Summary
As the primary site of T cell development, the thymus plays a key role in the generation of a strong yet self-tolerant adaptive immune response, essential in the face of the potential threat from pathogens or neoplasia. As the importance of the role of the thymus has grown, so too has the understanding that it is extremely sensitive to both acute and chronic injury. The thymus undergoes rapid degeneration following a range of toxic insults, and also involutes as part of the aging process, albeit at a faster rate than many other tissues. The thymus is, however, capable of regenerating, restoring its function to a degree. Potential mechanisms for this endogenous thymic regeneration include keratinocyte growth factor (KGF) signaling, and a more recently described pathway in which innate lymphoid cells produce interleukin-22 (IL-22) in response to loss of double positive thymocytes and upregulation of IL-23 by dendritic cells. Endogenous repair is unable to fully restore the thymus, particularly in the aged population, and this paves the way towards the need for exogenous strategies to help regenerate or even replace thymic function. Therapies currently in clinical trials include KGF, use of the cytokines IL-7 and IL-22, and hormonal modulation including growth hormone administration and sex steroid inhibition. Further novel strategies are emerging in the pre-clinical setting, including the use of precursor T cells and thymus bioengineering. The use of such strategies offers hope that for many patients, the next regeneration of their thymus is a step closer.
Keywords: Thymus damage, Aging, Tissue Regeneration
Introduction
The thymus is the primary site of T cell development. As other reviews in this volume have highlighted, the specialized thymic microenvironment supports the development of a broad but self-tolerant T cell repertoire. This is vital to the development of a strong adaptive immune response against pathogens and tumours, without leading to autoimmune disease.
The importance of the thymus, however, must be reconciled with the potential for loss of thymic function over a lifetime, and the ensuing detrimental effects. The thymus is exquisitely sensitive to a range of acute insults. It is important to stress that these insults should not be considered in isolation, as significant potential exists for coincidental conditions to impair thymic function in the clinical setting. Hematopoietic stem cell transplantation (HSCT), for example, may acutely damage the thymus through the chemotherapy, radiotherapy and antibody therapy of the conditioning regime. This may be compounded by infections acquired by the immunosuppressed patient, and in the case of allogeneic HSCT, thymic graft versus host disease (GVHD). Following resolution of the acute insult, the thymus is, however, capable of intrinsic recovery.
In addition to acute degeneration, thymic decline also occurs as an inevitable chronic process, in which the thymus gland undergoes involution with age. Thymic involution differs from aging in other organs and cannot be reversed. Furthermore, the aging process impairs the ability of the thymus to regenerate from acute damage. There is thus an increasing recognized need for exogenous strategies that can rejuvenate the aged or damaged thymus. We review the most promising therapeutic avenues, some of which are now entering clinical trials. There are caveats to such approaches, however. There may be potential detrimental consequences to rejuvenating an organ that has been evolutionarily selected to involute with age. Nevertheless, thymic regeneration undoubtedly offers much therapeutic potential, and the ability to harness this epitomises the exciting intersection between regenerative medicine and immune biology.
Causes and targets of acute thymic damage
Notwithstanding its importance for generating a diverse T cell repertoire, the thymus is extremely sensitive to negative stimuli (Figure 1). However, despite this sensitivity, thymic regeneration can occur following resolution of the insult; although this ability is blunted with increasing age (1). Acute thymic damage can cause significant morbidity and mortality in conditions where active recovery of thymopoiesis is required to sustain immune competence, such as after clinically induced immune depletion (2), and has been directly linked to opportunistic infections and an adverse clinical outcome in recipients of allogeneic HSCT (3).
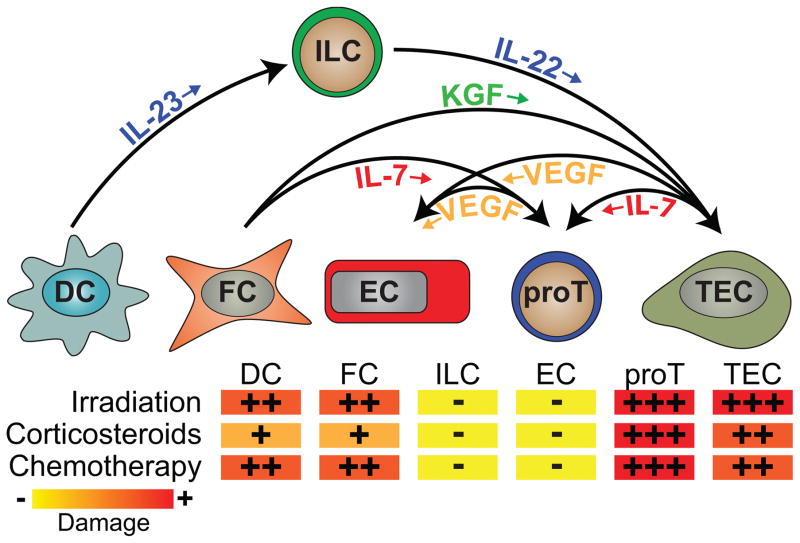
Figure 1. Targets of acute thymic damage and pathways of endogenous regeneration.
The thymus is extremely sensitive to damage, typically in the form of irradiation, cytoreductive chemotherapy or stress-induced (or administered) corticosteroids. While most of these insults target the T cell progenitors (most prominently CD4+CD8+ DP thymocytes), TECs are also notably targeted by both irradiation and cytoreductive chemotherapy. Corticosteroids specifically target thymocytes and so other cell populations including TECs, dendritic cells (DCs), fibroblasts (FC), innate lymphoid cells (ILCs) and endothelial cells (ECs) are relatively untouched initially (although due to crosstalk there is a decline in the numbers of cTECs and mTECs after the thymocyte depletion). ILCs and ECs, and to a lesser extent FC and DC, are remarkably resistant to acute damage. After injury the thymus has a remarkable capacity to regenerate itself. While the mechanisms underlying this regeneration remain poorly understood, in the past few years several pathways have been revealed. These include the IL-23/IL-22 and KGF pathways, which targets TECs; IL-7, which can be produced by both TECs and FCs and target early T cell progenitors; and VEGF, which can be produced by TECs and some thymocytes and targets ECs to induce angiogenesis, a crucial step during organ regeneration.
Cytoablative therapies
Although primarily directed against malignant cells, chemotherapy can target the haematopoietic system, including the T cell compartment (4, 5). Such T cell immunodeficiency results from ablation of both thymic and peripheral T cell subsets. Within the thymus, alkylating agents such as cyclophosphamide have been shown to deplete all thymocyte subsets (6, 7). While thymic stromal cells were traditionally thought to be resistant to damage caused by chemotherapy or radiation, there is now considerable evidence to suggest that such agents can also damage the thymic stroma (8). Thymic epithelial cells (TECs) expressing the highest levels of MHC class II are particularly vulnerable to the effects of chemotherapy, particularly those located in the medulla (mTECs) (8), likely due to their higher rate of proliferation (9, 10). Since these cells play a key role in negative selection (11), this selective depletion may have profound implications for causing a lack of tolerance to self, following such treatments, although this remains hypothetical at present.
Following chemotherapy, homeostatic expansion of the peripheral T cell pool can facilitate CD8+ T cell recovery within months in both young and old patients (12), although clearly the repertoire diversity is impacted (13). However, while in younger patients, peripheral expansion is augmented by recovery of thymic function in older patients there is evidence that chemotherapy induced thymic damage may take years, if ever, to recover (5, 14, 15).
Similarly, radiation can induce acute thymic damage and loss of cellularity (16). Although all thymocyte populations are typically affected, the double positive (DP) thymocyte population is particularly sensitive to irradiation (17). However, like chemotherapy, radiation can also lead to stromal damage with considerable reduction in TEC numbers (16, 18). It is notable that certain cell populations, such as endothelial cells and innate lymphoid cells (ILCs), are relatively radio-resistant (19, 20), and there cells can play a role in endogenous thymic regeneration.
Steroid hormones
Glucocorticoid hormones, acting through the nuclear glucocorticoid receptor (GR), exert a wide range of immunosuppressive and anti-inflammatory effects, providing the rationale for their use in autoimmune disease, allergic and inflammatory disorders, allograft rejection, GVHD and lymphoid malignancies (21). Similarly, elevated levels of endogenous glucocorticoids, as produced in stress, starvation, infection and Cushing disease, may also lead to immunosuppression (22). In the thymus, glucocorticoids induce apoptosis of DP thymocytes, which preferentially express the GR (23), in an Apaf-1 and caspase-9 dependent manner, leading to acute involution (24, 25). In view of the wide range of conditions associated with their elevated levels, glucocorticoids thus represent a common pathway mediating many episodes of acute atrophy of the thymus. Interestingly, the thymic epithelium itself can also produce glucocorticoids (26), and this may have subtle effects on steady-state thymopoiesis. For instance, thymus-derived glucocorticoids can inhibit T cell receptor (TCR)-mediated deletion of DP thymocytes, and thus modulate which TCR avidities result in positive or negative selection (27, 28).
Increased levels of the sex steroid hormones, testosterone, progesterone and estrogen, likewise act through their nuclear receptors causing thymic involution. Their effect is most obviously manifest during puberty, during which the rate of thymic involution increases rapidly (29). Similarly, pregnancy is associated with an acute transient involution of the thymus (30). Sex steroids have multiple effects to reduce the lymphocyte pool within the thymus. Although sex steroids can directly induce apoptosis of thymocytes (31), studies using chimeras and androgen-resistant testicular feminization mice have demonstrated that the primary mediator of sex steroid-induced thymic involution are the non-hematopoietic stromal cells (32). TECs express a functional androgen receptor (AR), and AR knock-down in this population partially abrogates sex steroid-induced thymic involution, further implicating TECs as important targets of androgens (33, 34). More recently it has been shown that androgens directly inhibit thymopoietic factors in TECs, including CCL25, a ligand critical for entry of T cell progenitors into the thymus, and Delta-like ligand 4 (Dll4), a Notch ligand crucial for the commitment and differentiation of T cell progenitors in a dose-dependent manner (35, 36). Furthermore, through their effects on both HSCs and the bone marrow microenvironment, sex steroids decrease lymphoid differentiation, reducing the number of available T cell progenitors in the thymus (37, 38).
Infection
Although previously thought to be an immune-privileged site (39), protected from infections and immune responses, increasing evidence over the last decade has highlighted that the thymus is indeed a target for a range of pathogens, including bacteria, viruses, fungi and parasites (40). The resulting lymphostromal disruption causes acute thymic involution and defects in thymic export of newly generated naïve T cells, leading to impaired immune responses against the pathogen (41). Thymic damage as a result of acute infection may be caused directly by the pathogen within the thymus or by the systemic effects invoked by infection.
Systemic pathogen specific factors most notably include bacterial lipopolysaccharide (LPS) released from gram-negative bacteria such as Escherichia coli (42, 43). LPS leads to severe acute thymic atrophy that peaks within 3–5 days, characterized by loss of DP thymocytes (44). In addition to the systemic effects of LPS, many infections also trigger a stress response and a surge in glucocorticoid levels, inducing thymocyte apoptosis as described above (25, 45). Other inflammatory mediators may rise during infection, leading to thymic damage, and again in particular depletion of the DP population. These include tumour necrosis factor α, the release of which can be triggered by infection with Francisella tularenis (46) and Trypanosoma cruzi infection (47); as well as the release of interferon γ caused by Salmonella enterica infection (48).
Pathogens may directly invade the thymus. HIV, the most well studied example, directly infects CD4+ SP thymocytes and progenitors, and induces apoptosis of uninfected thymocytes through secretion of viral products (49, 50). In addition, HIV infects thymic stromal cells, including TECs and dendritic cells (51–53). This leads to disruption of thymic architecture and degradation of the thymic microenvironment. Similar effects on TECs are observed in other chronic viral infections, such as CMV (54). Direct infection of the thymus by Trypanosoma cruzi can also alter the thymic environment by increasing deposition of fibronectin and laminin and increasing the expression of the chemokine ligands CXCL12 and CCL4 by stromal cells. In addition to causing structural disruption to the thymus, these changes promote the migration of DP thymocytes, leading to their premature release from the thymus and further thymic involution (55).
Although thymic atrophy in the context of infection may be coincidental, the conservation of this phenomenon across a diverse range of pathogens suggests a greater significance. Thus, induction of thymic involution may be a deliberate virulence strategy employed by pathogens in order to subvert immune responses, and propagate survival within the host. However, interestingly there is also emerging evidence in the context of mycobacterial infection that thymic infection in the absence of atrophy can result in the generation of naïve T cells tolerant to the pathogen (56). Thus, although it compromises T cell immunity, thymic atrophy may in fact represent a deliberate strategy employed by the host, to avoid the generation of an immune repertoire tolerant to certain pathogens. A further understanding of this process is therefore essential in the context of potential strategies to rejuvenate the damaged thymus.
Graft versus host disease
GVHD is a complication of allogeneic HSCT, characterised by three distinct phases: (1) tissue damage from conditioning therapy or other causes, (2) activation of alloreactive donor T cells by host antigen-presenting cells, and (3) target tissue damage mediated by soluble and cellular effectors (57, 58).
Although acute GVHD has been traditionally viewed as a disease of gut, liver and skin, there is now a large body of evidence demonstrating that the thymus is an extremely sensitive target of alloreactive T cells (59–62). Clinical manifestations of GVHD are associated with changes to both the lymphocyte and stromal compartments of the thymus, leading to acute involution. These include loss of cortical and medullary thymocytes, loss of TECs, decreased demarcation of the cortico-medullary junction and disrupted architecture of the gland (63, 64). Such changes have been shown to lead to decreased thymic output of naïve T cells, as indicated by TCR excision circles (TRECs), and a distorted TCR repertoire (65).
More recently, murine models of GVHD have demonstrated that loss of thymic cellularity is primary due to loss of the large DP subset, as a consequence of both a block in differentiation block in early DN thymocytes and also increased apoptotic cell death in the DP population (59). TECs have likewise been identified in these models as direct targets for alloreactive T cells, but in addition to acting as targets, there is also evidence that given their expression of MHCI and MHCII, these TECs can act as antigen presenting cells sufficient in themselves to prime alloreactive T cells (60). Together with the fact that the thymus is extremely sensitive to GVHD-mediated damage (62), this raises the distinct possibility that GVHD can be restricted to the thymus even during subclinical GVHD, and thus in many cases may not be detected in the clinical setting yet still have detrimental consequences for T cell reconstitution.
Murine models of GVHD have also highlighted that thymic damage in such cases is associated with impaired negative selection of thymocytes and Treg development, which may be potential contributory factors to the development of chronic GVHD and autoimmunity post HSCT (66, 67).
Moreover, given that corticosteroids are often used as a first line treatment for GVHD (58), and that they induce thymic involution in and of themselves, their use will likely compound the detrimental effects on thymic function during GVHD. Thus, the treatment of thymic GVHD is a conundrum that may require alternate therapeutic approaches.
Endogenous thymic regeneration
Endogenous thymic regeneration is a crucial function that allows for renewal of immune competence following immunodepletion caused by cytoreductive chemotherapy or radiation, acute infection and stress (Figure 1). Although the potential for the thymus to regenerate itself has been known for some time, surprisingly little is known about the mechanisms involved with this regeneration.
One of the first studies to identify a potential pathway of endogenous regeneration focused on fibroblast growth factor 7 (FGF7, also known as KGF). In this study, it was found that although KGF was redundant for steady-state thymopoiesis, it was crucial for regeneration after injury such as that caused by TBI, as mice deficient for KGF exhibited significantly worse thymic recovery compared to wildtype controls (68). KGF targets thymic epithelial cells and promotes their proliferation and differentiation (69, 70). Furthermore, KGF has been used in several studies and clinical trials as a means of boosting thymus function after damage (see below).
Another pathway of endogenous thymic regeneration identified is centered on ILCs and their production of interleukin-22 (IL-22), a recently identified cytokine predominantly associated with maintenance of barrier function at mucosal surfaces. This study proposed that 1) the depletion of CD4+CD8+ DP thymocytes triggers, 2) upregulation of IL-23 by dendritic cells (DCs), which induces 3) the production of IL-22 by intrathymic ILCs. IL-22 directly promotes the proliferation and survival of TECs, therefore this cascade of molecular and cellular events leads to regeneration of the supporting microenvironment and, ultimately, to rejuvenation of thymopoiesis (19). Additionally, a subsequent study correlated the increased expression of IL-22 after damage with increased expression of Foxn1 (71). Foxn1 is a molecule critically involved with thymus ontogeny and is also clearly important for thymic maintenance and regeneration (72, 73). In fact, forced expression of Foxn1 leads to regeneration of the aged thymus (74, 75) and thus activation of Foxn1 after damage could represent a potent pathway of endogenous or exogenous thymic regeneration. Although it is tempting to think that IL-22 may be directly promoting expression of Foxn1, currently there is no clear evidence of such a link.
It is also very likely that molecules involved in steady state thymopoiesis also contribute towards thymic regeneration. These include IL-7, CXCL12, SCF, CCL25, and the notch ligand DLL4. In fact, many of these have been identified as part of the mechanistic pathways for exogenous thymic regeneration through such systemic means as sex steroid ablation, which crucially needs IL-7 (but not KGF) to mediate regeneration (76), and induces expression of the Notch ligand Dll4 (36), CCL25, which induces importation of progenitors (35), and VEGF, which promotes vascularization and endothelial cell function and is also likely involved in endogenous thymic regeneration (77, 78).
Chronic thymic damage: Involution of the thymus with age
Aging is an inevitable process occurring in all living organisms, associated with the progressive decline of function in tissues and increased susceptibility to disease (79). Specific to the immune system, advancing age is associated with an array of defects in both innate and adaptive immunity, collectively termed immunosenescence, which impairs the ability to respond to both pathogens and vaccinations (80, 81). Elderly subjects demonstrate increased incidence of a range of bacterial and viral infections, and exhibit increased mortality from these diseases in comparison to younger patients (82). Furthermore, immunosenescence may also lead to a loss of tumour immune surveillance, increasing the propensity for neoplastic disease in the elderly (83). There may also be immune dysregulation, such that older subjects experience increasing autoimmune disease (84). A key feature of immunosenescence is involution of the thymus, characterized by both a progressive decrease in thymic cellularity and a loss of tissue organization (29, 85). This leads to profound age related defects, both quantitative and qualitative, in the T cell compartment.
Consequences of thymic immunosenescence
Involution of the thymus with age ultimately causes a decrease in the thymic output of naïve T cells and subsequently a constriction of the peripheral TCR repertoire. As T cell production decreases, there is homeostatic expansion of existing peripheral T cells, which leads to a skewing towards an increased proportion of memory T cells, and a consequent reduction in the diversity of TCR repertoire (86, 87). However, even those naïve T cells that are produced in the aged thymus are functionally impaired; expressing higher levels of senescence markers such as CD57 and exhibiting limited proliferation in response to antigen stimulation (88). Furthermore, they have reduced homing receptors such as CD62L and CCR7, interfering with their mobilization to relevant sites of action (89). The overall consequence of these changes is a defective adaptive immune response to neo-antigens, including new pathogens, vaccinations and tumour-associated antigens (90).
Thymic involution also contributes to the development of autoimmune disease in older age. The thymus provides a site where naïve T cells reactive to self are deleted through negative selection, both by interaction with thymic epithelial cells (TECs) and thymic dendritic cells. As these thymic cells are lost with age, the ability to mediate this central tolerance is impaired, and there is a greater chance that auto-reactive T cells will be released into the periphery (91, 92).
It is important to note, however, that despite thymic involution and alterations to the T cell compartment, some residual thymic function does persist into old age. Significant levels of naïve T cells can be detected in centenarians (93) and high levels of TRECs can still be detected in elderly subjects (94). Similarly, TDT expression within the thymus can be shown in aging persons (95). This residual function highlights that the thymus gland does not become a vestigial remnant in later life, but instead continues to function, albeit at a lower rate. Nevertheless, this low level function may be readily lost in the face of the acute insults described above, many of which are more common in the elderly population. This is compounded by a reduced ability of the aged thymus to endogenously regenerate following acute damage, although the reasons for this are not clear. The need to develop strategies to rejuvenate or replace thymic function, as discussed below, is thus particularly relevant to the aging population.
Mechanisms of age related thymic involution
There are phenotypic changes within both lymphoid and stromal compartments during age-related thymic involution, at least some of which will be exacerbated due to the extensive support offered by crosstalk interactions (96). The most prominent change in the thymus with age is the significant progressive decrease in the number of thymocytes within the organ. It has been estimated that in mice, the thymus of a 24 month-old mouse contains <1% of the T cells found in a neonatal equivalent.
Thymopoiesis is characterized by the stepwise differentiation of pro-T cells, the most primitive which is the early T-lineage progenitor (ETPs), itself a direct descendent of the circulating BM-derived progenitor (97). With age, although the number of bone marrow hematopoietic stem cells (HSCs) increases, their function, particularly towards lymphoid differentiation, declines considerably (98, 99). Although the ability of aged progenitors to get in to the thymus, and the ability of the thymus to accept these circulating progenitors, remains unchanged with age (100), this decrease in the supply of BM-derived progenitors results in a significant reduction in the number of ETPs in older individuals (101).
In addition to these numerical deficiencies, functionally, ETPs exhibit reduced proliferation and differentiation potential in the aged thymus (101). Similarly, later stages of DN thymocyte development are also affected, including differentiation blocks at the DN2, DN3 and DN4 stages (102, 103) and the accumulation of an abnormal population of CD44+CD24−CD3+ DN cells in the thymus of older mice (102, 104). Although the significance of these cells remains unclear, a similar population has been found in the aged bone marrow, associated with a reduction in haematopoiesis (105). Aged DP and single positive (SP) thymocytes express reduced levels of CD3, possibly impairing TCR-signalling, and exhibit a decrease in Concanavalin A-induced proliferation (104).
Such defects in thymocyte proliferation and differentiation at all stages may represent intrinsic abnormalities within lymphocytes derived from an aged bone marrow. However, thymocyte development is also instructed by the thymic microenvironment as part of the lymphocyte-stromal cross talk. Thus, defective thymocyte development is also due in part to abnormal extrinsic signalling from the altered thymic environment. Highlighting these extrinsic factors influencing thymic involution, young ETPs, administered by intrathymic injection, are capable of developing in young mice but fail to do so in older recipients (106). Architecturally, the non-hematopoietic stromal microenvironment of the thymus undergoes marked structural changes with age, primarily within the TEC compartment. With age, the cortical and medullary thymic epithelial regions become irregular and atrophied, with loss of the definition of the cortico-medullary junction (107); there is diminution of the thymic epithelial space and enlargement of the perivascular space (29); and increased adiposity (108, 109). Underlying some of these changes within the TEC compartment is the reduced expression of the transcription factor FOXN1, a key regulator of TEC differentiation in the fetal and postnatal thymus (72, 110). Indeed, induced expression of FOXN1 in murine models has been shown to delay age related thymic involution (75), and can also robustly rejuvenate the aged thymus through expansion of the TEC compartment (74). In addition, TECs exhibit decreased MHC class II expression, attenuating their ability to interact with developing thymocytes (9), and their production of the thymopoietic cytokine IL-7 by TECs is also reduced with age (111). Similar to the aging of many other organs, there is also an accumulation of fibroblasts in the aged thymus, leading to progressive fibrosis with age and again impeding the normal architecture of the gland (107). The accumulation of abnormal mesenchymal cells may also lead to abnormal signalling, such as adipokine secretion by adipocytes, which may further interfere with thymopoiesis, and warrants further investigation (109). The overall consequence of these structural changes is a progressive loss of thymic niche spaces, and thus a reduction in thymocyte development.
Underlying causes of age related thymic involution
Although aging affects all aspects of an organism, the thymus exhibits a unique pattern of aging, distinct from other tissues and organs (112). Thymic involution occurs much earlier than other acknowledged features of aging, beginning in early childhood and peaking at puberty (29). Following this initial phase, thymic tissue is estimated to be lost at approximately 3% per year until middle age and then subsequently at 1% per year (113). There is, however, individual variation, and in addition a sexual dimorphism exists, such that the rate of involution is greater in males than in females (114). Thus, although a full discussion of the causes of aging in general are beyond the scope of this review, below we focus on those aetiological factors which are particularly relevant to this pattern of involution observed in the thymus.
The variation in thymic involution between different strains of mice highlights how genetic polymorphisms may influence this process. This area has been investigated further through the use of recombinant-inbred (RI) mice (115). C57BL/6 and DBA/2 mice were used to generate 18 strains of RI mice, termed BXD. Using mathematical modelling, these mice could be classified into four groups on the basis of their initial thymic mass and the subsequent rate of thymic involution. A higher rate of thymic involution was shown to be in part due to a block in thymocyte development and thus decreased thymopoiesis. Quantitative trait loci influencing the rate of thymic involution could be identified most strongly on chromosome 9. The genes in this region thus represent a future target for further investigation.
Hormones, produced through the hypothalamic-pituitary axis, can influence age related thymic involution. Several studies have shown that growth hormone (GH) can rejuvenate the aged thymus (see below). The corollary of this has been to invoke the decline in serum GH concentration with age as playing a role in thymic involution.
The thymus is known to be acutely sensitive to sex steroids (see above), and similar lines of correlative evidence have strongly linked changes in sex steroid levels, especially androgens, to thymic degeneration with age. Notably, the greatest rate of thymic involution with age is observed at puberty (29), and sex steroid ablation is found to rejuvenate the aged thymus (see below). Furthermore, the increased level of androgens in males offers an explanation for the increased rate of thymic involution evident in males (114).
However, caveats exist to ascribing age related thymic involution solely to the changes in hormones with age. As discussed, the initiation of thymic involution begins in childhood, prior to the fall in serum GH or puberty associated rise in sex steroids. Furthermore, thymic involution continues with age even when sex steroid levels fall (116). Thus, further aetiological factors are required to explain the pattern of aging observed in the thymus.
The accumulated damage from oxygen free radicals is a recognised contributor to the aging process in many organ systems, leading to chronic damage evident in later life. Such free radical damage may play a key role in the aging of the thymus. This is supported through the finding that reducing metabolic activity, for example by caloric restriction (117) or modulation of IGF signalling (118), can reduce thymic involution. It is notable that the rapid course of involution demonstrated in the thymus would be difficult to reconcile with chronic free radical damage. However, it has recently been shown that thymic stromal cells are deficient in the enzyme catalase, making these cells much more sensitive to damage by oxidative by-products and thus offering an explanation as to why thymic involution proceeds more rapidly than aging in other organs (119).
Is there a physiological purpose of age related thymic involution?
The unique response of the thymus to aging, both in terms of age at initiation and rate of involution, raises the question that there may be a hitherto yet undiscovered biological purpose to thymic involution. This concept is supported by the finding that thymic involution with age is an evolutionary conserved process, evident across most species.
It has been shown that with age, there is decreased influx into the thymus of lymphoid progenitor cells from the bone marrow, and this is associated with a risk of developing T cell leukaemia, due to excessive prolongation of the time developing thymocytes reside in thymic niches (120). It is thus speculated that thymic involution is needed to prevent the development of a pro-leukemic environment within the thymus (121). Alternatively, thymic involution may represent a trade-off in terms of bioenergetics. In youth, it is important to develop broad TCR diversity for the purposes of a strong adaptive immune system. Once developed, however, an organism may need to give priorities to other functions, such as reproductive capability, and thus reducing the energy consumption associated with thymic function is a requisite to achieve this.
Thus, although rejuvenation of the aged thymus undoubtedly offers therapeutic potential, there are caveats to be considered. Novel strategies to enhance thymic function in the aged individual will need to be performed carefully, both at the appropriate time and in the correct context.
Exogenous strategies to enhance thymic recovery
Rejuvenation of immune function remains a prominent unmet need in several clinical situations. Over the past decades, the mechanisms that regulate thymic recovery after injuries and the development of strategies that can boost its endogenous repair have been the focus of intensive research. Here we summarize preclinical and clinical studies of promising strategies that have the potential to be translated into novel regenerative therapies.
Keratinocyte Growth Factor (KGF)
Keratinocyte Growth Factor (KGF), also know as fibroblast growth factor 7 (FGF-7), is a protein with a predicted molecular weight of 22.5kDa that belongs to the fibroblast growth factor family. It is primarily secreted in a paracrine fashion from mesenchymal cells and promotes proliferation of epithelial cells (122). KGF binds to an epithelial cell–specific splice variant of the fibroblast growth factor receptor-2 (FgfR2-IIIb) expressed on TECs within the thymus. The FgfR2-IIIb receptor is activated not only by KGF but also by other close members of the family (FGF-1, FGF-3, and FGF-10) (123, 124). Although mice that are deficient for KGF show no signs of defective steady-state thymopoiesis, KGF KO mice show a deficit in thymic recovery after immune insults, such as sub-lethal radiation and HSCT.
As a result of these studies exogenous administration of recombinant KGF has been tested for its ability to enhance thymic regeneration, particularly in the setting of HSCT. KGF protects epithelial cells from several thymic injuries, including radio- and chemotherapy, allogeneic or syngeneic HSCT and GVHD (125–127). The mechanism by which KGF acts appears to be in promoting TEC proliferation, as BrdU incorporation in TECs increases up to 10 fold when mice are treated with KGF for 3 days (128). Ultimately this increase in TEC proliferation leads to increased thymocyte expansion and enhanced T cell export. Mechanistically, engagement of the FgfR2-IIIb promotes stimulation of the p53 and NF-kB pathways, and results in the upregulation of BMP2, BMP4, Wnt5b, and Wnt10b in young mice (128). A recent study in a non-human primate model showed that adult rhesus macaques, receiving KGF after autologous HSCT, had accelerated hematopoietic recovery, improved thymopoiesis (as evaluated by TREC analysis) and enhanced naïve T cell recovery following transplant. However, the increase in T cell recovery was not associated with an improved immunity against CMV reactivation or an improved response to tetanus toxoid vaccination (129). However, while KGF can also promote the expansion of young and old thymi in mice (68), there is some debate as to its effectiveness in promoting steady-state thymopoiesis in non-human primates (129).
Human recombinant KGF (Palifermin) is an FDA approved drug for the prevention of mucositis in recipients of high dose chemotherapy. However there are as yet no clinical studies designed to evaluate the sole efficacy of KGF in enhancing thymus function and T cell reconstitution in immunocompromised patients (127).
Interleukin-7 (IL-7)
IL-7 is a common gamma-chain (γ-chain) cytokine of 25-kDa involved in several adaptive immune processes including thymopoiesis, B cell development, and lymph node organogenesis (130). It also represents a pro-survival factor for ILCs. In the thymus, IL-7 is primarily produced by cTECs, and to a lesser extent by fibroblasts. The IL-7 receptor (IL-7R) is a heterodimer complex consisting of the IL-7Rα (CD127) and the CD132 common γ-chain receptor. Although the γ-chain is expressed on all hematopoietic cells, IL-7Rα is almost exclusively expressed on lymphoid cells. In fact, IL-7 is a critical non-redundant cytokine for both T and B cell lymphopoiesis, and in the thymus IL-7R stimulation promotes proliferation, differentiation and survival of the developing thymocytes. It has been suggested that the engagement of the IL-7R induces cell differentiation through Jak-Stat signalling, and cell proliferation and survival through the PIK3/Akt pathway (131). Consistent with this, a defect in the IL-7Rα chain or the common γ chain in humans results in the severe combined immunodeficiency syndrome (SCID) (132, 133).
Given its critical role in thymopoiesis and in promoting pro-survival signals to peripheral lymphocytes, IL-7 has been extensively studied for its potential to enhance recovery after immune-insults (130). Several studies have demonstrated the beneficial effects of exogenous administration of IL-7, which enhances thymopoiesis and export of recent thymic emigrants, in addition to increasing the homeostatic proliferation of mature peripheral T cells (134, 135). Such effects ultimately lead to accelerated T cell recovery after syngeneic and allogeneic HSCT (136–138). Furthermore, administration of IL-7 has also been reported to increase antigen-specific T cell responses to vaccination and viral infections (139, 140). A more recent report described the results of a phase 1 clinical trial of recombinant human hIL-7 (CYT107) in recipients of T cell depleted allogeneic HSCT (NCT00684008). This study shows that CD3, CD4 and CD8 counts are increased in hIL-7 treated patients, and while no significant effects were reported on thymic output as measured by analysis of recent thymic emigrants, patients receiving hIL-7 did show a broader TCR beta repertoire diversity compared to untreated patients (141).
Interleukin-22 (IL-22)
IL-22 is a cytokine that belongs to the IL-10 cytokine family. It has gained increasing interest recently for its potential tissue protective effect (142). In particular, IL-22 has been primarily implicated in promoting epithelial integrity and antimicrobial immunity at mucosal surfaces. IL-22 is mainly produced by Th17 cells and innate lymphoid cells (ILCs) and it binds to a heterodimeric cell surface receptor, IL-22R, composed of IL-10R2 and IL-22R1 subunits (143–148). IL-22R is expressed on cells of epithelial origin, such as keratinocytes, intestinal or lung epithelial cells, and hepatocytes, and it is absent on cells of the immune system (142, 149). The key role of IL-22 in mediating endogenous recovery of thymus function after acute damage is described in detail above, and this provides the rationale to use IL-22 treatment as a potential therapeutic option, to stimulate thymic recovery in immunocompromised patients (19).
Based on these findings, a phase IIa clinical study has recently been initiated to evaluate the safety and tolerability of human recombinant IL-22 (hrIL-22) in conjunction with systemic corticosteroids in the treatment of gastrointestinal acute GVHD in patients receiving HSCT (NCT02406651). Importantly, peripheral T cell counts will be evaluated as a part of the study, allowing for further investigation of hrIL-22 as an immune-boosting therapy.
Growth hormone (GH) and Insulin-like growth factor 1 (IGF-1)
Several neuroendocrine hormones have been shown to mediate important effects on the immune system. Growth hormone (GH), also known as somatotropin, is a peptide hormone primarily secreted by the anterior pituitary gland (150); although interestingly, a previous study has also reported that GH can be produced by ex-vivo isolated human thymocytes and TECs (151). The potent effects of GH on thymic function have been extensively investigated using GH deficient mice, which exhibit defects in T cell development. However, given that the GH-receptor (GHR) is expressed by TECs as well as developing thymocytes (151), it is unclear which are the primary targets of these effects of GH. In addition, exogenous administration of recombinant GH has been found to promote thymus regrowth in several preclinical mouse models (152). GH administration promotes improved thymic cellularity, increased TCR diversity and enhanced recovery of the hematopoietic compartment in immunocompromised and aged animals (153, 154). Several intrinsic and extrinsic mechanisms have been identified for the beneficial effects of GH on thymic function such as enhanced proliferation of TECs and trafficking of common lymphoid progenitors (CLPs) into the thymus (155–157).
Mechanistically, it has been shown that GH activates the JAK2/Stat5 pathway leading to the expression of several downstream genes. Insulin-like growth factor 1 (IGF-1) has been described as the principal mediator of the biological effects of GH (158–160). IGF-1, sometimes referred as somatomedin C, is a protein with a structure similarity to insulin. IGF-1 is expressed by thymic stromal compartment, in particular by TECs and fibroblasts, while the IGF-1 receptor is expressed not only by the stromal component (mainly by the epithelial cells and fibroblasts) but also by the hematopoietic compartment (161).
Encouraging results have been generated in several clinical studies. Recombinant human GH enhances thymic recovery and immune reconstitution in HIV-infected patients (162). More recently, a phase 1 clinical trial was performed to test the safety and efficacy of GH in improving immune reconstitution post unrelated cord blood transplant (NCT00737113).
Sex steroid inhibition (SSI)
Surgical or chemical sex steroid inhibition (SSI) is a well-described approach to promote thymic growth in several pre-clinical and clinical studies (163, 164). In fact, several reports have shown that SSI promotes thymic enlargement in young as well as in old mice and promotes accelerated thymic recovery after immune insults, such as radio- and chemotherapy, syngeneic and allogeneic HSCT and GVHD (7, 76, 165, 166). Androgen deprivation has been the focus of extensive research for the treatment of prostate cancer patients, and in the past years several pharmacological treatments have been developed to block directly the effects of the androgen receptor on the target cells or to suppress the hypothalamus-pituitary-gonadal axis that systemically promotes the release of androgens from the gonads. Several of those therapeutic treatments have been investigated for their potential to reversibly inhibit sex steroids and promote thymic regrowth and immune reconstitution in mice and humans. Clinical studies using LHRH-agonists, which desensitize the LHRH-receptor and ultimately lead to the inhibition of luteinizing hormone (LH) and follicle stimulating hormone (FSH) release, showed that the treatment promoted accelerated engraftment post-HSCT and enhanced T cell reconstitution in autologous and allogeneic HSCT recipients (165). As a functional read out of the impact of the LHRH-agonist treatment on the thymic recovery, it was shown that the peripheral T cell repertoire was more diverse in patients receiving the agonist therapy (165).
Although the effects of SSI on thymic regrowth have been known for more than one century (167, 168), the exact mechanisms underlying these regenerative effects are still not completely understood, although recent studies have demonstrated that SSI can directly promote the expression of CCL25 (35) and the Notch ligand DLL4 (36), as well as promoting the function of hematopoietic stem and progenitor cells (37, 38, 169). Further work is needed to understand these mechanisms, as transient ablation of sex steroids clearly represents a feasible and appealing immune regenerative strategy. Currently, two clinical trials are on going to test the effects of the LHRH-Agonist (Lupron) alone or in combination with KGF (Palifermin) in promoting immune recovery of allo-HSCT patients (NCT01746849 and NCT01338987).
Precursor T cells
Generation of large numbers of precursor T (pre-T) cells, readily available to be infused at the time of HSCT, represents an interesting therapeutic approach to accelerate immune recovery in immunocompromised patients. The development of newly generated thymic-derived T cells can take several months after HSCT and several conditions, such as aging and GVHD, can delay this process even further (2). With the advent of the robust OP9-DL1 system for generating precursor T cells ex vivo (170), the concept of using these ex-vivo generated pre-T cells to expand and mature in the thymus thereby shortening the duration of immunodeficiency has been a promising one. Previous work has shown that pre-T cells can be generated using ex vivo co-culture of HSCs with ectopically transduced OP9-DLL1 or DLL4, two critical factors for thymocyte proliferation and commitment to T cell fate (171). Importantly, similar results were also obtained using a cell-free culture condition where recombinant DLL1 or DLL4 were immobilized on the culture dishes (172, 173).
The adoptive transfer of pre-T cells with T cell depleted BM or purified LSK into lethally irradiated recipients increases thymic cellularity, improves T cell chimerism and enhances peripheral T and NK reconstitution (174). While clearly pre-T cells help T cell reconstitution by providing a ready source of T cell progenitors, there is a also a recently reported secondary effect whereby pre-T cells, through the process of thymic crosstalk, can actually enhance thymic stromal function long after the ex vivo pre-T have transited through the thymus (175). Functionally, pre-T cell administration after HSCT can enhance resistance to L. monocytogenes and promote increased immune clearance to the A20 tumor cell line (174). Importantly, pre-T cells represent an “off the shelf” therapeutic strategy, which can be administered across MHC barriers, since the immature cells will be educated in the thymus of the recipients, and can also be used as vehicles for chimeric antigen receptors (176). In addition, pre-T cells can be also genetically engineered to recognize virus-specific as well as tumor-specific antigens for tumor immunotherapy.
Thymus bioengineering
In addition to the identification of strategies that can boost the recovery of residual thymic functionality, substantial progress has also been made over the past years to the engineering of a transplantable artificial thymus. In addition to representing a clinically relevant alternative for patients with minimal residual functionality (for example as a result of aging or repeated cycles of immunosuppressive treatments), thymus transplantation represents one of the few therapeutic options for patients with particular congenital immune deficiencies that result in complete athymia (such as Digeorge syndrome) (177–179). There is thus a significant clinical need for the development of ex vivo generated thymic tissue, rather than solely relying on endogenous thymus rejuvenation.
Immense effort has been invested in the past decades in order to characterize and rebuild in vitro the complex 3D structure that confers the thymus its specialized microenvironment. A particularly important area of investigation is the identification of biomaterials that can reproduce the 3D artificial matrix able to support cell-to-cell interactions. However, while the proof of concept has been demonstrated that 3D matrices seeded with thymic stromal cells can partially support T cell development from precursor hematopoietic cells (180, 181), the field has been limited by a lack of a sustaining source of epithelial cells to seed. However, recent studies have used several approaches that could overcome this barrier, including 1) identifying endogenous thymic epithelial progenitor cells (TEPC), 2) driving differentiation of embryonic stem cells or iPS cells into TEPC, and 3) transdifferentiation of other cell lineages into TEC-like, T cell supporting cells.
Although TEPC were identified in the developing thymus almost 15 years ago (182, 183), and the presence of an adult progenitor did indeed exist (184), it is only in the past two years that significant progress has been made into identifying the postnatal TEPC (185,186). Recent work has also demonstrated methods that could be used to drive primitive stem cells into thymic epithelial progenitors cells by precise regulation of crucial factors involved in TEC commitment and maturation, including FOXN1, HOXA3, TGFβ, BMP4, Wnt, Shh, and FGF signaling (187–190), which could be a promising source of cells for a bioengineered thymus. Finally, an innovative alternate approach was taken to generate TECs by direct reprogramming of mouse embryonic fibroblasts (MEFs) through the forced expression of FOXN1 (191). These FOXN1-induced TECs (iTECs) promote T cell development in vitro and in vivo when transplanted into nude mice and could be used to seed an artificial bioengineered thymus.
Another technique that may hold potential to generate organs in vitro is based on tissue decellularization methods. In this technique, all cells are removed from the organ, leaving the extracellular matrix intact. It has been reported that decellularization of the thymus, followed by reconstitution with thymic stromal cells and lineage negative BM progenitors, led to formation a functional thymus when transplanted into the kidney capsule of nude mice (192).
Conclusions and future directions
The thymus is an outlier, which behaves out of keeping with many other organs. Extremely sensitive to acute damage, it can rapidly involute, but it initially has immense ability to recover from such insults. However, with age, this ability to regenerate is lost, and in fact aging of the thymus proceeds at a faster rate than other tissues. A greater understanding of these processes, in close collaboration with other branches of regenerative medicine and immunology, will pave the way to new therapeutic options. Indeed, such work has already led to the establishment of clinical trials examining cytokines, growth factors and hormonal therapy (Table 1). The challenge for the future is to continue development of new strategies, and to ensure that those with potential undergo rigorous evaluation in the clinical environment.
Table 1.
| Strategy | Regenerative Targets | Stage of Development | References |
|---|---|---|---|
| IL-7 | BM HSPCs Thymocytes |
In trials | (136, 138, 141, 193–197) |
| IL-12 | Thymocytes | Pre-clinical | (198, 199) |
| IL-21 | Thymocytes | Pre-clinical | (200) |
| IL-22 | TECs | In trials | (19) |
| Flt3L | BM HSPCs Thymocytes |
Pre-clinical | (201–205) |
| IGF-1 | TECs | Pre-clinical | (158, 206) |
| GH/Ghrelin | Thymocytes | In trials | (207, 208) |
| KGF | TECs | In trials | (68, 69, 126–129) |
| SCF | Thymocytes | Pre-clinical | (209) |
| Sex steroid inhibition | TECs BM HSPCs Thymocytes |
In trials | (6, 36–38, 76, 164–166, 210) |
| Precursor T | Thymocytes | IND pending | (174, 176, 211) |
| HSPCs | Thymocytes | Pre-clinical | (212) |
| ex-vivo TECs | TECs Thymocytes |
Pre-clinical | (187, 190, 191, 213, 214) |
| Thymus bioengineering | Mature T cells | Pre-clinical | (192, 215) |
Acknowledgments
This research was supported by National Institutes of Health award numbers R00-CA176376 (J.A. Dudakov), R01-HL069929 (M.R.M. van den Brink), R01-AI080455 (M.R.M. van den Brink), R01-AI101406 (M.R.M. van den Brink), P30 CA008748 (Thompson), Project 4 of P01-CA023766 (M.R.M. van den Brink), R01-HL124112 (Jenq), and R01HL123340-01A1 (Cadwell). Support was also received from The Lymphoma Foundation, The Susan and Peter Solomon Divisional Genomics Program, and MSKCC Cycle for Survival. This project has received funding from the European Union’s Seventh Programme for research, technological development and demonstration under grant agreement No [602587]. J.A. Dudakov was supported by a CJ Martin fellowship from the Australian National Health and Medical Research Council, a Scholar Award from the American Society of Hematology, and the Mechtild Harf Award from the DKMS Foundation for Giving Life. M.S. Chaudhry was supported by a Lady Tata International Fellowship.
Footnotes
Conflict of interest: The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review. Patent applications have been filed on the therapeutic use of IL-22 (US 61/487,517; US 61/901,151) with J.A.D, A.M.H, and M.R.M vdB listed as inventors.
References
- 1.Hakim FT, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005;115:930–939. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol. 2012;19:324–335. doi: 10.1097/MOH.0b013e328353bc7d. [DOI] [PubMed] [Google Scholar]
- 3.Wils E-J, et al. Insufficient recovery of thymopoiesis predicts for opportunistic infections in allogeneic hematopoietic stem cell transplant recipients. Haematologica. 2011;96:1846–1854. doi: 10.3324/haematol.2011.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Oncologist. 1999;4:370–378. [PubMed] [Google Scholar]
- 5.Mackall CL, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 6.Heng TSP, Goldberg GL, Gray DHD, Sutherland JS, Chidgey AP, Boyd RL. Effects of castration on thymocyte development in two different models of thymic involution. J Immunol. 2005;175:2982–2993. doi: 10.4049/jimmunol.175.5.2982. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg GL, et al. Sex steroid ablation enhances immune reconstitution following cytotoxic antineoplastic therapy in young mice. The Journal of Immunology. 2010;184:6014–6024. doi: 10.4049/jimmunol.0802445. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher AL, et al. Ablation and regeneration of tolerance-inducing medullary thymic epithelial cells after cyclosporine, cyclophosphamide, and dexamethasone treatment. The Journal of Immunology. 2009;183:823–831. doi: 10.4049/jimmunol.0900225. [DOI] [PubMed] [Google Scholar]
- 9.Gray DHD, et al. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 2006;108:3777–3785. doi: 10.1182/blood-2006-02-004531. [DOI] [PubMed] [Google Scholar]
- 10.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. Journal of Experimental Medicine. 2007;204:2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of theT cell repertoire: what thymocytes see(and don’t see) Nature Reviews Immunology. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackall CL, et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood. 1997;89:3700–3707. [PubMed] [Google Scholar]
- 13.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 14.Storek J, Witherspoon RP, Storb R. T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant. 1995;16:413–425. [PubMed] [Google Scholar]
- 15.Weinberg K, Annett G, Kashyap A, Lenarsky C, Forman SJ, Parkman R. The effect of thymic function on immunocompetence following bone marrow transplantation. Biology of Blood and Marrow Transplantation. 1995;1:18–23. [PubMed] [Google Scholar]
- 16.Williams KM, Mella H, Lucas PJ, Williams JA, Telford W, Gress RE. Single Cell Analysis of Complex Thymus Stromal Cell Populations: Rapid Thymic Epithelia Preparation Characterizes Radiation Injury. Clinical and Translational Science. 2009;2:279–285. doi: 10.1111/j.1752-8062.2009.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gentil Dit Maurin A, Lemercier C, Collin-Faure V, Marche PN, Jouvin-Marche E, Candéias SM. Developmental regulation of p53-dependent radiation-induced thymocyte apoptosis in mice. Clin Exp Immunol. 2015;179:30–38. doi: 10.1111/cei.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sano S, et al. Stat3 in thymic epithelial cells is essential for postnatal maintenance of thymic architecture and thymocyte survival. Immunity. 2001;15:261–273. doi: 10.1016/s1074-7613(01)00180-7. [DOI] [PubMed] [Google Scholar]
- 19.Dudakov JA, et al. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 2012;336:91–95. doi: 10.1126/science.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang SL, et al. Chemokine treatment rescues profound T-lineage progenitor homing defect after bone marrow transplant conditioning in mice. Blood. 2014;124:296–304. doi: 10.1182/blood-2014-01-552794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976;84:304–315. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]
- 22.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 23.Purton JF, et al. Expression of the glucocorticoid receptor from the 1A promoter correlates with T lymphocyte sensitivity to glucocorticoid-induced cell death. J Immunol. 2004;173:3816–3824. doi: 10.4049/jimmunol.173.6.3816. [DOI] [PubMed] [Google Scholar]
- 24.Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function*. Annu Rev Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 25.Dooley J, Liston A. Molecular control over thymic involution: from cytokines and microRNA to aging and adipose tissue. Eur J Immunol. 2012;42:1073–1079. doi: 10.1002/eji.201142305. [DOI] [PubMed] [Google Scholar]
- 26.Vacchio MS, Papadopoulos V, Ashwell JD. Steroid production in the thymus: implications for thymocyte selection. J Exp Med. 1994;179:1835–1846. doi: 10.1084/jem.179.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittelstadt PR, Monteiro JP, Ashwell JD. Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J Clin Invest. 2012;122:2384–2394. doi: 10.1172/JCI63067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vacchio MS, Lee JY, Ashwell JD. Thymus-derived glucocorticoids set the thresholds for thymocyte selection by inhibiting TCR-mediated thymocyte activation. J Immunol. 1999;163:1327–1333. [PubMed] [Google Scholar]
- 29.Steinmann GG, Klaus B, Müller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985;22:563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 30.Dixit VD, Sridaran R, Edmonsond MA, Taub D, Thompson WE. Gonadotropin-releasing hormone attenuates pregnancy-associated thymic involution and modulates the expression of antiproliferative gene product prohibitin. Endocrinology. 2003;144:1496–1505. doi: 10.1210/en.2002-220955. [DOI] [PubMed] [Google Scholar]
- 31.Patiño JAG, Marino MW, Ivanov VN, Nikolich-Zugich J. Sex steroids induce apoptosis of CD8+CD4+ double-positive thymocytes via TNF-alpha. Eur J Immunol. 2000;30:2586–2592. doi: 10.1002/1521-4141(200009)30:9<2586::AID-IMMU2586>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 32.Olsen NJ, Watson MB, Henderson GS, Kovacs WJ. Androgen deprivation induces phenotypic and functional changes in the thymus of adult male mice. Endocrinology. 1991;129:2471–2476. doi: 10.1210/endo-129-5-2471. [DOI] [PubMed] [Google Scholar]
- 33.Lai KP, et al. Targeting thymic epithelia AR enhances T-cell reconstitution and bone marrow transplant grafting efficacy. Mol Endocrinol. 2013;27:25–37. doi: 10.1210/me.2012-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen NJ, Olson G, Viselli SM, Gu X, Kovacs WJ. Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology. 2001;142:1278–1283. doi: 10.1210/endo.142.3.8032. [DOI] [PubMed] [Google Scholar]
- 35.Williams KM, et al. CCL25 increases thymopoiesis after androgen withdrawal. Blood. 2008;112:3255–3263. doi: 10.1182/blood-2008-04-153627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Velardi E, et al. Sex steroid blockade enhances thymopoiesis by modulating Notch signaling. Journal of Experimental Medicine. 2014 doi: 10.1084/jem.20131289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dudakov JA, Goldberg GL, Reiseger JJ, Chidgey AP, Boyd RL. Withdrawal of sex steroids reverses age- and chemotherapy-related defects in bone marrow lymphopoiesis. The Journal of Immunology. 2009;182:6247–6260. doi: 10.4049/jimmunol.0802446. [DOI] [PubMed] [Google Scholar]
- 38.Dudakov JA, Goldberg GL, Reiseger JJ, Vlahos K, Chidgey AP, Boyd RL. Sex steroid ablation enhances hematopoietic recovery following cytotoxic antineoplastic therapy in aged mice. The Journal of Immunology. 2009;183:7084–7094. doi: 10.4049/jimmunol.0900196. [DOI] [PubMed] [Google Scholar]
- 39.Raviola E, Karnovsky MJ. Evidence for a blood-thymus barrier using electron-opaque tracers. J Exp Med. 1972;136:466–498. doi: 10.1084/jem.136.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savino W. The thymus is a common target organ in infectious diseases. PLoS Pathog. 2006;2:e62. doi: 10.1371/journal.ppat.0020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nunes-Alves C, Nobrega C, Behar SM, Correia-Neves M. Tolerance has its limits: how the thymus copes with infection. Trends in Immunology. 2013;34:502–510. doi: 10.1016/j.it.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baroni CD, De Franceschi GS, Uccini S, Adorini L, Cnen GD, Ruco L. Biological effects of Escherichia coli lipopolysaccharide (LPS) in vivo. I Selection in the mouse thymus of killer and helper cells. Immunology. 1976;31:217–224. [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuji T, Asano Y, Handa T, Honma Y, Ichinose Y, Yokochi T. Induction of apoptosis in lymphoid tissues of mice after intramuscular injection of enterotoxigenic Escherichia coli enterotoxin. Immunobiology. 2000;201:377–390. doi: 10.1016/s0171-2985(00)80092-3. [DOI] [PubMed] [Google Scholar]
- 44.Hick RW, Gruver AL, Ventevogel MS, Haynes BF, Sempowski GD. Leptin selectively augments thymopoiesis in leptin deficiency and lipopolysaccharide-induced thymic atrophy. J Immunol. 2006;177:169–176. doi: 10.4049/jimmunol.177.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gruver AL, Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. J Leukoc Biol. 2008;84:915–923. doi: 10.1189/jlb.0108025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen W, Kuolee R, Austin JW, Shen H, Che Y, Conlan JW. Low dose aerosol infection of mice with virulent type A Francisella tularensis induces severe thymus atrophy and CD4+CD8+ thymocyte depletion. Microb Pathog. 2005;39:189–196. doi: 10.1016/j.micpath.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pérez AR, et al. Thymus atrophy during Trypanosoma cruzi infection is caused by an immuno-endocrine imbalance. Brain Behav Immun. 2007;21:890–900. doi: 10.1016/j.bbi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Deobagkar-Lele M, Chacko SK, Victor ES, Kadthur JC, Nandi D. Interferon-γ- and glucocorticoid-mediated pathways synergize to enhance death of CD4(+) CD8(+) thymocytes during Salmonella enterica serovar Typhimurium infection. Immunology. 2013;138:307–321. doi: 10.1111/imm.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su L, et al. HIV-1-induced thymocyte depletion is associated with indirect cytopathogenicity and infection of progenitor cells in vivo. Immunity. 1995;2:25–36. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 50.Fang RHT, Colantonio AD, Uittenbogaart CH. The role of the thymus in HIV infection: a 10 year perspective. AIDS. 2008;22:171–184. doi: 10.1097/QAD.0b013e3282f2589b. [DOI] [PubMed] [Google Scholar]
- 51.Stanley SK, et al. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmitt N, et al. Differential susceptibility of human thymic dendritic cell subsets to X4 and R5 HIV-1 infection. AIDS. 2006;20:533–542. doi: 10.1097/01.aids.0000210607.63138.bc. [DOI] [PubMed] [Google Scholar]
- 53.Rozmyslowicz T, Murphy SL, Conover DO, Gaulton GN. HIV-1 infection inhibits cytokine production in human thymic macrophages. Exp Hematol. 2010;38:1157–1166. doi: 10.1016/j.exphem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mocarski ES, Bonyhadi M, Salimi S, McCune JM, Kaneshima H. Human cytomegalovirus in a SCID-hu mouse: thymic epithelial cells are prominent targets of viral replication. Proc Natl Acad Sci US A. 1993;90:104–108. doi: 10.1073/pnas.90.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendes-da-Cruz DA, Silva JS, Cotta-de-Almeida V, Savino W. Altered thymocyte migration during experimental acute Trypanosoma cruzi infection: combined role of fibronectin and the chemokines CXCL12 and CCL4. Eur J Immunol. 2006;36:1486–1493. doi: 10.1002/eji.200535629. [DOI] [PubMed] [Google Scholar]
- 56.Nobrega C, et al. Dissemination of mycobacteria to the thymus renders newly generated T cells tolerant to the invading pathogen. The Journal of Immunology. 2010;184:351–358. doi: 10.4049/jimmunol.0902152. [DOI] [PubMed] [Google Scholar]
- 57.Ferrara JLM, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nature Reviews Immunology. 2012;12:443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krenger W, Rossi S, Hollander GA. Apoptosis of thymocytes during acute graft-versus-host disease is independent of glucocorticoids. Transplantation. 2000;69:2190–2193. doi: 10.1097/00007890-200005270-00040. [DOI] [PubMed] [Google Scholar]
- 60.Hauri-Hohl MM, et al. Donor T-cell alloreactivity against host thymic epithelium limits T-cell development after bone marrow transplantation. Blood. 2007;109:4080–4088. doi: 10.1182/blood-2006-07-034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krenger W, Holländer GA. The immunopathology of thymic GVHD. Semin Immunopathol. 2008;30:439–456. doi: 10.1007/s00281-008-0131-6. [DOI] [PubMed] [Google Scholar]
- 62.Na I-K, et al. The cytolytic molecules Fas ligand and TRAIL are required for murine thymic graft-versus-host disease. J Clin Invest. 2010;120:343–356. doi: 10.1172/JCI39395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seddik M, Seemayer TA, Lapp WS. T cell functional defect associated with thymic epithelial cell injury induced by a graft-versus-host reaction. Transplantation. 1980;29:61–66. doi: 10.1097/00007890-198001000-00013. [DOI] [PubMed] [Google Scholar]
- 64.Lapp WS, Ghayur T, Mendes M, Seddik M, Seemayer TA. The functional and histological basis for graft-versus-host-induced immunosuppression. Immunol Rev. 1985;88:107–133. doi: 10.1111/j.1600-065x.1985.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 65.Przybylski GK, Kreuzer K-A, Siegert W, Schmidt CA. No recovery of T-cell receptor excision circles (TRECs) after non-myeloablative allogeneic hematopoietic stem cell transplantation is correlated with the onset of GvHD. J Appl Genet. 2007;48:397–404. doi: 10.1007/BF03195239. [DOI] [PubMed] [Google Scholar]
- 66.Wu T, et al. Thymic damage, impaired negative selection, and development of chronic graft-versus-host disease caused by donor CD4+ and CD8+ T cells. The Journal of Immunology. 2013;191:488–499. doi: 10.4049/jimmunol.1300657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dertschnig S, Hauri-Hohl MM, Vollmer M, Holländer GA, Krenger W. Impaired thymic expression of tissue-restricted antigens licenses the de novo generation of autoreactive CD4+ T cells in acute GVHD. Blood. 2015;125:2720–2723. doi: 10.1182/blood-2014-08-597245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alpdogan O, et al. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood. 2006;107:2453–2460. doi: 10.1182/blood-2005-07-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Erickson M, et al. Regulation of thymic epithelium by keratinocyte growth factor. Blood. 2002;100:3269–3278. doi: 10.1182/blood-2002-04-1036. [DOI] [PubMed] [Google Scholar]
- 70.Dooley J, Erickson M, Larochelle WJ, Gillard GO, Farr AG. FGFR2IIIb signaling regulates thymic epithelial differentiation. Dev Dyn. 2007;236:3459–3471. doi: 10.1002/dvdy.21364. [DOI] [PubMed] [Google Scholar]
- 71.Pan B, et al. Acute ablation of DP thymocytes induces up-regulation of IL-22 and Foxn1 in TECs. Clin Immunol. 2014;150:101–108. doi: 10.1016/j.clim.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Chen L, Xiao S, Manley NR. Foxn1 is required to maintain the postnatal thymic microenvironment in a dosage-sensitive manner. Blood. 2009;113:567–574. doi: 10.1182/blood-2008-05-156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rode I, Martins VC, Küblbeck G, Maltry N, Tessmer C, Rodewald H-R. Foxn1 Protein Expression in the Developing, Aging, and Regenerating Thymus. The Journal of Immunology. 2015;195:5678–5687. doi: 10.4049/jimmunol.1502010. [DOI] [PubMed] [Google Scholar]
- 74.Bredenkamp N, Nowell CS, Blackburn CC. Regeneration of the aged thymus by a single transcription factor. Development. 2014;141:1627–1637. doi: 10.1242/dev.103614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zook EC, et al. Overexpression of Foxn1 attenuates age-associated thymic involution and prevents the expansion of peripheral CD4 memory T cells. Blood. 2011;118:5723–5731. doi: 10.1182/blood-2011-03-342097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldberg GL, et al. Enhanced immune reconstitution by sex steroid ablation following allogeneic hemopoietic stem cell transplantation. J Immunol. 2007;178:7473–7484. doi: 10.4049/jimmunol.178.11.7473. [DOI] [PubMed] [Google Scholar]
- 77.Park H-J, et al. Up-regulation of VEGF expression by NGF that enhances reparative angiogenesis during thymic regeneration in adult rat. Biochim Biophys Acta. 2007;1773:1462–1472. doi: 10.1016/j.bbamcr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 78.Cuddihy AR, et al. Rapid thymic reconstitution following bone marrow transplantation in neonatal mice is VEGF-dependent. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2012;18:683–689. doi: 10.1016/j.bbmt.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nature Publishing Group. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nature Immunology. 2013;14:428–436. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gavazzi G, Krause K-H. Ageing and infection. Lancet Infect Dis. 2002;2:659–666. doi: 10.1016/s1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- 83.Pawelec G, Derhovanessian E, Larbi A. Immunosenescence and cancer. Crit Rev Oncol Hematol. 2010;75:165–172. doi: 10.1016/j.critrevonc.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 84.Montoya-Ortiz G. Immunosenescence, aging, and systemic lupus erythematous. Autoimmune Dis. 2013;2013:267078. doi: 10.1155/2013/267078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lynch HE, Goldberg GL, Chidgey A, van den Brink MRM, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends in Immunology. 2009;30:366–373. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Surh CD, Sprent J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J Exp Med. 2000;192:F9–F14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murray JM, et al. Naive T cells are maintained by thymic output in early ages but by proliferation without phenotypic change after age twenty. Immunol Cell Biol. 2003;81:487–495. doi: 10.1046/j.1440-1711.2003.01191.x. [DOI] [PubMed] [Google Scholar]
- 88.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nature Reviews Immunology. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 89.Mo R, et al. T cell chemokine receptor expression in aging. J Immunol. 2003;170:895–904. doi: 10.4049/jimmunol.170.2.895. [DOI] [PubMed] [Google Scholar]
- 90.Haynes L, Swain SL. Why aging T cells fail: implications for vaccination. Immunity. 2006;24:663–666. doi: 10.1016/j.immuni.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006;5:136–139. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 92.Coder BD, Wang H, Ruan L, Su D-M. Thymic involution perturbs negative selection leading to autoreactive T cells that induce chronic inflammation. The Journal of Immunology. 2015;194:5825–5837. doi: 10.4049/jimmunol.1500082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bagnara GP, et al. Hemopoiesis in healthy old people and centenarians: well-maintained responsiveness of CD34+ cells to hemopoietic growth factors and remodeling of cytokine network. J Gerontol A Biol Sci Med Sci. 2000;55:B61–6. doi: 10.1093/gerona/55.2.b61. discussion B67–70. [DOI] [PubMed] [Google Scholar]
- 94.Douek DC, Koup RA. Evidence for thymic function in the elderly. Vaccine. 2000;18:1638–1641. doi: 10.1016/s0264-410x(99)00499-5. [DOI] [PubMed] [Google Scholar]
- 95.Steinmann GG, Müller-Hermelink HK. Immunohistological demonstration of terminal transferase (TdT) in the age-involuted human thymus. Immunobiology. 1984;166:45–52. doi: 10.1016/S0171-2985(84)80142-4. [DOI] [PubMed] [Google Scholar]
- 96.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nature Reviews Immunology. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 97.Zhang SL, Bhandoola A. Trafficking to the thymus. Curr Top Microbiol Immunol. 2014;373:87–111. doi: 10.1007/82_2013_324. [DOI] [PubMed] [Google Scholar]
- 98.Beerman I, Maloney WJ, Weissmann IL, Rossi DJ. Stem cells and the aging hematopoietic system. Curr Opin Immunol. 2010;22:500–506. doi: 10.1016/j.coi.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nature Reviews Immunology. 2013;13:376–389. doi: 10.1038/nri3433. [DOI] [PubMed] [Google Scholar]
- 100.Gui J, Zhu X, Dohkan J, Cheng L, Barnes PF, Su D-M. The aged thymus shows normal recruitment of lymphohematopoietic progenitors but has defects in thymic epithelial cells. Int Immunol. 2007;19:1201–1211. doi: 10.1093/intimm/dxm095. [DOI] [PubMed] [Google Scholar]
- 101.Min H, Montecino-Rodriguez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J Immunol. 2004;173:245–250. doi: 10.4049/jimmunol.173.1.245. [DOI] [PubMed] [Google Scholar]
- 102.Thoman ML. The pattern of T lymphocyte differentiation is altered during thymic involution. Mech Ageing Dev. 1995;82:155–170. doi: 10.1016/0047-6374(95)01597-s. [DOI] [PubMed] [Google Scholar]
- 103.Aspinall R. Age-associated thymic atrophy in the mouse is due to a deficiency affecting rearrangement of the TCR during intrathymic T cell development. J Immunol. 1997;158:3037–3045. [PubMed] [Google Scholar]
- 104.Aw D, Silva AB, Palmer DB. Is thymocyte development functional in the aged? Aging (Albany NY) 2009;1:146–153. doi: 10.18632/aging.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sykes M. Unusual T cell populations in adult murine bone marrow. Prevalence of CD3+CD4−CD8− and alpha beta TCR+NK1.1+ cells. J Immunol. 1990;145:3209–3215. [PubMed] [Google Scholar]
- 106.Zhu X, Gui J, Dohkan J, Cheng L, Barnes PF, Su D-M. Lymphohematopoietic progenitors do not have a synchronized defect with age-related thymic involution. Aging Cell. 2007;6:663–672. doi: 10.1111/j.1474-9726.2007.00325.x. [DOI] [PubMed] [Google Scholar]
- 107.Aw D, Silva AB, Maddick M, Zglinicki von T, Palmer DB. Architectural changes in the thymus of aging mice. Aging Cell. 2008;7:158–167. doi: 10.1111/j.1474-9726.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 108.Yang H, et al. Axin expression in thymic stromal cells contributes to an age-related increase in thymic adiposity and is associated with reduced thymopoiesis independently of ghrelin signaling. J Leukoc Biol. 2009;85:928–938. doi: 10.1189/jlb.1008621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dixit VD. Thymic fatness and approaches to enhance thymopoietic fitness in aging. Curr Opin Immunol. 2010;22:521–528. doi: 10.1016/j.coi.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ortman CL, Dittmar KA, Witte PL, Le PT. Molecular characterization of the mouse involuted thymus: aberrations in expression of transcription regulators in thymocyte and epithelial compartments. Int Immunol. 2002;14:813–822. doi: 10.1093/intimm/dxf042. [DOI] [PubMed] [Google Scholar]
- 111.Aspinall R, Andrew D. Thymic atrophy in the mouse is a soluble problem of the thymic environment. Vaccine. 2000;18:1629–1637. doi: 10.1016/s0264-410x(99)00498-3. [DOI] [PubMed] [Google Scholar]
- 112.Palmer DB. The effect of age on thymic function. 2013:1–6. doi: 10.3389/fimmu.2013.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.George AJ, Ritter MA. Thymic involution with ageing: obsolescence or good housekeeping? Immunol Today. 1996;17:267–272. doi: 10.1016/0167-5699(96)80543-3. [DOI] [PubMed] [Google Scholar]
- 114.Gui J, Mustachio LM, Su D-M, Craig RW. Thymus Size and Age-related Thymic Involution: Early Programming, Sexual Dimorphism, Progenitors and Stroma. Aging Dis. 2012;3:280–290. [PMC free article] [PubMed] [Google Scholar]
- 115.Hsu H-C, et al. Age-related thymic involution in C57BL/6J x DBA/2J recombinant-inbred mice maps to mouse chromosomes 9 and 10. Genes Immun. 2003;4:402–410. doi: 10.1038/sj.gene.6363982. [DOI] [PubMed] [Google Scholar]
- 116.Griffith AV, Fallahi M, Venables T, Petrie HT. Persistent degenerative changes in thymic organ function revealed by an inducible model of organ regrowth. Aging Cell. 2012;11:169–177. doi: 10.1111/j.1474-9726.2011.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang H, Youm Y-H, Dixit VD. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. The Journal of Immunology. 2009;183:3040–3052. doi: 10.4049/jimmunol.0900562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vallejo AN, Michel JJ, Bale LK, Lemster BH, Borghesi L, Conover CA. Resistance to age-dependent thymic atrophy in long-lived mice that are deficient in pregnancy-associated plasma protein A. Proc Natl Acad Sci US A. 2009;106:11252–11257. doi: 10.1073/pnas.0807025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Griffith AV, et al. Metabolic Damage and Premature Thymus Aging Caused by Stromal Catalase Deficiency. Cell Reports. 2015;12:1071–1079. doi: 10.1016/j.celrep.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martins VC, et al. Thymus-autonomous T cell development in the absence of progenitor import. Journal of Experimental Medicine. 2012;209:1409–1417. doi: 10.1084/jem.20120846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boehm T, Swann JB. Thymus involution and regeneration: two sides of the same coin? Nature Reviews Immunology. 2015:1–8. doi: 10.1038/nri3534. [DOI] [PubMed] [Google Scholar]
- 122.Finch PW, Rubin JS. Keratinocyte growth factor/fibroblast growth factor 7, a homeostatic factor with therapeutic potential for epithelial protection and repair. Advances in cancer research. 2004;91:69–136. doi: 10.1016/S0065-230X(04)91003-2. [DOI] [PubMed] [Google Scholar]
- 123.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ornitz DM, et al. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- 125.Alvarez I, et al. Central T cell tolerance: Identification of tissue-restricted autoantigens in the thymus HLA-DR peptidome. Journal of Autoimmunity. 2015;60:12–19. doi: 10.1016/j.jaut.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 126.Rossi S, et al. Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease. Blood. 2002;100:682–691. doi: 10.1182/blood.v100.2.682. [DOI] [PubMed] [Google Scholar]
- 127.Min D, et al. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood. 2002;99:4592–4600. doi: 10.1182/blood.v99.12.4592. [DOI] [PubMed] [Google Scholar]
- 128.Rossi SW, et al. Keratinocyte growth factor (KGF) enhances postnatal T-cell development via enhancements in proliferation and function of thymic epithelial cells. Blood. 2007;109:3803–3811. doi: 10.1182/blood-2006-10-049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wils EJ, et al. Keratinocyte growth factor and stem cell factor to improve thymopoiesis after autologous CD34+ cell transplantation in rhesus macaques. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2012;18:55–65. doi: 10.1016/j.bbmt.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 130.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nature Reviews Immunology. 2011;11:330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Crawley AM, et al. Jak/STAT and PI3K signaling pathways have both common and distinct roles in IL-7-mediated activities in human CD8+ T cells. J Leukoc Biol. 2014;95:117–127. doi: 10.1189/jlb.0313122. [DOI] [PubMed] [Google Scholar]
- 132.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 133.Noguchi M, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. [PubMed] [Google Scholar]; The Journal of Immunology. 2008;181:5817–5827. [PubMed] [Google Scholar]
- 134.Alpdogan O, van den Brink M. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends in Immunology. 2005 doi: 10.1016/j.it.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 135.van Lent AU, et al. IL-7 enhances thymic human T cell development in “human immune system” Rag2-/-IL-2Rgammac-/- mice without affecting peripheral T cell homeostasis. J Immunol. 2009;183:7645–7655. doi: 10.4049/jimmunol.0902019. [DOI] [PubMed] [Google Scholar]
- 136.Alpdogan O, et al. Administration of interleukin-7 after allogeneic bone marrow transplantation improves immune reconstitution without aggravating graft-versus-host disease. Blood. 2001;98:2256–2265. doi: 10.1182/blood.v98.7.2256. [DOI] [PubMed] [Google Scholar]
- 137.Abdul-Hai A, et al. Stimulation of immune reconstitution by interleukin-7 after syngeneic bone marrow transplantation in mice. Exp Hematol. 1996;24:1416–1422. [PubMed] [Google Scholar]
- 138.Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood. 2001;97:1491–1497. doi: 10.1182/blood.v97.5.1491. [DOI] [PubMed] [Google Scholar]
- 139.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Colombetti S, Levy F, Chapatte L. IL-7 adjuvant treatment enhances long-term tumor-antigen-specific CD8+ T-cell responses after immunization with recombinant lentivector. Blood. 2009;113:6629–6637. doi: 10.1182/blood-2008-05-155309. [DOI] [PubMed] [Google Scholar]
- 141.Perales MA, et al. Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood. 2012;120:4882–4891. doi: 10.1182/blood-2012-06-437236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dudakov JA, Hanash AM, van den Brink MRM. Interleukin-22: Immunobiology and Pathology. Annu Rev Immunol. 2015;33:747–785. doi: 10.1146/annurev-immunol-032414-112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nature Immunology. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 144.Sawa S, et al. ROR[gamma]t+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nature Immunology. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 145.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takatori H, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. Journal of Experimental Medicine. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hughes T, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32:803–814. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sawa S, et al. Lineage Relationship Analysis of ROR≥t+ Innate Lymphoid Cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 149.Lindemans CA, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weigent DA. Lymphocyte GH-axis hormones in immunity. Cell Immunol. 2013 doi: 10.1016/j.cellimm.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 151.de Mello-Coelho V, et al. Growth hormone and its receptor are expressed in human thymic cells. Endocrinology. 1998;139:3837–3842. doi: 10.1210/endo.139.9.6199. [DOI] [PubMed] [Google Scholar]
- 152.Murphy WJ, Durum SK, Anver MR, Longo DL. Immunologic and hematologic effects of neuroendocrine hormones. Studies on DW/J dwarf mice. J Immunol. 1992;148:3799–3805. [PubMed] [Google Scholar]
- 153.Redelman D, Welniak LA, Taub D, Murphy WJ. Neuroendocrine hormones such as growth hormone and prolactin are integral members of the immunological cytokine network. Cell Immunol. 2008;252:111–121. doi: 10.1016/j.cellimm.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen BJ, Cui X, Sempowski GD, Chao NJ. Growth hormone accelerates immune recovery following allogeneic T-cell-depleted bone marrow transplantation in mice. Exp Hematol. 2003;31:953–958. doi: 10.1016/s0301-472x(03)00196-6. [DOI] [PubMed] [Google Scholar]
- 155.Knyszynski A, Adler-Kunin S, Globerson A. Effects of growth hormone on thymocyte development from progenitor cells in the bone marrow. Brain Behav Immun. 1992;6:327–340. doi: 10.1016/0889-1591(92)90032-j. [DOI] [PubMed] [Google Scholar]
- 156.Aiuti A, et al. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lympho-hematopoietic progenitors. Eur J Immunol. 1999;29:1823–1831. doi: 10.1002/(SICI)1521-4141(199906)29:06<1823::AID-IMMU1823>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 157.Tsuji Y, Kinoshita Y, Hato F, Tominaga K, Yoshida K. The in vitro proliferation of thymus epithelial cells stimulated with growth hormone and insulin-like growth factor-I. Cell Mol Biol (Noisy-le-grand) 1994;40:1135–1142. [PubMed] [Google Scholar]
- 158.Alpdogan O, et al. Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation. 2003;75:1977–1983. doi: 10.1097/01.TP.0000070167.81584.A2. [DOI] [PubMed] [Google Scholar]
- 159.Clark R. The somatogenic hormones and insulin-like growth factor-1: stimulators of lymphopoiesis and immune function. Endocr Rev. 1997;18:157–179. doi: 10.1210/edrv.18.2.0296. [DOI] [PubMed] [Google Scholar]
- 160.Montecino-Rodriguez E, Clark R, Dorshkind K. Effects of insulin-like growth factor administration and bone marrow transplantation on thymopoiesis in aged mice. Endocrinology. 1998;139:4120–4126. doi: 10.1210/endo.139.10.6263. [DOI] [PubMed] [Google Scholar]
- 161.Chu YW, et al. Exogenous insulin-like growth factor 1 enhances thymopoiesis predominantly through thymic epithelial cell expansion. Blood. 2008;112:2836–2846. doi: 10.1182/blood-2008-04-149435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Napolitano LA, et al. Growth hormone enhances thymic function in HIV-1-infected adults. J Clin Invest. 2008;118:1085–1098. doi: 10.1172/JCI32830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hince M, Sakkal S, Vlahos K, Dudakov J, Boyd R, Chidgey A. The role of sex steroids and gonadectomy in the control of thymic involution. Cell Immunol. 2008;252:122–138. doi: 10.1016/j.cellimm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 164.Sutherland JS, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 165.Sutherland JS, et al. Enhanced immune system regeneration in humans following allogeneic or autologous hemopoietic stem cell transplantation by temporary sex steroid blockade. Clin Cancer Res. 2008;14:1138–1149. doi: 10.1158/1078-0432.CCR-07-1784. [DOI] [PubMed] [Google Scholar]
- 166.Goldberg GL, et al. Luteinizing hormone-releasing hormone enhances T cell recovery following allogeneic bone marrow transplantation. The Journal of Immunology. 2009;182:5846–5854. doi: 10.4049/jimmunol.0801458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Henderson J. On the relationship of the thymus to the sexual organs: I. The influence of castration on the thymus. The Journal of physiology. 1904;31:222–229. doi: 10.1113/jphysiol.1904.sp001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Goodall A. The post-natal changes in the thymus of guinea-pigs, and the effect of castration on thymus structure. The Journal of physiology. 1905;32:191–198. doi: 10.1113/jphysiol.1905.sp001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Khong DM, et al. Enhanced hematopoietic stem cell function mediates immune regeneration following sex steroid blockade. Stem Cell Reports. 2015;4:445–458. doi: 10.1016/j.stemcr.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 171.La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 172.Dallas MH, Varnum-Finney B, Delaney C, Kato K, Bernstein ID. Density of the Notch ligand Delta1 determines generation of B and T cell precursors from hematopoietic stem cells. J Exp Med. 2005;201:1361–1366. doi: 10.1084/jem.20042450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mohtashami M, Shah DK, Nakase H, Kianizad K, Petrie HT, Zuniga-Pflucker JC. Direct comparison of Dll1- and Dll4-mediated Notch activation levels shows differential lymphomyeloid lineage commitment outcomes. The Journal of Immunology. 2010;185:867–876. doi: 10.4049/jimmunol.1000782. [DOI] [PubMed] [Google Scholar]
- 174.Zakrzewski JL, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 175.Awong G, et al. Human proT-cells generated in vitro facilitate hematopoietic stem cell-derived T-lymphopoiesis in vivo and restore thymic architecture. Blood. 2013;122:4210–4219. doi: 10.1182/blood-2012-12-472803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zakrzewski JL, et al. Tumor immunotherapy across MHC barriers using allogeneic T-cell precursors. Nature biotechnology. 2008;26:453–461. doi: 10.1038/nbt1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Markert ML, et al. Transplantation of thymus tissue in complete DiGeorge syndrome. N Engl J Med. 1999;341:1180–1189. doi: 10.1056/NEJM199910143411603. [DOI] [PubMed] [Google Scholar]
- 178.Markert ML, et al. Postnatal thymus transplantation with immunosuppression as treatment for DiGeorge syndrome. Blood. 2004;104:2574–2581. doi: 10.1182/blood-2003-08-2984. [DOI] [PubMed] [Google Scholar]
- 179.Markert ML, et al. Review of 54 patients with complete DiGeorge anomaly enrolled in protocols for thymus transplantation: outcome of 44 consecutive transplants. Blood. 2007;109:4539–4547. doi: 10.1182/blood-2006-10-048652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Marshall D, et al. T cell generation including positive and negative selection ex vivo in a three-dimensional matrix. J Hematother Stem Cell Res. 2003;12:565–574. doi: 10.1089/152581603322448277. [DOI] [PubMed] [Google Scholar]
- 181.Pinto S, Schmidt K, Egle S, Stark HJ, Boukamp P, Kyewski B. An organotypic coculture model supporting proliferation and differentiation of medullary thymic epithelial cells and promiscuous gene expression. The Journal of Immunology. 2013;190:1085–1093. doi: 10.4049/jimmunol.1201843. [DOI] [PubMed] [Google Scholar]
- 182.Gill J, Malin M, Hollander GA, Boyd R. Generation of a complete thymic microenvironment by MTS24(+) thymic epithelial cells. Nature Immunology. 2002;3:635–642. doi: 10.1038/ni812. [DOI] [PubMed] [Google Scholar]
- 183.Bennett AR, Farley A, Blair NF, Gordon J, Sharp L, Blackburn CC. Identification and characterization of thymic epithelial progenitor cells. Immunity. 2002;16:803–814. doi: 10.1016/s1074-7613(02)00321-7. [DOI] [PubMed] [Google Scholar]
- 184.Bleul CC, Corbeaux T, Reuter A, Fisch P, Mönting JS, Boehm T. Formation of a functional thymus initiated by a postnatal epithelial progenitor cell. Nature. 2006;441:992–996. doi: 10.1038/nature04850. [DOI] [PubMed] [Google Scholar]
- 185.Ucar A, Ucar O, Klug P, Matt S, Brunk F, Hofmann TG, Kyewski B. Adult thymus contains FoxN1(−) epithelial stem cells that are bipotent for medullary and cortical thymic epithelial lineages. Immunity. 2014;41:257–269. doi: 10.1016/j.immuni.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wong K, et al. Multilineage potential and self-renewal define an epithelial progenitor cell population in the adult thymus. CellReports. 2014;8:1198–1209. doi: 10.1016/j.celrep.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 187.Parent AV, et al. Generation of functional thymic epithelium from human embryonic stem cells that supports host T cell development. Cell Stem Cell. 2013;13:219–229. doi: 10.1016/j.stem.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sun X, et al. Directed differentiation of human embryonic stem cells into thymic epithelial progenitor-like cells reconstitutes the thymic microenvironment in vivo. Cell Stem Cell. 2013;13:230–236. doi: 10.1016/j.stem.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 189.Soh C-L, et al. FOXN1GFP/w Reporter hESCs Enable Identification of Integrin-β4, HLA-DR, and EpCAM as Markers of Human PSC-Derived FOXN1+ Thymic Epithelial Progenitors. Stem Cell Reports. 2014;2:925–937. doi: 10.1016/j.stemcr.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Su M, et al. Efficient in vitro generation of functional thymic epithelial progenitors from human embryonic stem cells. Scientific reports. 2015;5:9882. doi: 10.1038/srep09882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bredenkamp N, Ulyanchenko S, O’Neill KE, Manley NR, Vaidya HJ, Blackburn CC. An organized and functional thymus generated from FOXN1-reprogrammed fibroblasts. Nature cell biology. 2014;16:902–908. doi: 10.1038/ncb3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fan Y, et al. Bioengineering Thymus Organoids to Restore Thymic Function and Induce Donor-Specific Immune Tolerance to Allografts. Molecular therapy: the journal of the American Society of Gene Therapy. 2015;23:1262–1277. doi: 10.1038/mt.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Alpdogan O, et al. IL-7 enhances peripheral T cell reconstitution after allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2003;112:1095–1107. doi: 10.1172/JCI17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Andrew D, Aspinall R. Il-7 and not stem cell factor reverses both the increase in apoptosis and the decline in thymopoiesis seen in aged mice. J Immunol. 2001;166:1524–1530. doi: 10.4049/jimmunol.166.3.1524. [DOI] [PubMed] [Google Scholar]
- 195.Fry TJ, et al. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood. 2003;101:2294–2299. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]
- 196.Jacobs SR, Michalek RD, Rathmell JC. IL-7 is essential for homeostatic control of T cell metabolism in vivo. The Journal of Immunology. 2010;184:3461–3469. doi: 10.4049/jimmunol.0902593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Phillips JA, Brondstetter TI, English CA, Lee HE, Virts EL, Thoman ML. IL-7 gene therapy in aging restores early thymopoiesis without reversing involution. J Immunol. 2004;173:4867–4874. doi: 10.4049/jimmunol.173.8.4867. [DOI] [PubMed] [Google Scholar]
- 198.Li L, et al. IL-12 inhibits thymic involution by enhancing IL-7- and IL-2-induced thymocyte proliferation. J Immunol. 2004;172:2909–2916. doi: 10.4049/jimmunol.172.5.2909. [DOI] [PubMed] [Google Scholar]
- 199.Chen T, Burke KA, Zhan Y, Wang X, Shibata D, Zhao Y. IL-12 facilitates both the recovery of endogenous hematopoiesis and the engraftment of stem cells after ionizing radiation. Exp Hematol. 2007;35:203–213. doi: 10.1016/j.exphem.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 200.Rafei M, Dumont-Lagace M, Rouette A, Perreault C. Interleukin-21 accelerates thymic recovery from glucocorticoid-induced atrophy. PLoS ONE. 2013;8:e72801. doi: 10.1371/journal.pone.0072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ceredig R, Rauch M, Balciunaite G, Rolink AG. Increasing Flt3L availability alters composition of a novel bone marrow lymphoid progenitor compartment. Blood. 2006;108:1216–1222. doi: 10.1182/blood-2005-10-006643. [DOI] [PubMed] [Google Scholar]
- 202.Fry TJ, et al. Flt3 ligand enhances thymic-dependent and thymic-independent immune reconstitution. Blood. 2004;104:2794–2800. doi: 10.1182/blood-2003-11-3789. [DOI] [PubMed] [Google Scholar]
- 203.Kenins L, Gill JW, Boyd RL, Hollander GA, Wodnar-Filipowicz A. Intrathymic expression of Flt3 ligand enhances thymic recovery after irradiation. Journal of Experimental Medicine. 2008;205:523–531. doi: 10.1084/jem.20072065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kenins L, Gill JW, Hollander GA, Wodnar-Filipowicz A. Flt3 ligand-receptor interaction is important for maintenance of early thymic progenitor numbers in steady-state thymopoiesis. Eur J Immunol. 2010;40:81–90. doi: 10.1002/eji.200839213. [DOI] [PubMed] [Google Scholar]
- 205.Wils EJ, et al. Flt3 ligand expands lymphoid progenitors prior to recovery of thymopoiesis and accelerates T cell reconstitution after bone marrow transplantation. J Immunol. 2007;178:3551–3557. doi: 10.4049/jimmunol.178.6.3551. [DOI] [PubMed] [Google Scholar]
- 206.Chu CH, et al. IGF-II/mannose-6-phosphate receptor signaling induced cell hypertrophy and atrial natriuretic peptide/BNP expression via Galphaq interaction and protein kinase C-alpha/CaMKII activation in H9c2 cardiomyoblast cells. J Endocrinol. 2008;197:381–390. doi: 10.1677/JOE-07-0619. [DOI] [PubMed] [Google Scholar]
- 207.Carlo-Stella C, et al. Use of recombinant human growth hormone (rhGH) plus recombinant human granulocyte colony-stimulating factor (rhG-CSF) for the mobilization and collection of CD34+ cells in poor mobilizers. Blood. 2004;103:3287–3295. doi: 10.1182/blood-2003-07-2428. [DOI] [PubMed] [Google Scholar]
- 208.Dixit VD, et al. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117:2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fewkes NM, Krauss AC, Guimond M, Meadors JL, Dobre S, Mackall CL. Pharmacologic modulation of niche accessibility via tyrosine kinase inhibition enhances marrow and thymic engraftment after hematopoietic stem cell transplantation. Blood. 2010;115:4120–4129. doi: 10.1182/blood-2009-10-248898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Goldberg GL, et al. Sex steroid ablation enhances immune reconstitution following cytotoxic antineoplastic therapy in young mice. The Journal of Immunology. 2010;184:6014–6024. doi: 10.4049/jimmunol.0802445. [DOI] [PubMed] [Google Scholar]
- 211.Holland AM, Zakrzewski JL, Goldberg GL, Ghosh A, van den Brink MR. Adoptive precursor cell therapy to enhance immune reconstitution after hematopoietic stem cell transplantation in mouse and man. Semin Immunopathol. 2008;30:479–487. doi: 10.1007/s00281-008-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Arber C, et al. Common lymphoid progenitors rapidly engraft and protect against lethal murine cytomegalovirus infection after hematopoietic stem cell transplantation. Blood. 2003;102:421–428. doi: 10.1182/blood-2002-12-3834. [DOI] [PubMed] [Google Scholar]
- 213.Lai L, et al. Mouse embryonic stem cell-derived thymic epithelial cell progenitors enhance T-cell reconstitution after allogeneic bone marrow transplantation. Blood. 2011;118:3410–3418. doi: 10.1182/blood-2011-03-340794. [DOI] [PubMed] [Google Scholar]
- 214.Lai L, Jin J. Generation of thymic epithelial cell progenitors by mouse embryonic stem cells. Stem cells. 2009;27:3012–3020. doi: 10.1002/stem.238. [DOI] [PubMed] [Google Scholar]
- 215.Chung B, et al. Engineering the human thymic microenvironment to support thymopoiesis in vivo. Stem cells. 2014;32:2386–2396. doi: 10.1002/stem.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]