Abstract
Staphylococcus aureus is one of the most significant clinical pathogen, as it causes infections to humans and animals. Even though several antibiotics and other treatments have been used to control S. aureus infections and intoxication, bacterium is able to adapt, survive and produces exotoxins. Licorice (Glycyrrhiza glabra L.) has been used traditionally in various medicinal (antimicrobial) preparations, and Glycyrrhizic acid (GA) is the major active constituents present in it. In the present investigation the effect of licorice extract on methicillin susceptible S. aureus (FRI 722) and methicillin resistant S. aureus (ATCC 43300) growth and toxin production was studied. The MIC of licorice extract was found to be 0.25 and 2.5 mg GA ml−1 against S. aureus FRI 722 and S. aureus ATCC 43300, respectively. Inhibition of biofilm formation was observed even at very low concentration (25 μg GA ml−1). Gradual decrease in expression and production of exotoxins such as α and β hemolysins and enterotoxin B was observed with the increasing concentrations of licorice extract, however, suboptimal concentration induced the expression of some of the virulence genes. This study indicated efficacy of licorice extract in controlling growth and pathogenicity of both methicillin susceptible and methicillin resistant S. aureus, however, the mechanisms of survival and toxin production at suboptimal concentration needs further study.
Keywords: Staphylococcus aureus, Glycyrrhizic acid, Biofilm, Western blot, Exotoxins
Introduction
The use of higher plants and the plant preparations for the treatment of infections is an age-old practice, especially in developing countries, where there is a dependency on traditional medicine for the treatment of variety of diseases (Ahmad et al. 1998; Cock and van-Vuuren 2015). Licorice (Glycyrrhiza glabra L.) is one of the most widely used herb, and finds mention in the ancient medical history of Ayurveda. Licorice grows in the sub-tropical and warm temperate regions of the world, chiefly in India and Mediterranean countries. Roots and stolon parts of licorice plants are used in mixed herbal preparations to promote digestion and vitality. Licorice root extract has been studied for its anti-cancer and anti-viral activities and its ability to promote the healing of gastric ulcers (Khalsa and Tierra 2008). Pharmacological investigations indicate that licorice root extracts have antioxidant, antibacterial and anti-inflammatory activities (Vaya et al. 1997). The main ingredient of licorice roots glycyrrhizin or glycyrrhizic acid (GA), a triterpinoid saponin, is used for the control of cough, asthma, bronchitis, peptic ulcer, arthritis and allergic reactions (Stormer et al. 1993).
Staphylococcus aureus is a Gram positive, facultative anaerobe and clinically most important pathogen, which can cause many types of infections such as septicemia, meningitis, toxic shock syndrome, skin abscesses and food-borne illness (Brumfitt and Hamilton-Miller 1989). Staphylococcal intoxication is one of the most prevalent causes of gastroenteritis worldwide resulting from ingestion of one or more preformed staphylococcal enterotoxins (SEs) (Jablonski et al. 1997). Staphylococci secrete 20 serologically different staphylococcus super-antigens including TSST 1, the staphylococcal enterotoxins and staphylococcal enterotoxin like toxins (Smith et al. 2010), which are responsible for its pathogenicity. S. aureus also has the ability to produce a variety of extracellular and cell wall-associated proteins, such as α-hemolysin, β-hemolysin, coagulase, protein A and fibronectin binding protein, which contributes for its pathogenicity (Morteza et al. 2010). S. aureus produces biofilm (Shin et al. 2013) and is reported to be resistant to several antibiotics (Sharma and Anand 2002). Shen et al. (2015) reported that licochalcone A, a major phenolic compound of Glycyrrhiza inflate was effective against biofilm producing S. aureus, and it altered the expression of genes encoding pathogenic factors and toxin genes. Due to the widespread emergence of multiple drug resistant, S. aureus has become one of the most feared pathogens (Hossein et al. 2010). As the reports of bacterial resistant to synthetic chemicals are on rise, there is a need to look for alternatives for the control of this pathogen. Therefore, the objectives of the present investigation was to study the effect of licorice root extract on growth and biofilm formation by methicillin susceptible S. aureus (FRI 722) and methicillin resistant S. aureus (ATCC 43300). Further, the pathogenicity traits of bacteria in terms of expression of toxin genes and production of exotoxins were also determined.
Materials and methods
Bacterial cultures
Methicillin-sensitive S. aureus (MSSA) strain FRI 722 was obtained from Public Health Laboratory (Tilburg, The Netherlands) and Methicillin-resistance S. aureus (MRSA) strain ATCC 43300 was from American Type Culture Collection (Manassas, Virginia, USA). Escherichia coli MTCC 108, a non-biofilm former strain was purchased from Microbial Type Culture Collection (MTCC, Chandigarh, India). Bacteria were sub-cultured and maintained in Brain Heart Infusion (BHI, Hi- Media, Mumbai, India) agar slants at 4 °C.
Extraction, identification and characterization of glycyrrhizic acid in licorice extract
Glycyrrhizic acid was extracted from the dried root of Glycyrrhiza glabra (S. S. Herbals, New Delhi, India). Roots were washed with water and air dried. To 500 g of dried root, 1000 mL of water was added and refluxed at 90 °C for 5–6 h, and it was concentrated to 20 % of original volume in a flash evaporator (Buchi, Germany). The crude concentrate (pH 8.5) was treated with sulphuric acid (Qualigens, Mumbai, India) until the pH reached 2 and neutralized by the addition of ammonia (Qualigens, Mumbai, India). The precipitate thus obtained was collected by filtration, dried and converted to powder form. The powder was re-dissolved in methanol and concentration of glycyrrhizic acid (GA) in powder was determined as specified previously (Hong et al. 2007). In brief, the HPLC (Agilent Technologies 1100 series, USA) and an ODS column (4.6 × 250 mm, ID5 μm) with an injection volume of 20 μL and the mobile phase of acetonitrile (MERCK, Mumbai, India) and water (65:35) containing 2 % acetic acid (Qualigens, Mumbai, India) were used and the peak was detected at 254 nm by isocratic elution. GA was also characterized by LC-MS analysis. LC-MS analysis was performed on an Agilent 1100 LC/MS analyzer (Agilent Technologies, USA). MS data with PI-ESI detection (negative ion detection with electrospray ionization) was acquired in the m/z range of 200–1000, with a step size of 0.1 U. The collision-induced dissociation (CID) voltage was 100 V and the electrospray voltage (ESV) was set to 4000 V.
Antibacterial susceptibility testing
Anti-staphylococcal activity was determined by disc diffusion method (CLSI 2009). Sterile discs (6 mm diameter, Hi-Media, Mumbai) impregnated with different concentrations of licorice extract (0.000625–0.625 mg of GA) were placed over pre inoculated (105 cells of MSSA or MRSA) BHI agar culture plates and incubated at 37 °C for 24 h. Sterile discs with sterilized water served as negative control. The anti-staphylococcal effect of licorice extract was determined by measuring the diameter of the zone of inhibition in triplicates. Reference antibiotics disc such as Methicillin (5 μg/disc), Ampicillin (10 μg/disc), Novabiocin (30 μg/disc) and Vancomycin (30 μg/disc) purchased from Hi-Media were used for comparative studies.
Minimum inhibitory concentrations (MIC)
MIC was determined by agar dilution method as described by (Negi et al. 1999) with slight modification. In brief, culture suspensions were prepared by suspending overnight grown cultures in sterile saline solution and it was diluted serially to achieve bacterial suspension of 105 CFU mL−1. One mL of bacterial suspension was plated on BHI agar containing licorice extract with different GA concentrations ranging from 0.025 mg - 25 mg mL−1 and incubated at 37 °C for 24–48 h. The MIC value was defined as the lowest concentration of licorice extract at which complete inhibition of bacterial growth was observed. All the tests were performed in triplicates.
Growth kinetics
The growth kinetics of bacteria treated with/without different concentrations of licorice extract (0.025 mg to 25 mg GA mL−1) was determined by pour plate method (Marino et al. 2001). Overnight grown cultures were serially diluted to106 CFU mL−1 and transferred to 9 mL BHI broth containing different concentrations of GA to achieve cell density of 105 CFU mL−1 and incubated aerobically at 37 °C. Viable cell count was determined by withdrawing the samples at 0, 4, 18, 24 and 48 h. Killing curves were constructed by plotting the log10 CFU mL−1 verses growth period. Three independent trials were conducted for the experiment and the mean ± SD values were used for plotting the killing curve.
Hemolytic activity
Hemolytic activity of bacteria was determined by agar well diffusion method with rabbit erythrocytes, which are more sensitive to S. aureus α-toxin than human erythrocytes (Perez et al. 1990). In brief, cultures were grown in BHI broth supplemented with various GA concentrations (0.025 mg – 0.125 mg mL−1 of broth) till OD600 nm reached 2.5, and 100 μL culture was transferred to the wells (diameter 0.8 cm) on blood agar plates and incubated at 37 °C for 18–24 h. Lysis of red blood cells (zone formation) indicated the haemolytic activity of the test organism. Efficacy of lysis of red blood cells by MSSA and MRSA was determined by measuring zone diameter in triplicate.
Biofilm formation
The biofilm formation of S. aureus and E. coli (negative control) was performed in 96 well flat bottom polystyrene microtiter plates (AXYGEN Life sciences, California, USA) (Wei et al. 2006). The bacterial suspensions were prepared from the overnight grown culture and the turbidity of the suspension was adjusted to 0.5 OD600 (106 CFU mL−1). Ninety μL of licorice extract (0.025 mg – 2.5 mg GA mL−1) prepared in tryptone soya broth (TSB: Hi-Media, Mumbai, India) supplemented with 0.5 % glucose was added into the 96 well flat bottom polystyrene microtiter plates and aliquots of 10 μL of the bacterial suspension was added to each well. Ten μL inoculums in 90 μL fresh TSB with 0.5 % glucose served as a positive control and the wells containing only TSB with 0.5 % glucose without inoculum served as a blank and incubated at 37 °C for 24 h. The biofilms were fixed with methanol for 30 min, and stained with 0.1 % (w/v) crystal violet for 10 min. Biofilm formation was quantified by the absorbance at 595 nm using a microplate reader (BIO-RAD, Japan).
Surface coating assay
Effect of licorice extract on biofilm formation was confirmed by surface coating assay (Bendaoud et al. 2011). A volume of 10 μL of licorice extract (0.025 mg – 2.5 mg GA mL−1) or 10 μL of sterile water (control) was transferred to the center of a flat bottom polystyrene microtiter plates. The plate was incubated at 37 °C for 2 h to allow complete evaporation of the liquid. The wells were then filled with 200 μL of TSB containing 104 to 105 CFU/mL of MSSA, MRSA or E. coli. After 18 h, biofilms were rinsed with water and stained with 1 mL of Gram’s crystal violet. Stained biofilms were rinsed with water, dried, and the wells were photographed.
In-vitro transcription and translation of toxin determination by RT-PCR and SDS-PAGE and Western blot analysis
Gene expression of toxin genes hla, hlb and seb of MSSA and MRSA was determined by inoculating in BHI containing 0.025 mg- 2.5 mg GA mL−1 or in BHI alone. Samples were incubated at 37 °C till the OD600 nm reached 2.5, and centrifuged at 5000 × g for 10 min to collect cell pellet and supernatants for RNA and protein extraction. RNA was extracted and converted to cDNA for reverse transcriptase PCR (RT-PCR) (Rohinishree and Negi 2012) using primers listed in Table 1. The relative quantification of genes was determined by changes in the expression of hla, hlb and seb transcripts relative to expression in untreated bacteria. Expressions were normalized using housekeeping gene 16S rRNA. Data were expressed as change in transcript level after treatment with GA.
Table 1.
Nucleotide sequences and size of the RT-PCR products for the S. aureus gene-specific primers
| Target gene | Primer sequence | Annealing Temp (°C) | Product size (bp) |
|---|---|---|---|
| seb | F 5′ TCGCCTTATGAAACGGGATA 3′ R 5′ ACAAATCGTTAAAAACGGCG 3’ |
50 | 411 |
| hla | F5’ATGATGAAAATGAAAACACGTATAGTC 3′ R 5’ ATTTGAGCTACTTCATTATCAGGTAGTTG 3’ |
48 | 375 |
| hlb | F 5’TGTTAATAAAGGCACTCCAGAGTTC 3′ R 5′ CTTTGATTGGGTAATGATCTGAAAA 3’ |
49 | 292 |
| 16S rRNA | F 5’ AGAGTTTGATCCTGGCTCAG 3′ R 5′ AAGGAGGTGATCCAGCCGCA 3’ |
55 | 1500 |
For protein estimation culture supernatants were precipitated by adding trichloroacetic acid (Qualigens, Mumbai, India) to a final concentration of 10 % and incubated at 4 °C overnight. The precipitate was centrifuged at 15,000 × g for 20 min at 4 °C and finally washed three times with ice-cold ethanol. The aggregated proteins were dried and dissolved in 0.5 mL of 0.1 M Tris. The protein concentrations were determined by Bradford assay with BSA (Sigma Aldrich, Bangalore, India) as a standard.
Western blot analysis for S. aureus enterotoxins was done, and the production of α-hemolysin and Staphylococcal enterotoxin B (SEB) in MSSA and MRSA was detected by incubation with antibodies. Antibodies to α-hemolysin and SEB were purchased from Sigma-Aldrich and diluted to 1:10,000 and 1:8000, respectively. Anti-rabbit antiserum was used as a secondary antibody with a dilution of 1:10,000. The blots were developed using NBT/BCIP (Roche, Germany) ready to use tablets and documented using an imaging system (Isogen Proxima, The Netherlands).
Statistical analysis
Inhibition of biofilm formation and the fold change expression of toxin genes by licorice treatment were compared with untreated cells and significance was analysed using student’s t-test to determine statistical differences.
Results
Purity of extracts
The presence of GA in licorice extract was detected preliminary by HPLC with the aid of ODS C18 column by using 80 % of GA as an external standard (Fig. 1). GA was further characterized by LC-MS. The standard was directly injected into the TOF mass spectrometer to optimize the ESI conditions. Their mass spectra acquired by HPLC/TOFMS in negative ion mode are shown in Fig. 1. The ion patterns of sample was same as that of GA standard spectra of [M + H]− ions at m/z 822.037. The presence of GA in purified licorice extract was found to be 30.52 % on dry weight basis by HPLC analysis. For further experiments, the concentration of GA in licorice extract was adjusted by taking account of its purity.
Fig. 1.
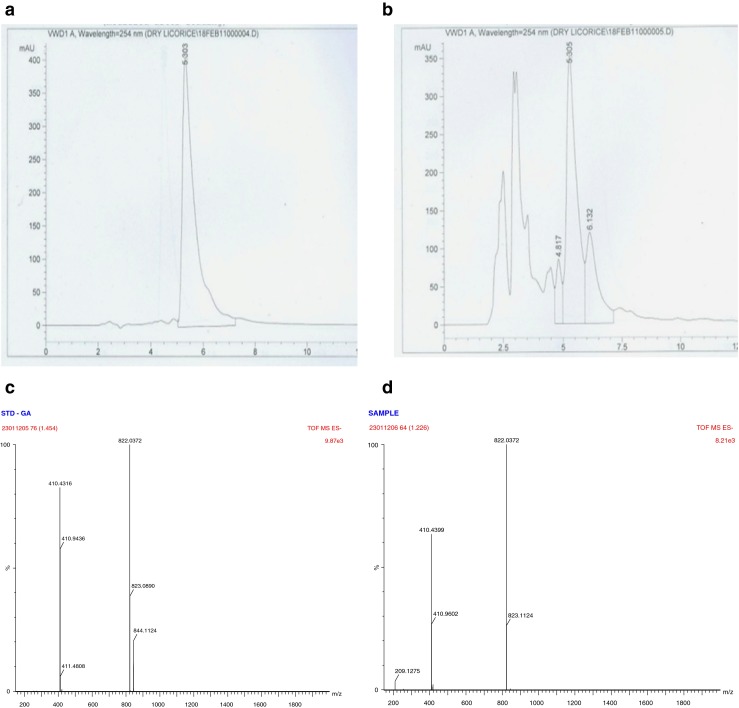
HPLC and LC-MS Chromatogram of glycyrrhizic acid
Effect of licorice extract on MSSA and MRSA
MIC determination
MSSA was found to be susceptible to Methicillin (5 μg/disc), Novabiocin (30 μg/disc) and Vancomycin (30 μg/disc) with mean diameter of 23.45 mm, 31.27 mm and 16.72 mm zone of inhibition, respectively, but resistant to ampicillin (10 μg/disc) with 27 mm zone of inhibition. Whereas, MRSA was susceptible to novobiocin (23 mm) and resistant to methicillin (14 mm), vancomycin (12 mm) and ampicillin (6 mm). Licorice extracts containing GA at 0.0625 and 0.625 mg/disc produced an inhibitory zone with a mean diameter of 18 and 22 mm for MSSA, and 8 and 16 mm for MRSA, respectively. In Agar dilution method, the licorice extract exhibited a significant antibacterial activity against MSSA and MRSA with MIC values of 0.25 and 2.5 mg mL−1 of GA, respectively.
Time-kill kinetic studies
The time-kill kinetic studies of licorice extract (0.025 to 25 mg GA mL−1) against MSSA and MRSA were performed, and MRSA cells were found to be more resistant to licorice extract as compared to MSSA. Licorice extracts at 2.5 and 25 mg mL−1 GA concentrations caused rapid decline of MSSA and MRSA viable cell count, and the count was reduced almost to 0 at 4 h growth phase (Fig. 2a). At 0.25 mg mL−1 of GA treatment, MRSA viable cell count was reduced from 5 log to 2.7 log CFU mL−1 at 4 h and cell count was constant till 48 h which show a bacteriostatic effect. Whereas, MSSA cells reduced to 2 log CFU mL−1 at 4 h and complete inhibition of cells was observed at 24 h. When MRSA and MSSA treated with 0.125 mg mL−1 of GA, viable cell was reduced to 3.7 log and 4.9 log CFU mL−1 respectively at 4 h, but cells were able to survive and grow up to the level of 8 log CFU mL−1 at the end of 24 h. On the other hand, at 0.025 mg mL−1 of GA, both MRSA and MSSA cells grew at a uniform growth rate, which was similar to that observed for control cells (without GA) showing a sigmoid growth curve (Fig. 2a).
Fig. 2.
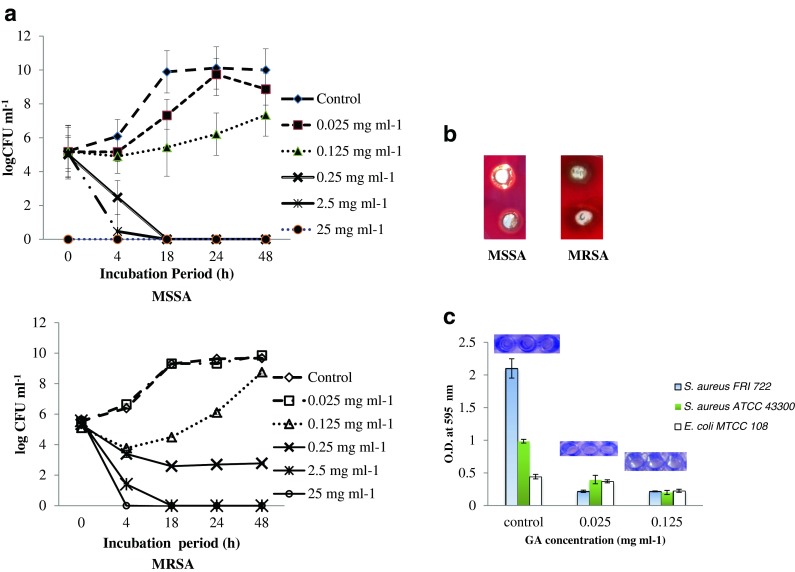
a Effect of different concentrations of GA on growth of MSSA and MRSA. b Effect of GA on haemolytic activity of MSSA and MRSA. c Effect of GA on biofilm formation of MSSA, MRSA and E.coli (negative control)
Haemolytic activity
Hemolysins secreted by S. aureus can promote hemolysis of rabbit erythrocytes, and hemolysis assay was used to investigate the effect of licorice on hemolytic activity of S. aureus cultures. Untreated cells of MRSA and MSSA showed strong haemolytic activity on rabbit blood agar with a wide zone of 30 mm around the agar wells. When treated with licorice extract of 0.025 mg, the zone of haemolysis for MSSA and MRSA was 30 mm and 20 mm, respectively (Fig. 2b).
Anti-biofilm formation activity
Biofilm formation for untreated control MSSA, MRSA and E. coli (negative control) cells were found to be 2.18, 0.96 and 0.53, at OD595 nm respectively. Licorice extract significantly (p ≤ 0.001) inhibited the biofilm formation by MRSA and MSSA even at low concentrations (0.025 mg and 0.125 mg GA mL−1), as all the treatments showed OD values less than the negative control, which indicates complete inhibition of biofilm formation. The complete inhibition of biofilm formation was further confirmed by surface coating assay (Fig. 2c).
Transcription of hla, hlb and seb in S. aureus
Effect of licorice extract on toxin-encoding genes (seb, hla and hlb) expression was determined by RT-PCR. Treatment at sub-inhibitory concentrations of licorice extract (0.025 mg and 0.125 mg GA mL−1) showed <2- fold change in the transcripts of hla and hlb genes, however it was statistically significant (p < 0.0001 for hla and p < 0.05 for hlb for MSSA and p < 0.05 for hla and p < 0.001 for hlb for MRSA). A complete suppression of seb expression was observed at all concentration tested in both MSSA and MRSA. In MRSA strain at 0.25 mg GA mL−1, expression of hla gene was statistically similar (p < 0.05) to untreated cells (0.98- folds) at similar concentration. Higher expression (> 2- fold) of hlb gene was seen at 0.025 mg GA mL−1, which was significantly (p < 0.001) higher than untreated cells. As both MRSA and MSSA strains were unable to survive at concentrations higher than 0.25 mg GA mL−1, the presence of genes and their expression could not be analyzed. This indicated that under suboptimal preservative treatments, the organisms were able to survive and express toxin genes in a dose dependent manner (Table. 2; Fig. 3).
Table 2.
Relative fold expression of virulence genes of MSSA (FRI 722) and MRSA (ATCC 43300) at different concentrations of GA
| Concentrations of GA (mg mL−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.025 | 0.125 | 0.25 | |||||||
| seb | hla | hlb | seb | hla | hlb | seb | hla | Hlb | |
| MSSA | * | 1.7 ± 0.09d | 1.4 ± 0.14a | * | 1.2 ± 0.19 | 1.3 ± 0.11a | * | * | * |
| MRSA | * | 1.1 ± 0.11a | 2.1 ± 0.15c | * | 1.3 ± 0.11b | 1.5 ± 0.02d | * | 0.98 ± 0.01 | * |
Values are means ± SD (n = 3)
Statistical significance of the relative expression ratio (to untreated cells) is indicated as a p < 0.05, b p < 0.01, c p < 0.001, and d p < 0.0001
*Not transcribed in treated cells
Fig. 3.
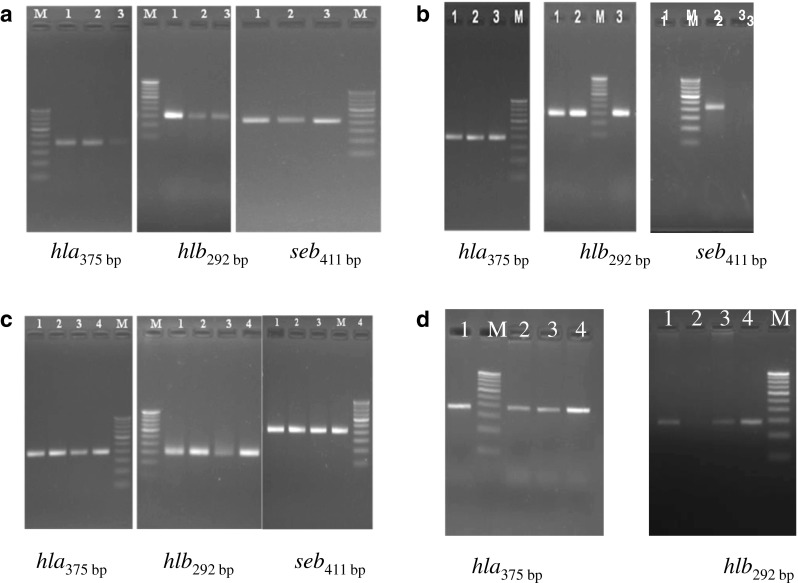
Agarose gel electrophoresis of PCR and RT-PCR amplification of toxin genes
Effect on α-hemolysin and seb protein production
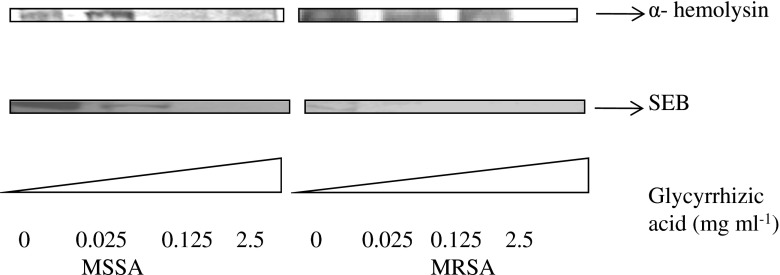
Western blot analysis was used to determine α- hemolysin and SEB production in MSSA and MRSA culture fluids. As the concentrations of licorice extract increased, toxin production was reduced. Exposure of MSSA and MRSA cells to 0.025 and 0.125 mg GA mL−1 did not show a significant decrease of α-hemolysin, however no α-hemolysin production was observed at higher concentrations. Decrease in SEB toxin production in MSSA and MRSA was observed even with the treatment at lowest concentrations of licorice extract (0.025 mg GA mL−1) (Fig. 4).
Fig. 4.
Western blot analysis of hla and seb proteins
Discussion
Emergence of multiple drug resistant S. aureus strains is a major public health threat. Due to the unavailability of effective antibacterial agents against these strains, the threat has been compounded over the last few decades (Qazi et al. 2006). Consequently, the development of alternative therapeutic strategies to combat S. aureus infections continues to be an active research area. S. aureus produces a large number of virulence factors which are responsible for the pathogenesis. Therefore, the clinical performance of antimicrobial agents employed for the treatment of S. aureus infections not only depends on their bacteriostatic and bactericidal effects but also on the ability to prevent virulence factors secretion by dying or stressed cells (Bernardo et al. 2004). Studies on various natural compounds reveal the antibacterial activity and inhibition of expression of virulence factors against methicillin susceptible and methicillin resistant S. aureus bacteria (Machado et al. 2003; Choi et al. 2010). The reduction in virulence of S. aureus after treatment with licochalcone A was attributed to the reduction in expression of virulence genes (Shen et al. 2015). The reduction in expression of virulence genes by treatment with licorice extract observed in the present study may also reduce toxigenic potential of S. aureus. The plate-hole diffusion assay of Australian plants extract demonstrated the active inhibition on growth of six MRSA strain with bactericidal and bacteriostatic activity (Palombo and Semple 2002). On the other hand, MIC of 200 mg mL−1 of licorice extract against S. aureus was reported by Jafarian et al. (2007). MIC of 18-β- glycyrrhetinic acid (GRA), a hydrolyzed product of GA was found to be 60 μg mL−1, and GA was not able to inhibit the growth of MRSA even at the highest concentration (125 μg mL−1) studied (Long et al. 2013). However, in our study, licorice extract (2.5 mg mL−1 of GA and above) was able to completely inhibit the growth of S. aureus ATCC 43300, and when treated with licorice extract containing 0.25 mg mL−1 of GA, a reduction in viable cell up to 18 h and bacteriostatic effect thereafter was observed (Fig. 2). Similar observation of concentration dependent growth inhibition of S. aureus ATCC 43300 was reported after treatment with Paenimacrolidin, a secondary metabolite of Bacillus species (Wu et al. 2011).
Earlier reports indicate that various plant extracts inhibit the growth of S. aureus, and repress the production of α, β-toxins and SEB in S. aureus (Aqil et al. 2005; Nimsha et al. 2011). in- vitro model study showed decrease in the expression of saeR, hla, mecA, and sbi genes in MRSA after treatment with GRA (Kao et al. 2010) and decrease in saeR expression is known to downregulate the hla and sbi genes (Long et al. 2013). In the present study, licorice extract (0.25 mg mL−1 of GA) inhibited the growth and toxin production (α- hemolysin and enterotoxins B) of MSSA and MRSA. Whereas, at higher concentrations (2.5 mg mL−1 of GA) cell growth was inhibited (Fig. 2a), and suboptimal concentrations (0.025 mg to 0.125 mg mL−1 of GA) had no effect on growth. Biofilm formation by S. aureus is reported to reduce the efficacy of antibacterial treatment (Raja et al. 2011) and biofilms formed by Pseudomonas aeruginosa are reported to impart it resistance against antibacterial compounds (Pagedar and Singh 2014). In the present investigation, licorice extract showed an inhibitory effect on biofilm formation even at the lowest concentration of GA (0.025 mg mL−1) (Fig. 2c), much lower to Acetyl-1, 1-keto-β-boswellic acid (AKBA), which showed 50 % inhibition the biofilm formation of S. aureus at 4 MIC level (Zhou et al. 2012). To our knowledge, this is the first report showing the evidence that Glycyrrhizic acid from Glycyrrhiza glabra has biofilm inhibition activity, however, in a recent report Shen et al. (2015) also observed reduction in biofilm formation by treatment with licochalcone A, a major phenolic compound of Glycyrrhiza inflate.
The production of hemolysins has been regarded as one of the strong evidence for pathogenic potential in S. aureus. Licorice extract containing GA at a concentration of 0.25 mg mL−1 and above inhibited exotoxin gene expression/protein production (hemolysins and enterotoxin B) in MSSA and MRSA as indicated by RT-PCR and Western blot analysis (Table. 2, Figs. 3, and 4). However, under suboptimal preservative treatments, the organisms were able to survive and express toxin genes in a dose dependent manner. It is reported that licochalcone E from licorice root can reduce the production of α-toxin in both MSSA and MRSA in dose dependent manner even at sub-inhibitory concentrations. Studies with synthetic antibiotics (erythromycin and streptomycin) have also reported inhibition of the hemolytic activity of bacteria by acting on the ribosomes responsible for hemolysin production (Witte 1975). During stress conditions, organisms are reported to enhance their defense mechanism by expression of virulence or metabolic genes (Rohinishree and Negi 2012) and in the present study also higher expression of hla and hlb genes were observed (Table. 2, Fig. 3) at sub-inhibitory concentrations (0.025 mg and 0.125 mg GA mL−1). However, at sub-inhibitory concentration (0.025 mg GA mL−1), the inhibition of the expression of seb was observed similar to seb inhibition by cerulenin (Rajan and Novick 2005). Probably, GA also interferes with the transcription of seb as cerulenin, and inhibits its expression.
The use of plants and plant products for the treatment of infections/diseases is on rise due to increasing drug resistance in several bacteria including S. aureus. This research provides evidence that use of licorice extract containing GA as a natural preservative is effective for the control of S. aureus by inhibiting its growth, the biofilm formation and toxin production. The extract containing GA was found to act at genetic level by blocking the transcription of virulence genes. Our results and earlier reports indicate that the natural products seem to be a good choice for the development of new strategies against pathogenic organisms and licorice extract could be rationally applied to inactivate the growth and toxins production of S. aureus. Although seb gene expression was inhibited at suboptimal preservative conditions, the survival of cells, hla and hlb expression and toxin production was increased at lower concentration treatments. Therefore GA at optimum concentration can be used as a preservative for food preservation, although the mechanisms of stress regulation at suboptimal concentration need further study.
Acknowledgments
Authors are grateful to Director, CSIR-CFTRI for constant encouragement. YSR acknowledges Council of Scientific and Industrial Research, New Delhi for Senior Research Fellowship for Ph. D. programme.
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest.
Footnotes
Research Highlights
• Licorice extract had 30.52 % Glycyrrhizic acid on dry weight basis and it showed antistaphylococcal activity
• Licorice extract showed complete growth inhibition of MSSA at 0.25 mg mL−1 GA and MRSA at 2.5 mg mL−1 GA
• Licorice extract suppressed expression of toxin genes and production of toxins, and inhibited biofilm formation
References
- Ahmad I, Mehmood Z, Mohammad F. Screening of some Indian medicinal plants for their antimicrobials properties. J Ethnopharmacol. 1998;62:183–193. doi: 10.1016/S0378-8741(98)00055-5. [DOI] [PubMed] [Google Scholar]
- Aqil F, Khan MS, Owais M, Ahmad I. Effect of certain bioactive plant extracts on clinical isolates of β-lactamase producing methicillin resistant Staphylococcus aureus. J Basic Microbiol. 2005;45:106–114. doi: 10.1002/jobm.200410355. [DOI] [PubMed] [Google Scholar]
- Bendaoud M, Vinogradov E, Balashova NV, Kadouri DE, Kachlany SC, Kaplan JB. Broad-spectrum biofilm inhibition by kingella kingae exopolysaccharide. J Bacteriol. 2011;193:3879–3886. doi: 10.1128/JB.00311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo K, Pakulat N, Fleer S, Schnaith A, Utermöhlen O, Krut O. Subinhibitory concentrations of linezolid reduce Staphylococcus aureus virulence factor expression. Antimicrob Agents Chemother. 2004;48:546–555. doi: 10.1128/AAC.48.2.546-555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumfitt W, Hamilton-Miller Methicillin-resistant Staphylococcus aureus. New Engl J Med. 1989;320:1188–1196. doi: 10.1056/NEJM198905043201806. [DOI] [PubMed] [Google Scholar]
- Choi JG, Kang OH, Chae HS, Obiang-Obounou B, Lee YS, Oh YC, Kim MS, Shin DW, Kim JA, Kim YH, Kwon DY. Antibacterial activity of hylomecon hylomeconoides against methicillin-resistant Staphylococcus aureus. Appl Biochem Biotechnol. 2010;160:2467–2474. doi: 10.1007/s12010-009-8698-5. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute- CLSI (2009) Performance standards for antimicrobial disk susceptibility tests (10th edition).
- Cock IE, van-Vuuren SF. South African food and medicinal plant extracts as potential antimicrobial food agents. J Food Sci Technol. 2015 [Google Scholar]
- Hong YY, Jian-Jun CAI, Rong-ji DAI, Deng Y-L, Yang K, Wei-Wei M. Separation and determination of glycyrrhizic acid and liquiritin in licorice using SPE-RP-HPLC. China: In Complex medical engineering. Beijing; 2007. pp. 1856–1860. [Google Scholar]
- Hossein M, Hadis M, Tahere S. Determining of antibiotic resistance profile in Staphylococcus aureus isolates. Asian Pac J Trop Med. 2010;3:734–737. doi: 10.1016/S1995-7645(10)60176-9. [DOI] [Google Scholar]
- Jablonski L, Gregory M, Bohach A. Staphylococcus aureus, fundamentals and frontiers. In: Doyle P, Beuchat LR, Montville TJ, editors. In food microbiology. Washington DC: ASM Pres; 1997. p. 353. [Google Scholar]
- Jafarian MM, Jafarian G, Ghazvini K. In vitro susceptibility of Helicobacter pylori to licorice extract. Iran J Pharm Res. 2007;6:69–72. [Google Scholar]
- Kao TC, Shyu MH, Yen GC. Glycyrrhizic acid and 18 betaglycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3beta signaling and glucocorticoid receptor activation. J Agric Food Chem. 2010;58:8623–8629. doi: 10.1021/jf101841r. [DOI] [PubMed] [Google Scholar]
- Khalsa KPS, Tierra M. The way of ayurvedic herbs. WI, USA: Lotus Press; 2008. [Google Scholar]
- Long DR, Mead J, Hendricks JM, Hardy ME, Voyich JM. 18β-glycyrrhetinic acid inhibits methicillin-resistant Staphylococcus aureus survival and attenuates virulence gene expression. Antimicrob Agents Chemother. 2013;57:241–247. doi: 10.1128/AAC.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado TB, Pinto AV, Pinto MCFR, Leal ICR, Silva MG, Amaral ACF, Kuster RM, Netto-dosSantos KR. In-vitro activity of Brazilian medicinal plants, naturally occurring naphthoquinones and their analogues, against methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 2003;21:279–284. doi: 10.1016/S0924-8579(02)00349-7. [DOI] [PubMed] [Google Scholar]
- Marino M, Bersani C, Comi G. Impedance measurements to study the antimicrobial activity of essential oils from lamiaceae and compositae. Int J Food Microbiol. 2001;67:187–195. doi: 10.1016/S0168-1605(01)00447-0. [DOI] [PubMed] [Google Scholar]
- Morteza SM, Susan M, Esmaeil D, Hossein M, Seyyed MSN. Antibacterial activity of eight Iranian plant extracts against methicillin and cefixime restistant staphylococcous aureus strains. Asian Pac J Trop Med. 2010;34:262–265. [Google Scholar]
- Negi PS, Jayaprakash GK, Jaganmohan Rao L, Sakaraih KK. Antimicrobial activity of turmeric oil: a by-product from curcumin manufacture. J Agric Food Chem. 1999;47:4297–4300. doi: 10.1021/jf990308d. [DOI] [PubMed] [Google Scholar]
- Nimsha SW, Smith WM, Mikkelsen D, Waanders J, Kerven G, Nola C, Gary DA, Mark TS. Purified 1-acetoxychavicol acetate (1- ACA) from galangal spice affects membrane fatty acid composition and triggers a cell envelope stress response in Staphylococcus aureus. Int J Antimicrob Agents. 2011;39:263–272. doi: 10.1016/j.ijantimicag.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Pagedar A, Singh J. Evaluation of antibiofilm effect of benzalkonium chloride, iodophore and sodium hypochlorite against biofilm of Pseudomonas aeruginosa of dairy origin. J Food Sci Technol. 2014 doi: 10.1007/s13197-014-1575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo EA, Semple SJ. Antibacterial activity of Australian plant extracts against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) J Basic Microbiol. 2002;42:444–448. doi: 10.1002/1521-4028(200212)42:6<444::AID-JOBM444>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Perez C, Paul M, Bazerque P. Antibiotic assay by agar well diffusion method. ACTA Biol Exp. 1990;15:113–115. [Google Scholar]
- Qazi S, Middleton B, Muharram SH, Cockayne A, Hill P, O'Shea P, Chhabra SR, Camara M, Williams P. Nacylhomoserine lactones antagonize virulence gene expression and quorum sensing in Staphylococcus aureus. Infect Immun. 2006;74:910–919. doi: 10.1128/IAI.74.2.910-919.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja AF, Khan FA, Khan IA, Shawl AS, Arora DS, Shah BA, Taneja SC. Antistaphylococcal and biofilm inhibitory activities of acetyl-11-keto-beta-boswellic acid from boswellia Serrata. BMC Microbiol. 2011;11:54. doi: 10.1186/1471-2180-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan PA, Novick RP. Subinhibitory cerulenin inhibits staphylococcal exoprotein production by blocking transcription rather than by blocking secretion. Microbiol. 2005;151:3059–3066. doi: 10.1099/mic.0.28102-0. [DOI] [PubMed] [Google Scholar]
- Rohinishree YS, Negi PS. Multiplex reverse transcription polymerase chain reaction to study the expression of virulence and stress response genes in Staphylococcus aureus. J Food Sci. 2012;77:M95–M101. doi: 10.1111/j.1750-3841.2011.02542.x. [DOI] [PubMed] [Google Scholar]
- Sharma M, Anand SK. Characterization of constitutive microflora of biofilms in dairy processing lines. Food Microbiol. 2002;19:627–636. doi: 10.1006/fmic.2002.0472. [DOI] [Google Scholar]
- Shen F, Tang X, Wang Y, Yang Z, Shi X, et al. Phenotype and expression profile analysis of Staphylococcus aureus biofilms and planktonic cells in response to licochalcone a. Appl Microbiol Biotechnol. 2015;99:359–373. doi: 10.1007/s00253-014-6076-x. [DOI] [PubMed] [Google Scholar]
- Shin K, Yun Y, Yi S, Lee HG, Cho JC, et al. Biofilm forming ability of Staphylococcus aureus strains isolated from skin. J Dermatol Sci. 2013;71:130–137. doi: 10.1016/j.jdermsci.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Smith K, Gould KA, Ramage G, Gemmell CG, Hinds J, Lang S. Influence of tigecycline on expression of virulence factors in biofilm-associated cells of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:380–387. doi: 10.1128/AAC.00155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormer FC, Reistad R, Alexander J. Glycyrrhizinic acid in liquorice - evaluation of health hazard. Food Chem Toxicol. 1993;31:303–312. doi: 10.1016/0278-6915(93)90080-I. [DOI] [PubMed] [Google Scholar]
- Vaya J, Belinky PA, Aviram M. Antioxidant constituent from licorice roots. Free Radic Biol Med. 1997;23:302–313. doi: 10.1016/S0891-5849(97)00089-0. [DOI] [PubMed] [Google Scholar]
- Wei GX, Campagna AN, Bokek LA. Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J Antimicrob Agents. 2006;57:1100–1109. doi: 10.1093/jac/dkl120. [DOI] [PubMed] [Google Scholar]
- Witte W. Control of alpha-haemolysin formation by plasmids in distinct strains of S. aureus, influence of erythromycin, rifampicin and streptomycin. In: Jeljaszewicz J, editor. Staphylococci and staphylococcal infection. Stuttgart: Gustar Fisher Verlag; 1975. pp. 298–303. [Google Scholar]
- Wu X, Qian C, Fang H, Wen Y, Zhou J, Zhan ZJ, Ding R, Li O, Gao H. Paenimacrolidin, a novel macrolide antibiotic from paenibacillus sp. F6-B70 active against methicillin-resistant Staphylococcus aureus. Microb Biotechnol. 2011;4:491–502. doi: 10.1111/j.1751-7915.2010.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Deng X, Qiu J. Antimicrobial activity of licochalcone E against Staphylococcus aureus and its impact on the production of staphylococcal alpha-toxin. J Microbiol Biotechnol. 2012;22:800–805. doi: 10.4014/jmb.1112.12020. [DOI] [PubMed] [Google Scholar]