Abstract
Histoplasmosis is endemic to the Midwestern United States, but cases have been reported nearly worldwide. A 1970 study found 3.8% skin test sensitivity to Histoplasma capsulatum in Uganda but no systemic study of histoplasmosis exposure has occurred since the onset of the human immunodeficiency virus (HIV) pandemic. This study investigated the seroprevalence of H. capsulatum and sought previously undetected cases of histoplasmosis in Kampala, Uganda. Serum, cerebrospinal fluid (CSF) and/or urine specimens were obtained from HIV-infected persons with suspected meningitis. Specimens were tested for H. capsulatum IgG and IgM by enzyme immune assay and Histoplasma antigen. 147 of the 257 subjects who were enrolled had cryptococcal meningitis. Overall, 1.3% (2/151) of subjects were serum Histoplasma IgG positive, and zero of 151 were IgM positive. Antigen was not detected in any serum (n = 57), urine (n = 37, or CSF (n = 63) samples. Both subjects with serum Histoplasma IgG positivity had cryptococcal meningitis. Histoplasma capsulatum IgG was detected at low levels in persons with HIV/AIDS in Kampala, Uganda. Histoplasmosis is not widespread in Uganda but microfoci do exist. There appears to be no cross-reactivity between Cryptococcus neoformans and Histoplasma antigen testing, and cryptococcosis appears to be at most, a rare cause of positive Histoplasma IgG.
Keywords: Histoplasmosis, Histoplasmosis capsulatum, Diagnostic Techniques and Procedures, Validation Studies, Human Immunodeficiency Virus, seroepidemiologic studies
Introduction
Histoplasmosis commonly occurs in the Midwestern United States and areas of Canada along the St. Lawrence Seaway and Great Lakes, but is also present in much of the world, including Mexico, Latin America, Asia, Southeast Asia, and sub-Saharan Africa. 1 , 2 A distinct variety, Histoplasma capsulatum var. duboisii occurs only in sub-Saharan Africa. The understanding of global distribution of disease due to Histoplasma is incomplete. 1
Cases of histoplasmosis have been reported in Uganda, notably a recent focal outbreak was reported among a group of international biology students who traveled to a Ugandan rainforest to conduct a field study. 3 Although histoplasmosis occurs in Uganda, the overall risk is not well understood. In 1970, a study of skin sensitivity to histoplasmin, including a total of 1,144 subjects and roughly equal proportions of adults and children, was conducted in six regions of Uganda. 4 Skin test positivity to Histoplasmin was noted in 3.8% of persons (95% confidence interval (CI), 2.8–5.1%) with positivity varying by region from 0 to 12% and the highest prevalence on the Nile River near Lake Victoria. 4 In the capital, Kampala 5 of 148 (3.3%) persons tested were sensitive by skin test. 4 This study was carried out prior to the widespread recognition of human immunodeficiency virus (HIV).
Disseminated Histoplasma capsulatum infection is frequently diagnosed with urine or serum antigen detection; however, cross-reactivity with other mycoses does limit certainty to some degree. 5–7 Positive results for both Histoplasma and cryptococcal antigen occasionally are observed in clinical practice, raising the question whether the polysaccharide antigens detected in these infections are cross-reactive. In one study by Zhuang and colleagues 29 serum samples from subjects with known histoplasmosis and 25 serum samples from subjects with known cryptococcosis were tested by EIA for Histoplasma antigen (MiraVista Diagnostics, Indianapolis, IN, USA) and latex agglutination (Meridian biosciences, Cincinnati) for cryptococcal antigen. 8 Samples from persons with histoplasmosis did not cross-react with cryptococcal testing, and samples from subjects with cryptococcosis did not cross-react with testing for histoplasmosis. While skin testing has traditionally been used to measure exposure to histoplasmosis 4 , histoplasmin skin material is no longer available. As a result, immunoglobulin G (IgG) antibody testing may be a way to assess exposure. 9 The specificity of the MiraVista EIA used to detect response to histoplasmosis in this study has been shown to be 95% in patients from an endemic area with non-fungal infections and healthy subjects from non-endemic and endemic areas. 10
Further information on Histoplasma prevalence in Uganda would be useful to gauge potential risk for persons living with AIDS. 11 In this study, we quantify seropositivity for histoplasmosis among persons in Kampala Ugandan with advanced HIV/AIDS and use antigen detection to attempt to identify undiagnosed histoplasmosis. A secondary objective was to determine if cross-reaction occurred between glucoxylomannan polysacrhide detected in the cryptococcal lateral flow antigen assay (LFA) or latex agglutination assay (IMMY Inc., Norman, OK, USA) and the galactomannan detected in the MiraVista Histoplasma EIA system. 8 It would not be expected that a person with histoplasmosis would cause a false positive in cryptococcal antigen testing.
Methods
HIV-infected persons were prospectively enrolled at the Infectious Disease Institute and at Mulago National Referral Hospital in Kampala, Uganda. From May 2006 until December 2013, HIV-infected persons with CD4<200 cells/μl who had either no active opportunistic infection at time of initiating ART or cryptococcal meningitis were enrolled as described previously. 12–16 Cryptococcal meningitis was diagnosed by cerebrospinal fluid (CSF) cryptococcal antigen (IMMY Inc., Norman OK) and/or quantitative fungal culture. 13 , 14 The IMMY Inc. latex agglutination text was used prior to 2012, whereas the LFA was used after this point. CSF and urine were collected at presentation with meningitis (cryptococcal or aseptic), and longitudinal serum, CSF, and urine samples were collected and cryopreserved (−80°C). At time of serum collection, the median duration of antiretroviral therapy (ART) was 26 weeks (interquartile range [IQR], 8 to 28 weeks).
The samples were subsequently sent to MiraVista Laboratories where enzyme immunoassays (EIA) were performed for anti- H capsulatum IgG and immunoglobulin M (IgM) using serum; and Histoplasma antigen using serum, CSF, and urine. 10 , 17 The antibody EIA was presented at the American Society for Microbiology General Meeting in 2014. 10 The EIA system used microplates coated with 100 ul of proprietary MVista® Histoplasma antigen prepared from a clinical isolate of Histoplasma capulatum . Sera were diluted 1:1000. Then, 100 ul of diluted sera was added to each well and detected with biotinylated goat anti-human IgG antibody or biotinylated goat anti-human IgM (mu chain specific) antibody (Vector Laboratories; Burlingame, CA) and after additional treatments with tetramethylbenzadine and sulfuric acid were read in a microplate reader at 450 nm with a 620 nm reference filter. Results were expressed as EIA units by comparison to the standard curve. Reproducibility was investigated via duplicate testing. Both IgG and IgM used pre-established cut-offs as determined by receiver operator characteristic analysis. Established sensitivity for IgG is 87.5% with 95% specificity, while IgM provides 67.5% sensitivity and 97% specificity. Combined sensitivity is 88.8% and specificity 91.9%. 10 The basis for diagnosis in the histoplasmosis cases in establishing these values was a positive culture and/or histopathology (proven case) and detection of antigen by enzyme immunoassay or antibody by immunodiffusion and/or complement fixation. 18
Statistical analysis was performed using SPSS version 22 (IBM Corporation, Armonk, NY). Percent IgM and IgG positivity was summarized using frequency, and H. capsulatum EIA positivity was compared to known cryptococcal meningitis status to assess for cross-reactivity. In addition, Histoplasma antigen detection frequency was calculated for serum, CSF, and urine and again, assessed for cross-reactivity with Cryptococcus neoformans.
Institutional review board approvals were obtained from the University of Minnesota, Makerere University, and the Uganda National Council of Science and Technology (UNCST). All participants or their surrogates provided written informed consent.
Results
Patient characteristics
Samples were obtained from 257 HIV-infected persons living with AIDS in total. Among these 257 participants, 147 (57%) had cryptococcal meningitis, and 110 (43%) did not have an active opportunistic infection when initiating ART. CSF was available for 71 subjects, urine for 37 subjects, and serum for 151 subjects. A sufficient volume of serum for antigen testing in addition to antibody testing was present in 57 subjects. Table 1 describes the numbers of each specimen type tested via each testing modality. Overall, 47% (120/257) were women. The average age was 35 years of age (SD ± 9 years). Among the 197 participants with CD4 counts measured, the median pre-ART nadir CD4 T cell count was 26 cells/μl (IQR, 10 to 79 cells/μl). Nadir CD4 T cell counts were significantly lower in persons with cryptococcal meningitis (median 18, IQR 7–41 cells/μl) than in persons without cryptococcal meningitis (median 93, IQR 49–149 cells/μl, P < .001). At time of serum collection, the median duration of ART was 6 months with a median CD4 count of 138 cells/μl. Among participants with cryptococcal meningitis, the median Cryptococcus growth on CSF culture was 51,600 colony forming units (CFU)/ml of CSF (IQR 4,350–246,000 CFU/ml) among 129 participants with cryptococcal meningitis.
Table 1.
Description of samples tested.
| Specimen type | Total | Antigen | Antibody |
|---|---|---|---|
| Cerebrospinal fluid | 71 | 63 (39) | 0 |
| Urine | 37 | 37 (0) | 0 |
| Serum | 151 | 57 (51) | 151 (104) |
Note: 257 subjects were enrolled of whom 147 had cryptococcal meningitis and 110 had no opportunistic infection. To the right of specimen type all columns describe numbers of each specimen type available for testing with numbers with cryptococcal meningitis in parentheses.
Antibody testing
Overall, 1.3% (2/151) of serum samples were IgG positive by EIA testing (95% CI, 0.2–4.7%) with individual values of 17 and 26 units and a cut-off for positivity of 15 units. None of the 151 samples tested for IgM by EIA were positive (95% CI 0–2.4%). Both of the Histoplasma IgG positive persons had prior cryptococcal meningitis. Among the 151 patients in whom serum antibodies were tested, 104 patients had cryptococcal meningitis and Cryptococcus CSF quantitative culture was positive in 89 subjects with a median value of 25,000 CFU/ml (IQR 1700–132,000 CFU/ml). Figure 1 displays a scatter plot of IgG and IgM values and the cut-off points for each test conducted on serum.
Figure 1.
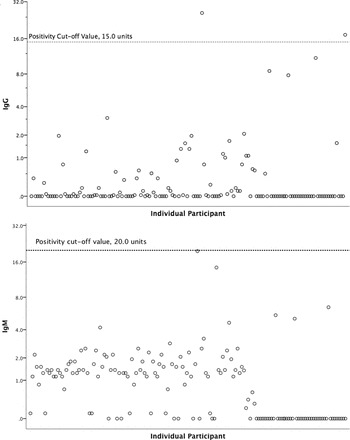
Scatter plots of serum Histoplasma Enzyme Immunoassay IgG and IgM results. Note : Figure 1a displays the results of serum EIA Histoplasma IgG testing with the positive cutoff noted by a horizontal line at 15 units and the positive results at 26 and 17 units. Figure 1b displays serum IgM EIA results with the positive cutoff noted by a horizontal line at 20 units.
Histoplasma antigen testing
Histoplasma antigen testing was performed on CSF in 63 subjects, and none were positive. Among those 63 persons, 39 persons had cryptococcal meningitis with a median quantitative CSF culture of 128,000 Cryptococcus CFU/ml (IQR 25,475–460,000 CFU/ml). Serum from 57 persons (51 of whom had cryptococcal meningitis) and urine from 37 persons (of whom none had cryptococcal meningitis) was tested for Histoplasma antigen, and none were positive. Neither of the two serum samples that tested positive for IgG by EIA had sufficient volume to allow for Histoplasma antigen testing.
Discussion
Histoplasma antigen was not detected in any patients, suggesting that histoplasmosis is rare in patients with AIDS presenting to hospitals in Kampala. A similar study performed in Tanzania identified 1% probable cases (9/907) among persons who were tested for Histoplasma antigen. 2 Yet in Uganda, the occurrence of 13 cases among foreign students who entered a hollow tree containing large amounts of bat guano is consistent with the presence of “microfoci” containing Histoplasma rather than widespread distribution in Uganda. 3
Cross-reactivity between polysaccharide antigens detected in the body fluids of patients with cryptococcosis and histoplasmosis was not demonstrated. Given that patients with advanced HIV often have multiple infections, diagnostics must be able to distinguish between mycoses. Further, the IMMY LFA and latex agglutination cryptococcal antigen detection assays have not been tested for cross-reaction with histoplasmosis (although this is unlikely) nor has the MiraVista EIA system been tested for cross-reaction in persons with known Cryptococcus. In our study, Histoplasma antigen was not detected in the serum of 51 patients, or CSF of 39 patients with cryptococcal meningitis. In all subjects with cryptococcal meningitis in whom serum, or CSF were available, all had extremely high median cryptococcal glucoxylomannan antigen titers, yet none tested positive Histoplasma antigen, this speaks against cross-reactivity between the antigens detected in these two mycoses.
Information is limited on cross-reactivity with the EIA antibody detection system, although cross-reactivity has been noted with enzyme-linked immunosorbent assay (ELISA) antibodies to histoplasmin and Histoplasma ribosomes with blastomycosis, coccidioidomycosis, paracoccidioidomycosis, aspergillosis, candidiasis, and interestingly, cryptococcosis. 19 Cross-reaction with penicilliosis, paracoccidioidomycosis, coccidioidomycosis, and blastomycosis would be geographically unlikely in Africa. 20–23 Infections due to Cryptococcus occur frequently among persons with advanced HIV in Uganda, and so the lack of significant cross-reactivity between testing for Cryptococcus and Histoplasma is useful information.
Among hospitalized HIV-infected Ugandans, we found a 1.3% IgG seroprevalence for histoplasmosis. This study is not representative of Uganda as a whole or Uganda patients with HIV as a whole but rather is a study of Ugandans presenting with an illness and advanced HIV in Kampala and the results should be interpreted with this in mind. This is the first study in ∼44 years to investigate prevalence of histoplasmosis exposure among Ugandans. 4 The previous study found 3.8% exposure through histoplasmin skin test positivity. 4 Although skin testing has been traditionally used to measure exposure to H. capsulatum , the FDA-approved histoplasmin for use in skin testing is no longer available in the United States. Our study may reflect a survivor bias (i.e., low nadir CD4 counts) and/or a nonstatistical difference in exposure prevalence. Serologic detection has a number of potential advantages as compared with skin testing. Skin testing is time intensive, requiring multiple visits to a healthcare facility and remains positive for life in 90% of persons whereas serologic testing is rapid, requires one healthcare facility visit and positivity often dissipates in months to years suggesting more recent infection. 9 As an example of the usefulness of IgM and IgG detection by EIA in other epidemiologic studies, Schlech and colleagues found a significant association between Histoplasma IgM positivity was associated with closer proximity to the presumed site of exposure. 24 In the current study only 1.3% of the serum specimens from patients with cryptococcal meningitis were reactive to the Histoplasma capsulatum IgG EIA. Thus, cryptococcosis would appear to be, at most, a rare cause for positive results in the Histoplasma antibody IgG EIA in this HIV-infected population although the possibility that the reactivity seen the two positive samples were due to cross-reactivity with cryptococcal antigen can not be excluded.
Given the patient population tested (HIV-infected with AIDS and low CD4 counts), it is possible that a number of persons were unable to mount an antibody response to the EIA antibody assays. However, studies in patients with confirmed disseminated histoplasmosis and AIDS found that the MiraVista IgG assay exhibited a decreased sensitivity (73%, n = 15) as opposed to studies of patients with acute pulmonary histoplasmosis and presumed normal immune systems (n = 65, sensitivity 88–90%, Richer et al., unpublished). Despite decreased sensitivity, the ability of this system to detect exposure to histoplasmosis among those with AIDS is important as very little is known about the incidence of histoplasmosis in Uganda. Further, it is exactly this population (those persons with advanced HIV) who are at higher risk for dissemination, making an ability to detect exposure crucial.
In conclusion, we provide evidence that histoplasmosis is relatively rare in Uganda among patients with advanced HIV/AIDS. We found similar seroprevalence by IgG for H. capsulatum among HIV-infected persons in Kampala, Uganda as compared to the last study of exposure (via skin testing) in this location ∼44 years prior. In addition, our findings agree with prior smaller studies that did not find cross-reaction between cryptococcal antigen and the MiraVista EIA antigen detection system. 8 Thus, in persons with advanced HIV or other immunosuppressive conditions, one should consider positive testing for cryptococcosis and histoplasmosis as evidence of each distinct disease, rather than potential cross-reaction of the diagnostic tests. Histoplasmosis in Kampala, Uganda remains rare, though present.
Acknowledgments
The authors thank Drs. Reuben Kiggundu, Abdu Musubire, Henry Nabeta, and Lillian Tugume for clinical care, Samuel Okurut for specimen management and the laboratory staff at MiraVista Diagnostics for sample testing.
Funding
Clinical cohorts were enrolled with support from the National Institute of Neurologic Diseases and Stroke (NINDS), National Institute of Allergy and Infectious Diseases (NIAID), and Fogarty International Center (R01NS086312, R25TW009345, U01AI089244, K24AI00024511, T32AI055433).
Declaration of Interest
Authors Wheat, Richer, Swartzentruber, and Jarrett are employees of MiraVista Diagnostics. The other authors have no conflicts of interest to declare.
References
- 1.Antinori S. Histoplasma capsulatum: more widespread than previously thought. Am J Trop Med Hyg. 2014;90:982–983. doi: 10.4269/ajtmh.14-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lofgren SM, Kirsch EJ, Maro VP, et al. Histoplasmosis among hospitalized febrile patients in northern Tanzania. Trans R Soc Trop Med Hyg. 2012;106:504–507. doi: 10.1016/j.trstmh.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cottle LE, Gkrania-Klotsas E, Williams HJ, et al. A multinational outbreak of histoplasmosis following a biology field trip in the Ugandan rainforest. J Travel Med. 2013;20:83–87. doi: 10.1111/jtm.12012. [DOI] [PubMed] [Google Scholar]
- 4.Bezjak V, Farsey SJ. Prevalence of skin sensitivity to histoplasmin and coccidioidin in varous Ugandan populations. Am J Trop Med Hyg. 1970;19:664–669. doi: 10.4269/ajtmh.1970.19.664. [DOI] [PubMed] [Google Scholar]
- 5.Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–825. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 6.Kuberski T, Myers R, Wheat LJ, et al. Diagnosis of coccidioidomycosis by antigen detection using cross-reaction with a Histoplasma antigen. Clin Infect Dis. 2007;44:e50–54. doi: 10.1086/511684. [DOI] [PubMed] [Google Scholar]
- 7.Wheat J, Wheat H, Connolly P, et al. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin Infect Dis. 1997;24:1169–1171. doi: 10.1086/513647. [DOI] [PubMed] [Google Scholar]
- 8.Zhuang D, Hage C, De Jesus M, et al. Cryptococcal glucoxylomannan does not exhibit cross-reactivity in the MVista Histoplasma antigen enzyme immunoassay. Clin Vaccine Immunol. 2008;15:392–393. doi: 10.1128/CVI.00383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wheat J, French ML, Kohler RB, et al. The diagnostic laboratory tests for histoplasmosis: analysis of experience in a large urban outbreak. Ann Intern Med. 1982;97:680–685. doi: 10.7326/0003-4819-97-5-680. [DOI] [PubMed] [Google Scholar]
- 10.Richer SM, Smedema ML, Wheat LJ. Improvement of the Sensitivity and Specificity of Histoplasmosis Antibody Detection. Boston, MA: American Society for Microbiology; 2014. p. 132. American Society for Microbiology. [Google Scholar]
- 11.Nacher M, Adenis A, Blanchet D, et al. Risk factors for disseminated histoplasmosis in a cohort of HIV-infected patients in French Guiana. PLoS Negl Trop Dis. 2014;8:e2638. doi: 10.1371/journal.pntd.0002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulware DR, Meya DB, Bergemann TL, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7:e1000384. doi: 10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370:2487–2498. doi: 10.1056/NEJMoa1312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kambugu A, Meya DB, Rhein J, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis. 2008;46:1694–1701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson RD, Rolfes MA, Birkenkamp KE, et al. Predictors of neurocognitive outcomes on antiretroviral therapy after cryptococcal meningitis: a prospective cohort study. Metab Brain Dis. 2014;29:269–279. doi: 10.1007/s11011-013-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulware DR, Meya DB, Bergemann TL, et al. Antiretroviral therapy down-regulates innate antiviral response genes in patients with AIDS in sub-saharan Africa. J Acquir Immune Defic Syndr. 2010;55:428–438. doi: 10.1097/QAI.0b013e3181ef4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connolly PA, Durkin MM, Lemonte AM, et al. Detection of histoplasma antigen by a quantitative enzyme immunoassay. Clin Vaccine Immunol. 2007;14:1587–1591. doi: 10.1128/CVI.00071-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448–454. doi: 10.1093/cid/cir435. [DOI] [PubMed] [Google Scholar]
- 19.Raman C, Khardori N, Von Behren LA, et al. Evaluation of an ELISA for the detection of anti-Histoplasma ribosomal and antihistoplasmin antibodies in histoplasmosis. J Clin Lab Anal. 1990;4:199–207. doi: 10.1002/jcla.1860040311. [DOI] [PubMed] [Google Scholar]
- 20.Litvintseva AP, Marsden-Haug N, Hurst S, et al. Valley Fever: Finding New Places for an Old Disease: Coccidioides immitis Found in Washington State Soil Associated With Recent Human Infection. Clin Infect Dis. 2015;60:e1–3. doi: 10.1093/cid/ciu681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richer SM, Smedema ML, Durkin MM, et al. Development of a highly sensitive and specific blastomycosis antibody enzyme immunoassay using Blastomyces dermatitidis surface protein BAD-1. Clin Vaccine Immunol. 2014;21:143–146. doi: 10.1128/CVI.00597-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanittanakom N, Cooper CR, Jr., Fisher MC, et al. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19:95–110. doi: 10.1128/CMR.19.1.95-110.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira MS. Paracoccidioidomycosis. Paediatr Respir Rev. 2009;10:161–165. doi: 10.1016/j.prrv.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Schlech WF, 3rd, Wheat LJ, Ho JL, et al. Recurrent urban histoplasmosis, Indianapolis, Indiana. Am J Epidemiol. 1983;118:1980–1981. 301–312. doi: 10.1093/oxfordjournals.aje.a113637. [DOI] [PubMed] [Google Scholar]


