed in a large cohort of primary neuroblastoma tumors (n = 200). Finally, we performed GSEA on these ranked lists to assess the enrichment of LIN28B regulated genes among the genes negatively correlated with miR-26a-5p or miR-26b-5p expression in primary neuroblastoma tumors. As expected for miRNAs that regulate the expression of LIN28B, the GSEA analysis for both miRNAs results in strong negative enrichment for the LSL-Lin28b gene set (Fig. 3b–c). This suggests that the functions miR-26a-5p and miR-26b-5p in neuroblastoma are strongly related to those of LIN28B. Hence, the putative tumor-suppressors miR-26a-5p and miR-26b-5p affect the LIN28B expression levels in neuroblastoma and show inverse correlation to LIN28B-induced gene expression patterns.
Figure 3. Overexpression of miR-26a-5p or miR-26b-5p reduces LIN28B expression.
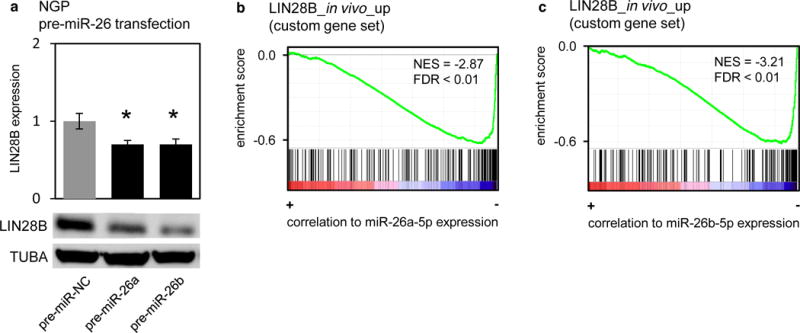
(a) mRNA (upper panel) and protein (lower panel) expression of LIN28B upon overexpression of miR-26a-5p or miR-26b-5p in NGP cells. T-test, pre-miR-26a: p = 2.7 × 10−2; pre-miR-26b: p = 3.5 × 10−2. (b–c) Result of GSEA. Red-blue color bars denote genes ranked based on correlation to miR-26a-5p (b) or miR-26b-5p (c) expression in primary neuroblastoma tumors (n = 200). Each vertical bar corresponds to genes upregulated in LSL-LIN28B tumors vs. normal adrenal glands. The green line represents the running enrichment score calculated in GSEA.
3.4. miR-26a-5p and miR-26b-5p are downregulated in MYCN-driven neuroblastoma
Given the decreased expression of miR-26a-5p and miR-26b-5p in neuroblastoma tumors with MYCN amplification as compared to neuroblastoma tumors that lack MYCN amplification [25], we sought to investigate whether miR-26a-5p and miR-26b-5p were regulated by the oncoprotein MYCN. To this end, we evaluated the expression of both miRNAs in MYCN-driven neuroblastoma models. First, we interrogated a murine neuroblastoma progression model, consisting of pre-tumorigenic lesions and advanced neuroblastoma tumors from the TH-MYCN+/+ mouse model, in combination with matching reference tissue from wild-type mice. Both miR-26a-5p and miR-26b-5p expression strongly decreased during TH-MYCN+/+ neuroblastoma development as compared to normal development (Fig. 4a–b). Next, these results were confirmed in a second, independent, murine MYCN-driven neuroblastoma model, the LSL-MYCN;Dbh-iCre model [26] (further referred to as the LSL-MYCN model) as both miRNAs were found to be lower expressed in LSL-MYCN tumors compared to normal adrenal tissue (Fig. 4c–d).
Figure 4. miR-26a-5p and miR-26b-5p are downregulated in MYCN-driven neuroblastoma.
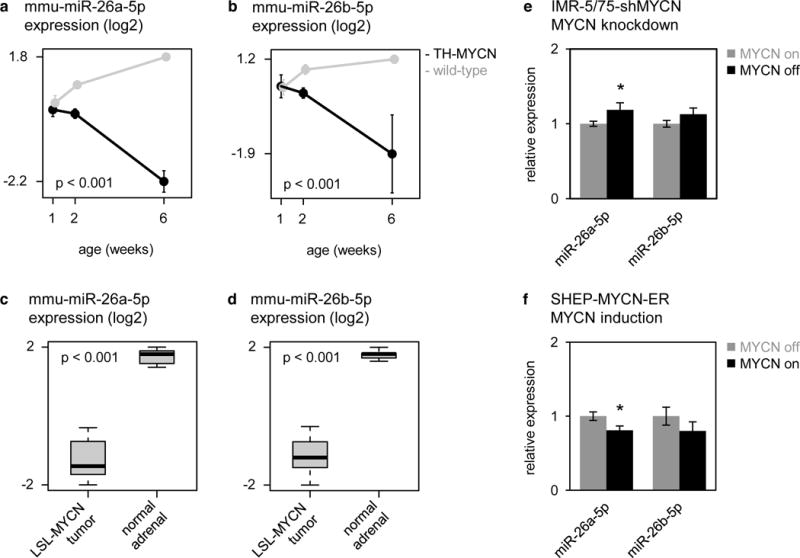
(a–b) Expression (log2) of mmu-miR-26a-5p (a) and mmu-miR-26b-5p (b) in sympathetic ganglia containing hyperplastic lesions, and advanced tumors from TH-MYCN+/+ mice at, respectively, 1 and 2 weeks and 6 weeks of age (black), and in normal sympathetic ganglia from wild-type mice at 1, 2 and 6 weeks of age (gray). Data are presented as mean ± standard deviation of four samples. Two-way ANOVA interaction, mmu-miR-26a-5p: p = 6.2 × 10−13; mmu-miR-26b-5p: p = 1.1 × 10−5. (c–d) Expression of miR-26a-5p (c) and miR-26b-5p (d) in LSL-MYCN tumors compared to normal adrenal tissue. T-test, miR-26a-5p: p = 7.6 × 10−10; miR-26b-5p: p = 8.0 × 10−10. (e) Relative expression of miR-26a-5p and miR-26b-5p in IMR-5/75-shMYCN cells before (gray bars) and after (black bars) silencing of MYCN. Data are presented as mean ± standard deviation of three replicate experiments. T-test, miR-26a-5p: p = 0.041; miR-26b-5p: p > 0.05.
To verify that the decreased expression of miR-26a-5p and miR-26b-5p in these in vivo models is a consequence of increased MYCN activity, we evaluated the expression of both miRNAs in multiple in vitro models of MYCN activity in neuroblastoma. First, the effect of MYCN knockdown on miRNA expression was evaluated in the IMR5-75-shMYCN model system. These cells stably express a tetracycline-inducible MYCN shRNA that, upon induction, reduced MYCN protein to 44% (Supplementary Fig. 3) [14]. We observed a modest but significant increase in the expression of miR-26a-5p upon silencing of MYCN, while the expression of miR-26b-5p did not change (Fig. 4e). Next, we assessed the effect of MYCN activation in the SHEP-MYCN-ER cell line. Here, miR-26a-5p expression level was decreased by 20% (Fig. 4f) upon 4-hydroxy-tamoxifen-mediated activation of the MYCN-ER fusion protein, whereas, again, no significant regulation of miR-26b-5p expression levels was observed (Fig. 4f). Together these in vivo and in vitro data support the hypothesis that MYCN could regulate the expression of miR-26a-5p in neuroblastoma cells. Furthermore, these data show that miR-26b-5p is downregulated during murine MYCN-driven neuroblastoma development; however, they do not provide evidence that this is a direct consequence of MYCN activity.
3.5. MYCN does not inhibit transcription of primary mir-26 transcripts in neuroblastoma cells
Both miR-26a-5p and miR-26b-5p are intronic miRNAs that are transcribed from three genomic regions, residing within the introns of three coding genes: CTDSP1, CTDSP2 and CTDSPL, host genes of mir-26b, mir-26a-2 and mir-26a-1 respectively; both mir-26a-1 and mir-26a-2 are processed into the mature miR-26a-5p. In order to evaluate whether transcriptional activity of MYCN directly regulates miR-26a-5p and miR-26b-5p, we measured the expression of the primary miRNA transcripts and the host genes in the IMR-5/75-shMYCN and SHEP-MYCN-ER cell lines. Knockdown of MYCN in IMR-5/75-shMYCN cells resulted in decreased expression of miR-26a-5p host genes CTDSP2 and CTDSPL, but did not affect the expression of pri-mir-26 transcripts (Fig. 5a–b). Induction of MYCN activity in the SHEP-MYCN-ER cells, did not affect miR-26 host gene expression levels, nor the expression of the pr-mir-26 transcripts (Fig. 5c–d). These data suggest that downregulation of miR-26a-5p by MYCN in neuroblastoma cells is not the consequence of transcriptional silencing of mir-26a-1 or mir-26a-2.
Figure 5. MYCN does not inhibit transcription of pri-mir-26a-1, pri-mir-26a-2 or pri-mir-26b in neuroblastoma cells.
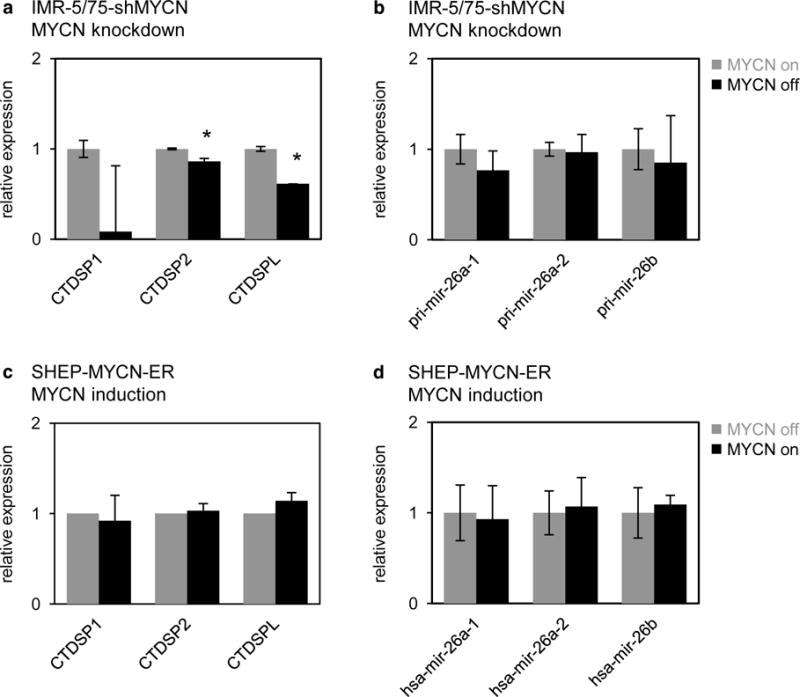
(a) Relative expression of pri-mir-26a-1, pri-mir-26a-2 and pri-mir-26b in IMR-5/75-shMYCN cells before (gray bars) and after (black bars) silencing of MYCN. Data are presented as mean ± standard deviation of three replicate experiments. T-test, pri-mir-26a-1: p > 0.05; pri-mir-26a-2: p > 0.05; pri-mir-26b: p > 0.05. (b) Relative expression of CTDSP1, CTDSP2 and CTDSPL in IMR-5/75-shMYCN cells before (gray bars) and after (black bars) silencing of MYCN. Data are presented as mean ± standard deviation of three replicate experiments. T-test, CTDSP1: p > 0.05; CTDSP2: p = 6.2 × 10−3; CTDSPL: p = 6.2 × 10−5. (c) Relative expression of pri-mir-26a-1, pri-mir-26a-2 and pri-mir-26b in SHEP-MYCNER cells before (gray bars) and after (black bars) induction of MYCN activity. Data are presented as mean ± standard deviation of three replicate experiments. T-test, pri-mir-26a-1: p > 0.05; pri-mir-26a-2: p > 0.05; pri-mir-26b: p > 0.05. (d) Relative expression of CTDSP1, CTDSP2 and CTDSPL in SHEP-MYCN-ER cells before (gray bars) and after (black bars) induction of MYCN activity. Data are presented as mean ± standard deviation of three replicate experiments. T-test, CTDSP1: p > 0.05; CTDSP2: p > 0.05; CTDSPL: p > 0.05.
3.6. MYCN induces LIN28B expression in neuroblastoma cells
Given that MYCN affects the expression levels of miR-26a-5p, and that both miR-26a-5p and miR-26b-5p regulate the expression of LIN28B, we postulated that MYCN affects LIN28B expression in neuroblastoma cells. To investigate this premise, we measured the effect of MYCN activation and silencing on LIN28B mRNA and protein expression in the above-mentioned in vitro models. In the SHEP-MYCN-ER cell line, LIN28B expression increased by 1.7-fold 48h upon activation of MYCN (Fig. 6a). Correspondingly, knockdown of MYCN resulted in a 2-fold decrease in LIN28B expression levels (Fig. 6b). In addition, we evaluated the relationship between MYCN and LIN28B expression levels in the murine MYCN-driven model systems. As expected from the in vitro data, LIN28B mRNA expression levels strongly increased during MYCN-driven murine neuroblastoma development in the TH-MYCN+/+ model compared to normal development (Fig. 6c). Accordingly, we detected LIN28B protein in murine neuroblastoma tumors, whereas expression was absent in wild-type sympathetic ganglia (Fig. 6d). In addition, LSL-MYCN tumors were characterized by 1.8-fold higher LIN28B mRNA levels as compared to normal adrenal tissue (Fig. 6e). Finally, LIN28B expression showed significant correlation to MYCN expression in a large cohort of primary neuroblastoma tumors (n = 200) from different clinical stages (Supplementary Fig. 4a) and was higher expressed in MYCN amplified neuroblastoma tumors (n = 40) versus MYCN non-amplified tumors (n = 160) (Supplementary Fig. 4b).
Figure 6. MYCN induces LIN28B expression in neuroblastoma cells.
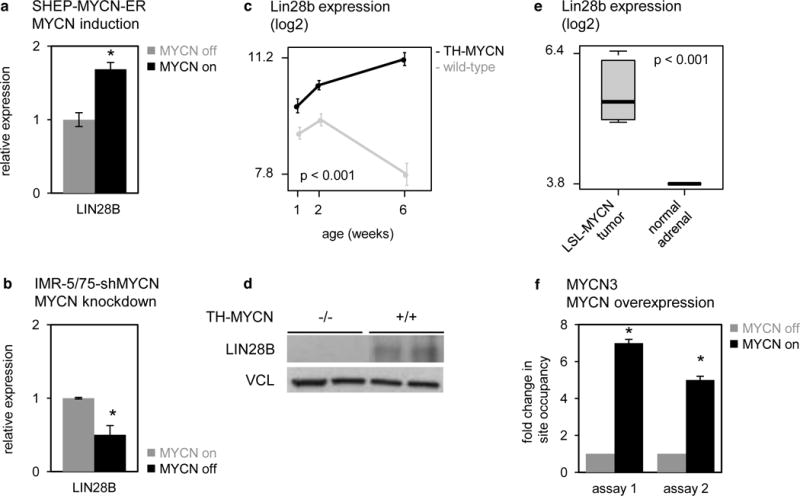
(a) Relative expression of LIN28B in SHEP-MYCN-ER cells before (gray bars) and after (black bars) induction of MYCN activity. Data represent average fold change of three independent biological replicates +/− standard deviation. T-test, p = 4.2 × 10−3. (b) Relative expression of LIN28B in IMR-5/75-shMYCN cells before (gray bars) and after (black bars) silencing of MYCN. Data are presented as mean ± standard deviation of three replicate experiments T-test, p = 5.7 × 10−3. (c) Lin28b expression (log2) in sympathetic ganglia containing hyperplastic lesions, and advanced tumors from TH-MYCN+/+ mice at, respectively, 1 and 2 weeks and 6 weeks of age (black), and in normal sympathetic ganglia from wild-type mice at 1, 2 and 6 weeks of age (gray). Data are presented as mean ± standard deviation of four samples. Two-way ANOVA, interaction p = 1.9 × 10−8. (d) Lin28b protein levels in advanced tumors from TH- MYCN+/+ mice at 6 weeks of age (indicated as “+/+”), and in normal sympathetic ganglia from wild-type mice at 6 weeks of age (indicated as “−/−”). (e) MYCN ChIP was performed on MYCN3 cell line either doxycycline treated (MYCN high) or untreated (MYCN low). Two assays (Supplementary Table 5) for MYCN binding site at LIN28B promoter was designed by analyzing MYCN ChIP-Sequencing database. Negative control IgG ChIP and Input material was also analyzed and used for normalizing the MYCN ChIP data. Two sets of control primers were used to confirm the specificity of immunoprecipitated genomic DNA. Fold enrichment in site occupancy was calculated by comparing normalized MYCN ChIP Cq values between treatments. ChIP-qPCR reactions were performed in triplicates and represented here as mean ± SEM. T-test, p < 0.001.
Thus far, our study supports a mechanistic model in which MYCN can enhance LIN28B expression in neuroblastoma via indirect repression of miR-26a-5p, which regulates LIN28B expression levels. Several studies, however, have reported that MYC, a family member of MYCN, can directly induce LIN28B expression via binding to an E-box sequence in its promoter region [27,28], and a similar mode of action for MYCN has been proposed [9,29]. To verify whether MYCN indeed binds the LIN28B promoter region and activates its transcription, we have evaluated direct interaction in the MYCN3 cell line, a neuroblastoma model of doxycycline-inducible MYCN overexpression. Here, overexpression of MYCN resulted in a 1.7-fold increase in LIN28B expression levels (Supplementary Fig. 5) and a > 5-fold increase in LIN28B promoter occupancy by MYCN as demonstrated by ChIP-qPCR with two independent assays (Fig. 6f). Together these data support an additional mechanistic model in which MYCN enhances LIN28B expression via induction of LIN28B transcription through direct promoter interaction.
4. Discussion
The elucidation of the LIN28B–let-7–MYCN axis in neuroblastoma and its role in sustaining proliferation and cell viability and suppression of differentiation of neuroblasts, offered exciting insights in MYCN-regulating pathways in neuroblastoma [3]. However, little is known about the regulation of LIN28B expression in neuroblastoma. Given the importance of miRNAs in regulating key oncogenes [30,31], we believe that the contribution of miRNAs to the regulation of LIN28B in neuroblastoma is underexplored. Here, we report on the first unbiased and genome-wide high-throughput miRNA target reporter screen to identify miRNAs targeting LIN28B. From the total of 28 LIN28B-targeting miRNAs identified in this study, 23 interactions were novel, whereas 5 out of 8 previously established LIN28B-targeting miRNAs were confirmed (Table 1). Subsequent filtering of the screen results through clinical data of neuroblastoma patients highlighted two miRNAs, miR-26a-5p and miR-26b-5p, for their potential tumor suppressive activity in neuroblastoma. To our knowledge, these miRNAs have not been associated to neuroblastoma tumor biology before.
The most studied LIN28B-targeting miRNAs are the let-7 family members [32]. In our 3′UTR library screen, we identified all let-7 family members as LIN28B-targeting miRNAs. Moreover, these miRNAs displayed negative correlation to LIN28B expression levels in primary neuroblastoma tumors (Table 1), thus confirming previous data and validating our 3′UTR library screen. However, in contrast to both miR-26a-5p and miR-26b-5p, the let-7 family members did not show association to patient outcome in a cohort of aggressive neuroblastoma tumors.
We revealed that induction or knockdown of MYCN activity in vitro respectively represses or induces the expression of miR-26a-5p, and that both miR-26a-5p and miR-26b-5p show decreased expression levels in murine MYCN-driven mouse tumors. The observed effects of MYCN perturbation on miR-26a-5p expression levels are modest (~ 20%); this could raise questions regarding the relevance of such regulation. However, it has been suggested that the capability of miRNAs to regulate multiple targets within the same pathway can amplify their biological effects [36]. As a result, even relatively small changes in miRNA expression may be biologically significant [37,38]. Therefore, it is our opinion that MYCN does affect the expression levels of miR-26a-5p and that this regulation could have significant biological consequences. However our observations do not fully support the regulation of miR-26b-5p expression levels: we observed no significant changes in miR-26b-5p expression levels upon perturbation of MYCN expression in in vitro models. Additional experimentation in cell culture systems may be useful to identify the exact role of miR-26b-5p in the proposed regulatory network.
Several reports have demonstrated that MYC, a MYCN family member, is capable of down-regulating expression of miR-26a-5p and miR-26b-5p in breast cancer [39] and hepatocellular carcinoma [40]. For miR-26a-5p, it has been demonstrated that this down-regulation occurs through a cooperative effect of MYC and EZH2 and the promoter region of its host genes CTDSPL (mir-26a-1 host gene) and CTDSP2 (mir-26a-2 host gene) in aggressive lymphomas [41]. However, our data do not support a similar mode of action in neuroblastoma as we did not observe direct regulation of the primary mir-26a-1, mir-26a-2 nor the mir-26b transcript by MYCN. This observation indicates that regulation of miR-26a-5p by MYCN is rather in keeping with an indirect mechanism, possibly via regulation of genes that contribute to or inhibit the stability of miR-26a-5p. For instance, nuclear modifiers that recognize unique motifs on pre-miR-26a can regulate the export of pre-miR-26a. If pre-miR-26a cannot be exported into cytoplasm and is retained in the nucleus, mature miR-26a-5p cannot be produced. Alternatively, Dicer processing can be blocked by cytoplasmic modifiers that bind to pre-miR-26a, or, as in the case of pre-let-7 regulation, Dicer processing efficiency can be decreased by TUT-mediated uridylation [42]. The discrepancy between pri-mir-26 and mature miR-26 levels may also result from the turnover rate of mature miR-26a-5p. Mature miRNAs have been thought to be very stable. However, studies have shown that extracellular signals can induce the rapid degradation of specific miRNAs [43,44]. Unknown modifiers may work on mature miR-26a-5p to affect its stability. Finding such modifiers of miR-26a-5p will be instrumental in understanding the nature of miR-26a-5p regulation. Further experimentation in cell culture systems may be useful to identify miR-26a-5p post-transcriptional regulators.
Our LIN28B-3′UTR library screen and subsequent validation experiments showed that miR-26a-5p regulate LIN28B expression levels in neuroblastoma. In addition, we showed that MYCN activation, both in vitro and in vivo, leads to decreased miR-26a-5p and increased LIN28B expression levels. From these observations, we concluded that MYCN can regulate LIN28B expression levels via indirect repression of miR-26a-5p. However, we further showed that MYCN regulates LIN28B mRNA levels via a second mechanism: MYCN can directly control LIN28B expression thorough binding of its promoter as shown by ChIP-analysis. This result is not unexpected as it had been reported that MYC induces LIN28B expression levels in B-cell lymphoma [27], while induction of LIN28B expression by MYCN in neuroblastoma had been suggested by several additional reports [9,29].
In conclusion, our integrated analyses uncovered a double regulatory feed-forward loop, enhancing the expression of both LIN28B and MYCN in neuroblastoma. The proposed molecular model consists of two alternative paths that are probably not mutually exclusive, but appear to differ between different cellular contexts, such as the different model systems analyzed here. The shortest path involves direct regulation of LIN28B by MYCN through binding at its promoter region (Fig. 7a). Subsequent induction of LIN28B expression results in reduced levels of mature let-7 miRNAs due to inhibition of miRNA processing by LIN28B. Finally, reduced let-7 levels results in loss of MYCN repression, which closes the proposed loop. The second path involves an intermediate step where MYCN indirectly represses the expression of the LIN28B-targeting miRNA miR-26a-5p (Fig. 7b). Again, subsequent induction of LIN28B expression results in reduced levels of mature let-7 miRNAs due to inhibition of miRNA processing by LIN28B.
Figure 7. Proposed working hypothesis for the regulation of LIN28B in neuroblastoma.
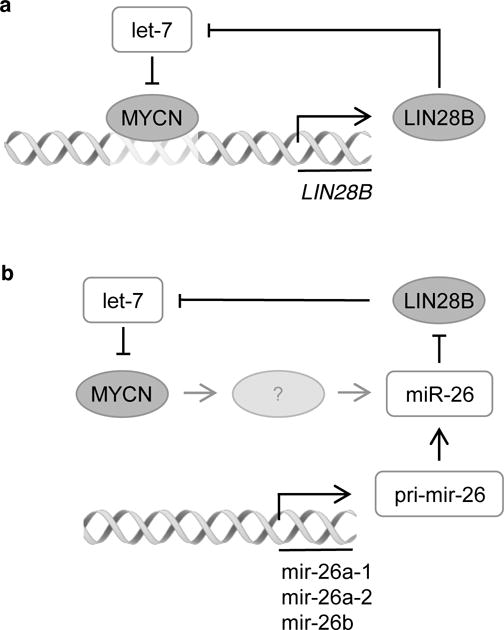
(a) MYCN enhances the expression of LIN28B via direct binding at the LIN28B promoter region. In turn, LIN28B inhibits the processing of the let-7 miRNAs, which results in loss of MYCN repression [3]. (b) MYCN indirectly represses the expression of miR-26a-5p via an unknown factor, indicated as “?”. Reduced miR-26a-5p expression levels results in increased LIN28B expression, which again, in turn, inhibits the processing of the let-7 miRNAs, resulting in loss of MYCN repression [3].
Understanding this regulatory mechanism upstream of LIN28B in neuroblastoma can open new perspectives for targeting the MYCN-LIN28B pathway in neuroblastoma tumors, a strategy that holds great promise. Moreover, the identification of additional target genes of the miRNAs in the described LIN28B-miRNA interactome might prove useful in the search for novel therapeutic targets.
Supplementary Material
Highlights.
The main messages/claims of our paper are
An unbiased LIN28B 3′UTR-miRNA library screen identifies 28 miRNAs targeting LIN28B
miR-26a-5p and miR-26b-5p are top candidate LIN28B-targeting miRNAs in neuroblastoma
Overexpression of miR-26a-5p or miR-26b-5p reduces LIN28B expression
miR-26a-5p and miR-26b-5p are downregulated in MYCN-driven neuroblastoma
MYCN does not inhibit transcription of primary mir-26 transcripts in neuroblastoma cells
MYCN induces LIN28B expression in neuroblastoma cells
Acknowledgments
We would like to thank the Ghent University Hospital (Ghent, Belgium), the University Children’s Hospital Essen (Essen, Germany) and the Hospital Clínico Universitario (Valencia, Spain) for collection of primary tumor samples. Further, we would like to thank F. De Vloed for excellent technical support.
Funding: This work was supported by the GOA (grant number 01G01910), by research grants from the European Union (FP7-ASSET project) to F.S., from the Belgian Foundation against Cancer (Stichting Tegen Kanker) [SCIE 2010-177 and 2012-199], from the Fund for Scientific Research Flanders (FWO) to F.S. [G.0530.12N] and the Belgian Childhood Cancer Fund (vzw Kinderkankerfonds); by a PhD grant from the Agency for Innovation by Science and Technology (IWT) to A.B. [IWT 101506]; an Emmanuel van der Schueren research grant from the Flemish League against Cancer (Vlaamse Liga tegen Kanker) to G.V.P.; a PhD grant from the Ghent University to G.V.P. [BOF 01D35609]; and a post-doc grant of the FWO to K.D.P. This work was further supported by Program Grants from the NHMRC Australia, the Cancer Institute NSW and the Cancer Council NSW to D.C., B.C. and G.M.; by grants from NIH (R01-CA174808), Alex’s Lemonade Stand Foundation and the Gillson-Longenbaugh Foundation to J.S.
Footnotes
Conflict of interest
The authors declare no conflict of interest
References
- 1.van de Bunt M, Gaulton KJ, Parts L, Moran I. The miRNA profile of human pancreatic islets and beta-cells and relationship to type 2 diabetes pathogenesis. PLoS ONE. 2013 doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shyh-Chang N, Daley GQ. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell. 2013;12:395–406. doi: 10.1016/j.stem.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molenaar JJ, Domingo-Fernández R, Ebus ME, Lindner S, Koster J, Drabek K, et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat Genet. 2012;44:1199–1206. doi: 10.1038/ng.2436. [DOI] [PubMed] [Google Scholar]
- 4.Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helwak A, Kudla G, Dudnakova T, Tollervey D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell. 2013;153:654–665. doi: 10.1016/j.cell.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Research. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 7.Liang L, Wong CM, Ying Q, Fan DNY, Huang S, Ding J, et al. MicroRNA-125b suppressesed human liver cancer cell proliferation and metastasis by directly targeting oncogene LIN28B2. Hepatology. 2010;52:1731–1740. doi: 10.1002/hep.23904. [DOI] [PubMed] [Google Scholar]
- 8.Guo Y, Chen Y, Ito H, Watanabe A, Ge X, Kodama T, et al. Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene. 2006;384:51–61. doi: 10.1016/j.gene.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Helland Å, Anglesio MS, George J, Cowin PA, Johnstone CN, House CM, et al. Deregulation of MYCN, LIN28B and LET7 in a molecular subtype of aggressive high-grade serous ovarian cancers. PLoS ONE. 2011;6:e18064. doi: 10.1371/journal.pone.0018064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotterman R, Knoepfler PS. N-Myc regulates expression of pluripotency genes in neuroblastoma including lif, klf2, klf4, and lin28b. PLoS ONE. 2009 doi: 10.1371/journal.pone.0005799.g006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Peer G, Lefever S, Anckaert J, Beckers A, Rihani A, Van Goethem A, et al. miRBase Tracker: keeping track of microRNA annotation changes. Database. 2014;2014:bau080–bau080. doi: 10.1093/database/bau080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimson A, Farh KKH, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Molecular Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, El Naqa IM. Prediction of both conserved and nonconserved microRNA targets in animals. Bioinformatics. 2008;24:325–332. doi: 10.1093/bioinformatics/btm595. [DOI] [PubMed] [Google Scholar]
- 14.Muth D, Ghazaryan S, Eckerle I, Beckett E, Pöhler C, Batzler J, et al. Transcriptional repression of SKP2 is impaired in MYCN-amplified neuroblastoma. Cancer Research. 2010;70:3791–3802. doi: 10.1158/0008-5472.CAN-09-1245. [DOI] [PubMed] [Google Scholar]
- 15.Schulte JH, Horn S, Otto T, Samans B, Heukamp LC, Eilers UC, et al. MYCN regulates oncogenic MicroRNAs in neuroblastoma. Int J Cancer. 2008;122:699–704. doi: 10.1002/ijc.23153. [DOI] [PubMed] [Google Scholar]
- 16.Shohet JM, Ghosh R, Coarfa C, Ludwig A, Benham A, Chen Z, et al. A genome-wide search for promoters that respond to increased MYCN reveals both new oncogenic and tumor suppressor microRNAs associated with aggressive neuroblastoma. Cancer Research. 2011;71:3841–3851. doi: 10.1158/0008-5472.CAN-10-4391. [DOI] [PubMed] [Google Scholar]
- 17.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 18.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biology. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002;3:R34. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu SD, Tseng YT, Shrestha S, Lin YL, Khaleel A, Chou CH, et al. miRTarBase update 2014: an information resource for experimentally validated miRNA-target interactions. Nucleic Acids Research. 2014;42:D78–85. doi: 10.1093/nar/gkt1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–486. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Y, Chen Y, Ito H, Watanabe A, Ge X, Kodama T, et al. Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene. 2006;384:51–61. doi: 10.1016/j.gene.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, El Naqa IM. Prediction of both conserved and nonconserved microRNA targets in animals. Bioinformatics. 2008;24:325–332. doi: 10.1093/bioinformatics/btm595. [DOI] [PubMed] [Google Scholar]
- 24.Mestdagh P, Lefever S, Pattyn F, Ridzon D, Fredlund E, Fieuw A, et al. The microRNA body map: dissecting microRNA function through integrative genomics. Nucleic Acids Research. 2011;39:e136–e136. doi: 10.1093/nar/gkr646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mestdagh P, Fredlund E, Pattyn F, Schulte JH, Muth D, Vermeulen J, et al. MYCN/c-MYC-induced microRNAs repress coding gene networks associated with poor outcome in MYCN/c-MYC-activated tumors. Oncogene. 2010;29:1394–1404. doi: 10.1038/onc.2009.429. [DOI] [PubMed] [Google Scholar]
- 26.Althoff K, Beckers A, Bell E, Nortmeyer M, Thor T, Sprüssel A, et al. A Creconditional MYCN-driven neuroblastoma mouse model as an improved tool for preclinical studies. Oncogene. 2014 doi: 10.1038/onc.2014.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang TC, Zeiteis LR, Hwang HW, Chivukula RR, Wentzel EA, Dews M, et al. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc Natl Acad Sci USA. 2009;106:3384–3389. doi: 10.1073/pnas.0808300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You X, Liu F, Zhang T, Lv N, Liu Q, Shan C, et al. Hepatitis B virus X protein upregulates Lin28A/Lin28B through Sp-1/c-Myc to enhance the proliferation of hepatoma cells. Oncogene. 2014;33:449–460. doi: 10.1038/onc.2012.618. [DOI] [PubMed] [Google Scholar]
- 29.Cotterman R, Jin VX, Krig SR, Lemen JM, Wey A, Farnham PJ, et al. N-Myc regulates a widespread euchromatic program in the human genome partially independent of its role as a classical transcription factor. Cancer Research. 2008;68:9654–9662. doi: 10.1158/0008-5472.CAN-08-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 31.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rybak A, Fuchs H, Smirnova L, Brandt C, Pohl EE, Nitsch R, et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nature Cell Biology. 2008;10:987–993. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 33.Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nature Publishing Group. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Lin S, Li JJ, Xu Z, Yao H, Zhu X, et al. MYC protein inhibits transcription of the microRNA cluster MC-let-7a-1~let-7d via noncanonical E-box. Journal of Biological Chemistry. 2011;286:39703–39714. doi: 10.1074/jbc.M111.293126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beckers A, van peer G, Carter DR, Mets E, Althoff K, Cheung BB, et al. MYCN-targeting miRNAs are predominantly downregulated during MYCN- driven neuroblastoma tumor formation. Oncotarget. 2014;5 doi: 10.18632/oncotarget.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calin GA, Croce CM. MicroRNA-cancer connection: the beginning of a new tale. Cancer Research. 2006;66:7390–7394. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- 37.Peltier HJ, Latham GJ. Normalization of microRNA expression levels in quantitative RT-PCR assays: identification of suitable reference RNA targets in normal and cancerous human solid tissues. Rna. 2008;14:844–852. doi: 10.1261/rna.939908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang K, Mestdagh P, Vandesompele J, Kerin MJ, Miller N. MicroRNA expression profiling to identify and validate reference genes for relative quantification in colorectal cancer. BMC Cancer. 2010;10:173. doi: 10.1186/1471-2407-10-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan S, Ding K, Li R, Zhang W, Li G, Kong X, et al. Identification of miR-26 as a key mediator of estrogen stimulated cell proliferation by targeting CHD1, GREB1 and KPNA2. Breast Cancer Research. 2014;16:R40. doi: 10.1186/bcr3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Y, Lu Y, Zhang Q, Liu JJ, Li TJ, Yang JR, et al. MicroRNA-26a/b and their host genes cooperate to inhibit the G1/S transition by activating the pRb protein. Nucleic Acids Research. 2012;40:4615–4625. doi: 10.1093/nar/gkr1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao X, Lwin T, Zhang X, Huang A, Wang J. Disruption of the MYC-miRNA-EZH2 loop to suppress aggressive B-cell lymphoma survival and clonogenicity. Leukemia. 2013 doi: 10.1038/leu.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z, Zha Y, Hu W, Huang Z, Gao Z, Zang Y, et al. The autoregulatory feedback loop of microRNA-21/programmed cell death protein 4/activation protein-1 (MiR-21/PDCD4/AP-1) as a driving force for hepatic fibrosis development. Journal of Biological Chemistry. 2013;288:37082–37093. doi: 10.1074/jbc.M113.517953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang HW, Wentzel EA, Mendell JT. A hexanucleotide element directs microRNA nuclear import. Science. 2007;315:97–100. doi: 10.1126/science.1136235. [DOI] [PubMed] [Google Scholar]
- 44.Krol J, Busskamp V, Markiewicz I, Stadler MB, Ribi S, Richter J, et al. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010;141:618–631. doi: 10.1016/j.cell.2010.03.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


