Abstract
Objective
This study aimed to describe the implementation of preoperative geriatric assessment (GA) in patients undergoing major cancer surgery and to determine predictors of postoperative delirium.
Summary Background Data
Geriatric surgical patients have unique vulnerabilities and are at increased risk of developing postoperative delirium.
Methods
Geriatricians at Memorial Sloan Kettering Cancer Center risk-stratify surgical patients with solid tumors, aged ≥ 75 years using preoperative GA, which includes basic and instrumental activities of daily living (ADLs, IADLs), cognition (Mini-Cog Test), history of falls, nutritional state, and comorbidities (Charlson Comorbidity Index [CCI]). The Geriatrics Service evaluates patients for postoperative delirium using the Confusion Assessment Method (CAM). A retrospective review was performed. The associations between GA and postoperative outcomes were evaluated. Univariate logistic regression analysis was performed to determine the predictive value of GA for postoperative delirium, and a multivariate model was built.
Results
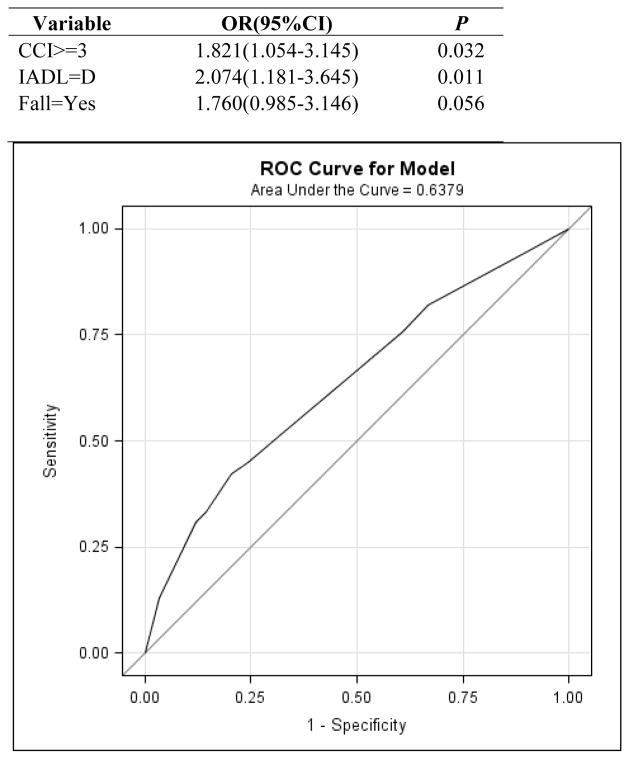
In total, 416 patients who received preoperative evaluation by the Geriatrics Service between September 1, 2010, and December 31, 2011, were included. Delirium occurred in 19% of patients. Patients with delirium had longer length of hospital stay (P<0.001) and greater likelihood of discharge to a rehabilitation facility (P<0.001). CCI score, history of falls, dependent on IADL, and abnormal Mini-Cog Test results predicted postoperative delirium on univariate analysis. Developed using a stepwise selection method, a multivariate model to predict delirium is presented including CCI score (P=0.032), dependence IADLs (P=0.011) and falls history (P=0.056).
Conclusions
Preoperative GA is feasible and may achieve a better understanding of older patients’ perioperative risks, including delirium.
INTRODUCTION
Among all known risk factors for developing cancer, the most powerful is growing old. Patients older than age 65 have an 11-fold increased cancer incidence and a 16-fold increase in cancer mortality than younger patients. The median age at a cancer diagnosis is 67 and the median age of cancer-related death is 73 years old.1 The population at risk is growing rapidly and by 2030, 20% of the US population will be over 65. 2
Cancer treatment in older adults can be challenging and complex. Aging is associated with an increasing prevalence of frailty, multiplicity of diseases, disabilities, decline of functional reserve, and progressive restriction in personal and social resources which result in greater vulnerability to clinically important outcomes such as functional decline, institutionalization, and falls.3 Older patients with cancer are less likely to be offered standard cancer treatments that have been shown to improve survival, in part because of concerns regarding their ability to tolerate treatment.4–6 One complication of treatment is delirium, a disturbance of consciousness with fluctuating symptoms, which is common among older hospitalized patients, and is associated with a significantly increased risk of other morbidities, longer hospitalizations and higher mortality rates.7–9 Delirium also predicts prolonged institutionalization and cognitive decline after discharge. 8–10
Chronological age alone does not accurately reflect remaining life-expectancy or treatment tolerance.11,12,13 Geriatric surgical patients have unique vulnerabilities that require assessment beyond the traditional preoperative evaluation 14 and the importance of geriatric assessment in predicting surgical outcomes on elderly patients has been previously reported. 15–19 Robinson and colleagues showed that markers for frailty, disability and comorbidities predicted post-discharge institutionalization and 6-month postoperative mortality.14 Recently, the American College of Surgeons in collaboration with the American Geriatric Society created best practice guidelines around optimal perioperative care of the surgical patient to identify high risk patients and prevent perioperative adverse outcomes. 20 However these may require significant resources and time to complete.20
A validated and brief preoperative evaluation tool that recognizes the unique physiologic vulnerabilities of the geriatric population and accurately predicts outcomes is greatly needed. The Memorial Sloan Kettering Cancer Center (MSKCC) Geriatric Service has incorporated selected elements of the comprehensive geriatric assessment into our daily clinical practice. This study will describe a short practical approach to preoperative geriatric assessment (GA) and determine the association between geriatric assessment variables and the risk of developing postoperative delirium and other outcomes in older cancer patients.
METHODS
The Geriatrics Service at MSKCC provides preoperative assessment of surgical patients ≥ 75 years old. Within the context of the preoperative assessment and as a standard of care, preoperative GA is performed by fellowship-trained geriatricians (MD) or geriatric Nurse Practitioners (NP) and Registered Nurses (RN) trained to perform geriatric assessment which is documented in a dedicated form in the electronic medical record. In addition to the usual components of a history, physical examination, medication review (including over-the-counter medications and supplements) and laboratory data, PGA uniquely captures: 1. Functional Status: assessed by activities of daily living (ADLs) or Katz’s Index21 which represents the ability of the patient to take care of him/herself, and instrumental activities of daily living (I-ADLs)22 which assess the ability of the patient to live independently in the community and history of falls in the last six months; 2. Cognitive status: assessed using the Mini-Cog Test (combined 3 item recall with a clock drawing test (CDT)) 23; 3. Nutritional Status: assessed by weight loss >10lbs in the last six months, albumin and BMI; 4. Comorbidities quantified by the Charlson Comorbidity Index (CCI). The American Society of Anesthesiologists (ASA) physical status score24 was obtained from the anesthesiology medical records. ADLs and IADLs are completed by the patient or caregiver. Mini-Cog is performed by the clinician.
Patients are medically optimized and risk-stratified for surgery. The subset of patients who are admitted to the hospital after the surgical procedure are evaluated daily by the MD and/or NP of the Geriatrics Service until discharge. As part of the daily postoperative follow up, every patient is evaluated for delirium using the Confusion Assessment Method (CAM).25 The CAM is a well-validated instrument for the diagnosis of delirium. It is completed by observing the patient for the presence of the following: 1) an acute change in mental status or fluctuating course of abnormal behavior; 2) inattention; 3) disorganized thinking; 4) altered level of consciousness. The CAM is considered positive for the delirium if the first 2 items and either the third or fourth item are present. Fluctuations in mental status between evaluations are monitored either by direct communication with the patients’ unit nurse or from nursing notes in the medical record.
Patients who underwent preoperative GA between 9/1/2010 and 12/31/2011 and were then admitted to MSKCC hospital after surgery were included in this analysis. Exemption from IRB/PB Review was obtained. Non-English speakers who were unable to perform the Mini Cog Test and patients who were admitted to the ICU were excluded from this analysis. Computerized databases maintained by the Geriatric and various surgical services as well as institutional databases were queried. A data set with preoperative variables was developed. Outcomes collected included: development of postoperative delirium, length of hospital stay (LOS), post-discharge disposition, visit to MSKCC Urgent Care Center (UCC) within 30 days, hospital readmission to MSKCC within 30 days and mortality rates (30 day and 6-month). A retrospective review of this surgical population was performed.
Statistical Analysis
The associations between potentially predictive clinical data (PGA measures, operative time, LOS, post-discharge disposition, visit to UCC within 30 days from time of first discharge, 30-day hospital readmission, and 6-month mortality) and post-operative delirium were evaluated by utilizing Fisher-Exact tests for categorical variables and Wilcoxon-Rank Sum tests for continuous variables. The relationship between 30 day readmission and 30 day UCC and the post-operative delirium after controlling for the discharge place is tested by using Cochran-Mantel-Haenszel test.
Univariate logistic regressions were performed to determine the predictive ability of the PGA for risk of developing post-operation delirium. Based on the univariate logistic analysis results, we used the p-value<0.1 as the criterion to choose the candidate variable for multivariate model building. The stepwise selection method was used in the multivariate logistic model selection based on the six variables whose significance exceeded our a priori cut-off of p<0.1 (“CCI”, “Falls in the last 6 months prior to surgery ”, ”IADL”, “CDT”, “Mini-Cog” and “ASA”). The Receiver Operating Characteristic (ROC) curve and the area under the curve (AUC) for the final multivariate model are provided as a measure of the model’s predictive accuracy. The ROC (receiver operating characteristic) curve is a commonly used summary plot for assessing the tradeoff between sensitivity and specificity. The AUC (area under the curve) is a quantitative summary which can range from 1.0 perfect discrimination) to 0.5 (discrimination no better than tossing a coin).
RESULTS
Between 9/1/2010 and 12/31/2011 416 cancer patients with a median age 80 (75–98) who underwent surgery for the treatment of solid tumors were followed postoperatively by the Geriatrics Service. Pre-treatment clinical and functional characteristics obtained during preoperative geriatric consultation are presented in Table 1. 45.9% were male and 93% were white. The majority of the patients had hepato-pancreatobiliary (20%), colorectal (18%), head/neck (13.6%) or urological (12.5%) cancer. The other 35% of the patients included a variety of cancers such as thoracic, gynecological, breast and mixed tumors. Most of these patients had good social support at home with family members involved in their care (92%).
Table 1.
Baseline Characteristics
| MEDIAN(RANGE) | # OF PATIENTS (%) | |
|---|---|---|
| BMI a | 26 (11.6–53.4) | |
| ALBUMIN ≤ 3.3a | 13 (3) | |
| WEIGHT LOSSb (> 10lbs in last 6 months) | 178 (47) | |
| NUMBER OF MEDICATIONS | 6(0–22) | |
| Charlson Comorbidity Index | 3(0–12) | |
| FALLS in the last 6 monthsc | 83 (20) | |
| Dependent on ADLs d (Score < 6) | 99 (24) | |
| Dependent on IADLsd (Score < 8) | 90 (22) | |
| Abnormal Mini Cog Teste (Score 0–3) | 123 (31) | |
| ASA c | ||
| score =2 | 54 (13) | |
| score=3 | 329 (80) | |
| score=4 | 26 (6) | |
| Type of Cancer | ||
| Hepatobiliary | 83 (20) | |
| Colorectal | 75 (18) | |
| Head and Neck | 57 (14) | |
| Urological | 52 (13) | |
| Other | 149 (35) | |
| LABORATORY DATA | ||
| Creatinine (mg/dl) | 1(0.4–3.1) | |
| Na (mEq/L) | 141 (124 – 147) | |
| Hb (g/dl) | 12.5 (6.7 – 18.1) | |
| Estimated Creatinine Clearance(ECC) (ml/min/1.73m2) | ||
| >60 | 253 (61) | |
| 30–60 | 154 (37) | |
| <30 | 9 (2) |
Data on the variable were missing for 2 patients.
Data on the variable were missing for 37 patients.
Data on the variable were missing for 7 patients.
Data on the variable were missing for 5 patients.
Data on the variable were missing for 16 patients.
Postoperative delirium (diagnosed by a positive CAM)25 occurred in 79 patients (19%). Table 2 shows the association between preoperative characteristics and postoperative outcomes and the development of postoperative delirium. Patients who developed delirium did not show a significant increase in their Urgent Care Clinic (UCC) visits within 30 days or 30 days readmission rate. Even after controlling for the discharging disposition, the readmission rate and the UCC visits rate within 30 days are not significantly associated with post-operative delirium (Cochran Mantel Haenszel p=0.864 for readmission and p=0.509 for UCC). 30 day mortality was too low for any statistical analysis; only four patients died within 3 days of surgery and 2 of them developed delirium. Even though 6-month mortality was higher in the group of patients that developed postoperative delirium (7.1% vs. 13.9%), the difference was not statistically significant (p=0.069).
Table 2.
Postoperative Characteristics and Outcome Measures
| Characteristic | Median (range) | Patients, no. (%) |
|---|---|---|
| Operative time, mina | 176 (8–1005) | — |
| Delirium (positive CAM) | — | 79 (19) |
| LOS, days | 6 (1–76) | — |
| Readmitted within 30 days | — | 47 (11) |
| Discharge disposition a | — | — |
| Home | — | 146 (36) |
| Home with services | — | 213 (52) |
| Skilled nursing facility | — | 48 (12) |
| UCC visit within 30 days | — | 67 (16) |
| Death within 30 days | — | 4 (1) |
| Death within 6 months | — | 35 (8) |
Data on the variable were missing for 9 patients.
As expected, patients experiencing delirium had significantly longer length of hospital stay (median 8 vs. 6 days) (p<0.001) and had greater risk of discharge to a rehabilitation facility (26.9 vs. 8.2%) (p<0.001). By univariate logistic regression, CCI score (p = 0.013), falls (p= 0.012), dependence in I-ADLs (p = 0.001), abnormal Mini-Cog test (p=0.046) and ASA score = 4 (p<0.047) were significant predictors of risk of developing post-operative delirium (Table 3). Based on these, using the stepwise selection with p=0.1 as criteria, a final multivariate model was developed which includes CCI score (p=0.032), dependence I-ADL (p=0.011) and falls (p=0.056) as significant predictors (Table 4). The Receiver Operating Characteristic (ROC) curve, is represented in figure1 with the AUC = 0.638.
Table 3.
Association Between Clinical Data and Postoperative Delirium
| Variables | Postoperative Delirium | P | |
|---|---|---|---|
|
| |||
| No | Yes | ||
|
| |||
| ALL n° | 337 | 79 | |
|
| |||
| AGE Median (Range) | 80 (75–98) | 80 (75–95) | 0.26 |
|
| |||
| GENDER n° (%) | |||
| Female | 183 (54) | 42 (53) | 0.9 |
| Male | 154 (46) | 37 (47) | |
|
| |||
| BMI Median (Range) | 26 (11.6 – 53.4) | 25.4 (18.1 – 41.2) | 0.345 |
|
| |||
| ALBUMIN n°(%) | |||
| >3.3 | 322(97) | 76 (96) | 0.721 |
| ≤3.3 | 10 (3) | 3 (4) | |
|
| |||
| Weight Loss n° (%) | |||
| No | 162 (53) | 39 (53) | 1 |
| Yes | 144 (47) | 34 (47) | |
|
| |||
| CCI N (%) | |||
| < 3 | 150 (45) | 23 (29) | 0.016 |
| ≥3 | 187 (55) | 56(71) | |
|
| |||
| Number of Medications | |||
| Median (Range) | 6 (0–22) | 7 (1–17) | 0.266 |
|
| |||
| Social Support n° (%) | |||
| No | 30 (9) | 3 (4) | 0.167 |
| Yes | 307 (91) | 76 (96) | |
|
| |||
| Falls in the last 6 months n°(%) | |||
| No | 272 (82) | 54 (69) | 0.018 |
| Yes | 59 (18) | 24 (31) | |
|
| |||
| Dependent on ADLs n°(%) | |||
| No | 257 (77) | 55 (70) | 0.146 |
| Yes | 75 (23) | 24 (30) | |
|
| |||
| Dependent on IADLs n°(%) | |||
| No | 270 (81) | 51 (65) | 0.002 |
| Yes | 62 (19) | 28 (35) | |
|
| |||
| Mini-Cog Test n°(%) | |||
| Normal | 231 (72) | 46 (60) | 0.054 |
| Abnormal | 92(28) | 31 (40) | |
|
| |||
| ASA n°(%) | |||
| Score = 2 | 49 (15) | 5 (6) | 0.078 |
| Score = 3 | 262 (79) | 67 (84) | |
| Score = 4 | 19 (6) | 7 (9) | |
|
| |||
| Creatinine Median (Range) | 1 (0.5 – 3.1) | 1 (0.4 – 1.9) | 0.96 |
|
| |||
| Sodium Median (Range) | 141 (124–147) | 140(130–147) | 0.185 |
|
| |||
| Hemoglobin Median (Range) | 12.5 (6.7–18.1) | 12.7 (7.8–16.4) | 0.823 |
|
| |||
| ECC n°(%) | |||
| >60 | 208 (62) | 45 (57) | 0.606 |
| 30–60 | 121 (36) | 33 (42) | |
| <30 | 8 (2) | 1 (1) | |
|
| |||
| Operative Time Median(Range) | 167 (8–815) | 191 (27–1005) | 0.155 |
|
| |||
| Length of Stay Median (Range) | 6 (1–55) | 8 (1–76) | <0.001 |
|
| |||
| Readmission in 30 days n°(%) | |||
| No | 299(89) | 70 (89) | 1 |
| Yes | 38 (11) | 9 (11) | |
|
| |||
| UCC Visits within 30days n°(%) | |||
| No | 281(83) | 68 (86) | 0.614 |
| Yes | 56 (17) | 11 (14) | |
|
| |||
| Death within 6 months n°(%)* | |||
| No | 313 (93) | 68 (86) | 0.069 |
| Yes | 24 (7) | 11 (14) | |
|
| |||
| Discharge Disposition n°(%) | |||
| Home | 136 (41) | 10 (13) | <0.001 |
| Home with Services | 166 (51) | 47(60) | |
| Skilled Nursing Facility | 27 (8) | 21(27) | |
landmark analysis 10 days post surgery
Table 4.
Univariate logistic regression for geriatric markers on postoperative delirium
| Variable | OR (95% CI)* | P |
|---|---|---|
| Age (increase by 1) | 1.03 (0.98–1.09) | 0.202 |
| Sex: male vs. female | 1.04 (0.64–1.71) | 0.855 |
| BMI (increase by 1) | 0.97 (0.93–1.02) | 0.394 |
| Weight loss: yes vs. no | 0.98 (0.58–1.63) | 0.941 |
| CCI: ≥ 3 vs. <3 | 1.95 (1.14–3.32) | 0.013 |
| Estimated creatinine clearance | ||
| 30–60 vs. >60 | 1.26 (0.76–2.08) | 0.366 |
| <30 vs. >60 | 0.58 (0.07–4.74) | 0.609 |
| No. of medications (increase by 1) | 1.02 (0.97–1.09) | 0.328 |
| Social support: yes vs. no | 2.47 (0.73–8.32) | 0.143 |
| Falls: yes vs. no | 2.04 (1.17–3.57) | 0.012 |
| ADL: dependent vs. independent | 1.49 (0.86–2.57) | 0.147 |
| IADL: dependent vs. independent | 2.39 (1.39–4.09) | 0.001 |
| Creatinine (increase by 1) | 0.83 (0.4–1.74) | 0.63 |
| Albumin: ≤ 3.3 vs. >3.3 | 1.27 (0.34–4.73) | 0.721 |
| Na (increase by 1) | 0.97 (0.90–1.04) | 0.432 |
| Hemoglobin (increase by 1) | 1.01 (0.87–1.16) | 0.867 |
| Mini-Cog Test: abnormal vs. normal | 1.69 (1.01–2.83) | 0.046 |
| CDT: abnormal vs. normal | 1.69 (0.97–2.93) | 0.063 |
| ASA | ||
| 3 vs. 2 | 2.50 (0.96–6.53) | 0.06 |
| 4 vs. 2 | 3.61 (1.02–12.77) | 0.047 |
The odds ratio (OR) is for developing postoperative delirium. CI, confidence interval.
Fig 1.
Multivariate model for predicting postoperative delirium and ROC curve for final multivariate model
Discussion
Patient specific treatment decision-making is a hallmark of surgical oncology, and arguably of heightened importance when treating elderly cancer patients. As many prior studies and existing guidelines make clear, consideration of function, cognition, geriatric-specific syndromes and life expectancy should thus be integrated in routine perioperative risk assessment.16 Our data are illustrative of the complexity in managing this patient population whose members commonly have multiple medical comorbidities and polypharmacy before surgery. Outcomes of our study show that preoperative GA is feasible and may promote better understanding of older patients’ perioperative risks including delirium.
The reported incidence of delirium ranges from 9% to 56% in general surgical patients with risk factors including age, cognitive impairment, illness severity, depression, medications use, and perioperative complications. 26 The incidence of delirium in our population was low at 19%. Increased number of comorbidities, previous falls, functional dependency and positive screening for cognitive decline were predictors of postoperative delirium. Prior studies have shown in patients ≥ 65 years undergoing radical cystectomy, the incidence of postoperative delirium was 29% and significantly associated with older age and a lower Mini Mental Status Examination score before surgery.27 After cardiac surgery, delirium is associated with significant declined in cognitive ability during the first year8 as well as greater risk of functional decline at one month.28 Previous studies have also demonstrated that preoperative functional limitations, cognitive impairment and increased number of comorbidities were associated with post-operative delirium. 14,27,29 It is of utmost importance to be able to predict delirium in order prevent postoperative delirium using well known non-pharmacological30 as well as less studied pharmacological methods.31 The low incidence of postoperative delirium in this cohort of older patients in our institution maybe related, in part, to the fact that the geriatric team provided pre and postoperative recommendations that could have triggered interventions that prevented the development of delirium in some of the patients.
Surgeons are poorly trained to independently manage time-consuming comprehensive geriatric assessments, and even a motivated primary care provider may not be equipped to provide such evaluations. We were able to integrate the different aspects of the comprehensive geriatric assessment into the preoperative evaluation. The preoperative GA was performed by the clinician (MD, RN or NP) and by the patient. The elements of the abbreviated GA that were found to be significant predictors of risk of developing postoperative delirium were either part of the elicited general history (falls, comorbidities), provided by the patient or companions (IADLs) or performed by a Geriatric NP or a trained RN (Mini-Cog) for all patients undergoing presurgical testing.
Lower IADLs, higher CCI and history of falls appear more sensitive than an abnormal Mini-Cog in the prediction of postoperative delirium. This may be due to the characteristics of this patient population which, in general, was a highly educated cohort. The slow development of dependency for the IADLs and family compensation for the deficits could go completely unnoticed unless specifically elicited during clinical encounters. Dependency for ADLs was mostly related to urinary incontinence (not rare in urologic cancer patients) and unsteady gait with the use of assistive devices for ambulation. Most cancer patients who are debilitated and functional dependent to the point of needing help for self-care would probably not be surgical candidates. Interestingly, dependency in IADLs and not in ADLs has also been shown to be predictive of chemotherapy toxicity in the older cancer patient.32
This study has limitations. First this is a homogeneous cohort of patients, predominantly white, largely with a good social support system, treated at a large academic cancer center. These patients were selected to be referred to a cancer center for treatment, possibly representing a healthier and fitter group than the general population of the same age. This may be the reason behind a relatively low incidence of postoperative delirium when compared to prior reports. Second, because of the fluctuating nature of this syndrome and by performing the CAM only once a day, we may have missed episodes of delirium (mostly in patients with hypoactive delirium) and we were unable to accurately document how long the episodes of delirium lasted. We would argue that, in older adults, the CAM should be part of the unit RN’s evaluation as one more vital sign, performed at multiple times points during the day. Third, we reported readmission rate and UCC visits to MSKCC; we did not survey patients to see if they were admitted locally or consulted the local Emergency Department.
In conclusion, the present analysis has demonstrated that it is possible and practical to evaluate older surgical patients preoperatively with an abbreviated geriatric assessment and this could be a crucial screening tool for risk of developing post-operative delirium. Post-operative delirium increases length of stay and cost to the health care system; moreover, it is a risk factor for institutionalization which, in turn, may not be an acceptable change in quality of life for the patient as well as an enormous source of anxiety and apprehension for the families. Older patients and their families should be educated of the possibility of developing postoperative delirium after cancer surgery so that they can be psychologically as well as practically prepared for the possibility. A prospective study is planned in order to validate this abbreviated geriatric assessment as an useful, practical predictor of postoperative delirium.
Acknowledgments
Financial Support: Department of Medicine, Memorial Sloan Kettering Cancer Center (research study assistantship to B.L.); Beatrice and Samuel Siever Foundation (B.K-G)
We would like to acknowledge the Beatrice & Samuel A. Seaver Foundation Corporation for the generous contribution to the Program on Cancer and Aging at the Memorial Sloan Kettering Cancer Center. We would like to thank Mr. David Sewell for his excellent editorial support.
Footnotes
COI Statement: The authors report no conflicts of interest.
Reprints will not be available from the author.
References
- 1.Muss HB. Cancer in the elderly: a societal perspective from the United States. Clin Oncol (R Coll Radiol) 2009;21:92–8. doi: 10.1016/j.clon.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:2758–65. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 4.Hurria A, Leung D, Trainor K, et al. Factors influencing treatment patterns of breast cancer patients age 75 and older. Critical reviews in oncology/hematology. 2003;46:121–6. doi: 10.1016/s1040-8428(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 5.Audisio RA, Bozzetti F, Gennari R, et al. The surgical management of elderly cancer patients; recommendations of the SIOG surgical task force. European journal of cancer (Oxford, England : 1990) 2004;40:926–38. doi: 10.1016/j.ejca.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Zbar AP, Gravitz A, Audisio RA. Principles of surgical oncology in the elderly. Clin Geriatr Med. 2012;28:51–71. doi: 10.1016/j.cger.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA : the journal of the American Medical Association. 1994;271:134–9. [PubMed] [Google Scholar]
- 8.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. The New England journal of medicine. 2012;367:30–9. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA : the journal of the American Medical Association. 2012;308:73–81. doi: 10.1001/jama.2012.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deschodt M, Braes T, Flamaing J, et al. Preventing delirium in older adults with recent hip fracture through multidisciplinary geriatric consultation. Journal of the American Geriatrics Society. 2012;60:733–9. doi: 10.1111/j.1532-5415.2012.03899.x. [DOI] [PubMed] [Google Scholar]
- 11.Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA : the journal of the American Medical Association. 2001;285:2987–94. doi: 10.1001/jama.285.23.2987. [DOI] [PubMed] [Google Scholar]
- 12.Polanczyk CA, Marcantonio E, Goldman L, et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Annals of internal medicine. 2001;134:637–43. doi: 10.7326/0003-4819-134-8-200104170-00008. [DOI] [PubMed] [Google Scholar]
- 13.Swaminathan V, Audisio R. Cancer in older patients: an analysis of elderly oncology. Ecancermedicalscience. 2012;6:243. doi: 10.3332/ecancer.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Annals of surgery. 2009;250:449–55. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 15.Fukuse T, Satoda N, Hijiya K, et al. Importance of a comprehensive geriatric assessment in prediction of complications following thoracic surgery in elderly patients. Chest. 2005;127:886–91. doi: 10.1378/chest.127.3.886. [DOI] [PubMed] [Google Scholar]
- 16.Audisio RA, Pope D, Ramesh HS, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Critical reviews in oncology/hematology. 2008;65:156–63. doi: 10.1016/j.critrevonc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Kristjansson SR, Nesbakken A, Jordhoy MS, et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Critical reviews in oncology/hematology. 2010;76:208–17. doi: 10.1016/j.critrevonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Robinson TN, Wallace JI, Wu DS, et al. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. Journal of the American College of Surgeons. 2011;213:37–42. doi: 10.1016/j.jamcollsurg.2011.01.056. discussion-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dale W, Hemmerich J, Kamm A, et al. Geriatric Assessment Improves Prediction of Surgical Outcomes in Older Adults Undergoing Pancreaticoduodenectomy: A Prospective Cohort Study. Annals of surgery. 2013 doi: 10.1097/SLA.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow WB, Rosenthal RA, Merkow RP, et al. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the american college of surgeons national surgical quality improvement program and the american geriatrics society. Journal of the American College of Surgeons. 2012;215:453–66. doi: 10.1016/j.jamcollsurg.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Katz S, Ford AB, Moskowitz RW, et al. STUDIES OF ILLNESS IN THE AGED. THE INDEX OF ADL: A STANDARDIZED MEASURE OF BIOLOGICAL AND PSYCHOSOCIAL FUNCTION. JAMA : the journal of the American Medical Association. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 22.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 23.Borson S, Scanlan JM, Chen P, et al. The Mini-Cog as a screen for dementia: validation in a population-based sample. Journal of the American Geriatrics Society. 2003;51:1451–4. doi: 10.1046/j.1532-5415.2003.51465.x. [DOI] [PubMed] [Google Scholar]
- 24.Cullen DJ, Apolone G, Greenfield S, et al. ASA Physical Status and age predict morbidity after three surgical procedures. Annals of surgery. 1994;220:3–9. doi: 10.1097/00000658-199407000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Annals of internal medicine. 1990;113:941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 26.Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. Journal of the American Geriatrics Society. 2006;54:1578–89. doi: 10.1111/j.1532-5415.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- 27.Large MC, Reichard C, Williams JT, et al. Incidence, risk factors, and complications of postoperative delirium in elderly patients undergoing radical cystectomy. Urology. 2013;81:123–8. doi: 10.1016/j.urology.2012.07.086. [DOI] [PubMed] [Google Scholar]
- 28.Rudolph JL, Inouye SK, Jones RN, et al. Delirium: an independent predictor of functional decline after cardiac surgery. Journal of the American Geriatrics Society. 2010;58:643–9. doi: 10.1111/j.1532-5415.2010.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson TN, Eiseman B. Postoperative delirium in the elderly: diagnosis and management. Clin Interv Aging. 2008;3:351–5. doi: 10.2147/cia.s2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. The New England journal of medicine. 1999;340:669–76. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 31.Breitbart W, Alici Y. Evidence-based treatment of delirium in patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:1206–14. doi: 10.1200/JCO.2011.39.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3457–65. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]