Abstract
This study aimed to determine bioactive components and radical scavenging capacity of black raspberry seed extracts as byproducts obtaining during the juice (FSE) and wine (WSE) making process. Cyanidin-3-O-rutinoside was identified as a major anthocyanin and the total anthocyanin contents of fresh and wine seed were 78.24 and 41.61 mg/100 g of dry weight, respectively. The total phenolic and flavonoid contents of FSE and WSE were 2.31 g gallic acid equivalent (GAE) and 360.95 mg catechin equivalent (CE), and 2.44 g GAE and 379.54 mg CE per 100 g dry weight, respectively. The oxygen radical absorbance capacity (ORAC) values were 1041.9 μM TE/g for FSE and 1060.4 μM TE/g for WSE. Pretreatment of the FSE and WSE inhibited the generation of intracellular reactive oxygen species (ROS), DNA and protein damage induced by hydroxyl radicals, and Fe3+/ascorbic acid-induced lipid peroxidation in a dose dependent manner. WSE more effectively protected from oxidative damage than FSE. Results from the current study suggest that black raspberry seeds as byproducts from juice and wine processing could be potential sources for natural antioxidants.
Keywords: Rubus coreanus Miq., Black raspberry seeds, Juice and winemaking, Byproducts, Seed extract, Antioxidants
Introduction
Living organisms produce reactive oxygen species (ROS) such as superoxide anion, hydroxyl radicals, and hydrogen peroxide through various metabolic pathways. Nucleic acids, proteins, or lipids in biotic systems may be converted to highly reactive free radicals and oxygen species by oxidation and can induce some degenerative diseases including cancer, inflammation, and aging (Abid-Essefi et al. 2004).
Dietary natural antioxidants are known to prevent important biological molecules from oxidative damage, which plays an essential role in human health (Arts and Hollman 2005; Shim and Lim 2013). Thus, the demands for novel food ingredients abundant in natural antioxidants and other valuable elements have been increasing for improving food quality and enhancing human health. Fruit seeds as major byproduct from fruit processing comprise antioxidant compounds including phenolic acids, flavonoids, and anthocyanin, etc., which contribute to developing antioxidant activities (Parry et al. 2006; Bushman et al. 2004). Therefore, fruit seeds have been effectively utilized for industrialized use of natural antioxidants (Peschel et al. 2006; Moure et al. 2001).
Korean black raspberry (Rubus coreanus Miq.), Bokbunja in Korean, is known to those aware of healthy food, and the consumption of Korean black raspberry has been continuously increasing. The concern about black raspberry may be related with its characteristic quality and medicinal activities including anti-inflammatory, anti-cancer, and anti-anaphylactic effects (Shin et al. 2002).
On account of their short shelf life, ripe black raspberry fruits have mostly been used to make processed food including jam, non-alcoholic and alcoholic beverages (Lee et al. 2009). The harvest quantity of Korean black raspberry has decreased as a result of the reduction of cultivation area due to the decline and aging of the rural population and reduced economic life due to climate change. According to the Ministry of Agricultural, Food and Rural Affairs (2013), from 2009 to 2012, the harvest quantity in Jeonbuk Province, one of the largest black raspberry producing districts in Republic of Korea, decreased from 6000 t to 3676 t. However, 64 % of the harvest quantity is used for liquor (54 %) and jam/beverage (10 %) processing, and the rate has been increasing steadily.
Especially, there is massive generation of solid waste such as seeds and pulp from black raspberry fruits during non-alcoholic and alcoholic beverage production. Seed and pulp account for 14 % and 6 % of the entire ripe fruits, respectively. A portion of seed and pulp has been used as animal feed, but most of the by-product has been discarded (Ku and Mun 2008). For that reason, exploration of seed waste as natural antioxidant material is worthwhile where the amount of processing of Korean black raspberry is annually increased.
Therefore, this study aimed to investigate antioxidant compounds and activities of seeds discarded from black raspberry juice and wine processing to increase the utilization of the byproducts. Various assays including oxygen radical absorbance capacity (ORAC), intracellular reactive oxygen species (ROS), DNA, protein, and lipid protection were carried out for measuring free radical scavenging capacities.
Materials and methods
Preparation of samples
Korean black raspberries, harvested and quick-freezed at producing district in June 2009 in Gochang, Republic of Korea, were purchased from local growers. They were divided into two groups. One group was crushed and juiced using a blender (MCH-602, Dongyang Magic Co., Seoul, Korea), and seeds were obtained from this processing. The other group of fruit was fermented by the traditional method to making homemade wine (i.e., black raspberry: sugar: 25 % alcohol =1: 0.2: 2.7, w/w/v), aged for approximately 100 days, and filtered through cheesecloth. Seeds collected from wine processing waste were washed with running water to eliminate fibrous constituents and pigments. The seeds were freeze-dried (Operon OPR-FDU-8603, Gimpo, Korea), powdered by a homogenizer (Polytron PT-MR2100, Kinematica AG, Luzern, Swizerland), sieved through 18 mesh (Testing sieve, Chunggye sanggongsa, Seoul, Korea), and subsequently kept in an air-tight container at −20 °C.
An aliquot amount of seeds (100 g) was defatted three times with 1 L petroleum ether having 0.05 % BHT for 1 h in an ultrasonicator (Bransonic 5510E-DTH, Danbury, CT, USA). After oil extraction, seed residue was extracted twice with 2 L of 70 % aqueous ethanol (ethanol: distilled water =70: 30, v/v) by sonicating for 2 h at room temperature and filtered through Whatman No. 2 filter paper (Whatman Laboratory Products, Clifton, NJ, USA). Crude ethanol extract should be kept in the refrigerator overnight for further dissolution in the solvent and was centrifuged at 3500 rpm for 30 min at 4 °C. The supernatant was evaporated under vacuum at 48 °C (EYELA rotary evaporator N-1000, Tokyo, Japan) to yield the ethanol extract. Hereafter, for simplicity, ethanolic extract of seeds from residues of juice processing and wine processing are denoted as FSE and WSE, respectively. The yields of ethanol extracts from FSE and WSE were 7.12 ± 0.42 and 7.53 ± 0.11 % in turn.
Identification and quantification of anthocyanins
Anthocyanin was analyzed by using a high performance liquid chromatography (HPLC) method (Ha et al. 2010). Freeze-dried fresh or wine seed powder (0.5 g) was extracted with 10 mL of 40 % methanol (0.1 % HCl) for 48 h at 4 °C in the dark and filtration was performed using a 0.45 μm cellulose acetate syringe filter (Millipore, Billerica, MA, USA) prior to injection. HPLC was carried out using a Dionex Ultimate 3000 systems (Thermo scientific, San Jose, CA, USA) and a Luna C18 column (250 × 4.6 mm i.d. Phenomenex, Torrance, CA, USA). The mobile phase contains 0.1 % formic acid in water (A) and 0.1 % formic acid in methanol (B). The gradient condition was as follow: a linear change from A: B (85: 15, v/v) to A: B (65: 35, v/v) for 30 min and then held for 10 min before returning to the initial conditions. The injection volume was 20 μL, flow rate was 0.4 mL/min, and wavelength was 520 nm. Anthocyanins standards were purchased from Extrasynthese (Genay, Cedex, France).
Determination of total phenolic content
The total phenolic content of the seed extract was estimated by the method of Kim and others (2009). Four hundred microliters of 10 % 2 N Folin-Ciocalteau reagents and 800 μL of 10 % sodium carbonate were added into 20 μL of diluted sample. The mixture was kept at room temperature for 1 h. The absorbance was measured at 750 nm by using SpectraMax (Molecular Devices, Sunnyvale, CA, USA). The total phenolic content was expressed as gallic acid equivalent g (GAE)/100 g dry weight.
Determination of total flavonoid content
The total flavonoid content of seed extract was evaluated by a colorimetric method described by Liu and others (2002) with slight modification. Each extract (0.25 mL) was added with 1.25 mL distilled water and 75 μL of 5 % sodium nitrite solution and kept at room temperature for 6 min. Subsequently, 150 μL of 10 % aluminum chloride hexahydrate solution was added and kept for a further 6 min. The reaction was completed by adding 0.5 mL of 1 M sodium hydroxide. Distilled water was added to get the total volume to 5 mL. The absorbance was measured at 510 nm using SpectraMax (Molecular Devices). The total flavonoid content in the sample was expressed as catechin equivalent g (CE)/100 g dry weight.
Oxygen radical absorbance capacity (ORAC) assay
The ORAC assay was adopted from the method described by both Ou et al. (2001) and Zulueta et al. (2009). In each well of 96 well plates, 50 μL of fluorescein (78 nM) and 50 μL of sample, blank, or standard were placed, and then 25 μL of 2,2′-azobis(2-aminopropane) dihydrochloride (AAPH) (221 mM) was added. After the addition, the fluorescence was measured immediately at a 485 nm (excitation wavelength) and a 535 nm (emission wavelength) using SpectraMax (Molecular Devices). The measurements were taken every 2 min until the relative fluorescence intensity (FI %) was less than 5 % of the initial value and expressed as μM trolox equivalents (μM TE).
Intracellular reactive oxygen species (ROS) assay
The intracellular ROS was measured by 2′, 7′-dichlorodihydrofluorescin diacetate (DCFH-DA) method (Han et al. 2011). Concisely, HepG2 cells seeded in a black 96-well were culture in incubator with 5 % CO2 and 37 °C. After 24 h, the media was removed from all wells, washed with Dulbecco’s phosphate buffered saline (DPBS), and then 100 μL of 20 μM DCFH-DA/DPBS solution was added. After pre-incubation for 20 min at 37 °C, 100 μL of diluted extracts with DPBS were added, and incubated for 1 h at 37 °C. The mixture of DCFH-DA and extracts was removed and washed with DPBS, and then treated with 200 μL of 500 μM H2O2. The fluorescent intensity was measured immediately for kinetic analysis at 15 min intervals up to 2 h at a 480 nm (excitation wavelength) and a 530 nm (emission wavelength) with a SpectraMax (Molecular Devices). The results were expressed as relative proportion to the oxidative stress of the control cells..
DNA damage protection assay
The protective effect of FSE and WSE on DNA damage induced by hydroxyl radicals in the plasmid pBR 322 was determined by the method of Li and Wang (2011). Briefly, the reaction mixture was composed of 0.5 μL plasmid pBR 322, 2 μL of 5 mM ferrous sulphate, 2 μL of 50 mM phosphate buffer, and 3 μL of samples. After mixing, 3 μL of 30 % H2O2 was added to each tube and the mixture was kept at 37 °C for 30 min. The DNA was analyzed on 1 % agarose gels, and DNA bands visualized by ethidium bromide staining were analyzed using a Molecular Imager Gel Doc system with Image Lab software (Bio-Rad, Hercules, CA, USA).
Protein damage protection assay
The effect of FSE and WSE on protein oxidation was measured by the method of Hu and others (2009b). Two hundred microliters of 4 mg/mL bovine serum albumin (BSA) was mixed with 200 μL of H2O or 1 mg/mL seed extracts. The mixture was kept at 37 °C for 3 h with 600 μL reagent solution (50 μM FeCl3, 1 mM H2O2, and 100 μM ascorbic acid). BSA without Fenton’s reagent was used as the positive control. After incubation, the mixture was mixed with a loading buffer and further incubated at 95 °C for 10 min. After 3 h, protein samples were subjected to sodium dodecylsulfate-polyacrylamide gel (SDS-PAGE) to quantify protein damage. The protein sample was analyzed on 12.0 % polyacrylamide gel and gel was stained with 0.15 % Coomassie brilliant blue R-250. Densitometric analysis was performed using a Molecular Imager Gel Doc system with Image Lab software software (Bio-Rad).
Lipid peroxidation inhibitory activity
Male ICR mice (n = 10), aged 6 weeks, purchased from Orient (Seongnam, Korea) and the protocols for animal experiment were approved by the Institutional Animal Care and Use Committee (IACUC) of Duksung Women’s University. Mice were provided with standard mouse diet and water ad libitum. After 1 week adaptation period, mice were killed by cervical dislocation and their liver tissues were rapidly removed. Liver tissue was homogenized with 5 mL 1 × lysis buffer (150 mM NaCl, 0.5 % TritonX-100, 0.1 mM EDTA, 10 mM Tris, pH 7.5) per gram tissue, centrifuged at 3500 rpm for 30 min, and tissue lysate supernatant was collected. According to the method of Yoshiyuki (1981), the reaction mixture was composed of 0.1 mL of liver homogenate, 0.25 mL of Tris-HCl buffer (pH 7.2), 0.05 mL of 4 mM FeCl3, 0.1 mM ascorbic acid, and samples. After the mixture was kept at 37 °C for 1 h, 0.5 mL of 0.1 N HCl, 0.2 mL of 9.8 % SDS, 0.9 mL of distilled water and 2 mL of 0.6 % thiobarbituric acid (TBA) were added and vigorously shaken. The tubes were placed in a boiling water bath at 100 °C for 30 min. After cooling, the flocculent precipitate was removed by adding 5 mL buthanol and centrifuged at 3000 rpm for 25 min. The absorbance of thiobarbituric acid reacting substance (TBARS) was measured at 532 nm using SpectraMax (Molecular Devices).
Statistical analysis
All experiments were conducted in triplicate (n = 3), and data were expressed as mean ± standard deviation (SD). The data were statistically analyzed by using statistical software, SAS version 9.1 (SAS institute Inc. Cary, NC, USA). One-way analysis of variance (ANOVA) was conducted, and significant difference was defined at p < 0.05 with Duncan’s multiple range test.
Results and discussion
Identification and quantification of anthocyanin in fresh and wine seed powder
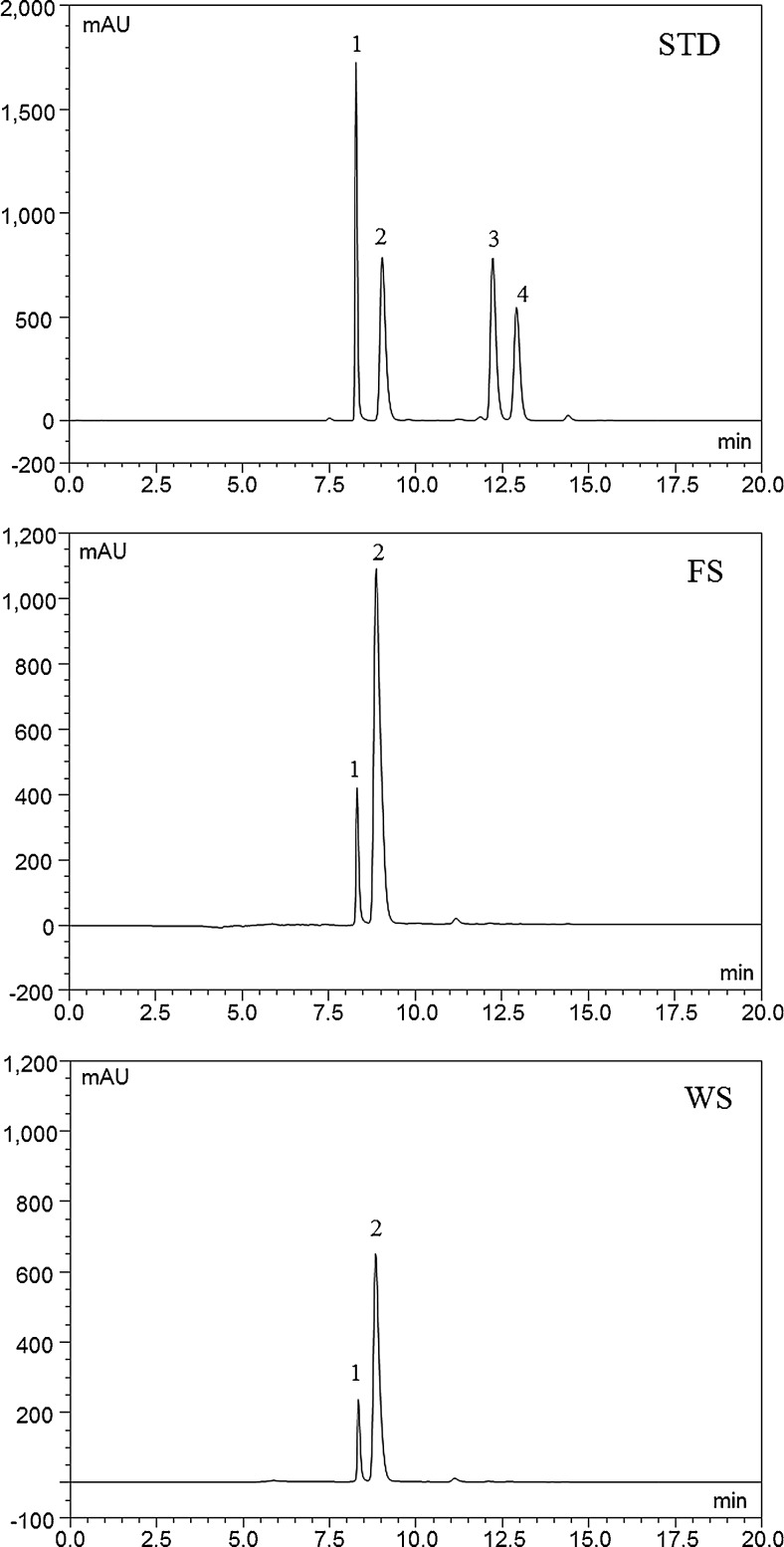
Many studies have shown anthocyanin to exhibit a substantial in vitro antioxidant capacity and a wide range of potential therapeutic effects including anti-inflammatory, anti-cancerogenic effect, and antiobesity (Wang and Stoner 2008; Kong et al. 2003). The HPLC chromatograms of anthocyanin standards, fresh seed, and wine seed were shown in Fig. 1. In both samples, three anthocyanin peaks were detected and a major peak was cyanidin-3-O-rutinoside (C-3-R, peak #2), which account for about 85 % of the total peak area. Cyanidin-3-O-glucoside (C-3-G, peak #1) and an unidentified minor peak (peak #3) was comprised less than 15 % of total peak area. The total anthocyanin content was expressed as the sum of C-3-R and C-3-G, and that of fresh and wine seed was 78.24 ± 0.44 and 41.61 ± 0.29 mg/g of seed powder, respectively. The total anthocyanin content of wine seed was relatively lower than that of fresh seed due to the loss of anthocyanin pigment during fermentation process.
Fig. 1.
HPLC chromatograms of anthocyanin standards (STD), fresh seed (FS), and wine seed (WS) at 520 nm. Peak #1, cyanidin-3-glucoside; #2, cyanidin-3-rutinoside; #3, peonidin-3-glucoside; #4, malvidin-3-gucoside
Total phenolic and flavonoid content of FSE and WSE
The total phenolic and flavonoid contents of FSE and WSE are shown in Table 1. The total phenolic content of WSE (2.44 ± 0.02 g GAE/100 g, dry weight) exhibited somewhat higher than that of FSE (2.31 ± 0.08 g GAE/100 g, dry weight. The total flavonoid content of FSE and WSE were 360.95 ± 15.12 and 379.54 ± 12.51 mg CE/100 g dry weight, respectively. Phenolic content of FSE and WSE were higher than those of reported for other berry fruit seeds, such as blueberry (1.58 g GAE/100 g, dry weight) and cranberry (1.46 g GAE/100 g, dry weight) (Parry et al. 2006), whereas total phenol contents of boysenberry (4.91 g GAE/100 g, dry weight), marion blackberry (5.03 g GAE/100 g, dry weight), and evergreen blackberry (5.44 g GAE/100 g, dry weight) were higher than those of FSE and WSE (Bushman et al. 2004). Ju et al. (2009) reported that the phenolic compound contents of Bokbunja increased as a result of the fermentation process. In addition, Puértolas et al. (2011) stated that phenolic compounds of red wines were changed during alcoholic fermentation. These changes of phenolic compounds might have been due to generation and increase of active compounds by bioconversion. Consequently, both total phenolic and flavonoid contents of WSE were slightly higher than those of FSE, but there were no statistically significant differences between fresh and wine seed of Korean black raspberry.
Table 1.
Total phenolic and flavonoid content of ethanolic extracts of fresh seed (FSE) and wine seed (WSE) of Korean black raspberry
| Sample1 | Total phenolic (g GAE/100 g, dry weight)2 |
Total flavonoid (mg CE/100 g, dry weight)2 |
|---|---|---|
| FSE | 2.31 ± 0.083a | 360.95 ± 15.12a |
| WSE | 2.44 ± 0.02a | 379.54 ± 12.51a |
1Abbreviation; FSE; ethanolic extract of seeds from residues of juice processing, WSE; ethanolic extract of seeds from residues of wine processing, GAE; gallic acid equivalent, CE; catechin equivalent
2Results are expressed as mean ± standard deviation (SD; n = 3)
3Values followed by the same superscript mark are not significantly different (p < 0.05)
Oxygen radical absorbance capacity
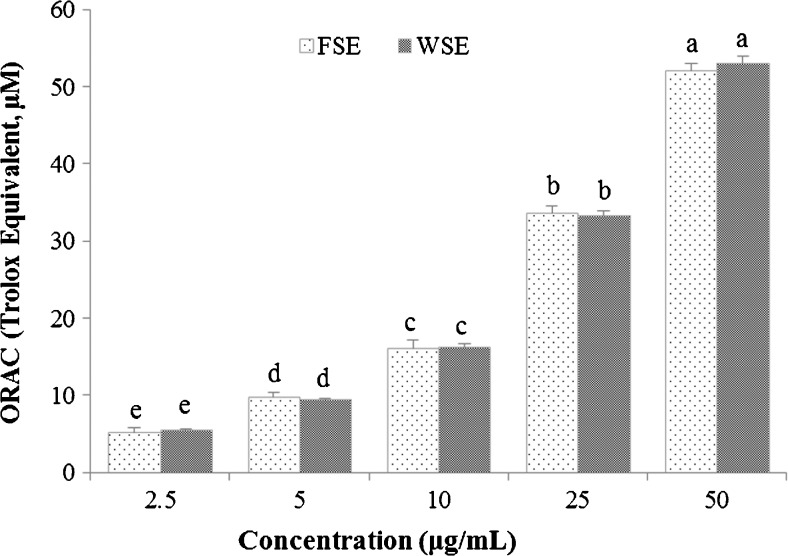
The ORAC assay is the most generally used a hydrogen atom transfer (HAT) process, which induced the oxidation of a fluorescent probe by peroxyl radicals (Ou et al. 2001). The peroxyl radical oxidation of the fluorescent probe was blocked by antioxidant substances existing in FSE and WSE until the antioxidant activity is terminated. The residual peroxyl radicals destroy the fluorescence of the fluorescent probe. The ORAC values of FSE and WSE showed siginificant increase in a concentration-dependent manner (p < 0.05), as shown in Fig.2. WSE was slightly higher than FSE, but there was no significant difference. At a concentration of 50 μg/mL, the ORAC values of FSE and WSE were 1041.9 and 1060.4 μM TE/g extract, respectively. The ORAC values of FSE and WSE were similar, this seems to be similar of their phenolic contents. Ehlenfeldt and Prior (2001) reported that ORAC value is increased in proportion to the total phenolic content. Parry et al. (2006) also reported that the ORAC values of 50 % acetone extract of cranberry, blueberry, black raspberry, and chardonnay grape seed were 110.5, 152.9, 296.2, and 1076 μM TE/g flour, respectively. These ORAC values were correlated to total phenolic content. These data suggest that fruit seeds including Korean black raspberry seeds are excellent dietary sources for oxygen radical absorbing components.
Fig. 2.
ORAC values corresponding to different concentrations of ethanolic extracts of fresh seed (FSE) and wine seed (WSE). Different letters above bars indicate significant differences between concentratons (p < 0.05)
Inhibitory effects on intracellular reactive oxygen species (ROS)
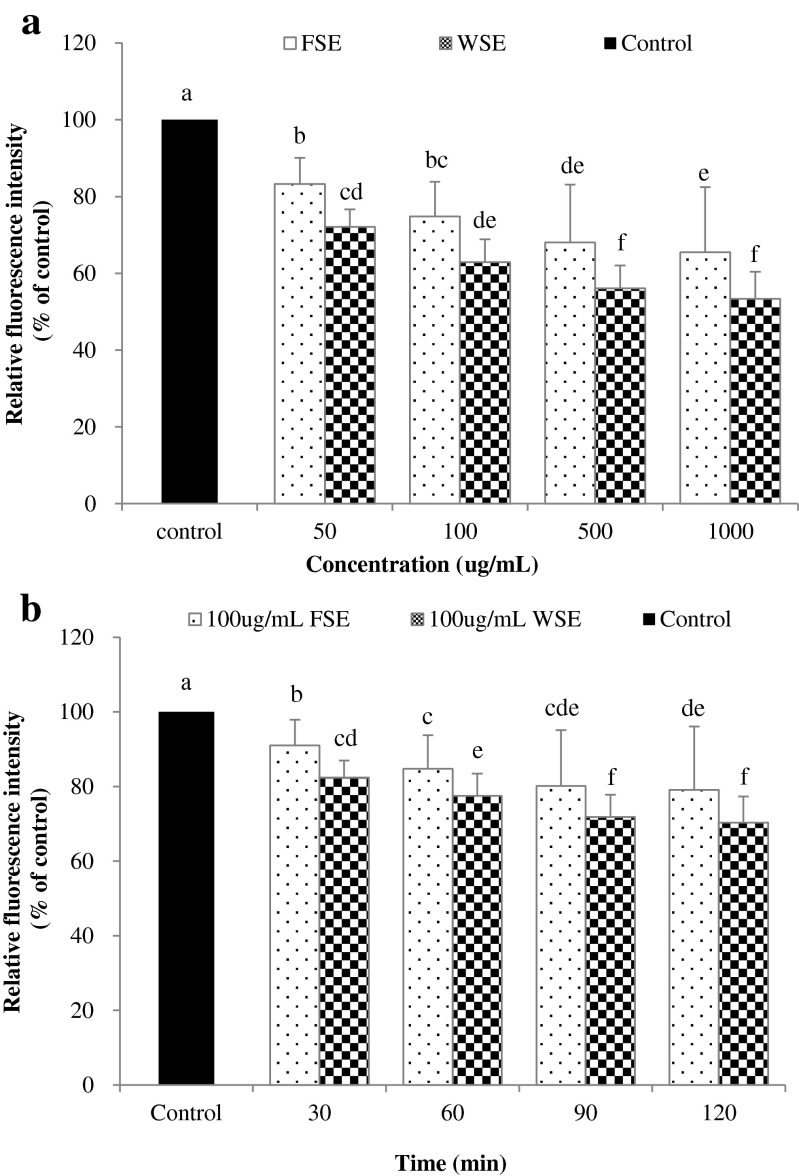
To determine the protective effect on H2O2-induced oxidative stress, a 2′, 7′-dichlorodihydrofluorescin diacetate (DCFH-DA) assay was conducted. Accumulation of ROS accompanied with an increase in oxidative stress has been associated with the development of several diseases. Free radicals and other reactive species generated in the human body caused oxidative damage to biomolecules including nucleic acids, proteins, and lipids (Jiang et al. 2011). The formation of intracellular ROS was measured by using oxidation sensitive dye, non-fluorescent DCFH-DA, as the substrate. It penetrates cells and is deacetylated by cellular esterases to non-fluorescent DCFH, which is rapidly oxidized to highly fluorescent DCF by ROS (Veerman et al. 2004). As shown in Fig. 3a, pretreatment with the FSE and WSE appeared to significantly inhibit the generation of intracellular ROS in a concentration-dependent manner (p < 0.05). The values of relative fluorescence intensity were reduced up to 65.5 and 53.4 % at 1000 μg/mL concentration of FSE and WSE, respectively. In a result of MTT assay, IC50 values of FSE and WSE were 117.10 and 156.02 μg/mL, respectively (Data not shown). In Fig. 3b, the formation of intracellular ROS was considerably inhibited up to 20.9 and 29.7 % of FSE and WSE, respectively, when treated with a concentration of 100 μg/mL based on MTT assay. It was indicated that WSE was more effective in the protection of cells from oxidative damage by ROS than FSE. Shahidi and Wanasundara (1992) reported that the antioxidant effect was associated with the contents of phenolic compounds existing in plants. The study suggested that phenolic hydroxyl groups of phenolic compounds and flavonoids provided reducing power by donating electron or hydrogen. Results from the current study imply that FSE and WSE can protect cells from oxidative damage by ROS and be developed into antioxidants to inhibit the formation of intracellular ROS.
Fig. 3.
Inhibitory effect of fresh seed extract (FSE) and wine seed extract (WSE) against intracellular reactive oxygen species generation in HepG2 cells as measured by DCFH-DA assay. HepG2 cells were labeled with DCFH-DA and treated with different concentrations of FSE and WSE (a) and treated with same concentration (100 μg/mL) of FSE and WSE (b). Fluorescence intensities of DCF due to oxidation of DCFH by cellular ROS generated by H2O2 were detected after 2 h (Ex = 485 nm and Em = 535 nm). Different letters above bars indicates significant different from control
Protective effects against DNA damage
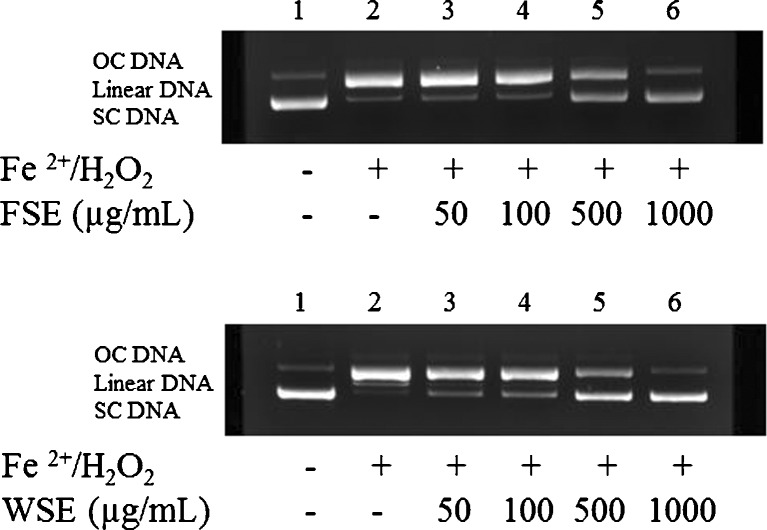
Martinez et al. (2003) reported that a DNA is another major sensitive biotarget of ROS mediated oxidative damage. DNA damage by ROS can initiate carcinogenesis or beget the pathogenesis of neurodegenerative diseases (Je et al. 2009). The protective effects of FSE and WSE against DNA damage caused by free radicals were estimated using plasmid pBR 322 in vitro. The pBR 322 DNA was broken into three forms– supercoiled (SC), open circular (OC), and linear form (Linear) – when it was exposed to the hydroxyl radical derived from the Fenton reaction. As shown in Fig. 4, FSE and WSE showed the protective effects against DNA damage induced by the hydroxyl radicals. The intact SC form of control (Line 1) was entirely converted to the OC form owing to the hydroxyl radical produced from the Fenton reaction by treatment of Fe2+/H2O2 (Line 2). Moreover, FSE and WSE dose-dependently protected the hydroxyl radical induced DNA damage (Line 3–6). The protective effect of WSE against DNA damage was more effective than that of FSE, which is due to the difference of polyphenol content. Hu et al. (2009a) reported that, as the total phenolic content of Kalopanax pictus Leaf were increased, the protective effect against DNA damage was larger. Hu et al. (2009b) also reported that methanolic extract from Duchesnea indica containing tannic acid was observed to protect SC DNA by 9.23, 71.4, and 92.3 % at the concentration of 100, 500, and 1000 μg/mL, respectively. Besides, Li and Wang (2011) showed that methanolc extract of peach blossom was more effective in protection of DNA damage by ROS than water extract of peach blossom, which had high content of total phenolic and flavonoid in the methanolic extract of peach blossom. Therefore, these results obviously explain the protective effect of the FSE and WSE against oxidative damage of DNA.
Fig. 4.
Agarose gel electrophoretic patterns of plasmid DNA breaks by ·OH generated from a Fenton reaction in the presence of ethanolic extracts of fresh seed (FSE) and wine seed (WSE). An amount of 0.5 μL of pBR 322 DNA was incubated 37 °C for 30 min in FeSO4 and 30 % H2O2 with the following additive combinations. Line 1, no addition (plasmid DNA); Line 2, FeSO4 and 30 % H2O2 (DNA damage control); Line 3–6, FeSO4 and 30 % H2O2 in the presence of FS and WS with concentrations of 50, 100, 500, and 1000 μg/mL, respectively
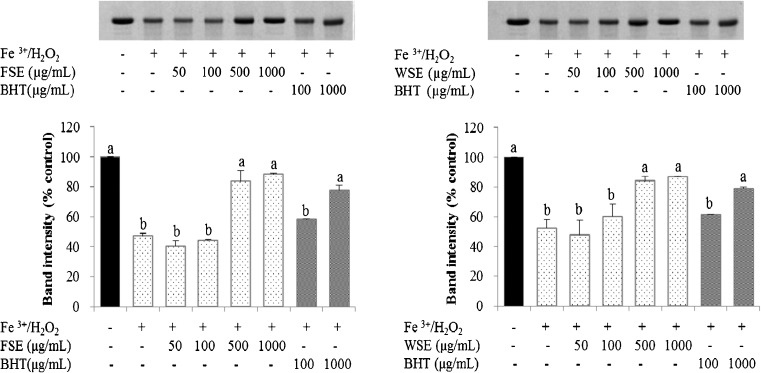
Protective effect against protein oxidation
It has been demonstrated that protein damage induced by free radicals provoked aging and the development of other diseases (Du and Gebicki 2004). This could be introduced by electron leakage, metal ion dependent reactions, and autoxidation of lipids and sugars (Bahramikia et al. 2009). In the present study, protein damage was induced by the Fe3+/H2O2/ascorbic acid system. Electrophoretic patterns of BSA in the treatment with the FSE, WSE, and BHT were shown in Fig. 5. The band intensity of BSA decreased to 47.1 % of the control in the Fe3+/H2O2/ascorbic acid system. A 500 and 1000 μg/mL of FSE repaired the BSA band intensity to 83.6 and 86.9 % of the initial level, respectively. The intensity of the BSA band was recovered to 84.1 and 88.3 % of the control group under the treatment of 500 and 1000 μg/mL concentration of WSE, individually. Similar to the protection against DNA damage, WSE showed somewhat greater protein protection than FSE. However, in the case of positive control, BHT showed approximately 78.2 % protection at 1000 μg/mL concentration. Hu et al. (2009b) also reported that the protection effect of BSA oxidative damage by methanolic extract from Duchesnea indica was even greater than water extract from Duchesnea indica and BHT. Results from the current study suggest that FSE and WSE were more effective than BHT in the protection of protein damage induced by Fe3+/H2O2/ascorbic acid.
Fig. 5.
Protective effect of BSA oxidative damage treated with Fe3+/H2O2 system in the presence of fresh seed extract (FSE) and wine seed extract (WSE). The densitometry analysis of protective effects at different concentrations of BHT (positive control) and seed extracts on protein. Relative band intensity was calculated % of control
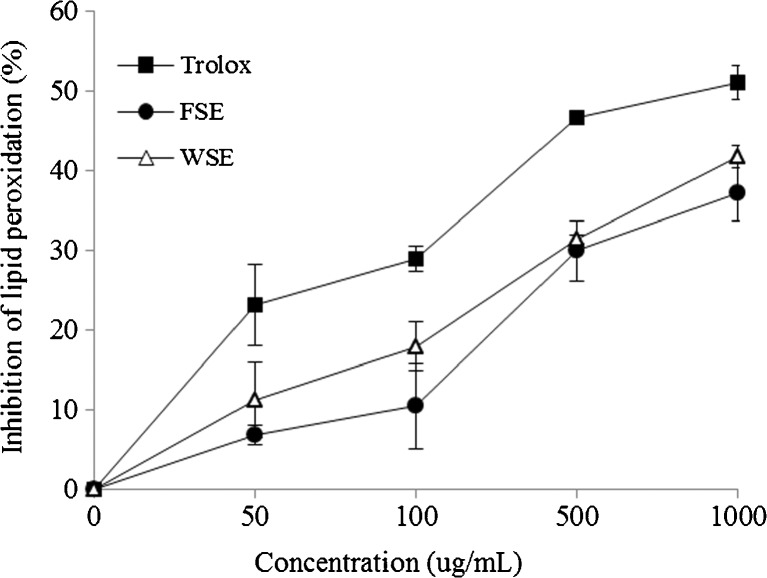
Inhibitory effect on lipid peroxidation
Unsaturated molecules such as unsaturated fatty acid abundant in membrane lipid are most subject to oxidative processes induced by the action of free radicals in cells and tissue, resulting in lipid peroxidation (Mayumi et al. 1993). Kim et al. (2000) reported that the tannins, ellagitannins, and condensed tannins from stem and leaf of Rubus coreanum showed potent antioxidative activities by TBARS. In the present study, the lipid peroxidation inhibitory effect of FSE and WSE in mouse liver homogenate induced by non-enzymatically with Fe3+/ascorbic acid was estimated by measuring the TBARS form. The lipid peroxidation inhibitory effects of FSE and WSE were in a dose-dependent manner (Fig. 6). The lipid peroxidation inhibition of Trolox showed nearly 28.9 and 51.0 % at a concentration of 100 and 1000 μg/mL, respectively. In the case of WSE, inhibition of lipid peroxidation exhibited 17.9 and 41.7 % at a concentration of 100 and 1000 μg/mL, respectively. However, FSE showed slightly lower activity as 37.2 % inhibition at a concentration of 1000 μg/mL than those of trolox and WSE. Also, the IC50 values of the inhibitory effect on lipid peroxidation of FSE and WSE were 1255.3 and 1132.1 μg/mL, respectively, indicating that WSE was more effective in the inhibition of lipid peroxidation induced by Fe3+/ascorbic acid than FSE. These results suggested that the WSE showed a significant anti-lipid peroxidation activity.
Fig. 6.
Inhibitory effect of ethanolic extracts of fresh seed (FSE) and wine seed (WSE) on lipid peroxidation. Trolox was used as a positive control. Each value is expressed as the mean (n = 3)
Conclusion
Massive quantities of seeds as byproducts are generated during black raspberry juice and winemaking procedure. The results presented here concluded that ethanolic extract of seeds contained significant amount of phenolic acids and flavonoids, resulting in scavenging cellular reactive oxygen species. It is suggested that the byproducts discarded from black raspberry juice and winemaking process may have considerable potential as natural antioxidant, diet nutrient and functional food materials. However, further studies are needed to elucidate specific mechanism by in vivo model.
Acknowledgments
This work was supported by the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0094017).
Footnotes
Research highlights
• Black raspberry seeds are produced during juice and winemaking procedure.
• Total phenolic content and flavonoids in seed extracts were determined.
• Pretreatment of seed extracts inhibited the generation of reactive oxygen species.
• Seed extracts protected DNA, protein, and lipid against cellular oxidative damage.
• Seeds as byproducts could have a potential as natural antioxidants.
Contributor Information
Soon-Mi Shim, Phone: +82-2-3408-3229, Email: soonmishim@sejong.ac.kr.
Gun-Hee Kim, Phone: +82-2-901-8496, Email: ghkim@duksung.ac.kr.
References
- Abid-Essefi S, Ouanes Z, Hasen W, Baudrimont I, Creppy EE, Bacha H. Cytotoxicity, inhibition of DNA and protein syntheses and oxidative damage in cultured cells exposed to zearalenone. Toxicol in Vitro. 2004;18:467–474. doi: 10.1016/j.tiv.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317–325. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- Bahramikia S, Ardestani A, Yazdanparast R. Protective effects of four Iranian medicinal plants against free radical mediated protein oxidation. Food Chem. 2009;115:37–42. doi: 10.1016/j.foodchem.2008.11.054. [DOI] [Google Scholar]
- Bushman BS, Phillips B, Isbell T, Ou B, Crane JM, Knapp SJ. Chemical composition of caneberry (rubus spp.) seeds and oils and their antioxidant potential. J Agric Food Chem. 2004;52:7982–7987. doi: 10.1021/jf049149a. [DOI] [PubMed] [Google Scholar]
- Du J, Gebicki JM. Proteins are major initial cell targets of hydroxyl free radicals. Int J Biochem Cell Biol. 2004;36:2334–2343. doi: 10.1016/j.biocel.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Ehlenfeldt MK, Prior RL. Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbush blueberry. J Agric Food Chem. 2001;49:2222–2227. doi: 10.1021/jf0013656. [DOI] [PubMed] [Google Scholar]
- Ha TJ, Lee MH, Park CH, Pae SB, Shim KB, Ko JM, Shin SO, Baek IY, Park KY. Identification and characterization of anthocyanins in yard-long beans (Vigna unguiculata ssp. Sesquipedalis L.) by high performance liquid chromatography with diode array detection and electrospray ionization/mass spectrometry (HPLC-DAD-ESI/MS) analysis. J Agric Food Chem. 2010;58:2571–2576. doi: 10.1021/jf903883e. [DOI] [PubMed] [Google Scholar]
- Han W, Hu W, Lee YM. Anticancer activity of human colon cancer (HT-29) cell line from different fraction of zanthoxylum schnifolium fruits. Korean J Pharmacogn. 2011;42:282–287. [Google Scholar]
- Hu W, Heo SI, Wang MH. Antioxidant and anti-inflammatory activity of kalopanax pictus leaf. J Korean Soc Appl Biol Chem. 2009;52:360–366. doi: 10.3839/jksabc.2009.064. [DOI] [Google Scholar]
- Hu W, Shen W, Wang MH. Free radical scavenging activity and protective ability of methanolic extract from duchesnea indica against protein oxidation and DNA damage. J Food Sci Nutr. 2009;14:277–282. doi: 10.3746/jfn.2009.14.4.277. [DOI] [Google Scholar]
- Je JY, Ahn CB, Oh MJ, Kang SY. Antioxidant activity off a red seaweed polysiphonia morrowii extract. Food Sci Biotechnol. 2009;18:124–129. [Google Scholar]
- Jiang L, Dai H, Sun Q, Geng C, Yang Y, Wu T, Zhang X, Zhong L. Ambient particulate matter on DNA damage in HepG2 cells. Toxicol Ind Health. 2011;27:87–95. doi: 10.1177/0748233710387001. [DOI] [PubMed] [Google Scholar]
- Ju HK, Cho EJ, Jang MH, Lee YY, Hong SS, Park JH, Kwon SW. Characterization of increased phenolic compounds from fermented bokbunja (rubus coreanus Miq.) and related antioxidant activity. J Pharm Biomed Anal. 2009;49:820–827. doi: 10.1016/j.jpba.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Kim KH, Lee YA, Kim JS, Lee DI, Choi YW, Kim HH, Lee MW. Antioxidative activity of tannins from rubus coreanum. Yakhak Hoeji. 2000;44:354–357. [Google Scholar]
- Kim SI, Sim KH, Ju SY, Han YS. A study of antioxidative and hypoglycemic activities of omija (schizandra chinensis baillon) extract under variable extract conditions. Korean J Food Nutri. 2009;22:41–47. [Google Scholar]
- Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923–933. doi: 10.1016/S0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- Ku CS, Mun SP. Antioxidant activities of ethanol extracts from seeds in fresh bokbunja (rubus coreanus Miq.) and wine processing waste. Bioresour Technol. 2008;99:4503–4509. doi: 10.1016/j.biortech.2007.08.063. [DOI] [PubMed] [Google Scholar]
- Lee JH, Whang JB, Youn NR, Lee SY, Lee HJ, Kim YJ, Koh KH. Antioxidant and oxygen radical scavenging capacities of the extracts of pear cactus, mulberry and Korean black raspberry fruit. J Food Sci Nutri. 2009;14:188–194. doi: 10.3746/jfn.2009.14.3.188. [DOI] [Google Scholar]
- Li C, Wang MH. Antioxidant activity of peach blossom extracts. J Korean Soc Appl Biol Chem. 2011;54:46–53. [Google Scholar]
- Liu M, Li XQ, Weber C, Lee CY, Brown J, Liu RH. Antioxidant and antiproliferative activities of raspberries. J Agric Food Chem. 2002;50:2926–2930. doi: 10.1021/jf0111209. [DOI] [PubMed] [Google Scholar]
- Martinez GR, Loureiro AP, Marques SA, Miyamoto S, Yamaguchi LF, Onuki J. Oxidative and alkylating damage in DNA. Mutat Res. 2003;544:115–127. doi: 10.1016/j.mrrev.2003.05.005. [DOI] [PubMed] [Google Scholar]
- Mayumi T, Schiller HJ, Bulkley GB. Pharmaceutical intervention for the prevention of post-ischemic reperfusion injury. In: Poli G, Albano E, Dianzani MU, editors. Free radicals-from basic science to medicine. 1st. Switzerland: Birkhäuser Verlag; 1993. pp. 438–457. [Google Scholar]
- Ministry of Agricultural, Food and Rural Affairs (2013) 2012-Current Status of Fruits Processing. http://library.mafra.go.kr/skyblueimage/16702.pdf. Accessed 15 April 2014
- Moure A, Cruz JM, Franco D, Domı’nguez JM, Sineiro J, Domı’nguez H, Nu’nez MJ. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- Parry J, Su L, Moore J, Cheng Z, Luther M, Rao JN, Wang JY, Yu LL. Chemical compositions, antioxidant capacities, and antiproliferative activities of selected fruit seed flours. J Agric Food Chem. 2006;54:3773–3778. doi: 10.1021/jf060325k. [DOI] [PubMed] [Google Scholar]
- Peschel W, Sanchez-Rabaneda F, Diekmann W, Plescher A, Gartzıa I, Jimenez D, Lamuela-Raventos R, Buxaderas S, Codina C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006;97:137–150. doi: 10.1016/j.foodchem.2005.03.033. [DOI] [Google Scholar]
- Puértolas E, Álvarez I, Raso J. Changes in phenolic compounds of Aragón red wines during alcoholic fermentation. Food Sci Technol Int. 2011;17:77–86. doi: 10.1177/1082013210368555. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Wanasundara PK. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- Shim SM, Lim SY. Texture properties and radical scavenging ability of porridge products based on beans, grains, and nuts. J Korean Soc Appl Biol Chem. 2013;56:77–82. doi: 10.1007/s13765-012-2219-x. [DOI] [Google Scholar]
- Shin TY, Kim SH, Lee ES, Eom DO, Kim HM. Action of rubus coreanus extract on systemic and local anaphylaxis. Phytother Res. 2002;16:508–513. doi: 10.1002/ptr.925. [DOI] [PubMed] [Google Scholar]
- Veerman EC, Nazmi K, Van’t Hof W, Bolscher JG, Den Hertog AL, Nieuw Amerongen AV. Reactive oxygen species play no role in the candidacidal activity of the salivary antimicrobial peptide histatin 5. Biochem J. 2004;15:447–452. doi: 10.1042/BJ20040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269:281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyuki K, Michinori K, Tadato T, Shigeru A, Hiromichi O. Studies on scutelariae Radix: IV. Effects on Lipid peroxidation in rat Liver Chem Pharm Bull. 1981;29:2610–2617. doi: 10.1248/cpb.29.2610. [DOI] [PubMed] [Google Scholar]
- Zulueta A, Esteve MJ, Frigola A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009;114:310–316. doi: 10.1016/j.foodchem.2008.09.033. [DOI] [Google Scholar]