Abstract
The chemical stability of the green tea (GT) preparation during refrigerated storage was investigated following the addition of mint (MS) or pomegranate (PS) syrups, a common habit in the Mediterranean countries that improves the savor of this popular beverage. The supernatants recovered by centrifuging GT supplemented or not with mint (GTMS) or pomegranate (GTPS) syrup were examined for their polyphenolic profiles using the high performance liquid chromatography with diode array detection and electrospray ionization-mass spectrometry. Following storage at 4 °C for 15 days, not-supplemented GT showed a significant decrease (≈92 %) of its phenolic content. However, the decrease was relatively lesser in GTPS (≈36 %) and in GTMS (≈40 %). The observed slight increase of the extractable polyphenolics in PS and MS during the storage might explain in part the relatively limited decrease of GTPS and GTMS total phenolic content. However, chromatographic examination proved that some tea compounds, particularly caffeine, were preserved following PS and MS supplementation. Likewise, syrups’addition to GT significantly (P < 0.5) limited the reduction of its antioxidant capacity as revealed by the DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-azino-bis-(3-ethylbenz-thialzoline-6-sulfonic acid)) assays. As expected, the antimicrobial trials showed that Gram (+) Staphylococcus aureus and Staphylococcus epidermidis were the most sensitive strains to tea polyphenols. The syrups supplementation noticeably preserved the tea bacteriostatic and bactericide activities during storage. The obtained analytical results demonstrate that MS or PS addition to green tea beverage stabilized its polyphenolic content and biofunctional properties during refrigerated storage, thus, scientifically supporting this popular practice in the Mediterranean countries.
Keywords: Antioxidant, Antimicrobial, Green tea, Mint syrup, Pomegranate syrup, Stability
Introduction
Without a doubt, the necessary biochemical reactions for life require intake of nutrients. During the last decades, bioactive molecules from fruits and vegetables have attracted a great deal of attention mainly due to their role in preventing acute and chronic diseases (Mansouri et al. 2005; Esfahanian et al. 2013). In this context, scientists took advantage of the innovative analytical methods and intensively focused their efforts to develop effective and safer agents starting from natural sources. Biomolecules, especially lipophilic flavonoids and phenolic acids, have been considered the main players in the beneficial effects on the human health (Amzad and Mizanur 2011). Hence, several polyphenols have been expected to play key roles in maintaining and improving our health. In addition, these compounds are thought to be sources of precursors for creating new and better therapeutic agents (Tao et al. 2010). Nowadays, food and beverage trends are ever more focused toward healthful and mindful consumers, a statement largely and extensively used in advertising and marketing. At the same time, scientific analytical reports continue emphasizing the advantages and benefits of some foods and beverages, especially traditional and folkloric nutritional products that are drawing local and international market tendencies.
Tea is the most widely consumed beverage in the world. Black, oolong, and green tea are produced from the leaf and buds of the tea plant (Camellia sinensis). Recent studies have shown that tea confers great beneficial effects to the health of consumers since reducing cholesterol and hypertension, and could act as antioxidant, antimicrobial, antiviral and may protect against some cardiovascular diseases and cancers (Saklar et al. 2015). To understand the mechanisms involved in these beneficial effects, a great deal of scientific efforts has been contributed to isolate and identify the active components in various tea preparations (Gramza and Korczak 2005).
Green tea consumption is predominant in Occidental and Asiatic countries as well as in Middle East and North Africa (Hamilton 1995; Yi et al. 2014; Dhaouadi et al. 2013). Typically, green tea decoction constitutes a popular beverage in the North African countries. This decoction, different from infusion, is prepared by cooking (boiling) the dried tea leaves for a relatively long period of time (Dhaouadi et al. 2009). In Mediterranean countries, including Tunisia, pomegranate or mint syrups are commonly added to green tea preparations. It is empirically known that pomegranate constitutes a medicinal and nutritional food source. Pomegranate pericarp (peel, rind) and seeds contain several high-value compounds, particularly punicic acid, punicalgins, flavones, flavonones, and flavanols, with beneficial health promoting potentials (Barberan et al. 2006). The pomegranate peel and seed extracts have been shown to prevent liver fibrosis and exhibit anti-inflammatory, anti-mutagenic, and antimicrobial activities (Çayır et al. 2011; Zahin et al. 2010; Endo et al. 2010). The diverse beneficial effects of these biomolecules stimulated their use in numerous novel functional food formulations that replies to the local and international market tendencies. Recently, pomegranate derived products, including juices and syrups, have become popular beverages and have been considered as good supplements for foods and dietetics (Lansky et al. 1998; Tezcan et al. 2009). Mint (Mentha spicata) leaves, commonly called “spearmint”, are extensively used as medicinal herb all over the world. This Lamiaceae is considered as stimulant, carminative, antispasmodic, stomachic and diuretic, and is used in the treatment of gas pain, rheumatism, toothache as well as muscle pain. Padmini et al. (2010) reported that mint possesses strong antioxidant properties due to the presence of active constituents like menthone, menthol, rosmarinic acid and carvone.
The common addition of mint and pomegranate syrup to green tea beverage in Tunisia is supposed not only to improve its organoleptic properties but also to strengthen its bioactive potentials, a popular assumption that has not been supported by scientific works. Hence, the aim of the present study was to determine the effect of the supplementation of mint and pomegranate syrups to green tea decoction, and to evaluate the stability of this popular beverage during refrigerated (4 °C) storage in terms of its functional properties.
Materials and methods
Samples preparations
The commercial green tea leaves (“Tea Garden” of the Tunisian Office of Commerce), mint and pomegranate syrups (70 °Brix) were purchased from local supermarkets. Green tea decoction was prepared by cooking 40 g of tea leaves in one liter of water for 15 min (Dhaouadi et al. 2009). The decoction was then filtered through 0.45 μm Teflon membrane (Millipore). Similarly, mint (MS) and pomegranate (PS) syrups were filtered prior to their use. Dark bottles were used to store 100 ml of each sample. GT samples consisted of green tea supplemented with distilled water (9 v/1v). Based on the same relative proportions, GTPS and GTMS were prepared by mixing GT (9 v) with PS (1v) and MS (1v), respectively. Mixtures (9v/1v) containing water/PS or water/MS were used as controls. All samples were stored in the refrigerator (4 ± 1 °C) during 15 days. Aqueous-acetone polyphenolic extracts were prepared at 0, 6 and 15 day of storage by mixing thoroughly 3 ml from each sample with 7 ml of cold acetone (−20 °C) (Fattouch et al. 2007). Following a centrifugation at 3500 rpm for 15 min at room temperature, the supernatant was collected and acetone was evaporated using a rotary evaporator (40 °C) until reaching 3 ml of final volume. The obtained polyphenolic extracts were immediately used or stored at −20 °C until further use.
Determination of the total phenolic compounds (TPC)
In order to estimate the total phenolic content (TPC) of the different samples prior to HPLC analysis, the colorimetric Folin-Ciocalteu method described by Yu et al. (2014) with slight modifications was used. To test tube containing 2 ml Folin-Ciocalteu reagent (Sigma-Aldrich, France) diluted 10 fold with distilled water (0.2 μS cm−1), 0.5 ml of the appropriately water-diluted polyphenolic extract were added, mixed thoroughly, and then incubated for 5 min. After addition of 2.5 ml of sodium carbonate (7.5 %, w/v) and incubation at room temperature for 30 min in the dark, the absorbance of the resulting solution was measured at 765 nm in an S-22 UV/VIS spectrophotometer (BOECO, Germany). TPC was expressed as gallic acid equivalent (GAE) per 100 ml of starting sample (GT, GTPS, GTMS, PS or MS).
Reversed phase HPLC-DAD-MS
The High-performance liquid chromatography (HPLC) separation of polyphenolic compounds was performed following the previously reported method of Dhaouadi et al. (2013) with some modifications. The used Agilent LC1100 series (Agilent Technologies, Inc.,Palo Alto,CA,USA) was controlled by the Chemstation Software and was equipped with pump, auto sampler, column oven and UV–Vis diode array detector (DAD). The HPLC instrument was coupled to an Esquire 3000 + (Bruker Daltonics, GmbH, Germany) mass spectrometer equipped with an electrospray ionization (ESI) source and ion-trap mass analyzer. The column used was a Merck Lichrospher 100 RP-18, 5 μm, 250 × 4 mm. The flow rate was set to 0.5 ml/min and the injection volume was 10 μl. Methanol/water gradient was used according to the following solvent program: starting from 95 % A (0.5 % formic acid in Milli-Q water)and 5 % B(methanol), reaching 75 % A at 10 min, 65 % A at 30 min, 55 % A at 35 min, 55 % A at 40 min, 50 % A at 45 min, 45 % A at 50 min, 30 % A at 53 min, 25 % A at 56 min, and 20 % A at 60 min, followed by a post-time isocratic plateau for 10 min at 95 % A before the next injection. Extracts were analyzed using a modified version of the method described by Dhaouadi et al. (2011). Mass spectrometry operating conditions were optimized in order to achieve maximum sensitivity values. The electrospray ionization-mass spectrometry (ESI) source was operated in negative mode to generate [M–H] ions using the following conditions: desolvation temperature at 250 °C and vaporizer temperature at 400 °C, dry gas (nitrogen) was set at 4.5 l/min probe voltage 4.5 kV, fragmentor voltage 20 V. The MS data were acquired as full scan mass spectra at 50–800 m/z by using 200 ms for collection of the ions in the trap. Identification of the main compounds was performed by HPLC-DAD analysis, comparing the retention time, UV spectra and MS data of the peaks in the samples with those of authentic standards. Quantitation of Epigallocatechin, Genistein, p-Coumaric acid, Chlorogenic acid, Catechin, Ferulic acid, Kaempferol, Gallocatechin gallate, Caffeine, Epigallocatechin gallate, Quercetin, Gallic acid, Caffeic acid and Ellagic acid content were performed using commercials standards (Sigma-Aldrich Milan, Italy). The software Chem Station for LC 3D (Agilent Technologies Life Sciences and Chemical Analysis, Waldbronn, Germany) was used for quantitation purposes. Quantitative evaluation of the compounds was performed by means of a five-point regression curve (R2 > 0.996), using external standards and evaluated at 280 nm monitoring wavelength.
Antioxidant activity
Scavenging ability on DPPH radical
The DPPH free radical-scavenging activity of each sample was determined according to the method described by Cherrat et al. (2013) with slight modifications. Freshly prepared by dissolving 3.7 mg of DPPH• (2,2-diphenyl-1-picrylhydrazyl, Sigma) in 100 ml of methanol. Diluted extract (40 μl) was added to 1960 μl of the DPPH and mixed. The absorbance at 517 nm was measured in a 1-cm glass cuvette at 0 min (start) and after 10 min of the reaction, incubated at room temperature, versus blank (40 μl of distilled water added to 1960 μl of DPPH preparation). The control sample was prepared in the following way: 40 μl of methanol were added to1960 μl of DPPH. All measures were made in triplicate and averaged. The TEAC (Trolox Equivalent Antioxidant Capacity) value was calculated from the equation determined from linear regression after plotting known solutions of Trolox (Fattouch et al. 2008) with different concentrations (0.05 to 0.8 mmol/l). The antiradical activity was also expressed as the inhibition percentage and was calculated using the following formula:
ABTS assay
The antiradical activity was also assessed using a second functional test based on the ABTS+ scavenging potential as described by Tuberoso et al. (2007) with slight modification. Briefly, the ABTS+ radical was generated by reacting 7 mM ABTS and 2.45 mM potassium persulphate. After incubation at room temperature in the dark for 16 h, the solution was diluted to get an absorbance of 0.70 ± 0.02 at 734 nm. The ABTS+ solution (1 ml) was added of the test sample (50 μl), mixed thoroughly and incubated for 30 min. The absorbance of the reactive mixture was measured at 734 nm and compared to the antioxidant potency of Trolox used as a reference. The results were expressed in terms of TEAC (μmole/100 ml).
Antimicrobial tests
To evaluate the antimicrobial activity of the extracts, the following microorganisms were used: four Gram (+) bacteria Staphylococcus aureus (ATCC 6538), Staphylococcus epidermidis (ATCC 106510), Bacillus cereus (ATCC11778) and Streptococcus faecalis (ATCC7830), one Gram (-) bacteria Escherichia coli (ATCC 8739). Sensibility of the test organism to the extract was determined by employing the standard disk diffusion technique using the method described by Laviola et al. (2013) with slight modifications. The bacterial suspension in nutritive broth, adjusted to 0.5 Mc Farland turbidity and evaluated using a serial 10-fold dilution method, was spread plated on count agar medium (MH) in order to give a population of 108 colony-forming units (cfu)/plate. For the disk diffusion test, sterile paper discs (6 mm Ø) were added of the test sample (25 μl) and placed onto the inoculated agar surface. After cultivation at 37 °C for 24 h, the resulting inhibition zones diameters (mm) were measured. The minimum inhibitory and bactericidal concentrations (MIC and MBC) were also determined using the broth microdilution method (Koneman 1995). A 100 μl amount of a given dilution of the polyphenolic extract and 100 μl of the bacterial suspensions (5 × 105 cfu/ml) were added in the micro wells. The plates were incubated aerobically at 37 °C for 24 h. Bacterial growth was revealed by the presence of turbidity and a ‘pellet’ on the well bottom. MICs were determined as the first well in ascending order that did not produce a pellet. To confirm MIC and establish MBC, 25 μl of the broth was removed from each well and inoculated on MH plates. After overnight incubation at 37 °C, the number of surviving organisms was determined; MBC was determined when 99.9 % of bacteria were dead (Fattouch et al. 2007).
Statistical analysis
All tests and analyses were run in triplicate and averaged. Quantitative presented data are means±standard deviations (SD). One-way analysis of variance with Dunnett’s post-test was performed using GraphPad Prism version 5.04 for Windows (Graph Pad Software, San Diego, CA, USA). Tukey’s test was used to determine the possible significant differences among mean values at the 5 % level. Difference of P < 0.05 were considered significant.
Results and discussion
Determination of the total phenolic content
The green tea decoction polyphenolic extract (715.66 ± 3.4 mg GAE per 100 ml) contained 1.2 times polyphenols in comparison to the green tea infusion. It seems that decoction, which is based on a boiling procedure for 15 min, increased the amount of phenolics in the aqueous-acetone extracts. This finding was in agreement with a previous report of Dhaouadi et al. (2013). In previous reports, the use of aqueous-acetone (3/7, v/v) solvent was shown to provide a good extraction of the main polyphenols from different foods, including fruits, leaves, juices and syrups (Fattouch et al. 2007; Dhaouadi et al. 2011). This simple procedure lead to high yields of phenolics (Menelaos et al. 2004) and removed interfering compounds, particularly cold acetone (−20 °C) precipitates proteins and peptides, which absorb at 280 nm and interfere with polyphenols in HPLC analysis. When using methanol, ethanol or water for the phenolics extraction, usually preparative chromatographic SPE or SPME steps are required. Hence, the cold acetone extraction might reduce the number of the steps, and consequently, would preserve the bioactive phenolics to analyze in their native forms. Accordingly, in the present work we preferentially used aqueous-acetone (3v/7v) to prepare polyphenolic compounds from the different samples. Using the colorimetric Folin-Ciocalteu method, the estimated total phenolic content of the analyzed green tea infusion was comparable to what reported by Pilar et al. (2008).At the starting of the experiment (day 0), the TPCs decreased significantly (p < 0.01) in the following order: GT>GTPS>GTMS. The green tea (GT) decoction polyphenolic extract contained higher polyphenolic content than GTPS (1.3times), GTMS (1.8 times), PS (8.2 times) and MS (9times). During the refrigerated storage, the TPCs showed decreasing trends in all the samples (Table 1). By the end of the experiment the TPC in GT extracts decreased by 11.3 times while both syrup-added GT, GTPS and GTMS, decrease by only 1.4 and 1.6 times, respectively, in comparison to their corresponding initial content before storage. These results are in agreement with the recent report of Xu et al. (2014) who worked on green tea infusion and found that storage at 4 °C during 80 days affected the solubility of many compounds including polyphenols, sugar, caffeine, flavones and proteins. At the 15th day of refrigerated storage, GTPS and GTMS polyphenolic extracts did not decreased noticeably when compared to green tea without syrups (GT). Compared to first day of the experiment MS and PS syrups incurred a slight increase in their TPCs estimated to reach 165.78 ± 2.7 and 171.08 ± 3.4 mg GAE per 100 ml, respectively. These results emphasize the preservative effect of both syrups, PS and MS, addition to green tea decoction on the TPC during low-temperature storage. Moreover, we observed the appearance of a black deposit on the bottom of GT, GTPS and GTMS bottles. The volume of the observed residue increased with the storage. This deposit was more noticeable in the GT bottle than in GTPS and GTMS bottles, especially at the end of the experiment. Same observations were reported by Xu et al. (2014) who suggested that the decrease in the concentrations of the chemical components, including polyphenols, was related to the formation of the sediments in green tea infusions.
Table 1.
Content of total phenolics (by the Folin–Ciocalteu method) and antioxidant activities
| Day number | |||
|---|---|---|---|
| 0 | 6 | 15 | |
| GT | |||
| TPC(mg GAE/100 ml) | 715.66 ± 3.4a | 440.09 ± 1.7b | 63.85 ± 5.11c |
| DPPH assay (mmol/100 ml) | 3792.15 ± 72.9a | 3392.30 ± 72.12a | 1141.51 ± 82.08b |
| ABTS assay (mmol/100 ml) | 3523.69 ± 12.53a | 3179.48 ± 27.36a | 935.14 ± 27.36b |
| GTPS | |||
| TPC(mg GAE/100 ml) | 544.78 ± 3.6a | 391.65 ± 8.5b | 373.49 ± 3.4b |
| DPPH assay (mmol/100 ml) | 3514.84 ± 63.84a | 3050.49 ± 9.12b | 2818.32 ± 9.12b |
| ABTS assay (mmol/100 ml) | 3463.88 ± 9.62a | 2861.3 ± 13.12b | 2712.82 ± 30.62b |
| GTMS | |||
| TPC(mg GAE/100 ml) | 390.96 ± 9.3a | 276.5 ± 9.5b | 237.34 ± 1.7c |
| DPPH assay (mmol/100 ml) | 3450.34 ± 27.36a | 2489.41 ± 91.2b | 2302.38 ± 9.12b |
| ABTS assay (mmol/100 ml) | 3105.67 ± 18.74a | 2310.69 ± 30.62b | 2143.66 ± 39.37b |
| PS | |||
| TPC(mg GAE/100 ml) | 87.95 ± 5.1a | 102.16 ± 8.8b | 171.08 ± 3.4c |
| DPPH assay (mmol/100 ml) | 1431.73 ± 91.2a | 1502.67 ± 9.12a | 1986.36 ± 91.2b |
| ABTS assay (mmol/100 ml) | 1274.44 ± 4.74a | 1432.206 ± 4.37b | 1744.62 ± 8.7c |
| MS | |||
| TPC(mg GAE/100 ml) | 78.19 ± 1.87a | 84.45 ± 3.23a | 165.78 ± 2.7b |
| DPPH assay (mmol/100 ml) | 1225.35 ± 18.24a | 1322.09 ± 63.84a | 1709.05 ± 63.84b |
| ABTS assay (mmol/100 ml) | 1082.65 ± 34.9a | 1218.76 ± 52.4b | 1506.43 ± 4.37c |
GT Green Tea, GTPS Green Tea with Pomegranate Syrup, GTMS Green tea with Mint Syrup, PS Pomegranate Syrup, MS Mint Syrup. TP total phenolics in mg gallic acid equivalent per 100 ml. TEAC value Trolox Equivalent Capacity (mmol of Trolox per 100 ml) determined by the ABTS and DPPH methods. (Means±S.D, n = 3). Means within the same row with different superscript are significantly different (p < 0.05)
Chromatographic profiles of the phenolic extracts
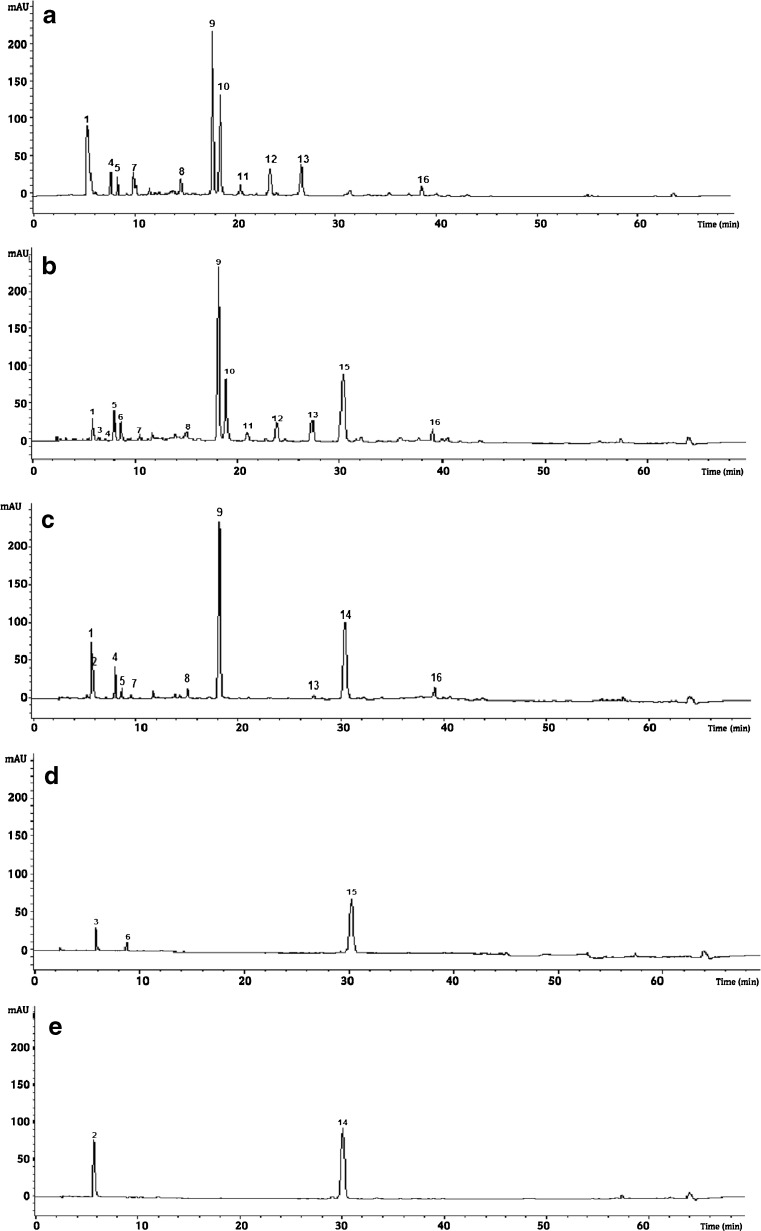
In this study, the combination of diode array detection (DAD) and negative electrospray ionization mass spectrometry (ESI−), coupled to HPLC using reverse-phase silica provided an accurate method for the structure elucidation of individual phenolics. Figures 1, 2 and 3 illustrate the obtained chromatograms at monitoring wavelength 280 nm of the different extracts prepared using the above described protocol. The repeatability of the chromatographic method was high, with respect to both retention times and peak areas. The mass spectra and UV characteristics of the identified phenolics in each extract are given in Table 2. About 16 different phenolic compounds were detected in the studied green tea, syrups and mixtures. Quantification was carried out by the external standard method from integrated peak areas of different tested samples. The quantitative polyphenolic characteristics of the five tested samples in 0, 6 and 15 day of refrigerated storage were illustrated in Tables 2, 4 and 5, respectively. Tea typical phenolic acids and flavonoids were detected in the herein studied decoctions, however, their presence and quantitation varied with stored days and syrup addition. Beside of the major polyphenolic compound Epigallocatechin 3 methyl gallate, green tea contained Epigallocatechin, Gallocatechin gallate, Catechin, Quercetin and Kaempferol (Table 2). Our results are in agreement with those obtained in the study of Atoui et al. (2005) who reported that catechin and their derivatives were the characteristic compounds of green tea. Mousavinejada et al. (2009) reported that ellagic acid was the most important compound in pomegranate juice. In the present study, pomegranate syrup (PS) contained mainly esters of ellagic followed by coumaric and ferrulic acids. No free forms of these organic acids were detected. Our data suggest that the organic acids were possibly esterified during the preparation process of the pomegranate syrup, a finding worthy of future deep investigation. In the other hand, the HPLC analysis of mint syrup showed the presence of caffeic acid and genestein. The latter was also found by Atoui et al. (2005) in Mint infusion. Sroka et al. (2005) worked on the aqueous polyphenolic extract of mint and reported the presence of caffeic acid (Tables 3, 4 and 5).
Fig. 1.
HPLC profiles (λ = 280 nm) of polyphenolic extracts of GT (a), GTPS(b), GTMS(c), PS(d)and MS(e) at the first day of experiment. Identified peaks as compared to standard compounds: 1, Epigallocatechin; 2, Genistein; 3, Ester ofcoumaric acid;4, Chlorogenic acid;5,Catechin;6,Ester of ferulic acid;7, kaempferol3-glucoside;8,Gallocatechin gallate; 9, Caffeine; 10, Epigallocatechin-3-methyl gallate; 11, Quercetin 3-glycoside; 12, Kaempferol 3-rutinoside;13, Ester of gallic acid; 14, Caffeic acid; 15, Esterof ellagicacid;16,Quercetin 3-diglycoside
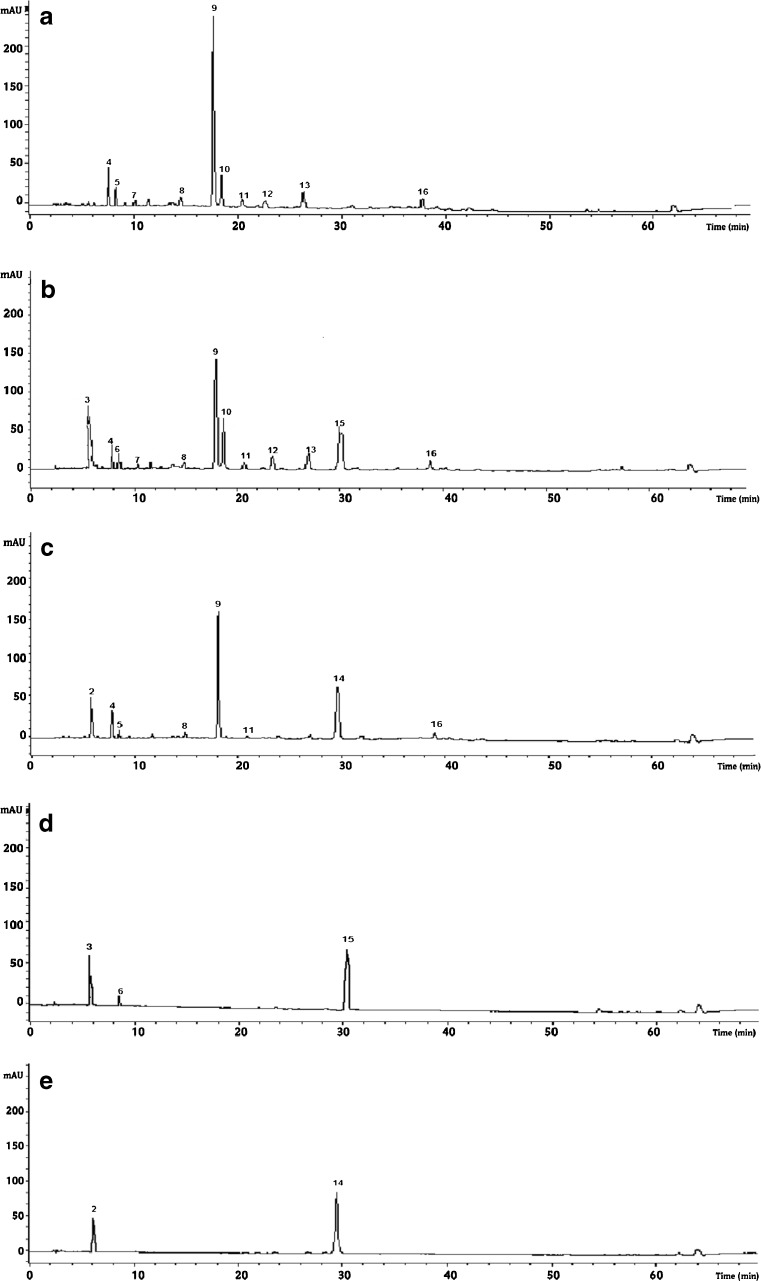
Fig. 2.
Chramatograms of polyphenolic extracts of Green tea (a), Green tea with pomegranate syrup (b), Green tea with pomegranate syrup (c), Pomegranate syrup(d) and Mint syrup(e) at the 6th day of experiment.
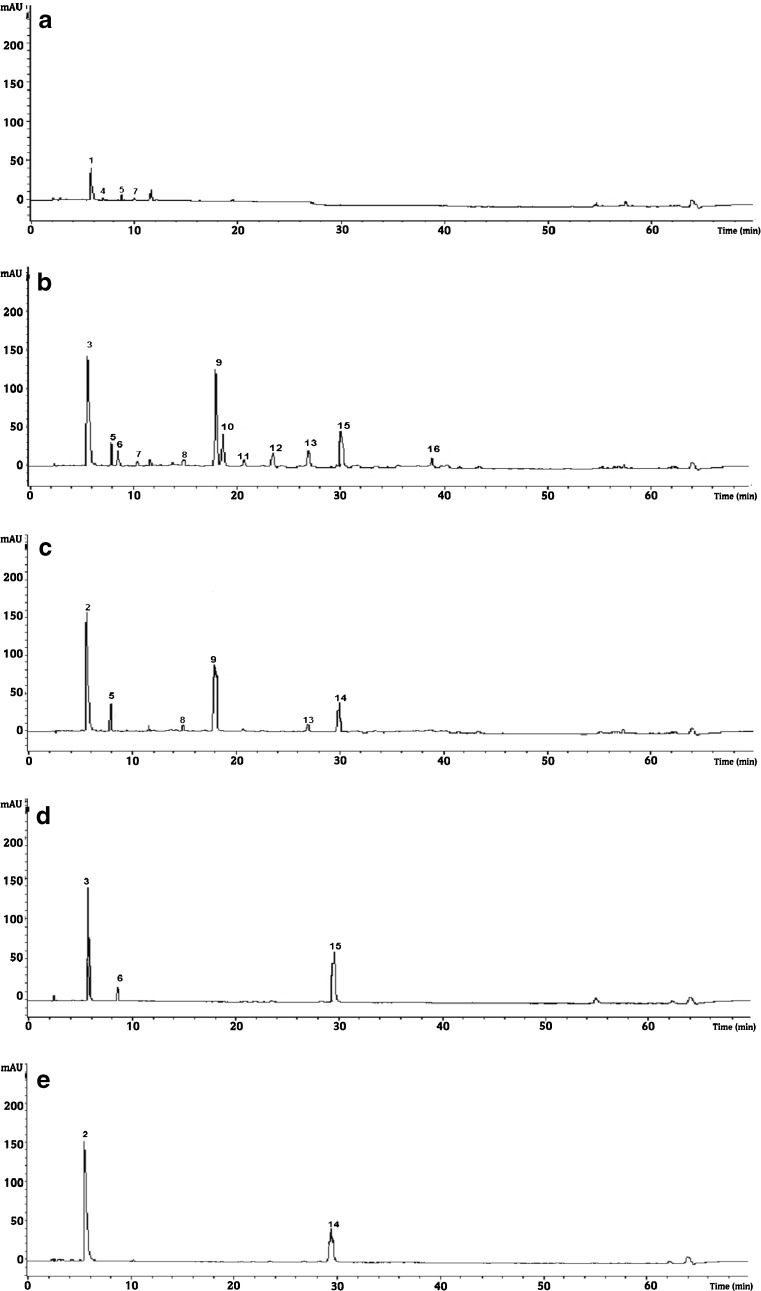
Fig. 3.
Chromatographic analysis (λ = 280 nm) of polyphenolic extracts Green tea (a), Green tea with pomegranate syrup (b), Green tea with pomegranate syrup (c), Pomegranate syrup(d) and Mint syrup(e) at the 15th day of experiment
Table. 2.
LC-ESI-MS characteristics of the identified polyphenols in aqueous-acetone extracts of different studied samples
| Peak no. | TR(min) | λmax | [M-H] | Identification |
|---|---|---|---|---|
| 1 | 5.90 | 270 | 305 | Epigallocatechin |
| 2 | 6.00 | 286 | 269 | Genistein |
| 3 | 6.10 | 298 | 495 | Ester of coumaric acid |
| 4 | 7.60 | 298 | 353 | Chlorogenic acid |
| 5 | 8.30 | 278 | 289 | Catechin |
| 6 | 8.90 | 294 | 507 | Ester of ferulic acid |
| 7 | 10.30 | 262 | 447 | Kaempferol 3-glucoside |
| 8 | 14.70 | 274 | 457 | Gallocatechin gallate |
| 9 | 18.20 | 270 | 193 | cafeine |
| 10 | 19.30 | 278 | 471 | Epigallocatechin 3 methyl gallate |
| 11 | 20.70 | 274 | 463 | Quercetin 3-glycoside |
| 12 | 23.60 | 350 | 593 | Kaempferol 3-rutinoside |
| 13 | 27.30 | 270 | 293 | Ester of gallic acid |
| 14 | 29.90 | 242 | 179 | Caffeic acid |
| 15 | 30.10 | 290 | 629 | Ester of ellagic acid |
| 16 | 39.30 | 254 | 611 | Quercetin 3-diglycoside |
Table 4.
Polyphenolic content (mg/100 ml) in GT, GTPS, GTMS, PS and MS extracts at the 6th day of experiment
| Peak no. | GT | GTPS | GTMS | PS | MS |
|---|---|---|---|---|---|
| 1 | – | – | – | – | – |
| 2 | – | – | 41.33±0.39 | – | 33.68±1.10 |
| 3 | – | 62.55±1.8 | – | 43.78±1.65 | – |
| 4 | 37.31±3.20 | 1.62±0.07 | 13.03±0.53 | – | – |
| 5 | 7.87±0.84 | – | 2.45±0.13 | – | – |
| 6 | – | 5.51±0.18 | – | 3.88±0.26 | – |
| 7 | 8.20±0.67 | 1.58±0.14 | – | – | – |
| 8 | 4.11±0.21 | 1.141±0.10 | 1.36±0.10 | – | – |
| 9 | 236.3±7.36 | 129.7±2.12 | 130±3.80 | – | – |
| 10 | 42.25±1.41 | 58.9±2.80 | – | – | – |
| 11 | 5.595±0.35 | 2.92±0.30 | 1.14±0.03 | – | – |
| 12 | 10.81±0.70 | 8.41±0.49 | – | – | – |
| 13 | 12.55±0.12 | 15.30±1.40 | – | – | – |
| 14 | – | – | 37.50±1.84 | – | 38.01±2.40 |
| 15 | – | 50.51±1.06 | – | 51.28±2.60 | – |
| 16 | 1.5±0.03 | 2.95±0.15 | 0.98±0.03 | – | – |
| Total | 366.55±14.87* | 341.12±11.15* | 227.83±6.81* | 98.95±4.50* | 71.69±3.5* |
GT Green Tea, GTPS Green Tea with Pomegranate Syrup, GTMS Green tea with Mint Syrup, PS Pomegranate Syrup, MS Mint Syrup. Data presented are means±SD (n = 3). *:p < 0.05
Table 5.
Quantitation of polyphenols (mg/100 ml) in GT, GTPS, GTMS, PS and MS extracts at the 15th day of storage period
| Peak no. | GT | GTPS | GTMS | PS | MS |
|---|---|---|---|---|---|
| 1 | 32.06±1.4 | – | – | – | – |
| 2 | – | – | 101.33±6.6 | – | 121.33±7.40 |
| 3 | – | 117.55±6.2 | – | 116.95±3.50 | – |
| 4 | 4.61±0.50 | – | – | – | – |
| 5 | 7.22±0.35 | 1.52±0.10 | 2.11±0.20 | – | – |
| 6 | – | 4.88±0.08 | – | 5.042±0.07 | – |
| 7 | 6.38±0.28 | 1.40±0.11 | – | – | – |
| 8 | – | 1.04±0.03 | 1.32±0.09 | – | – |
| 9 | – | 102.2±1.45 | 93.2±2.2 | – | – |
| 10 | – | 38.65±0.35 | – | – | – |
| 11 | – | 2.57±0.09 | – | – | – |
| 12 | – | 5.41±0.21 | – | – | – |
| 13 | – | 7.80±0.12 | 0.6375±0.03 | – | – |
| 14 | – | – | 26.43±1.60 | – | 27.18±1.30 |
| 15 | – | 44.01±1.06 | – | 45.16±0.56 | – |
| 16 | – | 2.95±0.12 | – | – | – |
| Total | 50.27±2.35* | 330.01±9.8* | 225.04±10.69* | 167.16±4.13* | 148.52±8.7* |
GT Green Tea, GTPS Green Tea with Pomegranate Syrup, GTMS Green tea with Mint Syrup, PS Pomegranate Syrup, MS Mint Syrup. Data presented are means±SD (n = 3).*: p < 0.05
Table 3.
Quantitation of polyphenols (mg/100 ml) in GT, GTPS, GTMS, PS and MS extracts at the first day of experiment
| Peak no. | GT | GTPS | GTMS | PS | MS |
|---|---|---|---|---|---|
| 1 | 65.43 ± 5.90 | 17.86 ± 2.40 | 37.93 ± 2.36 | – | – |
| 2 | – | – | 30.26 ± 1.12 | – | 18.23 ± 0.88 |
| 3 | – | 3.95 ± 0.70 | – | 14.08 ± 0.66 | – |
| 4 | 20.96 ± 1.90 | 3.60 ± 0.21 | 24.03 ± 1.23 | – | – |
| 5 | 18.11 ± 0.40 | 20.42 ± 1.20 | 7.5 ± 0.46 | – | – |
| 6 | – | 16.09 ± 2.12 | – | 7.88 ± 0.41 | – |
| 7 | 22.96 ± 1.01 | 3.40 ± 0.38 | 2.41 ± 0.23 | – | – |
| 8 | 7.763 ± 0.71 | 2.84 ± 0.10 | 3.03 ± 0.30 | – | – |
| 9 | 240.55 ± 7.99 | 203.7 ± 10.60 | 221.12 ± 7.17 | – | – |
| 10 | 219.42 ± 5.4 | 125.75 ± 3.53 | – | – | – |
| 11 | 7.17 ± 0.24 | 6.57 ± 0.40 | – | – | – |
| 12 | 41.26 ± 1.70 | 21.63 ± 1.15 | – | – | – |
| 13 | 48.03 ± 2.50 | 24.88 ± 0.60 | – | – | – |
| 14 | – | – | 43.10 ± 2.12 | – | 44.54 ± 2.42 |
| 15 | – | 58.86 ± 3.63 | – | 57.23 ± 3.85 | – |
| 16 | 4.7 ± 0.14 | 4.58 ± 0.17 | 1.66 ± 0.06 | – | – |
| Total | 696.27 ± 27.89* | 514.18 ± 27.19* | 371.08 ± 14.48* | 79.20 ± 4.90* | 62.77 ± 3.30* |
GT Green Tea, GTPS Green Tea with Pomegranate Syrup, GTMS Green tea with Mint Syrup, PS Pomegranate Syrup, MS Mint Syrup. Data presented are means±SD (n = 3). *: p < 0.05
At the end of the experiment, green tea shows the disappearance of the majority of its polyphenolic compounds while GTPS and GTMS preserve its composition. Several compounds such as gallocatechin gallate, epigallocatechin 3 methyl gallate, quercetin 3-glycoside, kaempferol 3-rutinoside, caffeic acid, quercetin 3-diglycoside were conserved in GTPS until the end of the experiment.
The quantitative analyses of extracts at different storage day, showed that the polyphenolic content in GT extract decreased from 696.27 ± 27.89 mg/100 ml, at the beginning of experiment, to 366.55 ± 14.87 mg/100 ml, after 6 days, and to 50.27 ± 2.35 mg/100 ml at the 15thth day. The local common habit of pomegranate or mint syrup addition seems to maintain the stability of the green tea decoction. In fact, in the first day, GTPS and GTMS contained respectively 514.18 ± 27.19 and 371.08 ± 14.48 mg/100 ml; whereas, they attained 330.01 ± 9.8 (GTPS) and 225.04 ± 10.69 (GTMS) mg/100 ml. The decrease of polyphenolic content in GT was important relatively to the slight decrease in GTPS and GTMS. This could be explained by the formation of an amount of sediment in GT relatively high when compared to that formed in GTPS and GTMS. As Xu et al. (2014) highlighted the presence of polyphenols, sugars, and caffeine in the sediment of green tea infusions during low temperature storage, the observation of the sediments in the herein studied green tea decoctions (GT, GTPS and GTMS) suggests that similar phenomenon could happen in our experiments. The concentrations of polyphenols, flavones, caffeine and protein significantly decreased with the extension of the storage duration, and the decrease mainly occurred in the first 10 days of storage (Xu et al. 2014). These authors stipulated that viscosity was found to help and improve the stability of green tea infusion during low temperature storage. In our study, due to their higher content in sugar (70°BX), PS and MS are supposed to increase the viscosity of GT decoction in GTPS and GTMS samples. High viscosity was also found to improve the stability of cloudy apple juice (Genovese and Lozano 2000). The viscosity and stability of apple juice concentrate have been increased by sugar addition in the work of Benitez et al.(2009).
Antioxidant activity
DPPH is a free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule (Soares et al. 1997). The herein used assay is based on the ability of the antioxidant to scavenge the radical anion DPPH•−. The reduction capability of DPPH radical was determined by the decrease in absorbance induced by the antioxidants. In the beginning of the experiment (0 day), GT exhibited the strongest activity (3792.15 ± 72.96 mmol TEAC/100 ml) followed by GTPS (3514.84 ± 63.84 mmol TEAC/100 ml) and GTMS (3450.34 ± 27.43 mmol TEAC/100 ml). After 15 days’ storage, as happened for phenolic compounds, the antioxidant activity was negatively influenced by refrigerated storage and presented a decrease of about: 3.3times for GT, 1.4 times for GTMS and 1.2 times for GMPS compared to the corresponding extracts at the start of the experiment (Table 1). The repeatability of the experiences was high and there was no significant differences (P > 0.05) between different extractions from the same sample. Moreover, a second functional assay was used based on the cationic ABTS▪+ radical-scavenging effects. Obtained data showed that at the first day of the experiment, all polyphenolic extracts exhibited strong antiradical potentials which were estimated to 3523.69 ± 12.5 (GT), 3463.88 ± 9.6 (GTPS) and 3105.67 ± 7.8 (GTMS) mmol TEAC/100 ml. Obtained results with both functional antiradical assays suggest that the analyzed extracts contain different antioxidant compounds able to scavenge cationic and anionic radicals. It has been found that DPPH is scavenged by antioxidant molecules such as ascorbic acid, tocopherol, flavonoids, and tannins essentially due to their hydrogen donating ability (Tuberoso et al. 2007). In contrast, ABTS+ is known to act as an electron donor for the reduction of oxo species (Swaran 2009). Both in vitro methods showed that the addition of pomegranate or mint syrup to green tea decoction improves and preserve its antioxidant activity. Xu et al. (2012) mentioned that high viscosity may help to improve stability of tea. The addition of PS and MS which contains high amount of sugar increase the stability of green tea decoction. This stability influenced the antioxidant activity which is related to total phenolic content. Our results agree with those found by Oliveira et al. (2012) who worked on total phenolic content and antioxidant activity of some malvaceae family species and founded a strong correlation between total polyphenol contents and antioxidant activity.
In vitro Antimicrobial tests
The polyphenol fractions of GT, GTPS, GTMS, PS and MS have been closely examined for their antimicrobial properties. Varying degree of bacteria sensitivity was observed, suggesting a differential intrinsic tolerance of microorganisms and/or the particular nature and combination of the phenolic compounds present in the extracts. Table 6 shows the antimicrobial activities of the different investigated fractions. The inhibition zones formed around the disks depended on the strain, the kind and the concentration of the extract. The strongest antibacterial activity of the polyphenolic extracts was recorded against Gram-positive Staph.aureus, Staph.epidermidis and B. cereus bacteria, whereas the lowest activity was against the Gram-negative E.coli. Our results are in concordance with those obtained in the study of Bong et al. (2007) who worked on antimicrobial effect of green tea and founds that Gram-positive Staph.aureus and Staph.epidermidis were the most sensitive bacteria to tea polyphenolic extract. The important antimicrobial activity of the different extracts can be due to the high catechin content present in tea. Several studies have shown that green tea catechins, especially, EGC and EGCG, inhibit the growth of many bacterial species (Pilar et al. 2008; Gramza and Korczak 2005).
Table 6.
Antibacterial activity of the GT, GTPS, GTMS,PS and MS polyphenolic extracts, Minimum Inhibitory Concentrations (MIC) and Minimum Bactericide Concentrations (MBC) values expressed in μg/ml
| IZ* | Escherichia coli (ATCC 8739) | Streptococcus faecalis (ATCC7830) | Staphylococcus epidermidis (ATCC 106510) | Staphylococcus aureus (ATCC 6538) | Bacillus cereus (ATCC11778) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | IZ* | MIC | MBC | IZ* | MIC | MBC | IZ* | MIC | MBC | IZ* | MIC | MBC | |||
| GT | 0 | 11.2±0.7a | 2460±12.7a | – | 15.1±0.7a | 1856±6.5a | – | 22.3±2.1a | 449.5±20.5a | 931.1±8.7a | 18.1±0.7a | 480±23a | 1077±11.7a | 21.5±1.3a | 449±20a | 931.1±8.7a |
| 6 | 8.8±0.2b | – | – | 10.8 ±0.7b | – | – | 17.8±0.7b | 522±4.6b | 1067±21.5b | 15.5±0.5b | 671±8.6b | 1525±14.3b | 16.5±0.5b | 522±4.6b | 1067±21.5b | |
| 15 | N | – | – | N | – | – | 10.3±0.3c | – | – | 8±0.5c | – | – | 9.7±0.2c | – | – | |
| GTPS | 0 | 9.6±0.7c | 2570±12.7a | – | 14.2±1a | 2141±6.09b | – | 18.2±0.7b | 490±3.3c | 1156±8.7b | 16.1±0.3b | 490±3.3a | 1156±8.7a | 17.6±0.2b | 490±3.3c | 1156±8.7b |
| 6 | 8.2±1.2b | – | – | 10.4 ±0.4b | 3410±17.2c | – | 16.7±0.3b | 625±8.05d | 1420±4.2c | 13±0.9d | 767±12c | 1420±4.2b | 15.9±0.9b | 767±12d | 1420±4.2c | |
| 15 | N | – | – | 13±0.7c | – | – | 14.2±0.4d | – | – | 11.4±1d | – | – | 12.1±0.7d | – | – | |
| GTMS | 0 | 8.7±0.9b | – | – | 9.7 ±0.7b | 2754±13.1d | – | 17±0.7b | 541.06±10b | 1545±4.3d | 14±0.3b | 541±10.9d | 1545±4.3b | 16.4±0.4b | 541±10b | 1545±4.3c |
| 6 | N | – | – | N | – | – | 15.8±0.2d | 662±13d | 1702±6.8e | 12.8±0.2d | 945±26.7e | 1702±6.8c | 14.8±0.5b | 662±13e | 1702±6.8d | |
| 15 | N | – | – | N | – | – | 13.3±1d | – | – | 11±0.7d | – | – | 11.4±0.4d | – | – | |
| PS | 0 | N | – | – | N | – | – | 8.8±0.8c | – | – | 7.8±0.2e | – | – | 7.6±0.2e | – | – |
| 6 | N | – | – | N | – | – | 10.6±0.7c | – | – | 9.5±0.5c | – | – | 9.6±0.7c | – | – | |
| 15 | N | – | – | N | – | – | 11.6±0.7c | – | – | 10±0.5c | – | – | 10.3±0.2c | – | – | |
| MS | 0 | N | – | – | N | – | – | 7.9±0.7e | – | – | 7.3±0.6e | – | – | 7±0.17e | – | – |
| 6 | N | – | – | N | – | – | 9.6±0.3c | – | – | 7.6±0.5e | – | – | 8.9±0.9c | – | – | |
| 15 | N | – | – | N | – | – | 11±1c | –– | – | 9.5±0.5c | – | – | 9.7±0.26c | – | – | |
| AMP | 12.7±1.5d | NT | NT | 15.5 ±0.3a | NT | NT | 13.5±0.5d | NT | NT | 11.2±0.5d | NT | NT | 19.2±0.7a | NT | NT | |
GT Green Tea, GTPS Green Tea with Pomegranate Syrup, GTMS GreenTea with Mint Syrup, PS Pomegranate Syrup, MS Mint Syrup. Values are given as means±SD of triplicate experiment. NT Not tested, N Not active, (-): No inhibition at the highest reached concentration. AMP Ampicilin.*IZ Diameter of inhibition zones including the diameter of Ø6 mm disk. Data presented are means±SD (n = 3).Means within the same column with different superscript are significantly different (p < 0.05)
In first day of the experiment, GT extract presented the highest antimicrobial activity followed by GTPS and GTMS extracts. In order to determine the MICs for all of the sensitive bacterial strains observed in the agar diffusion assay, the microdilution method was used, and results are shown in Table 6. The MIC values varied from 449.5 ± 20.5 to 2570 ± 12.7 μg phenolics per milliliter of medium culture. The extracts showed bacteriostatic and/or bactericide activities depending on the polyphenolic extract and the targeted bacterial strain (Table 6). At the first day of experiment, the lowest MICs were recorded with GT, GTPS and GTMS extracts tested against Staph. epidermidis, whereas the highest MICs were recorded with GTPS extract tested against E. coli strain. At the end of the experiment, the antimicrobial activity of different extracts against all bacterial strains significantly (p < 0.01) decreased to reach the following order of inhibitory potential: GTPS>GTMS>GT. At the end of experiment, no extracts exhibited bactericide effects against any tested bacteria (Table 6) with the highest concentrations in the assay conditions. This result suggests qualitative differences among the used extracts to affect bacterial growth in the medium cultures. This could be expected since obtained LC-MS data revealed the disappearance of the GT main polyphenolic compounds at the end of the experiment. Same trends were observed for GTPS and GTMS, even syrups roughly preserved their composition. During refrigerated storage of the studied tea preparations, the formed sediments, even though relatively high with GT in comparison to those formed in GTPS and GTMS, were at the origin of the loss of some effective compounds that seem to contribute to the antibacterial potential of these beverages.
Conclusion
This study contributes to the knowledge of the beneficial effect of the mint and pomegranate syrups addition to green tea decoction, which is a Tunisian commonly habit. The analytical characterization and evaluation of the exhibited biological activities of green tea supplemented or not with pomegranate and mint syrup could support the exploitation of these important sources of polyphenols in food and medicinal applications. Pomegranate and mint syrups can be used, in combination with other antimicrobial components or methods for stabilizing food products, as an alternative way of maintaining high functional properties without the need for conventional chemical food preservatives. Synergetic/antagonistic effects between different phenolic compounds should not be neglected in future works to deepen our knowledge about the behavior of the whole composition of the extracts, known as “totum”, so as we can accurately elucidate the phenomena behind the observed bioactivities.
Acknowledgments
This work was financially supported by the Tunisian–Portugal bilateral projects (05/TP/09 -TP/68/2012) and the Tunisian Ministry of Higher Education and Scientific Research. The authors would like to thank Engineer Walid MELLITI (FSB, University of Carthage, Tunis) for his kind help in microbiological interpretations.
Footnotes
Highlights
- Study of Green tea (GT) supplemented with Pomegranate (GTPS) or Mint (GTMS) syrups.
- RP-HPLC and LC-DAD-MS analysis of the polyphenolic composition of different extracts.
- Differential antioxidant and antimicrobial potentials of GT, GTPS and GTMS.
- Positive effect of syrups addition on GT during storage period.
References
- Amzad MH, Mizanur SMR. Isolation and characterisation of flavonoids from the leaves of medicinal plant Orthosiphon stamineus. Arab J Chem. 2011;8:218–221. [Google Scholar]
- Atoui A, Mansouri A, Boskou G, Kefalas P. Tea and herbal infusions: their antioxidant activity and phenolic profile. Food Chem. 2005;89:27–36. doi: 10.1016/j.foodchem.2004.01.075. [DOI] [Google Scholar]
- Barberan TFA, Seeram NP, Espin JC. Bioavailability of pomegranate polyphenols. In: Seeram NP, Schulman RN, Heber D, editors. Pomegranates: ancient roots to modern medicine. New York: CRC Press; 2006. pp. 45–60. [Google Scholar]
- Benitez EI, Genovese DB, Lozano JE. Effect of typical sugars on the viscosity and colloidal stability of apple juice. Food Hydrocoll. 2009;3:519–525. doi: 10.1016/j.foodhyd.2008.03.005. [DOI] [Google Scholar]
- Bong JA, Kwak JH, Son JH, Jung MP, Lee JY, Cheorun JO, Byun MW. Comparison study of the effect of green tea extract (GTE) on the quality of bread by instrumental analysis and sensory evaluation. Food Res Int. 2007;40:470–479. doi: 10.1016/j.foodres.2006.07.007. [DOI] [Google Scholar]
- Çayır K, Karadeniz A, Şimşek N, Yıldırım S, Karakuş E, Kara A, Akkoyun HT, Şengül E. Pomegranate seed extract attenuates chemotherapy-induced acute nephrotoxicity and hepatotoxicity in rats. J Med Food. 2011;14:1254–62. doi: 10.1089/jmf.2010.0286. [DOI] [PubMed] [Google Scholar]
- Cherrat L, Espina L, Bakkali M, Pagan R, Laglaoui A. Chemical composition, antioxidant and antimicrobial properties of Mentha pulegium, Lavandula stoechas and Satureja cala mintha Scheele essential oils and an evaluation of their bactericidal effect in combined processes. Innovative Food Sci Emerg Technol. 2013;22:221–229. doi: 10.1016/j.ifset.2013.12.016. [DOI] [Google Scholar]
- Dhaouadi K, Fattouch S, Hamdaoui MH (2009) Extraction, identification and quantification of the polyphenols of green and black tunisian tea decoctions commercialized as “garden of tea”. ISHS Acta Horticulturae 853: International Symposium on Medicinal and Aromatic Plants – SIPAM
- Dhaouadi K, Raboudi F, Estevan C, Barrajon E, Vilanova E, Hamdaoui MH, Fattouch S. Cell viability effects and antioxidant and antimicrobial activities of Tunisian date syrup (Rub El Tamer) polyphenolic extracts. J Agric Food Chem. 2011;59:402–406. doi: 10.1021/jf103388m. [DOI] [PubMed] [Google Scholar]
- Dhaouadi K, Jlassi H, Fattouch S, Hamdaoui MH (2013) Phenolic compounds content, antioxidant and antibacterial activities of green / black tea decoction as affected by cooking treatment. Metabolism & Endocrinology Abstracts; Proceedings of the Physiological Society; 37th Congress of IUPS (Birmingham, UK), Proc 37th IUPS, PCA327
- Endo EH, Cortez DA, Ueda-Nakamura T, Nakamura CV, Dias Filho BP. Potent antifungal activity of extracts and pure compound isolated from pomegranate peels and synergism with fluconazole against Candida albicans. Res Microbiol. 2010;161:534–40. doi: 10.1016/j.resmic.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Esfahanian Z, Behbahani M, Shanehsaz M, Hessami MJ, Nejatian MA. Evaluation of anticancer activity of fruit and leave extracts from virus infected and healthy cultivars of Vitis vinifera. Cell J. 2013;15:116–123. [PMC free article] [PubMed] [Google Scholar]
- Fattouch S, Caboni P, Coroneo V, Tuberoso CIG, Angioni A, Dessi S, Marzouki N, Cabras P. Antimicrobial activity of Tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. J Agric Food Chem. 2007;55:963–969. doi: 10.1021/jf062614e. [DOI] [PubMed] [Google Scholar]
- Fattouch S, Caboni P, Coroneo V, Tuberoso CIG, Angioni A, Dessi S, Marzouki N, Cabras P. Comparative analysis of polyphenolic profiles and antioxidant and antimicrobial activities of Tunisian pomefruit pulp and peel aqueous acetone extracts. J Agric Food Chem. 2008;56:1084–1090. doi: 10.1021/jf072409e. [DOI] [PubMed] [Google Scholar]
- Genovese DB, Lozano JE. Effect of cloud particle characteristics on the viscosity of cloudy apple juice. J Food Sci. 2000;65:641–645. doi: 10.1111/j.1365-2621.2000.tb16065.x. [DOI] [Google Scholar]
- Gramza A, Korczak J. Tea constituents (Camellia sinensis L.) as antioxidants in lipid systems. Trends Food SciTechnol. 2005;16:351–358. doi: 10.1016/j.tifs.2005.02.004. [DOI] [Google Scholar]
- Hamilton M (1995) Antimicrobial properties of tea (Camellia sinensis L.). Antimicrob Agents Chemother 2375–2377 [DOI] [PMC free article] [PubMed]
- Koneman EW (1995) Atlas of diagnostic microbiology. Delfino A ed, Italy, pp 549–560 and 638–659
- Lansky E, Shubert S, Neeman I. Pharmacological and therapeutical properties of pomegranate. In: Megarejo P, Martinez JJ, Martinez J, editors. Proceedings international symposium on pomegranate. Spain: CIHEAM - Option Mediterraneans; 1998. pp. 231–235. [Google Scholar]
- Laviola BG, Oliveira AMC, Bhering LL, Alves AA, Rocha RB, Lopes-Gomes BE, Cruz CD. Estimates of repeatability coefficients and selection gains in Jatropha indicate that higher cumulative genetic gains can be obtained by relaxing the degree of certainty in predicting the best families. Ind Crop Prod. 2013;51:70–76. doi: 10.1016/j.indcrop.2013.08.016. [DOI] [Google Scholar]
- Mansouri A, Embarek G, Kokkalouc E, Kefalasa P. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera) Food Chem. 2005;89:411–420. doi: 10.1016/j.foodchem.2004.02.051. [DOI] [Google Scholar]
- Menelaos P, Hans R, Annett M, Bernd H, Rudolf G. Identification andquantification of polyphenols in carob fruits (Ceratonia siliqua L.) and derivedproducts by HPLC-UV-ESI/MS. J Agric Food Chem. 2004;52:3784–3791. doi: 10.1021/jf030660y. [DOI] [PubMed] [Google Scholar]
- Mousavinejada G, Emam-Djomeha Z, Rezaei K, Khodaparast MH (2009) Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars. Food Chem 115:1274–1278
- Oliveira AMF, Pinheiro LS, Pereira CKS, Matias WN, Gomes RA, Chaves OS, Souza MFV, de Almeida RN, Assis TS. Total phenolic content and antioxidant activity of some Malvaceae family species. Antioxidants. 2012;1:33–43. doi: 10.3390/antiox1010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmini E, Valarmathi A, Usha RM. Comparative analysis of chemical composition and antibacterial activities of and Mentha spicata Camellia sinensis. Asian J Exp Biol Sci. 2010;1:772–781. [Google Scholar]
- Pilar M, Carbo R, Angel J, Lopez J, Gordon M. Antioxidant and antimicrobial activities of tea infusion. Food Chem. 2008;108:55–63. doi: 10.1016/j.foodchem.2007.10.040. [DOI] [Google Scholar]
- Saklar S, Erdal E, Ozdemir IS, Karadeniz B. Effects of different brewing conditions on catechin content and sensory acceptance in Turkish green tea infusions. J Food Sci Technol. 2015 doi: 10.1007/s13197-015-1746-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JR, Dins TCP, Cunha AP, Ameida LM. Antioxidant activity of some extracts of Thymus zygis. Free Radic Res. 1997;26:469–478. doi: 10.3109/10715769709084484. [DOI] [PubMed] [Google Scholar]
- Sroka Z, Fecka I, Cisowski W. Antiradical and anti-H2O2 properties of polyphenolic compounds from an aqueous peppermint extract. J Nat Res. 2005;60:826–832. doi: 10.1515/znc-2005-11-1203. [DOI] [PubMed] [Google Scholar]
- Swaran JSF. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid Med Cell Longev. 2009;4:191–206. doi: 10.4161/oxim.2.4.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Hideaki Y, Toshiro N, Yumi K, Hironori U, Kazuo R. Selective cytotoxicity of glycyrrhetinic acid against tumorigenic r/m HM-SFME-1 cells: potential involvement of H-Ras down regulation. Toxicol Lett. 2010;192:425–430. doi: 10.1016/j.toxlet.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Tezcan F, Gultekin-Ozguven M, Diken T, Ozcelik B, Erim FB. Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem. 2009;115:873–877. doi: 10.1016/j.foodchem.2008.12.103. [DOI] [Google Scholar]
- Tuberoso CIG, Kowalczyk A, Sarritzu E, Cabras P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007;103:494–1501. doi: 10.1016/j.foodchem.2006.08.014. [DOI] [Google Scholar]
- Xu YQ, Chen SQ, Yuan HB, Tang P, Yin JF. Analysis of cream formation in green tea concentrates with different solid concentrations. Food Sci Technol. 2012;49:362–367. doi: 10.1007/s13197-011-0281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YQ, Chenb GS, Du QZ, Yuan HB, Yin JF. Sediments in concentrated green tea during low-temperature storage. Food Chem. 2014;149:137–143. doi: 10.1016/j.foodchem.2013.10.084. [DOI] [PubMed] [Google Scholar]
- Yi D, Tan X, Zhao Z, Cai Y, Li Y, Lin X, Lu S, Chen Y, Zhang Q. Reduced risk of dyslipidaemia with oolong tea consumption: a population-based study in southern China. Br J Nutr. 2014;111:1421–1430. doi: 10.1017/S0007114513003644. [DOI] [PubMed] [Google Scholar]
- Yu XM, Zhu P, Zhong QP, Li MY, Ma HR. Subcritical water extraction of antioxidant phenolic compounds from Xi Lan olive fruit dreg. J Food Sci Technol. 2014 doi: 10.1007/s13197-014-1551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahin M, Aqil F, Ahmad I. Broad spectrum antimutagenic activity of antioxidant active fraction of Punica granatum L. peel extracts. Mutat Res. 2010;703:99–107. doi: 10.1016/j.mrgentox.2010.08.001. [DOI] [PubMed] [Google Scholar]