Abstract
Currently, many studies on neuropsychiatric disorders have utilized massive trio-based whole-exome sequencing (WES) and whole-genome sequencing (WGS) to identify numerous de novo mutations (DNMs). Here, we retrieved 17,104 DNMs from 3,555 trios across four neuropsychiatric disorders: autism spectrum disorder (ASD), epileptic encephalopathy (EE), intellectual disability (ID), schizophrenia (SCZ), in addition to unaffected siblings (Control), from 36 studies by WES/WGS. After eliminating non-exonic variants, we focused on 3,334 exonic DNMs for evaluation their association with these diseases. Our results revealed a higher prevalence of DNMs in the probands of all four disorders than the one in the controls (P < 1.3 × 10-7). The elevated DNM frequency is dominated by loss-of-function/deleterious single nucleotide variants and frameshift indels (i.e., extreme mutations, P < 4.5 × 10-5). With extensive annotation of these “extreme” mutations, we prioritized 764 candidate genes in these four disorders. A combined analysis of Gene Ontology, microRNA targets, and transcription factor targets revealed shared biological process and non-coding regulatory elements of candidate genes in the pathology of neuropsychiatric disorders. In addition, weighted gene co-expression network analysis (WGCNA) of human laminar-specific neocortical expression data showed that candidate genes are convergent on eight shared modules with specific layer-enrichment and biological process features. Furthermore, we identified that 53 candidate genes are associated with more than one disorder (P < 0.000001), suggesting a possibly shared genetic etiology underlying these disorders. Particularly, DNMs of the SCN2A gene are frequently occurred across all four disorders. Finally, we constructed a freely available NPdenovo database, which provides a comprehensive catalog of the DNMs identified in neuropsychiatric disorders.
Introduction
Over the last decade, next-generation sequencing (NGS) has become one of the most effective tools for identifying the genetic causes of Mendelian, complex, and undiagnosed diseases1-3. Recent whole-exome sequencing (WES) and whole-genome sequencing (WGS) studies of neuropsychiatric disorders have indicated that de novo mutations (DNMs) play prominent roles in these disorders4-20 despite their high heritabilities and genetic heterogeneities21, 22. DNMs including single nucleotide variants (SNVs), small insertions and deletions (indels), copy-number variants (CNVs), and structural variants (SVs) are extremely rare and regarded as more deleterious, having a stronger disruptive effect on biological functions due to less stringent evolutionary selection23, 24. Therefore, DNMs offer considerable insights into the genetic bases and clinical interpretations of sporadic cases in which inheritance seems to offer no explanation for disease etiology7, 10, 25. Trio-based WES/WGS is revolutionizing the identification of DNMs, and has been performed on more than 3,000 controls and patients with neuropsychiatric disorders, mostly including autism spectrum disorder (ASD), epileptic encephalopathy (EE), intellectual disability (ID), and schizophrenia (SCZ). These studies identified several dozen candidate genes harboring recurrent loss-of-function (LoF) DNMs that are crucial to pathogenesis of these disorders, such as CHD8, SCN2A, NTNG1 and KATNAL2 in ASD11-14, 26, 27, GABRB3, ALG13 and CACNA1A in EE9, DYNC1H1, STXBP1, SYNGAP1 and SCN2A in ID16, 17, and LAMA2, DPYD, TRRAP and VPS39 in SCZ18, 19. However, the genetic etiologies of these disorders remain difficult to decipher due to limited sample sizes, high genetic heterogeneity, and complex pathogenesis10, 22.
In addition, DNMs are so rare; it has been difficult to be statistically evaluated in terms of the relevance of most detected DNMs to these diseases. To facilitate DNM interpretation, we curated and cataloged all DNMs reported to date in ASD, EE, ID, SCZ, and unaffected siblings or controls by WES/WGS. We subjected all DNMs to consistent quality control standards to characterize the frequency of different classes of DNMs and prioritized genes associated with each disorder. Functional enrichment and co-expression network analysis revealed that some genetic etiologies are shared among these four neuropsychiatric disorders. In addition, the developed NPdenovo database here is a useful tool for future studies in elucidating the mechanisms and underlying the genetic etiologies of these diseases.
Materials and Methods
Data collection and annotation
In total, 3,555 trios from four disorders (ASD, EE, ID and SCZ) together with the unaffected siblings/controls were collected from currently available trios-based WES/WGS studies, in which 17,104 DNMs were identified (Figure 1 and Supplementary Table 1). Comprehensive annotation was performed for each DNM by ANNOVAR28 with RefSeq (hg19, from UCSC), including: 1) Gene information (gene region, effect, mRNA GenBank accession number, amino acid change, cytoband, et al.); 2) Functional prediction for missense mutations by twelve bioinformatics tools; 3) Allele frequency in different populations of public database (different version of dbSNP, 1000 Genomes, ESP6500 and CG69); 4) Disease-related database (ClinVar, HGMD, COSMIC, MGI, OMIM); and 5) Genome features for non-coding variations (segmental duplication, VISTA enhancer, transcription factor, DNase I hypersensitivity, chromatin state segmentation and non-coding RNA from ENCODE).
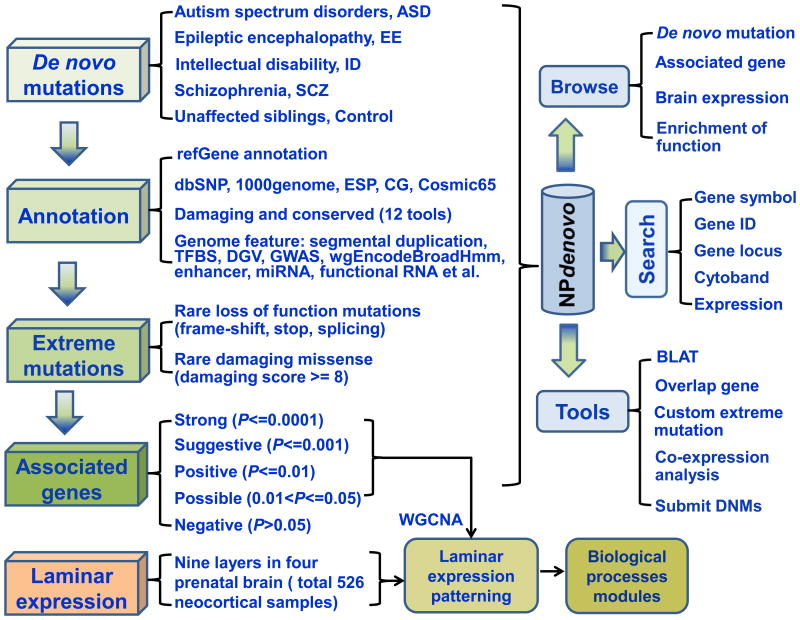
Figure 1. Flowchart of the NPdenovo database.
Totally six main parts are included in our analysis of DNMs in neuropsychiatric disorders: 1) Collection of DNMs; 2) Comprehensive annotation of DNMs; 3) Identification of extreme mutations (rare and damaging/LoF mutations); 4) Prioritization of candidate genes with statistic support; 5) Laminar expression patterning of candidate genes by WGCNA; and 6) Development of the NPdenovo database.
Identification of extreme mutations
To identify pathogenic mutations, firstly, we removed all DNMs with minor allele frequency (MAF) > 0.1% in dbSNP138, 1000-Genome (released in April, 2012), and ESP6500. Synonymous and non-frameshift mutations were eliminated due to their low possibility to contribute to disorders. The LoF mutations, such as nonsense/splicing SNVs, frameshift indels, were directly considered to be damaging. For missense mutations, which account for the majority of DNMs, though many tools or methods were developed to predict degree of damages based on evolutionary conservation or functional disruption, all of them have inevitable limitations and biases. A proposed solution for this is to use consensus prediction or majority vote of many methods29. Consequently, twelve generic tools and methods were applied for functional prediction of damaging missense mutations, expected with more robust statistical power than any single tool, including SIFT30, Polyphen2_hvar31, Polyphen2_hdiv31, MutationTaster32, MutationAssessor33, LRT34, FATHMM35, GERP++36, PhyloP37, SiPhy38, 39, RadialSVM and MetaLR. The predicted damaging scores of twelve tools were sourced from dbNSFP 2.0 database29 and integrated in ANNOVAR. Then, for each missense mutation, total damaging score is the summed number of tools predicted to be “deleterious” or “conserved”. Since missense mutations in cases were found to be more likely to have higher damaging scores (≥ 8) than controls, only the DNMs with a damaging score higher than eight are considered to be deleterious mutations (Supplementary Figure 1). The genes harboring rare LoF/deleterious SNVs and rare frame-shift indels, which we refer to as extreme mutations, were used for candidate genes prioritization (Figure 1).
Prioritize candidate genes
Our recently developed TADA program (Transmission And De novo Association)40, which can predict risk genes accurately on the basis of allele frequencies, gene-specific penetrance and mutation rate was used to calculate the P-value for the likelihood of each gene contributing to the corresponding disorders with default parameters. To gain a clear view on risk genes in each disorder, according to the P-value generated by the TADA program, we classified genes into five different types in each disorder: “strong” (PTADA ≤ 0.0001), “suggestive” (0.0001 < PTADA ≤ 0.001), “positive” (0.001 < PTADA ≤ 0.01), “possible” (0.01 < PTADA ≤ 0.05) and “negative” (PTADA > 0.05). Genes with PTADA ≤ 0.05 (i.e. candidate genes) were used for the analysis of functional enrichment and neocortical expression profiles (Figure 1).
Neocortical expression profiles
Human laser micro-dissection data (LMD) of four prenatal brains (15-21 post conceptual weeks, pcw) from BrainSpan Atlas (http://brainspan.org)41 were used to investigate the expression patterns of candidate genes42. In this data, neocortex was divided into ∼25 areas in each sample, delineating nine layers per area from subpial granular zone (SZ) to ventricular zone (VZ), corresponding to 526 neocortical samples (Supplementary Table 2). In this study, signed hybrid weighted gene co-expression network analysis (WGCNA) was performed across all neocortical samples42 using the standard method with power of 4 (as it was the smallest threshold that resulted in a scale-free R2 fit of 0.9; Figure 1).
Functional enrichment analysis
Functional enrichment of genes (such as Gene Ontology, transcription factor targets, MicroRNA targets, and KEGG pathways, et al.) were all performed by WebGestalt (http://bioinfo.vanderbilt.edu/webgestalt/) with default parameters43.
Results
To characterize the DNMs in neuropsychiatric disorders, we retrieved 17,104 DNMs identified from trio-based WES/WGS from ASD, EE, ID, SCZ and normal controls of 36 studies (Supplementary Table 1). After eliminating non-exonic variants, 3,334 exonic DNMs located in the coding DNA sequence (CDS) regions were left, comprising 3,112 de novo SNVs and 222 de novo indels.
High prevalence of extreme DNMs in neuropsychiatric disorders
Although several studies have documented that cases have a significant high rate of LoF DNMs related to controls11-14, they have not presented statistically compelling evidence due to the absence of a large sample set. With the combined data from 3,555 trios, we observed that disease groups have higher prevalence of DNMs (both de novo SNVs and de novo indels) than controls (two-sample Poisson rate test : ASD, OR = 1.36, corrected P = 5.36 × 10-10; EE, OR = 1.52, P = 2.84 × 10-9; ID, OR = 1.60, P = 1.52 × 10-9; SCZ, OR = 1.31, P = 1.3 × 10-7; Table 1 and Supplementary Figure 2A). Interestingly, we found that the significant increase of DNMs is dominated by LoF/deleterious SNVs and frameshift indels, rather than synonymous SNVs, tolerant missense and non-frameshift indels (Table 1 and Supplementary Figure 2B-E). In addition, the frequency of extreme mutations in cases and controls showed significant difference (two-sample Poisson rate test : ASD, OR = 1.80, adjusted P = 5.96 × 10-11; EE, OR = 2.54, P = 1.89 × 10-15; ID, OR = 3.03, P = 1.30 × 10-19; SCZ, OR = 1.50, P = 4.4 × 10-5; Table 1 and Supplementary Figure 2F), suggesting that these extreme DNMs are likely contributed to the pathogenesis of these four neuropsychiatric disorders.
Table 1. Odds ratio of functional classes of DNMs in coding region.
| Group | Trios | VS Control | Total DNM | De novo SNVs | De novo InDels | Extreme mutations | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Total SNVs | Damaging SNVs | Tolerant missense | Synonymous | Total | Frameshift | Non- frameshift | |||||
| ASD | 1038 | DNMs | 1040 | 967 | 329 | 394 | 244 | 73 | 63 | 10 | 364 |
| OR | 1.36 | 1.31 | 2.03 | 1.18 | 1.21 | 2.66 | 3.31 | 1.18 | 1.80 | ||
| 95% Cl | 1.24-1.50 | 1.19-1.45 | 1.67-2.48 | 1.02-1.38 | 1.00-1.47 | 1.68-4.33 | 1.94-5.94 | 0.42-3.45 | 1.51-2.16 | ||
| P-value | 1.34E-10 | 3.27E-08 | 6.69E-14 | 0.027 | 0.055 | 7.12E-06 | 1.76E-06 | 0.82 | 1.49E-11 | ||
| Pcorrected | 5.36E-10 | 1.31E-07 | 2.67E-13 | 0.11 | 0.22 | 2.85E-05 | 7.02E-06 | 1 | 5.96E-11 | ||
|
|
|
||||||||||
| EE | 291 | DNMs | 327 | 303 | 124 | 123 | 56 | 24 | 21 | 3 | 144 |
| OR | 1.52 | 1.47 | 2.73 | 1.32 | 0.99 | 3.11 | 3.94 | 1.26 | 2.54 | ||
| 95% Cl | 1.34-1.74 | 1.28-1.68 | 2.14-3.49 | 1.06-1.63 | 0.72-1.34 | 1.71-5.64 | 2.00-7.84 | 0.22-5.27 | 2.03-3.18 | ||
| P-value | 7.11E-10 | 5.30E-08 | 1.13E-15 | 0.01 | 1 | 0.000121 | 2.65E-05 | 0.722003 | 4.74E-16 | ||
| Pcorrected | 2.84E-09 | 2.12E-07 | 4.54E-15 | 0.042 | 1 | 0.00048 | 0.0001 | 1 | 1.89E-15 | ||
|
|
|
||||||||||
| ID | 220 | DNMs | 259 | 229 | 108 | 78 | 43 | 30 | 26 | 4 | 130 |
| OR | 1.60 | 1.47 | 3.15 | 1.10 | 1.00 | 5.15 | 6.45 | 2.23 | 3.03 | ||
| 95% Cl | 1.38-1.85 | 1.26-1.71 | 2.44-4.06 | 0.85-1.42 | 0.70-1.41 | 2.94-9.07 | 3.40-12.48 | 0.49-8.33 | 2.41-3.82 | ||
| P-value | 3.81E-10 | 1.16E-06 | 4.97E-18 | 0.433678 | 1 | 3.19E-09 | 2.12E-09 | 0.25 | 3.24E-20 | ||
| Pcorrected | 1.52E-09 | 4.64E-06 | 1.99E-17 | 1 | 1 | 1.28E-08 | 8.49E-09 | 1 | 1.30E-19 | ||
|
|
|
||||||||||
| SCZ | 1024 | DNMs | 986 | 917 | 275 | 416 | 226 | 69 | 54 | 15 | 299 |
| OR | 1.31 | 1.26 | 1.72 | 1.27 | 1.13 | 2.54 | 2.88 | 1.79 | 1.50 | ||
| 95% Cl | 1.19-1.44 | 1.14-1.40 | 1.41-2.11 | 1.09-1.47 | 0.93-1.38 | 1.60-4.16 | 1.66-5.21 | 0.72-4.90 | 1.25-1.81 | ||
| P-value | 3.29E-08 | 3.14E-06 | 4.00E-08 | 0.0016 | 0.20 | 2.09E-05 | 4.53E-05 | 0.21 | 1.10E-05 | ||
| Pcorrected | 1.31E-07 | 1.26E-05 | 1.60E-07 | 0.0066 | 0.81232 | 8.36E-05 | 0.00018 | 0.85 | 4.40E-05 | ||
|
|
|
||||||||||
| Control | 982 | DNMs | 722 | 696 | 153 | 315 | 191 | 26 | 18 | 8 | 191 |
In total, we collected 3,334 exonic DNMs from 3,555 trios, including 1040 DNMs from1038 ASD trios, 327 DNMs from 291 EE trios, 259 DNMs from 220 ID trios, 986 DNMs from 1,024 SCZ trios and 722 DNMs from 982 trios of control. In each group, exonic DNMs were classified into different functional classes. Compared to control group, P-values were calculated on the basis of two-sample Poisson rate test (Supplementary Materials and Methods). Bonferroni correction was used to counteract the problem of multiple comparisons. We referred to LoF/deleterious SNVs as damaging SNVs. The rare LoF/deleterious SNVs and rare frame-shift indels, which were regarded as as extreme mutations. P values below 0.005 are highlighted in bold.
Prioritization of candidate genes
Using the TADA program44, we identified a total of 764 potential candidate genes with PTADA ≤ 0.05 in four disorders: 330 genes for ASD, 109 for EE, 106 for ID, and 277 for SCZ (Table 2 and Supplementary Table 3). We identified six genes with strong associations in ASD, nine genes in EE, 18 genes in ID, and seven genes in SCZ (PTADA ≤ 0.0001), most of which harbor recurrent extreme DNMs. Many of the 764 candidate genes have been previously reported to be severely implicated in neuropsychiatric disorders: CHD8, SCN2A, RLEN, NRXN1, and NRXN2 in ASD; SCN1A, SCN2A, SCN8A, STXBP1, GABRB3, and CDKL5 in EE; SCN2A, DYNC1H1, CTCF, TCF4, and DEAF1 in ID; LAMA2, MIF, TRH, and HSPA8 in SCZ. In addition to those known candidate genes, we also identified several novel candidates, such as SUV420H1, KATNAL2, TBR1, NR3C2, TUBA1A, KIRREL3, and UBE3C in ASD; SLC35A2, THAP1, RAB5C, VPS37A, WDR45, IQSEC2, and CHD2 in EE; RB1, DEAF1, CNGA3, RBL2, PACS1, and FHDC1 in ID; TAF13, ESAM, RB1CC1, and MKI67 in SCZ.
Table 2. Number of associated genes in each neuropsychiatric disorder.
| Disorder | Strong (P≤0.0001) |
Suggested (P≤0.001) |
Positive (P≤0.01) |
Possible (P≤0.05) |
Total associated |
|---|---|---|---|---|---|
| ASD | 6 | 23 | 135 | 166 | 330 |
| EE | 9 | 22 | 77 | 1 | 109 |
| ID | 18 | 29 | 59 | 0 | 106 |
| SCZ | 7 | 19 | 115 | 136 | 277 |
We classified all 764 potential candidate genes (PTADA ≤ 0.05) into four different types in each disorder on the basis of P-value in TADA program. The detail information can be found in Supplementary Table 3.
Pathway shared across disorders
To characterize the function of the 764 identified candidate genes harboring extreme mutations in the four disorders, we conducted an enrichment analysis of Gene Ontology (GO) terms in each disorder (Supplementary Table 4). Although neuropsychiatric disorders have complex etiologies and are genetically heterogeneous, the overlaps suggested that some biological processes share in to all four neuropsychiatric disorders (such as nervous system development, and multicellular organismal signaling), indicating shared molecular pathologies may underlying this subtype of complex diseases. In addition, our results showed that candidate genes in each disorder are highly enriched in respective biological processes. For example, the terms transmission of nerve impulse, synaptic transmission and ion transport are over-represented in the set of EE genes.
Candidate genes that converge on these functional pathways from the neuropsychiatric disorders suggest that they are likely regulated by common special regulatory elements in their non-coding regions. Thus, we performed enrichment analysis of transcription factors (TF) and microRNA targets, which regulates the 5-upstream promoter region and downstream regulatory region (3′-UTR), respectively (Supplementary Table 4). Interestingly, we found that several TF binding sites and microRNA target sites of candidate genes are shared among four neuropsychiatric disorders. For instance, the transcription factor binding sites, “hsa_GGGAGGRR_V$MAZ_Q6”, and “hsa_CAGGTG_V$E12_Q6” are shared by all four disorders; the MicroRNA target sites, “hsa_TTGCACT”, and “hsa_TGAATGT” are shared by ASD and SCZ. In addition, each of the disorders was enriched with some special regulatory elements. For example, ID genes showed enrichment in the “hsa_AAGCCAT” site (P = 2.71 × 10-12, OR = 3.9), which is the target of miR-29A, miR-29B and miR-29C. It should be noted that brain-specific knockdown of miR-29 was found to result in neuronal cell death45. Our analysis thus demonstrated a plausible link between given transcription factors or microRNAs with these disorders, although the specific factors/microRNAs remain to be identified and verified.
Shared neocortical expression profiles
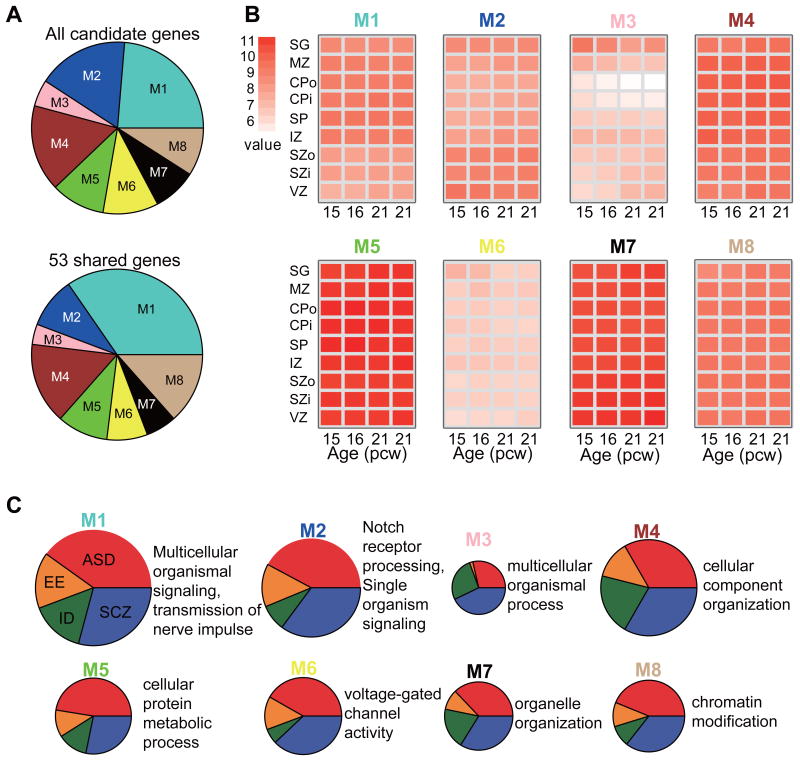
Human brain development involves complex regulation of cellular proliferation, signaling, and transcription pathways, and requires orchestration of many relevant genes46. We performed weighted gene co-expression network analysis (WGCNA) with our candidate genes in all 526 laminar neocortical samples (Supplementary Materials and Methods). The identified co-expression network includes eight modules of varying sizes from 36 to 170 genes with highly coordinated spatio-temporal expression patterns of each module in the human neocortex (M1-M8, Figure 2A). All the modules consist of candidate genes of all four disorders, each is presented with laminar-specific enrichment (Figure 2B and Supplementary Table 5) and distinct biological processes (Figure 2C and Supplementary Table 6). For example, the largest module (M1) included 170 genes enriched in the intermediate zone and was convergent on GO terms “multicellular organismal signaling” (P = 1.36 × 10-12, OR = 4.2) and “transmission of nerve impulse” (P = 4.07 × 10-12, OR = 4.16). Moreover, candidate genes associated with ASD, EE, ID, and SCZ contribute to all the modules, highlighting the shared genetic etiology underlying these disorders.
Figure 2. Laminar expression patterning of candidate genes.
(A) Distribution of all candidate genes and 53 shared genes in eight modules (M1-M8), which are clustered by WGCNA on the basis of laminar neocortical expression data. Each module is assigned with a color arbitrarily by WGCNA. (B) Module eigengene expression of eight modules in the cortical network. For each module, each box corresponds to the average expression level of genes across the nine layers of neocortex (rows) in four samples (columns). The nine layers correspond to SZ, MZ, CPo, CPi, SZ, IZ, SZo, SZi and VZ (Supplementary Table 2). The four samples correspond to the four high-quality mid-gestational brains, two from 15 and 16 pcw (post-conceptual weeks) and two from 21 pcw. White, low expression; red, high expression. (C) The distribution of candidate genes and their biological processes in four disorders (ASD, EE, ID and SCZ). Candidate genes in each module are shown as a pie chart in four disorders. The size of each pie chart is proportional to the number of genes in corresponding modules. The enrichment analysis of GO was performed by WebGestalt.
Shared genes in neuropsychiatric disorders
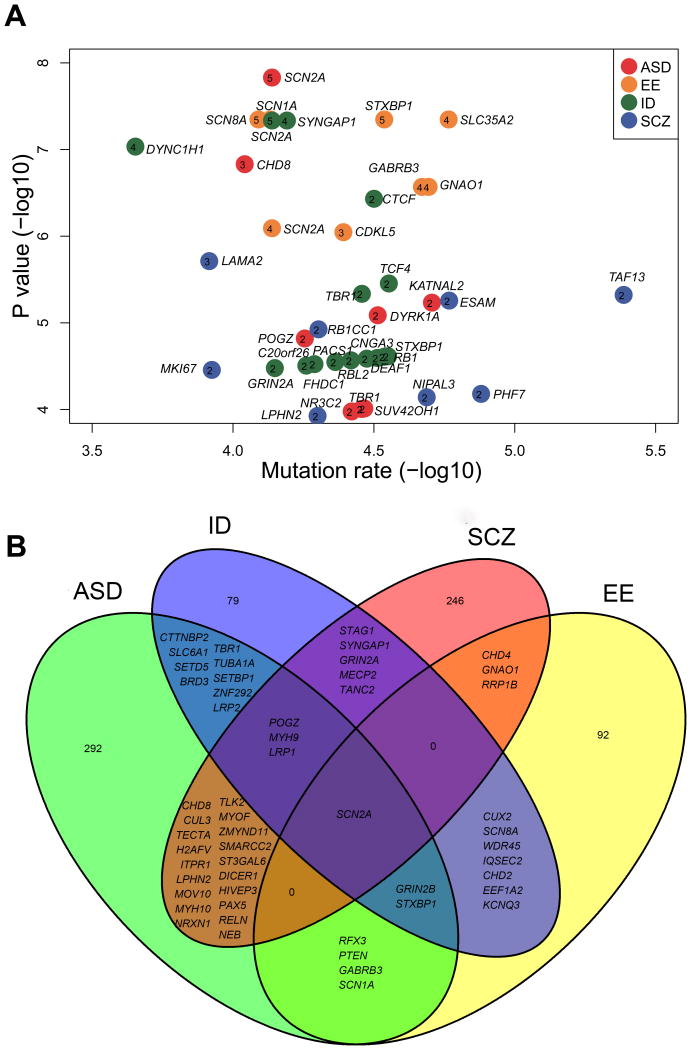
Among the 764 candidate genes, we identified 12 genes harboring recurrent DNMs in 1,038 ASD trios, eight from 1,024 SCZ trios, eight from 291 EE trios, and 15 from 220 ID trios (Figure 3A). Most of them have been previously linked with neuropsychiatric disorders. However, no single gene was found to harbor recurrent extreme DNMs in 982 controls. More significantly, we identified 53 genes that are associated with more than one disorder (permutation test, P < 0.000001 based on random resampling, Figure 3B, Supplementary Figure 3A, Supplementary Materials and Methods). On the contrary, the genes harboring extreme mutations in controls did not significantly correlate with ASD (P = 0.16, Supplementary Figure 3B), EE (P = 0.11, Supplementary Figure 3C), ID (P = 0.14, Supplementary Figure 3D), and SCZ (P = 0.31, Supplementary Figure 3E). And the extreme DNM-containing genes in the controls was also not significantly overlapped with the 53 shared genes in the four disorders (P = 0.12, Supplementary Figure 3F).
Figure 3. Candidate genes in neuropsychiatric disorders.
(A) Scatter diagram of candidate genes with recurrent DNMs. The numbers in each plot indicate the total quantity of extreme DNMs in different disorders. Y-axis represents the value of –log10 (P-value), indicating the predicted degrees of association. X-axis denotes –log10 (mutation rate in TADA program). (B) Intersections of candidate genes between each individual disorder. The overlap area in Venn diagram shows the common candidate genes between/among different disorders.
Furthermore, these 53 shared genes are significantly enriched in several neural function-associated GO terms, such as “regulation of transmission of nerve impulse”, “regulation of excitatory postsynaptic membrane potential”, and “regulation of neurological system process” (Supplementary Table 7 and Supplementary Table 8). Briefly, we found that 34% of shared genes (18 of 53) are included in the largest co-expression module M1 (hypergeometric test, P = 0.026), suggesting that these shared genes may account for the common etiology of all four neuropsychiatric disorders (Figure 2A, Supplementary Table 5).
More interestingly, different extreme DNMs of SCN2A were frequently identified in all four disorders, including five extreme DNMs in ASD (PTADA = 1.51 × 10-8), five in ID (PTADA = 8.26 × 10-7), four in EE (PTADA = 4.72 × 10-8), and one in SCZ (PTADA = 0.0047) (Figure 3B and Supplementary Table 9). Of the 15 extreme mutations, nine are frame-shift indels, stop-gain or splicing site SNVs (i.e., LoF DNMs), compared to zero rare LoF mutations of SCN2A in the ESP6500 database and controls (MAF < 0.01%, Supplementary Table 9). It is known that SCN2A encodes the alpha subunit of the voltage-gated sodium channel, which is responsible for generation and propagation of action potentials in excitable cells, such as nerve and neuroendocrine cells. SCN2A and its homologs such as SCN1A and SCN8A, have been found to be associated with ASD, ID, ataxia, and elevated sensitivity to pain47. In support of those previous discoveries, in the combined neocortex co-expression network, SCN2A was included in the largest module M1. This means that SCN2A is widely expressed in cortex, and is enriched in the intermediate zone (outer cortical plate, CPo; inner cortical plate, CPi; subplate zone, SP; Supplementary Figure 4). These observations suggested that SCN2A may involve common molecular pathways and contribute to overlapped phenotypes in these four disorders.
Moreover, we found that MYH9, LRP1 and POGZ were shared by ASD, ID, and SCZ; GRIN2B and STXBP1 were shared by ASD, EE and ID (Figure 3B). Several other candidate genes are shared by two disorders, such as CHD8, RELN, and NRXN1 shared by ASD and SCZ; SETBP1, SETD5, BRD3, CTNNBP2, LRP2, and SLC6A1 shared by ASD and ID, WDR45, IQSEC2, CHD2, KCNQ3, and SCN8A shared by EE and ID (Figure 3B). Despite the apparently distinct pathogenesis of these four neuropsychiatric disorders, functional association of these overlapping genes suggests that these disorders share some genetic architecture and molecular pathway features (Supplementary Table 8). For example, GRIN2B (glutamate receptor, ionotropic, N-methyl d-aspartate 2B) is involved in synaptic transmission and its mutations have been reported in patients with West syndrome and intellectual disability, or behavioral phenotypes48. SETBP1 (SET Binding Protein 1) is associated with ASD (c.2716delC, p.P906fs, PTADA = 0.004) and ID (c.1774A>T, p.K592X, PTADA = 0.0009). A recent study of more than 30,000 cases with developmental delay showed that SETBP1 is frequently mutated (both de novo SNVs/indels and de novo CNVs) in patients with intellectual disability and loss of expressive language49.
The NPdenovo database
To make our findings easily accessible to the research community, we have developed the NPdenovo database (http://122.228.158.106/NPdenovo/) for storage and retrieval of DNMs, candidate genes, and their brain expression patterns, and for exploring the genetic etiology of neuropsychiatric disorders (Supplementary Figure 5, Supplementary Materials and Methods).
Discussion
To gain insight into the biological implication of DNMs in diseases, datasets from multiple independent studies need to be integratively analyzed in a comprehensive source7, 50, 51. In doing so, we found that extreme mutations in the four neuropsychiatric disorders are significantly more frequent than in controls. Specially, EE and ID patients are more likely to harbor recurrent DNMs than ASD and SCZ, suggesting that ID and EE have lower heterogeneities than ASD and SCZ7. Numerous studies have revealed that patients with recurrent mutations are prone to present with similar clinically recognizable phenotypes8, 52-54. Thus, the identified recurrent DNMs may provide a “genotype-first” approach for complex disease-subtype diagnosis and therapy55. For example, patients with disruptive CHD8 (chromodomain helicase DNA binding protein 8) mutations were characterized as a subtype of autism with macrocephaly, distinct faces, and gastrointestinal complaints, responsible for 0.4% (15/3,730) of the patients with developmental delay or ASD56. ADNP (activity-dependent neuroprotector) is another ASD-associated gene, which is frequently mutated in 0.17% (10/5,776) of ASD cases with shared clinical phenotypes of intellectual disability and facial dysmorphisms57.
Next, by employing the TADA program40 to prioritize associated genes, we identified 764 genes with PTADA < 0.05, of which many were previously identified candidate genes as well as a large number of novel candidate genes. Although most of these candidate genes have not been verified or validated by in vitro experiments or animal models, our analysis provided meaningful reference and a downsized list of candidate genes for further studies of these DNMs in neuropsychiatric disorders. For example, SUV420H1 (suppressor of variegation 4-20 homolog 1) was found to harbor two damaging missense mutations (p.W264S and p.A513V) and one splicing site SNV (c.977+1G>A) in unrelated ASD cases, and was thus prioritized by this study as a strong candidate gene for ASD (PTADA = 0.00009). Furthermore, two damaging de novo missense mutations (p.Q264P and p.I228S) in DEAF1 (DEAF1 transcription factor) were described in two independent ID studies. DEAF1 is expressed in the neurons and is associated with anxiety and depression phenotypes and behavioral problems58, 59. In fact, numerous candidate genes harboring only one extreme DNM were identified with significant P-values. Additional experimental data may be required to determine their pathogenic role due to the heterogeneities in these disorders. For example, a dopamine transporter gene, SLC6A3 (solute carrier family 6 member 3) showed a significant P value (PTADA = 0.01). A recent study demonstrated that the de novo extreme mutation in this gene (c.1067C>T, p.T356M) can confer risk for ASD based on animal model60. Therefore, candidate genes from our study provide new avenue to build up etiological network of these complex neuropsychiatric disorder.
Recently, several studies documented that LoF DNMs in ASD are often involved in chromatin remodeling, wnt signaling, transcriptional regulation and synaptic function25-27. Besides, activity-dependent neuronal signaling networks61 and disruption of neuroplasticity62 also play a key role in the etiology of ASD. Moreover, DNMs in schizophrenia have been implicated in its etiological development in the fetal prefrontal cortical network20 and synaptic networks19. GO analysis in this study revealed that candidate genes in each disorder are enriched for some unique biological processes, but some are obviously shared, suggesting the overlapped molecular pathways in neuropsychiatric disorders. Our study provides a global view on the molecular etiologies of DNMs in neuropsychiatric disorders.
More importantly, some special regulatory elements are enriched in candidate genes. These elements include not only upstream control regions regulated by particular TFs, but also downstream regulatory regions mediated by particular microRNAs. Most previous studies have focused on the biological function of genes in coding regions. Given by the facts that mutations in non-coding regulatory regions are also involved in etiology of human diseases63, 64, regulation in non-coding regions is particularly important for brain development44, 65 and neuropsychiatric disorders66, 67, we strongly propose that DNMs located in transcription factor and microRNA target sites may participate in the pathology of these four disorders.
To investigate the underlying relationship between these disorders, we performed WGCNA on the 764 candidate genes and identified eight modules with the similar expression patterns in prenatal human neocortex, each module representing specific gene ontology biological processes. Previous studies have demonstrated that ASD51, 68, 69 and SCZ20 genes display specific co-expression networks in the human brain or neocortex. Our analysis showed that at least eight co-expression networks of candidate genes are associated with neuropsychiatric disorders. Some of these display superficial layer-enrichment or intermediate layer-enrichment consistent with previous studies68, 69. Others display extreme high/medial/low expression profiles in whole neocortex or deep layer-enrichment, which has not been detected in previous studies. However, no single module clearly represents an individual disorder, supporting the existence of interconnected molecular pathways in these four disorders7, 70, 71. This assumption is also consistent with the results of a previous protein–protein interaction (PPI) analysis72.
Previous studies have identified numerous genes associated with multiple neuropsychiatric diseases, such as SCN2A, GRIN2B, STXBP1, GABRB3, RELN, GABRA1 and MECP2. A recent trio-based WES study found that DNMs associated with chromatin modeling in schizophrenia (CHD8, MECP2, and HUWE1) overlap with ASD and ID73. In this study, we identified 53 genes that overlapped in all four disorders, support a genetic overlap between these diseases7, 70, 71. Only the extreme DNMs of SCN2A were identified in all four disorders. Based on our analysis, SCN2A is responsible for 0.5% (5/1038) of patients with ASD, 1.4% (4/291) of EE, 2.3% (5/220) of ID, and 0.1% (1/1024) of SCZ, making it one of the most frequently mutated genes in these neuropsychiatric disorders.
In summary, we have provided new insights into the shared genetic basis of DNMs in neuropsychiatric disorders. We also provide new evidence that some candidate genes, molecular pathways, regulatory elements, and expression profiles are shared among ASD, EE, ID, and SCZ (Supplementary Table 10). All these data can be easily discovered from our online NPdenovo database. In conclusion, our findings may improve understanding of their genetic etiology and facilitate the diagnosis and genetic counseling of these disorders in the future.
Supplementary Material
Supplementary Figure 1. Percentage of missenses with varying damaging scores in case and control. For each missense mutation, twelve tools were combined to predict the functional mutation, the total damaging score is the summed number of tools predicted to be “deleterious” or “conserved” (Materials and Methods). Based on damaging score, we classified missense mutations into three groups: [0, 3], [4, 7], [8, 12]. De novo missense mutations with high damaging score (≥ 8) are enriched in cases (four neuropsychiatric disorders) in comparison with controls. However, the other types of missense mutations with low damaging score (≥ 4) and medial damaging score (<4) are not enriched in cases.
Supplementary Figure 2. Odds ratio of functional classes of DNMs. Each bar plot corresponds to the odds ratio (OR) to control. Error bar represents the 95 % confidence interval (Cl) of OR in each disorder by two-sample Poisson rate test. This figure illustrates Table 1.
Supplementary Figure 3. Significance of the shared genes in four neuropsychiatric disorders. The histograms show the results of a randomly resampling (1,000,000 iterations). The observed value is shown by the vertical red line and corresponds to a P value by permutation test (Supplementary Materials and Methods). Six data sets were assessed: (A) shared genes in the four disorders; (B) ASD and control; (C) EE and control; (D) ID and control; (E) SCZ and control; and (F) the 53 shared genes and extreme DNMs-containing genes in control.
Supplementary Figure 4. Laminar transcriptional patterning of SCN2A. Heat map showing expression level of SCN2A at 15 pcw (A), 16 pcw (B) and 21 pcw (C, D) in nine layers and different sub–structures in human brain (Supplementary Table 2). Red indicates high expression, and white indicates low expression. The laminar-specific neocortical expression data contains four prenatal brains from BrainSpan Atlas: two from 15 and 16 pcw and two from 21 pcw (post conceptual week).
Supplementary Figure 5. Snapshot of NPdenovo database. NPdenovo provides numerous ways to browse, search, and analyze DNMs in neuropsychiatric disorders. All the DNMs and candidate genes are comprehensively annotated in NPdenovo database, such as the mutation information, gene information, and brain expression.
Acknowledgments
The project was funded by the National Natural Science Foundation of China (31171236/C060503), the National Basic Research Program of China (No. 2012CB517902 and 2012CB517904), the National “12th Five-Year” scientific and technological support projects (No. 2012BAI03B02), and the Special Funds of National Health and Family Planning Commission of China (No. 201302002).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nature Reviews Genetics. 2011;12(11):745–755. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein DB, Allen A, Keebler J, Margulies EH, Petrou S, Petrovski S, et al. Sequencing studies in human genetics: design and interpretation. Nature Reviews Genetics. 2013;14(7):460–470. doi: 10.1038/nrg3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacArthur D, Manolio T, Dimmock D, Rehm H, Shendure J, Abecasis G, et al. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014;508(7497):469–476. doi: 10.1038/nature13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ku C, Polychronakos C, Tan E, Naidoo N, Pawitan Y, Roukos D, et al. A new paradigm emerges from the study of de novo mutations in the context of neurodevelopmental disease. Molecular psychiatry. 2013;18(2):141–153. doi: 10.1038/mp.2012.58. [DOI] [PubMed] [Google Scholar]
- 5.Michaelson JJ, Shi Y, Gujral M, Zheng H, Malhotra D, Jin X, et al. Whole-genome sequencing in autism identifies hot spots for de novo germline mutation. Cell. 2012;151(7):1431–1442. doi: 10.1016/j.cell.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu TW, Chahrour MH, Coulter ME, Jiralerspong S, Okamura-Ikeda K, Ataman B, et al. Using whole-exome sequencing to identify inherited causes of autism. Neuron. 2013;77(2):259–273. doi: 10.1016/j.neuron.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoischen A, Krumm N, Eichler EE. Prioritization of neurodevelopmental disease genes by discovery of new mutations. Nature neuroscience. 2014;17(6):764–772. doi: 10.1038/nn.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krumm N, O'Roak BJ, Shendure J, Eichler EE. A de novo convergence of autism genetics and molecular neuroscience. Trends Neurosci. 2014;37(2):95–105. doi: 10.1016/j.tins.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phenome E, Consortium EK. De novo mutations in epileptic encephalopathies. Nature. 2013 doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veltman JA, Brunner HG. De novo mutations in human genetic disease. Nature Reviews Genetics. 2012;13(8):565–575. doi: 10.1038/nrg3241. [DOI] [PubMed] [Google Scholar]
- 11.Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74(2):285–299. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neale BM, Kou Y, Liu L, Ma'ayan A, Samocha KE, Sabo A, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485(7397):242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485(7397):246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485(7397):237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y-h, Yuen RK, Jin X, Wang M, Chen N, Wu X, et al. Detection of clinically relevant genetic variants in autism spectrum disorder by whole-genome sequencing. The American Journal of Human Genetics. 2013;93(2):249–263. doi: 10.1016/j.ajhg.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T, et al. Diagnostic exome sequencing in persons with severe intellectual disability. New England Journal of Medicine. 2012;367(20):1921–1929. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- 17.Rauch A, Wieczorek D, Graf E, Wieland T, Endele S, Schwarzmayr T, et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. The Lancet. 2012;380(9854):1674–1682. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- 18.Xu B, Ionita-Laza I, Roos JL, Boone B, Woodrick S, Sun Y, et al. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nature genetics. 2012;44(12):1365–1369. doi: 10.1038/ng.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014 doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulsuner S, Walsh T, Watts AC, Lee MK, Thornton AM, Casadei S, et al. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell. 2013;154(3):518–529. doi: 10.1016/j.cell.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gratten J, Visscher PM, Mowry BJ, Wray NR. Interpreting the role of de novo protein-coding mutations in neuropsychiatric disease. Nature genetics. 2013;45(3):234–238. doi: 10.1038/ng.2555. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan PF, Daly MJ, O'Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nature Reviews Genetics. 2012;13(8):537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crow JF. The origins, patterns and implications of human spontaneous mutation. Nature Reviews Genetics. 2000;1(1):40–47. doi: 10.1038/35049558. [DOI] [PubMed] [Google Scholar]
- 24.Eyre-Walker A, Keightley PD. The distribution of fitness effects of new mutations. Nature Reviews Genetics. 2007;8(8):610–618. doi: 10.1038/nrg2146. [DOI] [PubMed] [Google Scholar]
- 25.Krumm N, O'Roak BJ, Shendure J, Eichler EE. A de novo convergence of autism genetics and molecular neuroscience. Trends in neurosciences. 2014;37(2):95–105. doi: 10.1016/j.tins.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poultney CS, Samocha K, Kou Y, Liu L, Walker S, Singh T, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515(7526):209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iossifov I, O'Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515(7526):216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Jian X, Boerwinkle E. dbNSFP v2. 0: A Database of Human Non-synonymous SNVs and Their Functional Predictions and Annotations. Human mutation. 2013;34(9):E2393–E2402. doi: 10.1002/humu.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nature protocols. 2009;4(7):1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 31.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nature methods. 2010;7(4):248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nature methods. 2010;7(8):575–576. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 33.Reva B, Antipin Y, Sander C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic acids research. 2011;39(17):e118–e118. doi: 10.1093/nar/gkr407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chun S, Fay JC. Identification of deleterious mutations within three human genomes. Genome research. 2009;19(9):1553–1561. doi: 10.1101/gr.092619.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shihab HA, Gough J, Cooper DN, Stenson PD, Barker GL, Edwards KJ, et al. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Human Mutation. 2013;34(1):57–65. doi: 10.1002/humu.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davydov EV, Goode DL, Sirota M, Cooper GM, Sidow A, Batzoglou S. Identifying a high fraction of the human genome to be under selective constraint using GERP++ PLoS computational biology. 2010;6(12):e1001025. doi: 10.1371/journal.pcbi.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollard KS, Hubisz MJ, Rosenbloom KR, Siepel A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome research. 2010;20(1):110–121. doi: 10.1101/gr.097857.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garber M, Guttman M, Clamp M, Zody MC, Friedman N, Xie X. Identifying novel constrained elements by exploiting biased substitution patterns. Bioinformatics. 2009;25(12):i54–i62. doi: 10.1093/bioinformatics/btp190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindblad-Toh K, Garber M, Zuk O, Lin MF, Parker BJ, Washietl S, et al. A high-resolution map of human evolutionary constraint using 29 mammals. Nature. 2011;478(7370):476–482. doi: 10.1038/nature10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He X, Sanders SJ, Liu L, De Rubeis S, Lim ET, Sutcliffe JS, et al. Integrated model of de novo and inherited genetic variants yields greater power to identify risk genes. PLoS genetics. 2013;9(8):e1003671. doi: 10.1371/journal.pgen.1003671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sunkin SM, Ng L, Lau C, Dolbeare T, Gilbert TL, Thompson CL, et al. Allen Brain Atlas: an integrated spatio-temporal portal for exploring the central nervous system. Nucleic acids research. 2013;41(D1):D996–D1008. doi: 10.1093/nar/gks1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller JA, Ding S-L, Sunkin SM, Smith KA, Ng L, Szafer A, et al. Transcriptional landscape of the prenatal human brain. Nature. 2014 doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Duncan D, Shi Z, Zhang B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic acids research. 2013;41(W1):W77–W83. doi: 10.1093/nar/gkt439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wenger AM, Clarke SL, Notwell JH, Chung T, Tuteja G, Guturu H, et al. The enhancer landscape during early neocortical development reveals patterns of dense regulation and co-option. PLoS genetics. 2013;9(8):e1003728. doi: 10.1371/journal.pgen.1003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roshan R, Shridhar S, Sarangdhar MA, Banik A, Chawla M, Garg M, et al. Brain-specific knockdown of miR-29 results in neuronal cell death and ataxia in mice. rna. 2014;20(8):1287–1297. doi: 10.1261/rna.044008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nature Reviews Neuroscience. 2009;10(10):724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao Y, Anttonen A-K, Liukkonen E, Gaily E, Maljevic S, Schubert S, et al. SCN2A mutation associated with neonatal epilepsy, late-onset episodic ataxia, myoclonus, and pain. Neurology. 2010;75(16):1454–1458. doi: 10.1212/WNL.0b013e3181f8812e. [DOI] [PubMed] [Google Scholar]
- 48.Lemke JR, Hendrickx R, Geider K, Laube B, Schwake M, Harvey RJ, et al. GRIN2B mutations in West syndrome and intellectual disability with focal epilepsy. Annals of neurology. 2014;75(1):147–154. doi: 10.1002/ana.24073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coe BP, Witherspoon K, Rosenfeld JA, van Bon BW, Vulto-van Silfhout AT, Bosco P, et al. Refining analyses of copy number variation identifies specific genes associated with developmental delay. Nat Genet. 2014 doi: 10.1038/ng.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu L, Sabo A, Neale BM, Nagaswamy U, Stevens C, Lim E, et al. Analysis of rare, exonic variation amongst subjects with autism spectrum disorders and population controls. PLoS genetics. 2013;9(4):e1003443. doi: 10.1371/journal.pgen.1003443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ben-David E, Shifman S. Combined analysis of exome sequencing points toward a major role for transcription regulation during brain development in autism. Mol Psychiatry. 2012;10 doi: 10.1038/mp.2012.148. [DOI] [PubMed] [Google Scholar]
- 52.Schuurs-Hoeijmakers JH, Oh EC, Vissers LE, Swinkels ME, Gilissen C, Willemsen MA, et al. Recurrent de novo mutations in PACS1 cause defective cranial-neural-crest migration and define a recognizable intellectual-disability syndrome. American journal of human genetics. 2012;91(6):1122–1127. doi: 10.1016/j.ajhg.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carvill GL, Heavin SB, Yendle SC, McMahon JM, O'Roak BJ, Cook J, et al. Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat Genet. 2013;45(7):825–830. doi: 10.1038/ng.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338(6114):1619–1622. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stessman HA, Bernier R, Eichler EE. A genotype-first approach to defining the subtypes of a complex disease. Cell. 2014;156(5):872–877. doi: 10.1016/j.cell.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bernier R, Golzio C, Xiong B, Stessman HA, Coe BP, Penn O, et al. Disruptive CHD8 Mutations Define a Subtype of Autism Early in Development. Cell. 2014 doi: 10.1016/j.cell.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Helsmoortel C, Vulto-van Silfhout AT, Coe BP, Vandeweyer G, Rooms L, van den Ende J, et al. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat Genet. 2014;46(4):380–384. doi: 10.1038/ng.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Albert PR, Vahid-Ansari F, Luckhart C. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre-and post-synaptic 5-HT1A receptor expression. Frontiers in Behavioral Neuroscience. 2014;8:199. doi: 10.3389/fnbeh.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vulto-van Silfhout AT, Rajamanickam S, Jensik PJ, Vergult S, de Rocker N, Newhall KJ, et al. Mutations Affecting the SAND Domain of DEAF1 Cause Intellectual Disability with Severe Speech Impairment and Behavioral Problems. The American Journal of Human Genetics. 2014;94(5):649–661. doi: 10.1016/j.ajhg.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamilton PJ, Campbell NG, Sharma S, Erreger K, Hansen FH, Saunders C, et al. De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Molecular psychiatry. 2013;18(12):1315–1323. doi: 10.1038/mp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ebert DH, Greenberg ME. Activity-dependent neuronal signalling and autism spectrum disorder. Nature. 2013;493(7432):327–337. doi: 10.1038/nature11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ronemus M, Iossifov I, Levy D, Wigler M. The role of de novo mutations in the genetics of autism spectrum disorders. Nature Reviews Genetics. 2014;15(2):133–141. doi: 10.1038/nrg3585. [DOI] [PubMed] [Google Scholar]
- 63.Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337(6099):1190–1195. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khurana E, Fu Y, Colonna V, Mu XJ, Kang HM, Lappalainen T, et al. Integrative annotation of variants from 1092 humans: application to cancer genomics. Science. 2013;342(6154):1235587. doi: 10.1126/science.1235587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. Rna. 2003;9(10):1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maffioletti E, Tardito D, Gennarelli M, Bocchio-Chiavetto L. Micro spies from the brain to the periphery: new clues from studies on microRNAs in neuropsychiatric disorders. Frontiers in cellular neuroscience. 2014;8:75. doi: 10.3389/fncel.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu B, Hsu PK, Karayiorgou M, Gogos JA. MicroRNA dysregulation in neuropsychiatric disorders and cognitive dysfunction. Neurobiol Dis. 2012;46(2):291–301. doi: 10.1016/j.nbd.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parikshak NN, Luo R, Zhang A, Won H, Lowe JK, Chandran V, et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell. 2013;155(5):1008–1021. doi: 10.1016/j.cell.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willsey AJ, Sanders SJ, Li M, Dong S, Tebbenkamp AT, Muhle RA, et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell. 2013;155(5):997–1007. doi: 10.1016/j.cell.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hormozdiari F, Penn O, Borenstein E, Eichler E. The discovery of integrated gene networks for autism and related disorders. Genome research. 2014 doi: 10.1101/gr.178855.114. gr. 178855.178114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu X, Need AC, Petrovski S, Goldstein DB. One gene, many neuropsychiatric disorders: lessons from Mendelian diseases. Nat Neurosci. 2014;17(6):773–781. doi: 10.1038/nn.3713. [DOI] [PubMed] [Google Scholar]
- 72.Cristino A, Williams S, Hawi Z, An J, Bellgrove M, Schwartz C, et al. Neurodevelopmental and neuropsychiatric disorders represent an interconnected molecular system. Molecular psychiatry. 2013;19(3):294–301. doi: 10.1038/mp.2013.16. [DOI] [PubMed] [Google Scholar]
- 73.McCarthy SE, Gillis J, Kramer M, Lihm J, Yoon S, Berstein Y, et al. De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Mol Psychiatry. 2014;19(6):652–658. doi: 10.1038/mp.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Percentage of missenses with varying damaging scores in case and control. For each missense mutation, twelve tools were combined to predict the functional mutation, the total damaging score is the summed number of tools predicted to be “deleterious” or “conserved” (Materials and Methods). Based on damaging score, we classified missense mutations into three groups: [0, 3], [4, 7], [8, 12]. De novo missense mutations with high damaging score (≥ 8) are enriched in cases (four neuropsychiatric disorders) in comparison with controls. However, the other types of missense mutations with low damaging score (≥ 4) and medial damaging score (<4) are not enriched in cases.
Supplementary Figure 2. Odds ratio of functional classes of DNMs. Each bar plot corresponds to the odds ratio (OR) to control. Error bar represents the 95 % confidence interval (Cl) of OR in each disorder by two-sample Poisson rate test. This figure illustrates Table 1.
Supplementary Figure 3. Significance of the shared genes in four neuropsychiatric disorders. The histograms show the results of a randomly resampling (1,000,000 iterations). The observed value is shown by the vertical red line and corresponds to a P value by permutation test (Supplementary Materials and Methods). Six data sets were assessed: (A) shared genes in the four disorders; (B) ASD and control; (C) EE and control; (D) ID and control; (E) SCZ and control; and (F) the 53 shared genes and extreme DNMs-containing genes in control.
Supplementary Figure 4. Laminar transcriptional patterning of SCN2A. Heat map showing expression level of SCN2A at 15 pcw (A), 16 pcw (B) and 21 pcw (C, D) in nine layers and different sub–structures in human brain (Supplementary Table 2). Red indicates high expression, and white indicates low expression. The laminar-specific neocortical expression data contains four prenatal brains from BrainSpan Atlas: two from 15 and 16 pcw and two from 21 pcw (post conceptual week).
Supplementary Figure 5. Snapshot of NPdenovo database. NPdenovo provides numerous ways to browse, search, and analyze DNMs in neuropsychiatric disorders. All the DNMs and candidate genes are comprehensively annotated in NPdenovo database, such as the mutation information, gene information, and brain expression.