Abstract
In this study, the effect of commercial additives viz. cafodos and altesa employed to treat Indian octopus (Cistopus indicus) was examined during chilled and frozen storage. Shelf lives of treated and untreated octopus in ice were 6 and 8 days, respectively in ice. Treated and untreated frozen octopus had a shelf life of 40 days. Autolytic and microbiological changes were not controlled by the additives, as evidenced through rapid reduction in non-protein nitrogen (NPN) and α-amino nitrogen (α-AN) compounds; as well as accumulation of water soluble ammoniacal nitrogen and total volatile base- nitrogen (TVB-N) compounds. Loss of texture and colour were the major quality defects noticed in treated octopus as a result of enhanced protein solubility. Therefore, the additives approved for use in octopus neither enhanced the shelf life nor improved the sensory quality.
Keywords: Indian octopus, Chilled storage, Frozen storage, Additives, Shelf life
Introduction
Cephalopods viz. squid, cuttlefish, and octopus are the important marine resources that are rich in taste and have more edible parts (Sikorski and Kolodziejska 1986). They are considered as highly favoured seafood delicacy in European and Asian markets (Mohanan 2004), but currently only few species are traded on a large scale. Cephalopods caught in Indian waters are mainly exported in frozen form to overseas countries like Japan, USA, Spain, Italy, etc. contributing to about 1,47,951 tonnes of the total marine seafood exports in the year 2012–13 (MPEDA 2013). Of which, the share of octopus is 6 % in terms of quantity and 5 % in terms of value (INR 150 crore) (Anon 2014). In recent years, the prospect of the octopus export to overseas countries has increased due to the demand for baby octopus and this has resulted in their increased exploitation.
Cephalopods have unique spoilage pattern different from other species, dominated by autolysis, which results in shorter shelf life of 8–10 days, but have a very late microbial growth (Hurtado et al. 2001; Lapa-Guimaraes et al. 2002). Autolytic activity is around six times greater than fish; and in the case of octopus, it is as high as 10 times (Jimenez-Colmenero and Borderias 1983). The processing industries now face severe problems due to the rapid spoilage and off odour development in chilled and frozen octopus that has led to frequent rejection of commodities by the importing countries (FDA 2014). Octopus are often held in ice in the chill room, prior to freezing, until sufficient quantities become available for processing. Shelf life studies are widely available for the squid and cuttlefish, but there are only very few reports on octopus (Vaz-Pires and Barbosa 2004; Lougovois et al. 2008). To extend the shelf life of chilled cephalopods, sodium chloride is widely used to increase the firmness of the meat. Kulshrestha and Rhee (1996) reported that the sensory quality of squid increased upon treatment with sodium salts of ascorbate, lactate and phosphate.
“Cafodos”, a mixture of sodium citrate and hydrogen peroxide and “Altesa”, containing a mixture of sodium citrate and sodium bicarbonate are the two additives approved for use in chilled octopus prior to freezing. These compounds have multiple properties like pH buffering, sequestering, preservative and antioxidative effects (European Commission 2013). At present, these additives along with sodium chloride are used to treat the octopus. In this study, the effect of these commercial additives used by the seafood processing industries to treat the octopus was examined based on sensory, biochemical and microbiological characteristics held in chilled and frozen storage.
Material and methods
Thirty kilograms of fresh Indian octopus (Cistopus indicus) procured from the Thoothukudi Fishing Harbour, Tamilnadu, India were transported in insulated boxes and processed in chilled and frozen forms at M/s Theva Seafood Pvt. Ltd., Thoothukudi. They were washed with potable water and pre-processed by removing their gut contents and washed again with 2 ppm chlorinated water. The yield after pre-processing was 24 kg.
They were then divided into 2 groups of 8 kg and 16 kg, respectively to process them in chilled and frozen forms. For chilled storage, one set was treated with 2 % salt, 0.5 % cafodos and 0.2 % altesa for 3 h (TI) and the other left untreated to serve as control (CI). Additives were procured from M/s Frontline Food Ltd., Norton West UK by the company. They were separately placed in direct contact with flake ice (1:2) in thermocole boxes and kept in chilled storage set at 5 °C for a period of 12 days. Additional flake ice was added as and when required to compensate the melting loss. For frozen storage, one set was treated with 2 % salt, 0.2 % cafodos and 0.5 % altesa for 3 h (TF) and the other was left untreated to serve as control (CF). They were arranged in rectangular trays lined with 200 gauge polyethylene sheets, to which chilled glaze water was added to form a block of 1 kg net weight and then frozen in contact plate freezer at −40 °C for 2 h. They were then packed in cartons and held in a cold storage at −18 °C for a period of 4 months. Samples were periodically analyzed for the changes in sensory, biochemical and microbiological quality characteristics.
Sensory analysis was performed by a panel consisting of six trained assessors following the quality index method (QIM) (Vaz-Pires and Barbosa 2004). When the average overall score exceeds 9, the octopus were considered unacceptable. Autolytic activity was determined as tyrosinase value by the method of Hurtado et al. (2001), by incubating the muscle homogenate mixed with chilled 0.15 M NaCl in a water bath set at 40 °C for 1 h and reading the absorbance at 660 nm in a UV–Vis spectrophotometer (Jasco,V-530, Japan) using tyrosine as a standard. Non-protein nitrogen (NPN) was determined according to the AOAC method (1995), after deproteinization of the muscle homogenate with 7 % TCA, followed by digestion with conc. H2SO4 in a Kjeldhal digestion apparatus and subsequently determination of nitrogen using a Kelplus distillation apparatus (Pelican Instruments Pvt. Ltd., Chennai, India). Water soluble ammoniacal nitrogen was determined by the steam distillation method described in APHA (1992), by mixing the muscle homogenate with borate buffer (pH 8.4), followed by determination of nitrogen. Total volatile base nitrogen (TVB-N) was determined by the steam distillation method described by Antonacopoulos and Vyncke (1989), after deproteinization of the muscle homogenate with 6 % perchloric acid, followed by determination of nitrogen. Free fatty acids (FFA) were estimated by the method of Takagi et al. (1984), by extracting the fat from the muscle homogenate with three volumes of chloroform: methanol: isopropanol (2:1:2) followed by titration with standard alkali. The α-amino nitrogen (α-AN) was determined using the copper method described by Pope and Stevens (1939), by mixing the muscle homogenate with thymolphthalin indicator, 1 N NaOH and copper phosphate suspension, and then with potassium iodide and titrating against standard sodium thiosulphate in acidic condition using starch as indicator.
Total psychrophilic bacterial counts were determined as per the method described in APHA (1995). For which, 25 g of octopus mantle tissue was homogenized with 225 ml of 0.85 % sterile physiological saline and decimal dilutions were made with the same diluent. Spread plating was done on plate count agar (PCA). Total psychrophilic bacterial count was determined following incubation of plates at 5-7 °C for 5 days.
All analysis were carried out in triplicate and the results are expressed as average mean ± standard deviation. One and two way analysis of variance (ANOVA) were performed using standard statistical package (SPSS 10.0. Chicago, USA) to examine the statistical significance among the treatments and the storage period. Correlation coefficients were determined between each biochemical quality parameter and sensory quality scores using Microsoft Excel, 2007.
Result and discussion
The average quality index scores increased gradually in chilled octopus (TI and CI) until day 4 and later significantly (p < 0.05) reaching above 9 on day 12 (Table 1). Mantle of the TI octopus turned dull and pinkish on day 8 itself, while CI octopus turned pink on day 10. Pink colouration was also noticed in the mouth region. Pink discolouration occurs in octopus due to breakdown of chromatophore in the skin (Lapa-Guimaraes et al. 2002) as well as due to stacking and abuse handling (Sungsri-in 2010). In TI octopus, odour became sweetish on day 4 and later turned to acidic and slightly ammoniacal on day 8. Strong ammoniacal off odour has been described as the major reason for the sensory rejection of iced squid, Todaropsis ebalanae (Paarup et al. 2001). Storage studies carried out on chilled octopus have recorded that sensorial rejection occurred between day 6 and 7 at 2.5 °C (Hurtado et al. 1999); day 7 at 1–2 °C (Civera et al. 1999); and day 8 in ice (Vaz-Pires and Barbosa 2004), which were more or less similar to our observation. As the TI octopus developed pink colouration and ammoniacal odour much earlier than CI octopus, they were acceptable only up to day 6, while CI octopus up to day 8. An average QIM score of 9 correlated well with the sensory changes and hence, taken as a limit for sensory acceptance of chilled octopus.
Table 1.
Sensory changes in treated and control gutted octopus during iced storage
| Days | Sample | QIM score • |
|---|---|---|
| Day 0 | Control | 0 |
| Treated | 0 | |
| Day 2 | Control | 1.5 ± 0.15 |
| Treated | 1.5 ± 0.15 | |
| Day 4 | Control | 2.6 ± 0.15 |
| Treated | 2.9 ± 0.05 | |
| Day 6 | Control | 6.1 ± 0.05 |
| Treated | 6.9 ± 0.05 | |
| Day 8 | Control | 7.5 ± 0.20 |
| Treated | 8.4 ± 0.15 | |
| Day 10 | Control | 8.3 ± 0.10 |
| Treated | 9.4 ± 0.05 | |
| Day 12 | Control | 9.7 ± 0.10 |
| Treated | 10 |
•Total QIM Score is 10
Frozen octopus remained fresh with bright skin colour until day 40 in cold storage with scores below 2.25; and later, increased very gradually (Table 2). TF octopus had higher average sensory scores than CI octopus due to loss in texture and skin colour. Earlier studies have shown that frozen squid treated with ascorbic acid was of good quality with more shelf life (Selvaraj et al. 1991) and those treated with sodium ascorbate, lactate and phosphate had better sensory quality (Kulshrestha and Rhee 1996). Similarly frozen cuttlefish treated with 2 % salt and 0.2 % citric acid also had improved overall quality (Sophia and Sherief 2003) and those treated with 5 % NaCl and 0.3 % H2O2 had improved colour (Thanonkaew et al. 2008). In this study, the additives viz. cafodos and altesa did not improve the texture or colour of the frozen as well as chilled octopus. Further to support, Benjakul et al. (2012) had also observed no changes in the colour values during frozen storage of squid treated with 3 % NaCl containing 0.5 % H2O2 compared to control.
Table 2.
Sensory changes in frozen stored gutted Octopus
| days | Sample | QIM score • |
|---|---|---|
| Frozen | Raw | 0 |
| 0 day | Control | 0.25 ± 0.5 |
| Treated | 1.00 ± 0.8 | |
| 20 day | Control | 0.25 ± 0.5 |
| Treated | 1.25 ± 0.5 | |
| 40 day | Control | 2.25 ± 0.9 |
| Treated | 3.50 ± 1.0 | |
| 60 day | Control | 5.25 ± 2.6 |
| Treated | 5.25 ± 1.5 | |
| 80 day | Control | 7.55 ± 0.9 |
| Treated | 7.0 ± 1.0 |
•Total QIM Score is 10
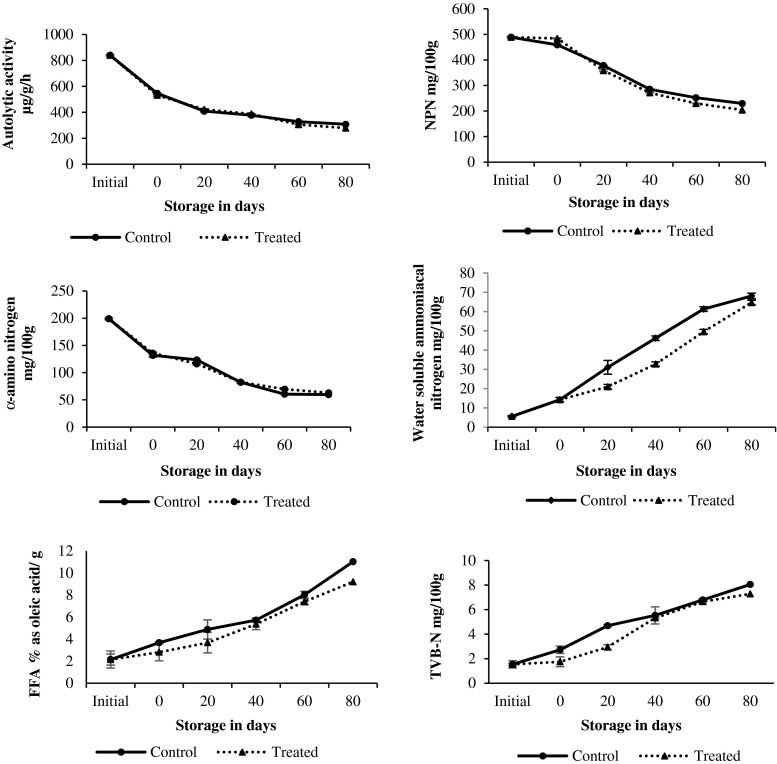
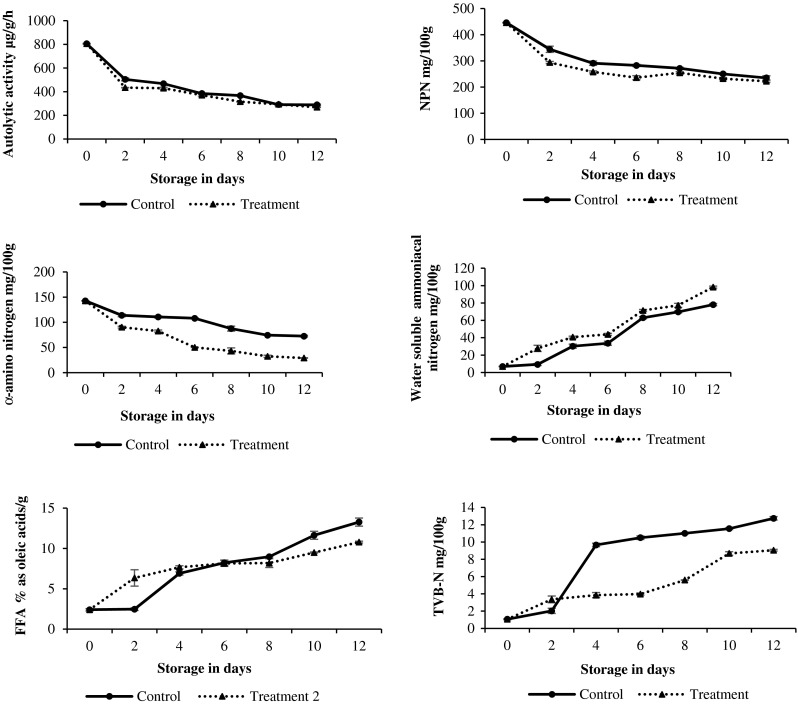
Autolytic activity reduced from about 800 to 550 g/g/h within day 2 in chilled octopus (p < 0.05) and later reduced very gradually (Fig.1). Autolytic activity also reduced in frozen octopus, but the TF octopus had slightly lower values. Lapa-Guimaraes et al. (2002) indicated that spoilage in cephalopods is generally dominated by autolysis that would lead to a shorter shelf life. In an earlier report, even application of high pressure had reduced the autolytic activity in octopus by around 68 % at the start of chilled storage (Hurtado et al. 2001). The additives examined in this study had sodium salts of citrate, chloride and carbonate, which did not suppress or enhance the autolysis as compared to control. The higher autolytic activity post-mortem produces an increased level of muscle derived nitrogen components (Hurtado et al. 1999). But, the NPN compounds in chilled octopus decreased significantly from about 440 to 340 mg/100 g (p < 0.05) and later gradually. Continuous reduction in the NPN compounds was also recorded in octopus (Dhananjaya and Venkatappa 2006) and other cephalopods (Vaz-Pires et al. 2008) on ice storage. Prafulla et al. (2000) have noticed about 79.5 % and 74.9 % reductions in NPN compounds during chilled storage of squids and cuttlefish. Significant reduction in the NPN at the initial phase of chilled storage implies the simultaneous onset of microbial spoilage and leaching. On the other hand, changes in NPN of frozen octopus were very minimal due to the limited microbial growth during cold storage (Fig. 2). As there were no significant differences in NPN values of control and treated octopus (p > 0.05), the additives were not effective against spoilage bacteria.
Fig. 1.
Changes in the biochemical changes in treated and control gutted octopus during iced storage
Fig. 2.
Changes in the biochemical changes in frozen stored gutted octopus
The α-amino nitrogen (α-AN) linearly reduced on storage in TI octopus than CI octopus, recording a loss of 50 % within 3 days (Fig. 1). Romo et al. (1996) reported that in squid (Dosidicus gigas), loss of α-AN was 40 % after 72 h of ice storage. It is further proven that the additives not only failed to control the growth of spoilage bacteria but also promoted the growth of spoilage bacteria, as evidenced by the significant differences in α-AN values of CI and TI octopus (p < 0.05). The α-AN also decreased significantly in frozen octopus until day 20 (p < 0.05). Joseph and Perigreen (1988) have also recorded a decrease in NPN and α-AN contents in frozen cuttlefish fillets (Sepia aculeate) similar to our findings (Fig. 2). There were also no remarkable differences in α-AN values of CF and TF octopus (p > 0.05).
Ammoniacal nitrogen formation is quite common in squids during chilled storage (Paarup et al. 2001). In addition, decrease in protein extractability during ice storage of squid and cuttlefish have also been reported (Ruiz-Capillas et al. 2006). As the estimated water soluble ammoniacal nitrogen includes soluble protein nitrogen, it serves as an index of protein solubility and ammonia formation. This nitrogen fraction was very low in fresh octopus, but increased gradually on storage (Fig.1). Significant differences were observed between day 2 and day 4 (p < 0.05) and between day 6 and day 8 (p < 0.05) in chilled octopus. In TI octopus, the initial increase was more rapid (p < 0.05). In frozen octopus, this fraction increased very gradually during storage (Fig. 2). In support to our findings, high protein solubility of 60 % was also recorded in 5 % NaCl treated frozen octopus held for 12 months in frozen storage by Moral et al. (2002). The additives viz. cafodos, altesa and salt had also enhanced the solubility of octopus myofibrillar protein leading to more water soluble ammoniacal nitrogen fraction. Among the various biochemical parameters, a positive correlation existed between the sensory scores and this fraction of frozen treated and untreated octopus.
FFA values increased from 2.3 % to 13.2 % oleic acid/g fat in chilled octopus. Increase in FFA values during ice storage of octopus and cuttlefish was reported by several authors (Dhananjaya and Venkatappa 2006; Sophia and Sherief 2003). Significant changes were noticed between day 2 and day 4 in CI octopus (p < 0.05) and within day 2 (p < 0.05) in TI octopus. Additives had thus promoted the lipid hydrolysis rapidly in chilled octopus. In frozen octopus also lipid hydrolysis proceeded in a similar manner as that of chilled octopus. (Fig. 2). Earlier studies have indicated that enzymatic reduction can continue in frozen fish held at -30 °C (Cassens 1994) and cause intrinsic chemical changes. Although sodium chloride has an ability to inactivate autolytic enzymes of marine species (Siringan et al. 2006), it had failed to control the action of lipase when used along with sodium citrate, sodium carbonate and hydrogen peroxide. FFA values have also shown good correlation with the sensory scores in CF octopus (r2 = 0.965) and TF (r2 = 0.996) octopus.
TVB-N values increased from day 4 itself exponentially reaching a maximum of 12.74 mg/100 g on day 12 in chilled octopus (Fig. 1), indicating the onset of microbial spoilage. Earlier, citric acid has been reported to reduce TVB-N and pH, but increase the toughness of the chilled squid (Agrafioti and Katsanidis 2012), while NaCl did not have any significant effect in the presence of other additives. TVB-N values of frozen octopus also increased but very gradually at the beginning and later, significantly (p > 0.05) as a result of the slow proliferation of psychrophilic spoilage microflora. An increase in TVB-N, TMA-N, PV and FFA values in NaCl treated and control cuttlefish during frozen storage was also observed by Sastry and Srikar (1985), similar to the present findings. A good correlation also existed between TVB-N values and sensory scores of treated and untreated octopus. (r2 = 0.997).
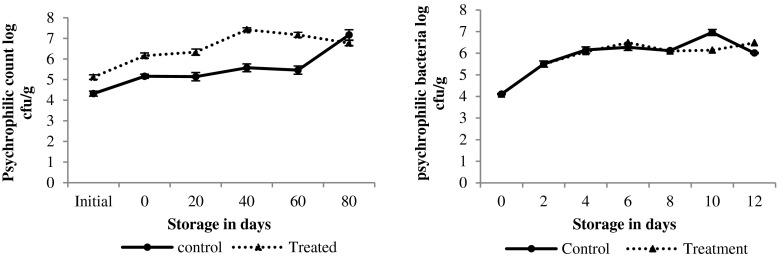
Total psychrophilic bacterial count in chilled octopus was initially 4.11 log cfu/g, which increased gradually until day 4 (p < 0.05), and therefore remained constant at 6 log cfu/g until day 12. Initial TPC of common octopus has been earlier reported to be as low as 3–4 logs cfu/g (Hurtado et al. 2001; Vaz-Pires and Barbosa 2004) similar to our observation. In an earlier report, TPC in aerobically stored squid had exceeded the maximum limit of 7 log cfu/g beyond day 8 in ice (Ohashi et al. 1991). In general, Pseudomonas and Shewanella spp. are the main spoilage bacteria that grow during aerobic storage in chilled seafood (Gram and Dalgaard 2002). Growth of psychrophilic bacteria in the frozen octopus was also noticed as the counts increased from 4 to 7 log cfu/g within day 80 (Fig. 3). On the contrary, Dhananjaya and Venkatappa (2006) had noticed a decrease in total mesophilic count from 5 log to 3 log cfu/g in battered and breaded squid and cuttlefish during 105 days of frozen storage. Since, the author had examined the total mesophilic bacteria, the results did not reflect the presence of total psychrophilic bacteria. TF octopus had one or two log higher counts than CF octopus, which further proved that these additives did not control the growth of psychrophilic spoilage bacteria. However, no good correlation existed between the total psychrophilic counts and sensory scores.
Fig. 3.
Changes in the microbiological changes in iced and frozen stored octopus
Conclusion
This study indicated that treatment of octopus with additives like cafodos, altesa and sodium chloride could not control the growth of psychrophilic bacteria instead can enhance the protein solubility leading to the production of soluble nitrogenous compounds for bacterial utilization. This has attributed to the loss of texture and colour in chilled and frozen octopus. Additives have also failed to control lipid hydrolysis that is predominant in frozen products. Among the biochemical indices, water soluble ammoniacal nitrogen and FFA correlated well with the sensory scores and therefore could serve as better quality indices. Further study can be undertaken to examine whether these additives affect the functional properties of the octopus protein fractions.
Acknowledgments
Authors wish to thank the Dean of this institution for providing necessary support to carry out this work. TANUVAS Merit fellowship awarded to the first author to undertake research work as part of his master degree programme is also hereby acknowledged. We would like to accord our thanks to M/s Theva Seafood Pvt. Ltd., Thoothukudi, for providing necessary facilities and support to undertake this work.
References
- Agrafioti PT, Katsanidis E. Effect of additives on the selected quality attributes and cooking yield of squid: modeling and optimization. Int J Food Prop. 2012;15:579–589. doi: 10.1080/10942912.2010.494755. [DOI] [Google Scholar]
- Anon, (2014) Octopuses-indian-seafood-exporters. http://articles.economictimes.indiatimes.com/2014-03-26/news/48595258_1_octopuses-indian-seafood-exporters-anwar-hashim. Accessed 6 June 2015
- Antonacopoulos N, Vyncke W. Determination of volatile bases in fish. Z LebensmUnters Forseh. 1989;189(3):309–316. doi: 10.1007/BF01683206. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 16th. Washington, D.C.: Association of official analytical chemists; 1995. [Google Scholar]
- APHA . Standard methods for the examination of water and wastewater. 18th. Washington, DC: Water Pollution Control Federation; 1992. [Google Scholar]
- APHA (1995) Compendium of methods for the microbiological examination of foods. APHA, New York 701
- Benjakul S, Sungsri-in R, Kijroongrojana K. Effect of treating of squid with sodium chloride in combination with oxidizing agent on bleaching, physical and chemical changes during frozen storage. Food Bioprocess Technol. 2012;5:2077–2084. doi: 10.1007/s11947-010-0460-z. [DOI] [Google Scholar]
- Cassens RG (1994) Meat preservation, preventing losses and assuring safety, 1st Edn., Food and Nutrition Press, Inc., Trumbull, Connecticut, USA., SBN:0917678346: 402–451
- Civera T, Grassi MA, Pattono D. Caractteristiche chimiche e microbiologiche di molluschi cefalopodi nel corso Della conservazione. Industrie Alimentari. 1999;38:933–937. [Google Scholar]
- Dhananjaya S, Venkatappa Studies on the ice storage characteristics of octopus, octopus membranaceus (quoy and gaimard) Fish Technol. 2006;43:154–161. [Google Scholar]
- European Commission (2013) Summary report of the standing committee on the food chain and animal health held in Brussels on 17 April 2013
- FDA, 2014 http://www.accessdata.fda.gov/cms_ia/importalert_ 49.html
- Gram L, Dalgaard P (2002) Fish spoilage bacteria: problems and solutions. Curr Opin [DOI] [PubMed]
- Hurtado JL, Borderias J, Montero P. Characteristics of proteolytic activity in octopus (octopus vulgaris) arm muscle. J Food Biochem. 1999;23:469–483. doi: 10.1111/j.1745-4514.1999.tb00031.x. [DOI] [Google Scholar]
- Hurtado JL, Montero P, Borderias J. Chilled storage of pressurized octopus (octopus vulgaris) muscle. J Food Sci. 2001;66:400–406. doi: 10.1111/j.1365-2621.2001.tb16117.x. [DOI] [Google Scholar]
- Jimenez-Colmenero F, Borderias AI. A study of the effects of frozen storage on certain functional properties of meat and fish protein. Food Technol. 1983;18:731–737. doi: 10.1111/j.1365-2621.1983.tb00311.x. [DOI] [Google Scholar]
- Joseph J, Perigreen PA. Studies on frozen storage of cuttlefish fillets. Fish Technol. 1988;25:32–35. [Google Scholar]
- Kulshrestha SA, Rhee KS. Precooked reduced - fat beef patties chemical and sensory qualities as affected by sodium ascorbate, lactate and phosphate. J Food Sci. 1996;61(5):1053–1057. doi: 10.1111/j.1365-2621.1996.tb10931.x. [DOI] [Google Scholar]
- Lapa-Guimaraes J, Aparecida AM, Edwardo FP, Contreras GE. Sensory, color and psychotropic bacterial analyses of squids (loligo plei) during storage in ice. Lebensm Wiss Technol. 2002;35:21–29. doi: 10.1006/fstl.2001.0783. [DOI] [Google Scholar]
- Lougovois VP, Kolovou MK, Savvaidis IN, Kontominas MG. Spoilage potential of ice-stored whole musky octopus (eledone moschata) Int J Food Sci Technol. 2008;43:1286–1294. doi: 10.1111/j.1365-2621.2007.01607.x. [DOI] [Google Scholar]
- Mohanan P (2004) Influence of Processing Variables on Protein Quality and Frozen Storage Stability of Two Commercially Important Species of Squid (Loligo duvaucelii and Dorgteuthis sibogae). Ph.D. Thesis, Cochin University of Science and Technology, Cochin
- Moral A, Morales J, Ruíz-Capillas C, Montero P. Muscle protein solubility of some cephalopods (pota and octopus) during frozen storage. J Sci Food Agric. 2002;2002(82):63–68. [Google Scholar]
- MPEDA Export of marine products from India. MPEDA newsletter. March. 2013;2013:5–7. [Google Scholar]
- Ohashi E, Okamoto M, Ozawa A, Fugita T. Characterization of common squid using several freshness indicators. J Food Sci. 1991;56(1):161–163. doi: 10.1111/j.1365-2621.1991.tb08001.x. [DOI] [Google Scholar]
- Paarup T, Sanchez JA, Moral A, Christensen H, Bisgaard M, Gram L. Sensory, chemical and microbiological changes during storage of ice squid (todaropsis eblanae) J Appl Microbiol. 2001;10:198–205. doi: 10.1046/j.1365-2672.2002.01604.x. [DOI] [PubMed] [Google Scholar]
- Prafulla V, Francis L, Lakshmanan PT. Effect of differebt methods of icing on the quality of squid and cuttlefish during storage. Fish Technol. 2000;37:81–88. [Google Scholar]
- Pope CG, Stevens MF. The determination of amino nitrogen using copper method. Biochem J. 1939;33:1070–1077. doi: 10.1042/bj0331070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo C, Astudillo J, Mun˜oz O, Contreras E. Determinacio’n de ı’ndices bioquı’micos y funcionales relevantes Para evaluar la conservacio’n de jibia (dosidicus gigas) a bordo. In: Proceedings of the Workshop on Fish and Mollusc Larviculture; 1996. pp. 197–213. [Google Scholar]
- Ruiz-Capillas C, Moral A, Morales J, Montero P. Characterization and functionality of frozen muscle protein in volador (illex coindetii), pota (todaropsis eblanae), and octopus (eledone cirrhosa) (pages 2164–2168) J Food Sci. 2006;68(7):2164–2168. doi: 10.1111/j.1365-2621.2003.tb05741.x. [DOI] [Google Scholar]
- Sastry HHC, Srikar LN (1985) Protein and related changes in cuttlefish (Sepia aculeate) during iced storage. In Harvest and Post-harvest Technology of Fish (Ed. K. Ravindran) society of fisheries Technologists, India, 386–388
- Selvaraj P, Jasmine JI, Jeyachandran P. Effect of ascorbic acid dip treatment on frozen storage of squid (loligo duvaucelii, oribigny) Fish Technol. 1991;2:117–121. [Google Scholar]
- Sikorski ZE, Kolodziejska H. The composition and properties of squid meat. J Food Chem. 1986;20:213–224. doi: 10.1016/0308-8146(86)90174-3. [DOI] [Google Scholar]
- Sophia MJ, Sherief PM. Effect of treatments on the chilled storage shelf life of cuttlefish (sepia aculeate) fillets. Fish Technol. 2003;40(I):32–35. [Google Scholar]
- Siringan P, Raksakulthai N, Yongsawatdigual J. Autolytic activity and biochemical characteristics of endogenous proteinases in Indian anchovy (stolephorus indicus) Food Chem. 2006;98:678–684. doi: 10.1016/j.foodchem.2005.06.032. [DOI] [Google Scholar]
- Sungsri-in R. Development of pink colour of squid and effect of chemical treatment on physic-chemical changes of squid during frozen storage. M.Sc Thesis: Prince of Songla University; 2010. p. 102. [Google Scholar]
- Takagi T, Hayashi K, Itabashi V. Toxic effects of free unsaturated fatty acids in mouse assay of diarrheic shellfish toxins by intra peritoneal injection. Bull Jpn Soc Sci Fish. 1984;50:1413–1418. doi: 10.2331/suisan.50.1413. [DOI] [Google Scholar]
- Thanonkaew A, Benjakul S, Visessanguan W, Decker AE. The effect of antioxidants on the quality changes of cuttlefish (sepia pharaonis) musle during frozen storage. Swiss Soc Food Sci Technol. 2008;41:161–169. [Google Scholar]
- Vaz-Pires P, Barbosa A. Sensory, microbiological, physical and nutrition properties of iced whole common octopus (octopus vulgaris) Lebensm Wiss Technol. 2004;37:105–114. doi: 10.1016/S0023-6438(03)00141-5. [DOI] [Google Scholar]
- Vaz-Pires P, Seixas P, Mota M, Lapa-Guimaraes J, Lindo A, Silva T. Sensory, microbiological, physical and chemical properties of cuttlefish (sepia officinalis) and broad tail short fin squid (illex coindetii) stored in ice. J Food Sci Technol. 2008;41:1655–1664. [Google Scholar]