Abstract
Leptin administration results in leptin resistance presenting a significant barrier to therapeutic use of leptin. Consequently, we examined two hypotheses. The first examined the relationship between leptin dose and development of physiological and biochemical signs of leptin resistance. We hypothesized lower doses of leptin would produce proportional reductions in body weight without the adverse leptin-induced leptin resistance. The second compared pulsed central leptin infusion to continuous leptin infusion. We hypothesized that pulsed infusion at specific times of the day would evoke favorable body weight reductions while tempering the development of leptin-induced leptin resistance. The first experiment examined leptin responsiveness, including food intake, body weight and hypothalamic STAT3 phosphorylation to increasing doses of viral gene delivery of leptin. Varying the dose proved inconsequential with respect to long-term therapy and demonstrated proportional development of leptin resistance. The second experiment examined leptin responsiveness to pulsed central leptin infusion, comparing pulsed versus constant infusion of 3 ug/day leptin or a 2 h morning versus a 2 h evening pulsed leptin infusion. Pulsed delivery of the supramaximal dose of 3 ug/day was not different than constant delivery. Morning pulsed infusion of the submaximal dose of 0.25 ug reduces food intake only over subsequent immediate meal period and was associated with body weight reductions, but results in cellular leptin resistance. Evening pulsed infusion did not decrease food intake but reduces body weight and maintains full leptin signaling. The positive benefit for pulsed delivery remains speculative, yet potentially may provide an alternative mode of leptin therapy.
Keywords: leptin pulsed delivery, leptin resistance, STAT3
1. Introduction
Leptin, product of the ob gene, is an adipocyte derived hormone exhibiting highly conserved homology between species (Zhang et al., 1994). The role of leptin in physiology is multi-faceted and has been expertly reviewed in several articles to mark the 20th anniversary of its discovery (Münzberg and Morrison, 2015; Park and Ahima, 2015; Sáinz et al., 2015); in brief, leptin is important in regulation of energy balance, reproduction, immune function, and bone metabolism among others (Mantzoros et al., 2011). Regulation of body weight through controlling food intake and energy expenditure occurs via binding of leptin to the long form of the leptin receptor (Fei et al., 1997). Expression of leptin receptors within the central nervous system (CNS) are highest in the hypothalamus (Hypo), (Elmquist et al., 1998; Scott et al., 2009) however other CNS nuclei express leptin receptors including hindbrain nuclei (Gautron and Elmquist, 2011). From the early evidence of leptin administration in murine models, there was a great hope in using leptin as a therapy to treat obesity in humans. However leptin therapy was shown to be ineffective at weight reduction in various randomized placebo controlled clinical trials (Heymsfield et al., 1999; Zelissen et al., 2005). Though leptin may not be an effective monotherapy for obesity, recently numerous studies have evaluated leptin in the treatment of diabetes type II (Mittendorfer et al., 2011; Moon et al., 2011), lipodystrophy (Chong et al., 2010; Diker-Cohen et al., 2015) and hypothalamic amenorrhea (Chou et al., 2011; Sienkiewicz et al., 2011) with promising results in selective outcome measures particularly in conditions where basal levels of leptin are low (Coppari and Bjorbaek, 2012). Additionally, efforts into sensitizing the effects of leptin have been undertaken (Roth et al., 2008), presumably such a strategy would enhance the effectiveness of leptin therapy where currently a leptin resistant state is present. Currently, there are numerous registered clinical trials ongoing investigating leptin or leptin in combination with complimentary therapeutic agents (Polyzos and Mantzoros, 2015). The continued investigation into leptin as a clinical treatment warrants a further understanding of the development of leptin resistance.
Levels of leptin are higher in obese rodents and humans and related to the observed adiposity (Maffei et al., 1995) thus common obesity exhibits a pattern of leptin resistance. The Fischer 344 x Brown Norway (F344BN) rat is a good model to study the effects of long-term leptin delivery due to the relative stability of body weight and adiposity as an adult (Altun et al., 2007), thus avoiding any potential development of leptin resistance in control animals over the course of lengthy experiments. Delivery of recombinant adeno-associated viral leptin (rAAV-leptin) into the third ventricle of F344BN rats produces a rapid decrease in body weight and food intake. The effect of rAAV-leptin however is not maintained and over time; there is an increase in weight gain despite continuous elevated central leptin levels (Matheny et al., 2011; Scarpace et al., 2003). In rats of the same strain following three months of high fat feeding, treatment with AAV-leptin produced no change in body mass or food intake compared to GFP treated controls (Wilsey et al., 2003). The high fat feeding elevates endogenous leptin thereby inducing leptin resistance (Knight et al., 2010), thus rendering the rats insensitive to rAAV-leptin gene delivery (Scarpace and Zhang, 2009).
Interestingly, leptin resistance may be selective and produce differential effects on various brain areas (Mark, 2013; Matheny et al., 2011; Münzberg et al., 2004). The ability of leptin administration to produce a leptin resistant state even in lean animals (Martin et al., 2000) generates a significant barrier to the use of leptin therapeutically, a condition termed leptin-induced leptin resistance. In light of these observations we set out to test two hypotheses. The first examined if there was a relationship between the levels of leptin and the development of both physiological and biochemical signs of resistance to leptin. We hypothesized that a lower dose of leptin would produce attenuated positive markers in relation to body weight and body composition but importantly not create the observed leptin-induced leptin resistance. The second compared pulsed leptin central infusion to continuous leptin infusion. We hypothesized that pulsed infusion at specific times of the day would evoke favorable body weight reductions while tempering the development of leptin-induced leptin resistance.
2. Materials and methods
2.1. Experimental animals
Six-month-old male F344BN rats were obtained from National Institute of Aging Colony. Animals were cared for in accordance with the principles of the Guide to the Care and Use of Experimental Animals and protocols were approved by the University of Florida Institutional Animal Care and Use Committee. Rats were singly-housed with a 12:12 h light-dark cycle (07:00 to 19:00 h) and maintained on chow (Diet 7912, 3.1 kcal/g; 17% kcal from fat, 25% kcal protein, Harlan Teklad; Madison, WI).
2.2. Experimental design
This study consists of two experiments; the first examining leptin responsiveness to increasing doses of viral gene delivery of leptin and the second examining leptin responsiveness to pulsed leptin icv infusion.
In the first experiment, rats were assigned to four treatment groups: Low-Dose Leptin (LD), Mid-Dose Leptin (MD), High-Dose Leptin (HD) or GFP-Control (Control) containing 10-12 animals per group. Rats were administered recombinant adeno-associated virus encoding either green fluorescent protein, or leptin by injection into the 3rd ventricle. Rats were provided ad libitum access to food and water, and food consumption and body weight were recorded daily. Body composition of animals was measured in 15 day intervals. Respiratory measures were conducted at experimental day 55. Half of rats were killed at day 60 for endpoint analysis while remaining rats continued to be monitored for long-term body weight effects for 4 additional months. Rats that were excluded from analysis displayed post-surgical complications.
The second experiment consisted of two parts, each with 6-7 rats per group. In the first, the amount of leptin delivered (3 ug) over a single day was held constant and the time of delivery was varied; either constant over the 24 h period (0.125 ug/h) or pulsed over a 4h period (0.75 ug/h) prior to the dark cycle. The third group was the control in which ACSF was infused. Body weight and food were recorded daily except for days 7 and 8 in which food was recorded separately during the light phase and the dark phase. In the second part of this experiment, leptin delivery was pulsed controlling both the amount (0.25 ug) and rate of delivery (0.125 ug/h), but varied the timing, with a 2 h delivery prior to the light phase compared to a 2 h delivery prior to the dark phase.
2.3. rAAV-vector administration
The pTR(2)ObW construct encoding leptin transgene under a chicken β-actin promoter linked to CMV enhancer was packaged into recombinant adeno-associated virus (rAAV) serotype 1 using previously describe methods (Zolotukhin et al., 2002), with the exception that the AAV1 helper plasmid pKRAP1A was used. Dot blot titer of rAAV1-leptin was 5.86×1012 viral genomes (vg) per milliliter. UF11 rAAV1, encoding green fluorescent protein (GFP) was used for the control group. The rAAV1-GFP titer was calculated by dot blot at 5.90×1012 vg/ml. Stock rAAV1-leptin vector was used as the high-dose leptin treatment. 1:100 dilutions of rAAV1-leptin were made in artificial cerebrospinal fluid (aCSF) to obtain the MD and LD treatments. 3 μl of rAAV1 vector was delivered into the third ventricle of animals according to the following coordinates 1.3 mm anterior to Bregma, 0.0 mm from midline, depth of 9.6 mm ventral from surface of skull, at an angle of 20° (Paxinos and Watson, 2005). Coordinates were verified previously in separate rats using injection of bromothymol blue dye.
2.4. Central leptin infusion
Leptin infusion, either constant or programmable, was provided as follows. For constant delivery, osmotic minipumps (Durect Corporation) were implanted subcutaneously into the rat and leptin or vehicle was constantly infused into the lateral ventricle via an implanted cannula as described previously (Scarpace et al., 2007). Programmable or pulsatile leptin delivery was accomplished by using a subcutaneously implanted Imprecio programmable infusion pump (Durect Corporation) via a cannula implanted in the lateral ventricle. Infusion protocols consisting of flow rates, delivery and stop times were constructed using the Imprecio software and downloaded to the pump prior to implantation.
2.5. Acute central leptin administration
Rats were anesthetized using a cocktail of ketamine and xylazine, mounted into a stereotaxic apparatus, and the 3rd ventricle was targeted for delivery of acute leptin peptide using the same coordinates as for initial vector treatment. Diluted leptin peptide (0.2 μg/μl in aCSF, Amgen, Thousand Oaks, CA) was delivered at a rate of 1 μl/min over 5 mins, resulting in a total injection volume of 5 μl and a resulting dose of 1 μg leptin per rat. Rats were killed one h post injection to assess leptin-mediated STAT3 signaling.
2.6. Tissue harvest and preparation
Rats were killed under anesthesia by thoracotomy and blood was taken by cardiac puncture and serum collected following centrifugation in serum separator tubes. Subsequently, rats were perfused with 30 ml of cold saline. The perirenal, retroperitoneal and epididymal white adipose depots along with interscapular brown adipose tissue (iBAT) were excised and weighed. An incision was made along the dorsal midline of the rat to remove the iBAT depot. Muscle and white adipose tissue that were attached to the iBAT was subsequently removed, and the remaining tissue weighed on an analytical scale. For measurement of UCP1 an approximately 20 mg sample was removed from the iBAT and homogenized in 0.300 ml homogenization buffer (10 mMTris-HCl, 2% SDS, pH 6.8) by sonication. Remaining cellular debris was removed by centrifugation. In order to remove lipid component, the supernatant was passed through a 0.45 μm syringe filter.
The mediobasal hypothalamus (MBH) was collected by cutting medially of the piriform lobes, caudal to the optic chiasm and rostral to the cerebral crus at a depth of 2 mm. Tissue was collected in 200 μl of homogenization buffer. Samples were briefly sonicated and concentration of all samples was determined by detergent compatible protein assay (Bio-Rad Laboratories, Hercules, CA).
2.7. Body Composition and Respiratory Measurements
Time-Domain Nuclear Magnetic Resonance (TDNMR) was used to perform measurements of fat mass in unanaesthetized animals using a minispec LF90 analyzer (Bruker Optics, The Woodlands, TX). Measurements were taken in duplicate for each animal at approximately 15 day intervals throughout the experiment. Fat mass was calculated as a percentage of body weight for each individual animal immediately prior to surgery, and the resulting value was used as the baseline value for all future calculations. Oxygen consumption and carbon dioxide production were assessed using a modular animal respirometry system consisting of an Oxygen Analyzer S-3A/I and Carbon Dioxide Analyzer CD-3A (AEI Technologies, Naperville, IL). Flow rates for each chamber were set to 1L/min.
2.8. Western analysis
Protein homogenate (30 μg, MBH; 5 ug, BAT) was separated on a SDS-PAGE gel and electro-transferred to nitrocellulose membranes (Scarpace et al., 2001). Immunoreactivity was assessed with antibodies specific to phospho-tyrosine 705 of STAT3 (Cell Signaling, Danvers, MA) or specific to UCP1 (Abcam, Cambridge, MA). Western blots for phosphorylated STAT3 were reprobed with antibodies specific to GAPDH (Abcam, Cambridge, MA) and the data presented is normalized to corresponding GAPDH. Western blots for UCP1 were reprobed with antibodies specific to beta-tublin (Abcam) and data presented is normalized to corresponding beta-tublin.
2.9. Statistical analysis
Data were analyzed using one-way ANOVA or two-way ANOVA. A post-hoc test (Tukey or Bonferroni) was applied to determine individual differences between means when a significant main effect was determined. A P-value of less that 0.05 was considered significant.
3. Results
3.1 Body weight and food consumption in response to dose-dependent leptin overexpression
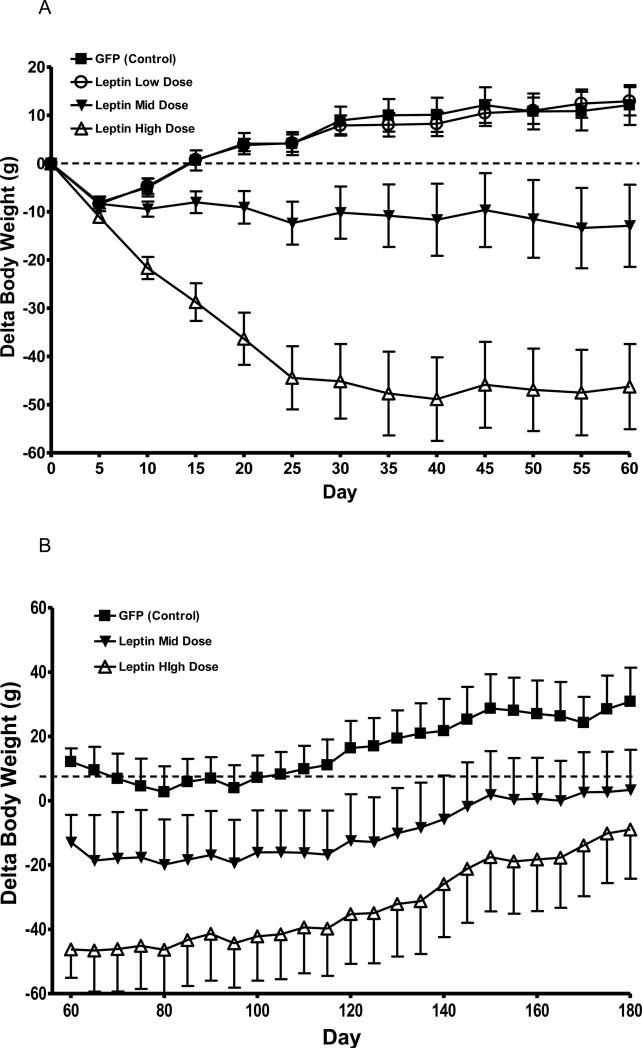
Leptin overexpression in the CNS was achieved by delivering three different titers of rAAV-leptin, either 1.76×106 viral genomes (vg)/rat (Low-Dose, LD), 1.76×108 vg/rat (Mid-Dose, MD),1.76×1010 vg/rat (High-Dose, HD) or GFP-Control (Control). As expected, body weight reduction over two months followed a predicted dose dependent response (Fig. 1 A). The greatest degree of weight loss was observed in the HD group over the first 25 days, and this weight loss was subsequently maintained throughout the 60 day experimental period. The MD of rAAV-leptin demonstrated a more gradual, but significant weight loss over the 60 days. In contrast, body weight in the LD rAAV-leptin group slowly increased over the experimental period and was not different from control (Fig. 1 A).
Fig. 1.
Change in body weight of rats following AAV-leptin mediated leptin overexpression. A: Body weight of control (squares) shows a surgical effect through day 5 followed by a return to normal growth. LD (open circles) exhibits the same surgical effect and growth resumption as control. MD (closed triangles) demonstrates no weight gain during the 60 day experimental period, while HD (open triangles) shows a decrease in body weight following treatment over the 60 day experimental period. B: Six rats in each group were killed at day 60 and remaining rats monitored for body weight from day 60 to 180. LD rAAV-leptin group is indistinguishable from control and not shown. At day 180, body weight was not different between MD and HD.
Data represents the mean ± S.E.M. of 10-12 (A) or 5-6 (B) rats per group. Delta BW was averaged over the 60 day period and analyzed by one-way ANOVA. P<0.0001 for difference with treatment; P<0.001 for difference between HD and each other group and P<0.01 between MD and each other group by Tukey post-hoc analysis.
Food consumption following rAAV-leptin treatment paralleled that of body weight in that there were reductions in food intake with the HD and MD over the initial 30 days of treatment. The average daily food consumption over the first 15 days was diminished by greater than 30% with the HD and by 12.5% in the MD from control (Table 1). The leptin-induced diminished food consumption waned during the next 15 day segment with the HD (14% decrease), but was maintained with the MD (11.6%). There were no significant differences in food intake during the remaining 30 days, and there were no differences between the LD rAAV-leptin and control (Table 1). The cumulative food intakes through the end of each segment were qualitatively similar to the daily averages (Table 1).
Table 1.
Food intake following rAAV-leptin dose response by 15 day segments.
| Average Daily Food Intake (g) | Cumulative Food Intake (g) | |||||||
|---|---|---|---|---|---|---|---|---|
| D: 0-15 | D: 15-30 | D: 30-45 | D: 45-60 | D: 0-15 | D: 0-30 | D: 0-45 | D: 0-60 | |
| Control | 16.56 (0.55) | 16.90 (0.31) | 16.82 (0.33) | 16.02 (0.33) | 245.45 (8.27) | 501.91 (12.40) | 754.23 (16.40) | 994.60 (19.11) |
| LD | 16.02 (0.33) | 16.57 (0.32) | 16.56 (0.40) | 16.49 (0.30) | 240.32 (5.00) | 488.81 (8.94) | 737.25 (14.08) | 984.62 (17.99) |
| MD | 14.48 (0.49)a | 14.93 (0.60) a | 15.92 (0.52) | 15.42 (0.37) | 217.21 (7.34) | 441.14 (15.57) | 679.95 (22.96) a | 911.20 (27.19) ab |
| HD | 11.61 (0.75) abc | 14.48 (0.70) ab | 16.57 (0.65) | 16.19 (0.65) | 174.13 (11.29) a | 391.34 (20.99) ab | 639.96 (29.97) ab | 882.76 (38.00) ab |
Data represents the mean ± S.E.M of 10-12 rats per group analyzed by two-way ANOVA with Bonferroni post-hoc significance testing. Average daily food intake by segment, main effects and interaction significant p<0.0001.
P<0.05 for difference from control.
P<0.05 for difference from LD.
P<0.05 for difference from MD.
3.2. Long-term body regulation
A small number of animals were continually monitored for body weight changes over the next 4 months. The control animals continued a gradual increase in body weight and LD rAAV-leptin was indistinguishable from the control group (LD rAAV-leptin data not shown). The MD rAAV-leptin continued a gradual increase in body weight parallel to that of control whereas the HF rAAV-leptin gained weight a slightly greater rate such that there was no longer a significant difference at 6 months post-treatment (Fig. 1 B).
3.3. Body composition and metabolic changes
Changes in body composition were assessed by TD-NMR initially and every 15 days during the first phase of experiment to day 60 (Table 2). There was a significant decrease in percent body fat by day 15 only in the HD rAAV-leptin compared with pre-experimental percent body fat. By day 30 both the MD and HD demonstrated decreases in percent body fat. Interestingly, between days 45 and 60 of the experiment, some of the lost fat in MD and HD was regained, and MD rAAV-leptin was no longer significant by day 60. There were no changes in percent body fat over the course of the experiment in either the control or LD rAAV-leptin (Table 2). Intra-abdominal fat depots were weighed at terminus of the experiment and paralleled the TD-NMR measurements. Only the HD rAAV-leptin displayed a significant loss in adiposity (Table 2).
Table 2.
Metabolic measures and fat depots following rAAV leptin treatment.
| Time point | GFP | LD | MD | HD |
|---|---|---|---|---|
| Change in % Fat Day 15 | −0.15 (0.33) | −0.38 (0.36) | −1.18 (0.25) | −3.31 (0.41)abc |
| Change in %Fat Day 30 | 0.04 (0.37) | −0.08 (0.48) | −1.30 (0.38)a | −2.48 (0.37)ab |
| Change in % Fat Day 45 | 0.32 (0.29) | 0.34 (0.51) | −1.11 (0.41)ab | −2.61 (0.47)abc |
| Change in % Fat Day 60 | 0.61 (0.31) | 0.65 (0.41) | −0.62 (0.59) | −1.89 (0.42)ab |
| Total adiposity (g) | 14.78 (1.64) | 15.81 (0.84) | 11.21 (2.34 | 4.96 (1.19)abc |
| UCP1 (% of control) | 100 (8.84) | 96.75 (9.15) | 114.8 (15.90) | 122.7 (12.91) |
| RQ | 0.86 (0.01) | 0.85 (0.01) | 0.86 (0.01) | 0.88 (0.01) |
Data represents the mean ± S.E.M. Change in % fat mass was calculated as the difference between the percentage of fat mass at the indicated day and the percentage of fat mass at day 0 for each individual animal. Respiratory quotient was measured at day 53. Adiposity represents the cumulative wet tissue weight of the epididymal, retroperitoneal and perirenal white adipose tissue depots. UCP1 levels for the control were set to 100 and the S.E.M. of groups adjusted accordingly. Data for change in % fat were analyzed by two-way ANOVA with Bonferroni post-tests. P<0.001 for interaction. Data for total adiposity (P<0.001), UCP1 (NS), and RQ (NS) were analyzed by one-way ANOVA with Tukey post-tests.
P<0.05 for difference from Control.
P<0.05 for difference from LD.
P<0.05 for difference from MD.
Western Blot: Top image represents UCP1 and bottom Beta-tublin.
![]()
Just before day 60, oxygen consumption and carbon dioxide production were assessed to calculate respiratory quotient (RQ). There was no significant change in RQ. Another indicator of energy expenditure, UCP1 in brown adipose tissue was determined at death. Brown adipose tissue UCP1 trended towards an increase with rAAV-leptin that, however, was not significant (Table 2).
3.4. Leptin signaling in response to acute leptin stimulation
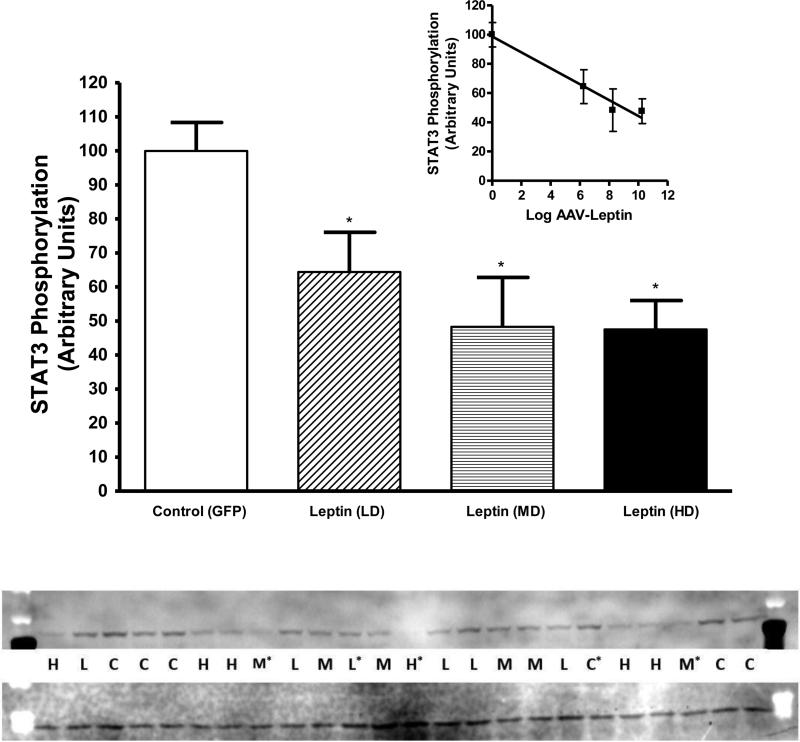
We previously demonstrated that continuous leptin treatment results in leptin resistance (Scarpace et al., 2002). Prior to death at day 60, leptin signaling was assessed in the mediobasal hypothalamus following acute injection of 1 μg of leptin into the third ventricle in order to assess cellular leptin resistance. This acute dose of leptin corresponded to a supra-maximal dose of leptin based on our previously determined full dose-response curve (Scarpace et al., 2001). All rats received leptin, thus maximal signaling was compared across groups. Leptin induced phosphorylated STAT3 (P-STAT3) was diminished two months after treatment with leptin vector in all the leptin treated groups compared with control (Fig. 2). Moreover, the degree of decrease was correlated with the magnitude of the leptin vector dose (Fig. 2 inset).
Fig. 2.
Phosphorylated STAT3 protein in the mediobasal hypothalamus two months after treatment with leptin vector following acute leptin stimulation. Leptin treated groups, LD (stippled), MD (horizontal lines) and HD (solid bar), showed a reduced ability to phosphorylate STAT3 in response to acute leptin stimulation compared with control (open bar). The value of phosphorylated STAT3 in control group is arbitrarily set to 100 with S.E.M. adjusted proportionally with remaining groups normalized to the level in control. Data represents the mean ± S.E.M. of 4-5 rats per group. Data were analyzed by one-way ANOVA (P=0.01) with Tukey post hoc test. *P<0.05 for difference between LD, MD or HD and control. Western Blot: Top image represents pSTAT3 and bottom GAPDH. *Excluded from study secondary to complications (death at stimulation or sickness during experiment).
Inset: Correlation between the dose of administered rAAV-leptin and leptin stimulated STAT3 phosphorylation (r2 =0.970, P=0.015).
3.5. Body weight and food consumption in response to constant vs. pulsed leptin
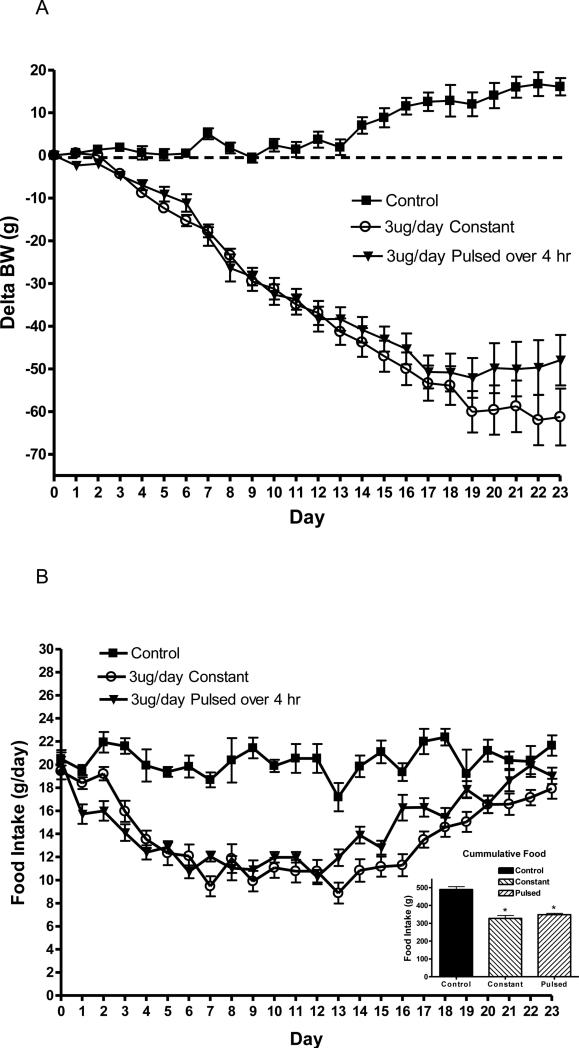
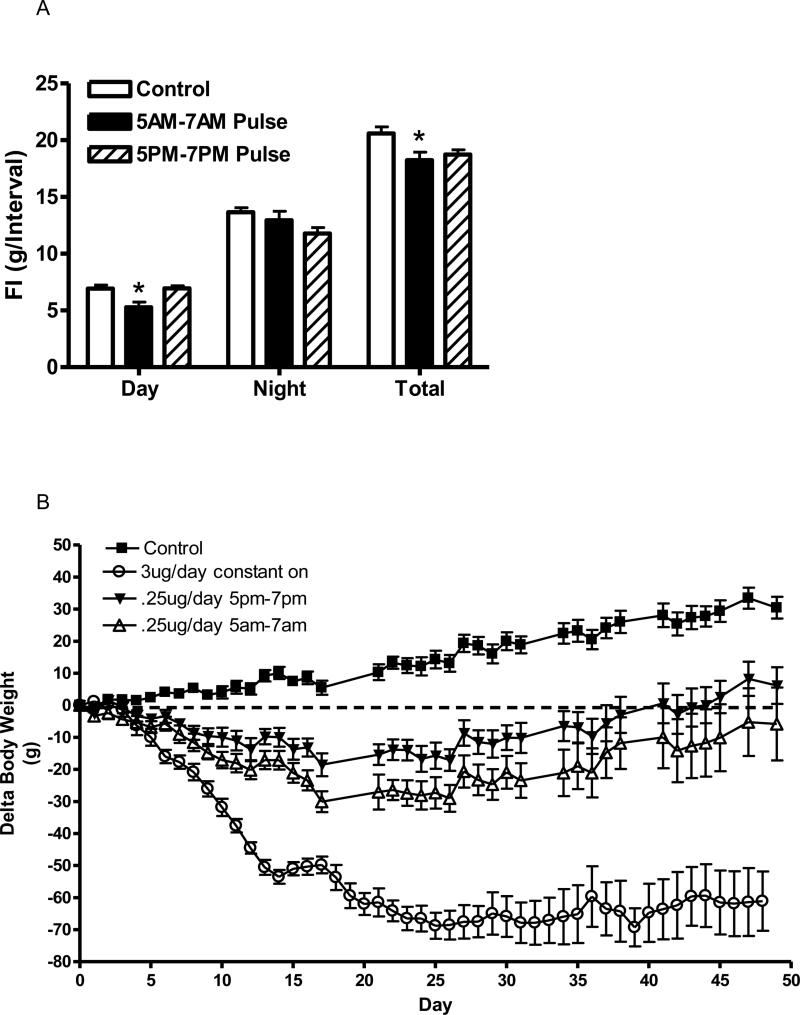
Prior to leptin infusion, body weight was not different between groups and averaged 397 ± 6g. As expected body weight rapidly decreased with the constant infusion of leptin compared with control. Surprisingly, there was no differences in delta body weight between the 4 h pulsed leptin (3 ug/day over 4 h) and the constant (3 ug/day over 24 h) infusions, although there was a trend towards a separation near the end of the experiment (Fig. 3 A).
Fig. 3.
Delta body weight (A) and daily food intake (B) in rats following a 3ug/day constant (open circles) or pulsed 3 ug/day (triangles) leptin infusion compared with ACSF infused rats (squares). Leptin was infused into the third ventricle and the pulsed delivery was over 4 h, starting two h prior to dark cycle. Data represents the mean ± S.E.M. of 6-7 rats per group. There are no significant differences in body weight between leptin treatments. B (Inset): Cumulative food consumption over the experimental treatment period. P<0.0001 for treatment by one-way ANOVA, *P<0.001 for difference from control by Tukey post-hoc analysis.
Food consumption paralleled the change in body weight. There was a rapid and efficacious decrease in food intake with both constant and pulsed leptin infusion, but again no differences between the two types of leptin treatment (Fig. 3 B). Cumulative food consumption over the experimental period indicated a significant decrease of approximately 30% with either leptin treatment, but no difference between the two types of treatment (Fig. 3 B inset). The circadian pattern of food consumption on days 7-8 of treatment indicated the greatest of amount of food was consumed during the dark phase (7 pm to 7 am), and this consumption was diminished by to a similar extent with either constant or intermittent leptin (Table 3). When shorter periods during the dark phase were examined, there was no distinguishing pattern between the two types of leptin infusions (data not shown).
Table 3.
Food intake during dark and light phases with constant versus pulsed leptin infusion.
| Control | Constant Leptin (3ug/day) | Pulsed Leptin (3ug/4h) | |
|---|---|---|---|
| Dark Phase (g) | 18.85 ± 1.14 | 7.67 ± 0.81a | 7.50 ± 0.61a |
| Light Phase (g) | 4.59 ± 0.68 | 2.23 ± 0.247 | 3.53 ± 0.68 |
| Total (g) | 21.44 ± 0.89 | 9.90 ± 0.89a | 11.0 ± 1.05a |
Food consumption was measured during days 7-8 of treatment. Dark phase is from 7 pm to 7 am. P<0.0001 for treatment by one-way ANOVA.
P<0.001 for difference from corresponding control by Tukey's post-hoc analysis.
3.6. Leptin signaling in response to acute leptin stimulation
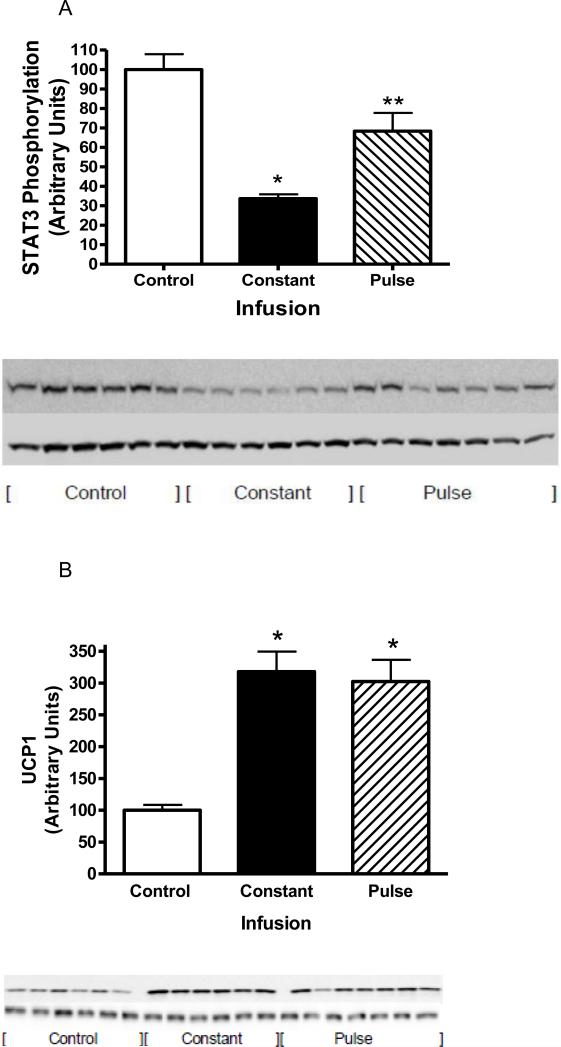
Prior to death, leptin signaling was assessed following acute injection of 1μg of leptin into the third ventricle in order to assess cellular leptin resistance. All rats received leptin, thus maximal signaling was compared across groups. Leptin induced phosphorylated STAT3 (P-STAT3) was diminished by greater than 30% with the pulsed leptin infusion compared with control. Moreover, with the constant infusion, P-STAT3 levels were decreased by nearly 70% compared to control and by 50% compared with the pulsed leptin infusion (Fig. 4 A).
Fig. 4.
A: STAT3 phosphorylation following a single i.c.v. injection of ACSF (open bars) or 1 μg of leptin in rats with constant leptin infusion (sold bars) or 4 h pulsed leptin infusion (hatched bars). STAT3 phosphorylation was assessed 1 h later in the MBH. Data represents the mean ± S.E.M. of 6-7 rats per group. The value control group is arbitrarily set to 100 with S.E.M. adjusted proportionally with remaining groups normalized to the level in control. P<0.0001 by one-way ANOVA, *P<0.001 for difference from Control; **P<0.05 for difference from control or constant infusion by Tukey post-hoc analysis. Western Blot: Top image represents pSTAT3 and bottom GAPDH.
B: UCP1 protein levels in BAT in control (open bars) and following constant leptin infusion (solid bars) or 4 h pulsed leptin infusion (hatched bars). Data represents the mean ± S.E.M. of 6-7 rats per group P<0.001 by one-way ANOVA, *P<0.001 (Constant infusion) or P<0.05 (Pulsed infusion) for difference from Control by Tukey post-hoc analysis. Western Blot: Top image represents pSTAT3 and bottom beta-tublin.
3.7. Brown Adipose Tissue UCP1
UCP1 protein levels in brown adipose tissue were determined as a measure of leptin-induced thermogenesis. As expected, UCP1 levels were elevated following both types of leptin infusion, but there was no significant difference between the constant infusion compared and the pulsed infusion (Fig. 4 B). When total UCP1 levels per BAT tissue were calculated, the same pattern was observed with an increase with either type of infusion but no difference among constant and pulsed infusions (100 ± 11.7, Control; 254.2 ± 29.2, Constant; 189.5 ± 12.9, Pulsed; P<0.001).
3.8. Body weight and food consumption in response to morning vs. night pulsed leptin
The initial experiment suggests that the dose of 3 ug/day may be too large to metabolize within one day. Therefore, we examined a lower dose of 0.25 ug/day delivered between 5 pm and 7 pm or between 5 am and 7 am, and assessed the effective duration of these doses. Two weeks after initiation of leptin infusions, food consumption was measured in the light period (7 am-7 pm) and dark period (7 pm-7 am) for six consecutive days. Pulsed leptin infusion between 5 am and 7 am significantly reduced food consumption during the light period but not the dark period (Fig. 5). Interestingly, while the leptin infusion from 5 pm to 7 pm only tended to reduce food intake in the dark period, this trend was not significant. Moreover, this PM infusion had no effect on food consumption in the light period (Fig. 5 A). When total daily food consumption was considered, during the first two weeks, food intake was significantly reduced only with the AM infusion, although there was trend for a decrease with the PM infusion (Fig. 5 A). After this initial two-week period, food consumption returned to control value with both the AM and PM infusions and was not different over the duration of the experiment (data not shown).
Fig. 5.
A: Food consumption during the light and dark phases and total daily food intake in control (open bars) and following a pulsed 0.25 ug/day infusion for 4 h starting 2 h before beginning of dark cycle (solid bars) or pulsed 0.25 ug/day infusion for 4 h starting 2 h before beginning of light cycle (hatched). Data represents the mean ± S.E.M. of 6-7 rats per group; P<0.01 (Day) for difference between AM Pulse and either Control or PM Pulse.
B: Delta body weight in rats following control (ACSF infused, squares),a 3 ug/day constant infusion (open circles), pulsed 0.25 ug/day infusion for 4 h starting 2 h before beginning of dark cycle (open triangles), or pulsed 0.25 ug/day infusion for 4 h starting 2 h before beginning of light cycle (closed circles). Data represents the mean ± S.E.M. of 6-7 rats per group. Two-way ANOVA of delta body weight indicated significance with time, treatment and interaction (P<0.001). Control was significantly different from AM pulse infusion starting at day 10 (P<0.01) and from PM infusion starting at day 16 (P<0.01) by post-hoc analysis. Note: The constant-on infused group was determined separately from the other groups and was not included in the statistical analysis.
When long term body weight was examined, both the AM and PM pulsed infusion demonstrated a substantial decrease in delta body weight compared with control. There was slight trend of a greater decrease with the AM infusion compared with the PM infusion but this was not significant (Fig. 5 B). Importantly, the magnitudes of the decreased delta body weight with either of these lower (0.25 ug/day) pulse doses was considerably less than the higher (3 ug/day) constant infusion (Fig. 5 B).
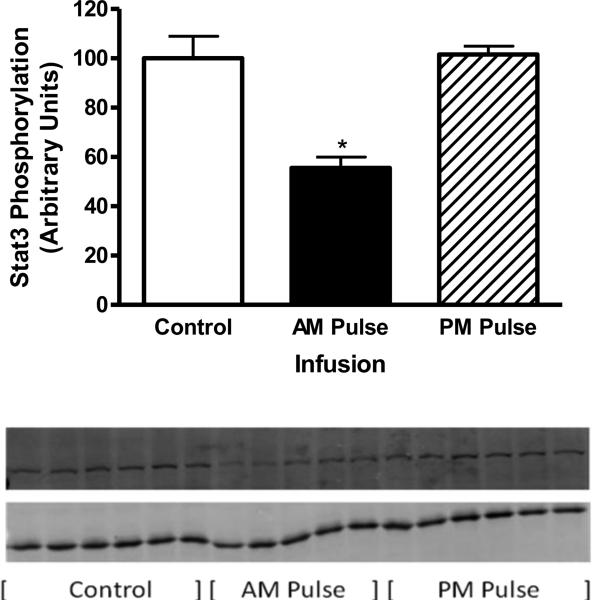
3.9 Leptin signaling in response to acute leptin stimulation
Prior to death, leptin signaling was assessed in order to assess cellular leptin resistance. All rats received leptin, thus pSTAT3 signaling was compared across groups. Interestingly, cellular leptin resistance was only observed following the AM pulsed dose (Fig. 6). Leptin signaling was unchanged with the PM pulsed infusion whereas signaling was diminished by 45% with the AM pulsed infusion. These contrasting differences in signaling were despite very similar decreases in delta BW with the AM and PM pulsed leptin treatment (Fig. 5 B).
Fig. 6.
STAT3 phosphorylation following a single i.c.v. injection of ACSF (open bars) or 200 ng of leptin in rats with AM leptin infusion (solid bars) or PM leptin infusion (hatched bars). STAT3 phosphorylation was assessed 1 h later in the MBH. Data represents the mean ± S.E.M. of 5-6 rats per group. The value control group is arbitrarily set to 100 with S.E.M. adjusted proportionally with remaining groups normalized to the level in control. P<0.0001 by one-way ANOVA, *P<0.001 for difference from Control; **P< .001 for difference from control or PM infusion by Tukey post-hoc analysis. Western Blot: Top image represents pSTAT3 and bottom GAPDH.
4. Discussion
Tolerance or resistance to leptin is the hallmark of prolonged leptin treatment, yet the underlying mechanism remains elusive. This study provides additional insight by examining unique treatment modalities, including pulsed delivery, morning versus evening delivery and dose-response leptin overexpression. With respect to the latter, we previously demonstrated that long-term leptin overexpression resulted in an attenuation of the anorexic and body weight reduction effect in a stepwise fashion characterized by a rapid reversal of the anorexic response and slower regain in body weight. In addition, we identified cellular leptin resistance (diminished leptin-mediated STAT3 phosphorylation) in multiple regions within hypothalamus as well as in the ventral tegmental area (VTA) even in the absence of high-fat influence (Matheny et al., 2011). The loss of leptin stimulated STAT3 phosphorylation is generally considered one marker of cellular leptin resistance; however this conjecture has not been conclusively established. The decrease in leptin signaling could be related to prolonged maximal stimulation, or simply reflect prolonged stimulation at any level or neither. We hypothesized that a prolonged treatment with a lower dose of leptin would not induce cellular leptin resistance and produce protracted anorexic responses and extended negative energy balance. The observed data did not support this hypothesis. Dose-response long-term central leptin overexpression decreased food consumption in the expected dose-response fashion, but resistance to the anorexic response was observed with all effective doses within similar time frames. Similarly, in the two higher doses, the body weight reductions were dose dependent over the first two months, whereas the long-term effectiveness was not different at 6 months of treatment. Interestingly, the cellular leptin resistance was correlated with the rAAV-leptin titer after two months of treatment. It is important to note, in past experiments after long-term high-dose central leptin overexpression, not only is there persistent cellular leptin resistance, but also the physiological responsiveness to exogenously administered leptin is blunted (Scarpace et al., 2003). These data suggest that the long-term effectiveness of leptin is not dependent on the dose whereas cellular leptin resistance is positively correlated with the leptin dose, at least after 2 months of treatment. Collectively, these data suggest that cellular leptin responsiveness is not a reliable marker for leptin therapeutic responses.
The failure to improve long-term physiological responses to leptin by varying the dose, prompted investigations using pulsed leptin delivery. We examined two types of pulsed delivery. In the first, the amount of leptin delivered (3 ug) over a single day was held constant and the time of delivery was varied; either constant over a 24 h period (0.125 ug/h) or pulsed over a 4 h period (0.75 ug/h) prior to the dark cycle. Both the constant and pulsed delivery deceased daily food consumption, but interestingly, the food reductions were only significant on dark phase consumption. Surprisingly, the overall anorexic responses and body weight reductions were essentially the same. The simplest explanation is that the dose of leptin delivered over the 4 h period was of significant magnitude that clearance failed to reduce it to a level that is sub-maximal with respect to physiological responses. This is supported by the leptin signaling data that indicates greater cellular leptin resistance (i.e., lower leptin signaling) with the constant infusion than with the pulsed. Our data from the dose-response experiment indicates that the degree of cellular leptin resistance is related to dose. In the pulsed group, the local concentrations of leptin will be lower than the level in the constant infusion for a certain portion of the 24 h period, thus imparting a less efficacious induction of cellular leptin resistance. The assumption is that the concentration of leptin with the constant infusion is maximal, thus the portion of time that the pulsed delivery results in a higher local leptin concentration will be no more efficacious than the constant delivery. That is, the degree of cellular resistance is proportional to the lowest level of leptin exposure. This is further supported by the induction of UCP1 which is greater with the constant delivery than the pulsed delivery.
The second type of pulsed delivery controlled both the amount and rate of delivery, but varied the timing, either a 4 h pulsed delivery prior to the light phase or a 4 h pulsed delivery prior to the dark phase. The amount of leptin in this experiment was less than one-tenth of that given in the previous experiment, potentially representing a sub-maximal dose. This apparently was the case as the reduction in body weight was considerably less than that observed with the 3 ug/day dose. There were two salient findings from this experiment. First, leptin pulsed prior to the light phase (AM pulse) reduced food consumption in the day but not at night. This further supports our conjecture that the 0.25ug/day dose is sub-maximal and suggests that the AM pulsed delivery has cleared to sub-therapeutic levels by the evening. The results with pulsed delivery prior to the dark phase (PM pulse) are less clear. In a corresponding fashion with the AM pulse, the PM pulse decreased food consumption in the dark phase, but this difference did not achieve significance. As expected the PM pulse did not alter food intake during the light phase. These data further support the finding from our first pulse experiment that the action of leptin is brief and only effective at threshold concentration levels. Furthermore, the data raises the possibility that the threshold for reducing dark phase consumption may be greater than that for the light phase. It is important to note that with the AM pulse, there was no rebound feeding during the subsequent dark phase. These data suggest that pulsed leptin therapy may be beneficial in reducing food consumption during particular meal periods.
The second salient finding from this pulsed experiment is that AM pulsed delivery induces cellular leptin resistance whereas PM pulsed delivery does not. This is despite very similar long-term body weight reductions with the AM and PM pulsed leptin infusions. The only observed differences in the AM and PM pulsed infusions is that the AM pulse selectively diminished the light-phase food consumption, whereas the PM pulse did not significantly decrease either dark-phase or light-phase consumption. Potentially, the AM pulse-induced anorexic response is related to the onset of cellular leptin resistance. An alternative explanation is that both the AM and PM pulse infusions induce short-term cellular leptin resistance during the period when the local levels of leptin are elevated and because the rats were killed in the morning, cellular leptin resistance was only observed with the AM pulse infusion. Collectively, these data suggest that pulsed infusion may be beneficial by decreasing body weight without inducing persistent cellular leptin resistance. In particular, the PM pulse leptin delivery appears to reduce body weight with minimal effect on food consumption while maintaining normal leptin signaling.
This concept is supported by αMUPA mice that exhibit prolonged elevated leptin. These mice demonstrate lifelong elevated leptin expression with a lean phenotype (Froy et al., 2011). This is in contrast to rodents with chronic rAAV-leptin gene delivery-mediated leptin overexpression that demonstrate leptin resistance and exacerbated weight gain when exposed to a high-fat diet (Scarpace and Zhang, 2009). The contrasting phenotypes displayed by these two models of leptin overexpression are likely due to the different temporal natures of the leptin overexpression. The rAAV-mediated leptin overexpression represents an unregulated and constant mode of overexpression, whereas leptin overexpression in αMUPA mice is circadian. Thus, constant leptin overexpression induces leptin receptor desensitization that disrupts energy homeostasis characterized by an obese phenotype, whereas circadian expression may permit persistent leptin receptor activity that maintains energy homeostasis. Leptin is currently being investigated for use in several conditions as outlined in the introduction (Polyzos and Mantzoros, 2015). In all of these conditions, treatment involves long-term leptin administration, in the form of Metreleptin. This drug is normally administered subcutaneous once a day and has a half life of about 4 h. This route of administration is in contrast to present study that employs central delivery. However, leptin resistance is observed with both central and subcutaneous administration of leptin as first described by Friedman (Halaas et al., 1997) and additionally with both low and high dose delivery of subcutaneous leptin in rats (Martin et al., 2000). It is unknown whether the therapeutic effect of subcutaneous leptin will be maintained with chronic treatment in humans. Consideration of the timing of leptin delivery may be critical for successful treatment.
In conclusion, this study investigated three types of long-term leptin therapies, including dose response with constant delivery, pulsed verses constant delivery, and AM versus PM pulsed delivery. Varying the dose proved inconsequential with respect to long-term therapy. The two pulsed delivery experiments suggested pulsed delivery of leptin prior to the light phase reduces food intake over limited meal periods and is associated with body weight reductions, but results in cellular leptin resistance In contrast, the PM pulsed infusion prior to the dark phase reduces body weight to a similar degree with minimal reduction in food consumption and maintains full leptin signaling. The long term consequences of either an AM or PM pulse delivery remain speculative, yet potentially may provide an alternative mode of leptin therapy.
Acknowledgements
Supported by the NIH DK091710.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Altun M, Bergman E, Edström E, Johnson H, Ulfhake B. Behavioral impairments of the aging rat. Physiology & Behavior. 2007;92:911. doi: 10.1016/j.physbeh.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53:27. doi: 10.1007/s00125-009-1502-9. [DOI] [PubMed] [Google Scholar]
- Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R, Brinkoetter MT, Gong H, Arampatzi K, Mantzoros CS. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A. 2011;108:6585. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppari R, Bjorbaek C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov. 2012;11:692. doi: 10.1038/nrd3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and Generalized Lipodystrophy: Comparison of Baseline Characteristics and Response to Metreleptin. The Journal of Clinical Endocrinology & Metabolism. 2015;100:1802. doi: 10.1210/jc.2014-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535. [PubMed] [Google Scholar]
- Fei H, Okano HJ, Li C, Lee G-H, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proceedings of the National Academy of Sciences. 1997;94:7001. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froy O, Sherman H, Bhargava G, Chapnik N, Cohen R, Gutman R, Kronfeld-Schor N, Miskin R. Spontaneous caloric restriction associated with increased leptin levels in obesity-resistant [alpha]MUPA mice. 2011;35:226. doi: 10.1038/ijo.2010.125. [DOI] [PubMed] [Google Scholar]
- Gautron L, Elmquist JK. Sixteen years and counting: an update on leptin in energy balance. The Journal of Clinical Investigation. 2011;121:2087. doi: 10.1172/JCI45888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci U S A. 1997;94:8878. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- Knight ZA, Hannan KS, Greenberg ML, Friedman JM. Hyperleptinemia is required for the development of leptin resistance. PLoS One. 2010;5:e11376. doi: 10.1371/journal.pone.0011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. 1995;1:1155. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, Hamnvik OP, Koniaris A. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301:E567. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark AL. Selective leptin resistance revisited, American Journal of Physiology - Regulatory. Integrative and Comparative Physiology. 2013;305:R566. doi: 10.1152/ajpregu.00180.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RL, Perez E, He Y-J, Dawson R, Jr, Millard WJ. Leptin resistance is associated with hypothalamic leptin receptor mRNA and protein downregulation. Metabolism. 2000;49:1479. doi: 10.1053/meta.2000.17695. [DOI] [PubMed] [Google Scholar]
- Matheny M, Shapiro A, Tümer N, Scarpace PJ. Region-specific diet-induced and leptin-induced cellular leptin resistance includes the ventral tegmental area in rats. Neuropharmacology. 2011;60:480. doi: 10.1016/j.neuropharm.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorfer B, Horowitz JF, DePaoli AM, McCamish MA, Patterson BW, Klein S. Recombinant Human Leptin Treatment Does Not Improve Insulin Action in Obese Subjects With Type 2 Diabetes. Diabetes. 2011;60:1474. doi: 10.2337/db10-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H-S, Matarese G, Brennan AM, Chamberland JP, Liu X, Fiorenza CG, Mylvaganam GH, Abanni L, Carbone F, Williams CJ, De Paoli AM, Schneider BE, Mantzoros CS. Efficacy of Metreleptin in Obese Patients With Type 2 Diabetes: Cellular and Molecular Pathways Underlying Leptin Tolerance. Diabetes. 2011;60:1647. doi: 10.2337/db10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzberg H, Flier JS, Bjørbæk C. Region-Specific Leptin Resistance within the Hypothalamus of Diet-Induced Obese Mice. Endocrinology. 2004;145:4880. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- Münzberg H, Morrison CD. Structure, production and signaling of leptin. Metabolism. 2015;64:13. doi: 10.1016/j.metabol.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H-K, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64:24. doi: 10.1016/j.metabol.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; San Diego: 2005. [Google Scholar]
- Polyzos SA, Mantzoros CS. Leptin in Health and Disease: Facts and Expectations at its Twentieth Anniversary. Metabolism. 2015;64:5. doi: 10.1016/j.metabol.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA. 2008;105:7257. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáinz N, Barrenetxe J, Moreno-Aliaga MJ, Martínez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64:35. doi: 10.1016/j.metabol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Tumer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience. 2001;104:1111. doi: 10.1016/s0306-4522(01)00142-7. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Zhang Y, Cheng KY, Tumer N. Leptin antagonist reveals an uncoupling between leptin receptor signal transducer and activator of transcription 3 signaling and metabolic responses with central leptin resistance. J Pharmacol Exp Ther. 2007;320:706. doi: 10.1124/jpet.106.112813. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Zhang Y, Shek EW, Prima V, Zolotukhin S, Tümer N. Leptin-Induced Leptin Resistance Reveals Separate Roles for the Anorexic and Thermogenic Responses in Weight Maintenance. Endocrinology. 2002;143:3026. doi: 10.1210/endo.143.8.8966. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Zolotukhin S, Tümer N, Zhang Y. Leptin-induced leptin resistant rats exhibit enhanced responses to the melanocortin agonist MT II. Neuropharmacology. 2003;45:211. doi: 10.1016/s0028-3908(03)00158-8. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Zhang Y. Leptin resistance: a prediposing factor for diet-induced obesity. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2009;296:R493. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienkiewicz E, Magkos F, Aronis KN, Brinkoetter M, Chamberland JP, Chou S, Arampatzi KM, Gao C, Koniaris A, Mantzoros CS. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism. 2011;60:1211. doi: 10.1016/j.metabol.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Wilsey J, Zolotukhin S, Prima V, Scarpace PJ. Central leptin gene therapy fails to overcome leptin resistance associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1011. doi: 10.1152/ajpregu.00193.2003. [DOI] [PubMed] [Google Scholar]
- Zelissen PMJ, Stenlof K, Lean MEJ, Fogteloo J, Keulen ETP, Wilding J, Finer N, Rössner S, Lawrence E, Fletcher C, McCamish M. Effect of three treatment schedules of recombinant methionyl human leptin on body weight in obese adults: a randomized, placebo-controlled trial. Diabetes, Obesity and Metabolism. 2005;7:755. doi: 10.1111/j.1463-1326.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ, Jr., Chiodo VA, Phillipsberg T, Muzyczka N, Hauswirth WW, Flotte TR, Byrne BJ, Snyder RO. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]