Abstract
This study aimed to identify using the infrared (IR) thermography data the injuries of guavas during cooling and storage at different temperatures. Three experiments were performed at three different temperatures with one storage time. The first experiment was done with static air in a refrigerator at 5 °C, the second experiment was conducted in a tunnel with forced air at 10 °C, and the third experiment was conducted in an air conditioned environment at 20 °C. Mechanical injuries caused by the impact of a pendulum were induced on guava surfaces. The surface temperatures were obtained for bruised and sound tissues during cooling and storage using an Infrared (IR) camera. With thermography, it was possible to distinguish the injured tissues of the fruits that were unaffected at temperatures of 5, 10 and 20 °C in first hours of cooling. The results suggest that the storage of guava fruits at 5 °C in static air resulted in cold-induced injury, while storage at 20 °C resulted in an altered activity pattern. The stored guava fruits were analyzed for mass loss, firmness, color, total sugars, total pectin and solubility. The parameters values were lower during the forced-air cooling and storage at 5 and 10 °C. When stored at 20 °C, there was fruit maturation that caused tissue softening, which makes the fruits more susceptible to deterioration and thermographic readings showed opposite trends.
Keywords: Psidium guajava, L. injury, Refrigeration, IR thermography
Introduction
The guava tree (Psidium guajava, L.) originates from tropical America and most likely from the region between southern Mexico and northern South America. This species is widely spread across tropical and subtropical regions worldwide. The guava crop is economically important in Brazil, and in 2011, guava fruit production reached 342,500 t and generated BRL 276 million with an average yield of 21.5 t ha−1. Guava is grown heavily in the states of São Paulo, Pernambuco, and Minas Gerais. Together, these states accounted for approximately 60 % of the domestic guava production in 2011 (Brazilian Institute of Geography and Statistics [INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA – IBGE] 2011).
Guava is a tropical climacteric fruit. During ripening, guava exhibits an increased respiratory rate and greater ethylene biosynthesis, which modulate the rate of fruit ripening. Guava fruit is very perishable with a maximum shelf life of only 8 days (Mowlah and Itoo 1983). This short shelf life hinders market availability and may lead to significant postharvest losses. Thus, techniques are required to increase the consumption potential of guava.
Among the causes of postharvest loss, mechanical injuries are particularly important and include impact, compression, and cutting injuries. These injuries cause irreparable damage and compromise the commercial value of the fruit, as observed in the Paluma and Pedro Sato varieties of guava (Mattiuz et al. 2002). These damages stimulate the production of ethylene, which accelerates the maturation process and reduces the shelf life of guava fruit (Neves et al. 2009). The main aspects of deterioration include softening, loss of green coloration and shine, and rotting.
Thermography is a non-destructible analytical tool that does not come in contact with the product. Thus, thermography does not mechanically damage or contaminate the study object (Veraverbeke et al. 2006). Food processing studies that use infrared thermography to control citrus surface drying by image analysis have been published by Fito et al. (2004). Baranowski and Mazurek (2009) detected physiological disorders and mechanical defects in apples using infrared (IR) thermography. Gan-Mor et al. (2011) used thermal analysis to develop postharvest precision steam-disinfection technologies for carrots. In addition, Vadivambal et al. (2007) employed thermal imaging to observe hot and cold spots in rye and oats during microwave heating. Furthermore, Veraverbeke et al. (2006) evaluated the surface quality of waxed apples with thermography.
Thermal imaging has been used in some studies that involve the quantification of plant material surface temperatures. In addition, this technique can be used to quantify or control fruit quality. Furthermore, studies have used thermography for injury detection on the surface of apples and tomatoes before the appearance of visible injuries (Varith et al. 2003; Veraverbeke et al. 2006; Baranowski and Mazurek 2009).
Refrigeration is one of the most efficient methods for postharvest quality maintenance. In addition, refrigeration is the most economical method for prolonged fruit and vegetable storage. The use of refrigeration prolongs the preservation of fruits. Forced-air cooling is one alternative postharvest technology for the guava production chain. Forced-air cooling cools the fruits as quickly as possible after harvest to preserve their quality and extend their shelf life. During the fast cooling process, the passage of air in contact with fruits results in mass flows and temperature and moisture gradients that differ at the intact surface in direct contact with the air and at surface with injuries. These specific sites are ideal for the onset and development of injuries caused by temperature and moisture variations. However, these gradients are difficult to quantify by traditional intrusive methods because sensors cannot quantify these differences. Therefore, it is reasonable to formulate a hypothesis that such variations on the fruit surface could be quantified and correlated to the injuries observed during the cooling and storage processes of unpackaged fruits. This study aims to obtain the infrared thermography data during cooling and cold-stored of guava at different temperatures for identify injuries on the guava fruit surfaces using a non-destructible analytical methodology.
Materials and methods
Sample preparation
The guava (Psidium guajava L.) fruits from the Pedro Sato cultivar were obtained from orchard in the municipality of Lavras - Minas Gerais. These fruits were collected by hand in the early morning and placed in previously sterilized polyethylene boxes before transporting to the laboratory. Fully mature fruits were selected with matte-green peel coloration, firm pulp, and absence of mechanical and physiological injuries. The fruits were washed under running water, weighted, and disinfected by submerging them in a sodium hypochlorite solution (0.01 g/L) at room temperature for 5 min.
Impact-induced injury
Injuries were caused by the impact of a pendulum consisting of a 60 cm long rod with a sphere on its end. The pendulum was initially placed at 45° relative to the sample position. The sphere had a radius of 1 cm and weighted approximately 180 g. This sphere was responsible for impacting a delineated region of approximately 4 cm2 in each sample. The shaft-sphere set weighted 705 g and was released from its initial position in free fall to generate an impact of approximately 1.00 J of energy on the guava fruits.
Cooling of fruits
Three experiments were performed with three different temperatures and one storage time. The first experiment was conducted in static air and in a refrigerator at 5 ± 3 °C and relative humidity of 70 %. The second experiment was conducted in a refrigeration chamber with forced air at 10 ± 1 °C, 90 % relative humidity (Fig. 1). Finally, the third experiment was conducted in an air-conditioned environment at 20 ± 2 °C, 70 % relative humidity. The relative humidity of the air in the three experiments was measured using a digital thermo-hygrometer (Hygrotherm max/min. TFA – Germany). The guavas were subjected to the control and induced injury treatments prior to storing for 9 days.
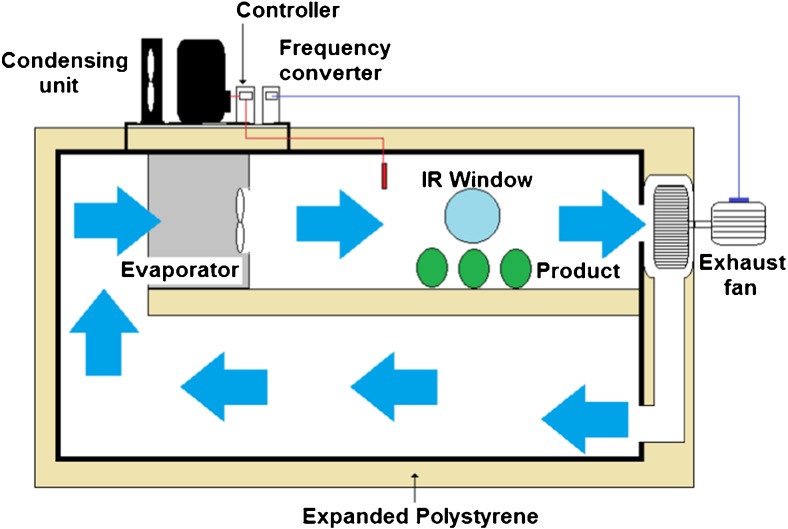
Fig. 1.
Forced-air cooling equipment
The temperature reductions of guava fruits were monitored using a signal conditioning system (National Instruments Mod. SCXI – Hungary) and the Lab View 8.5 software with 1-s intervals between each record. The temperature readings were obtained with resistance temperature devices (RTDs). Sensors were inserted in the surface and geometric center of the guava fruits. In addition, these sensors were placed in the forced-air stream at different locations to measure the air temperature inside the chamber. The data obtained by temperature sensors have been helpful in calibrating the IR camera. All experiments were performed in triplicates.
Use of infrared thermography
Thermal images were obtained with a thermal imaging camera (FLIR Systems, Inc. – model ETO T420, Wilsonville, OR, USA)) and were evaluated with the FLIR Tools software. These images were captured by the thermal imaging camera throughout the storage period in the air-conditioned environment and refrigerator with static air. To obtain thermal images in the forced-air chamber, an inspection window measuring 7.62 cm in diameter (3 in.) (FLIR – IRW 3C) was placed at the back of the chamber that allowed the passage of infrared rays (Fig. 1). Monitoring was performed every 6 h. For all experimental configurations, the IR camera was calibrated. Two guavas placed side by side were cooled from 30 to 5 °C and the temperatures were simultaneously recorded by the IR camera and by a flat thermocouple located on the surface of target.
Air velocity measurement
For the second experiment, the air velocity inside of the chamber was measured at different points in the section that was perpendicular to the air flow direction. In this chamber, the fan speed was controlled by a frequency converter (Vector inverter WEG – CFW500, Jaraguá do Sul, Brazil) throughout the entire experiment.
Analytical methods
The following physical and chemical analyses were performed from three replicates on the first and last days of the experiment. Mass loss was obtained as the difference between the initial and final fruit masses. Firmness was defined by measuring the maximum compression force required to introduce a 5-mm cylindrical tip (probe) to a depth of 1.0 cm in four replicates. Perforations were performed at the equatorial region of each fruit. These measurements were taken using a penetrometer (Instrutherm - model PTR300, São Paulo, Brazil), and the results are expressed in Newtons (N). Color was assessed at the equatorial region of the guava peel on four replicates. A colorimeter (Konica Minolta - model CM 5, Osaka, Japan) was used for the following parameters: L* (lightness), a* (red/green intensity), b* (yellow/blue intensity), and h (Hue). Total sugars were extracted with 70 % ethyl alcohol and determined by the Antrona method (Dische 1962). These results were expressed as grams of glucose per 100 g of pulp. Total pectin and soluble pectin concentrations were extracted by using the techniques standardized by McCready and McComb (1952). In addition, the total and soluble pectin concentrations were determined colorimetrically by following the methods presented by Bitter and Muir (1962). These results were expressed in g of galacturonic acid/100 g of pulp. The percentage solubility was obtained as the percentage of soluble pectin relative to total pectin.
Experimental design
The experiments were performed according to a completely randomized design (CRD) that was arranged in a 2 × 3 factorial scheme with 3 replicates. The first factor corresponds to the treatments (control and impact-induced injury), and the second factor corresponds to the storage temperatures of guava fruit (5, 10 and 20 °C). The analyzed physical and chemical parameters were mass loss, firmness, color, total sugars, total and soluble pectin. The results were subjected to an analysis of variance (ANOVA) using the R software. The means were compared by the Scott-Knott test at a 5 % significance level.
Results and discussion
Cooling curves
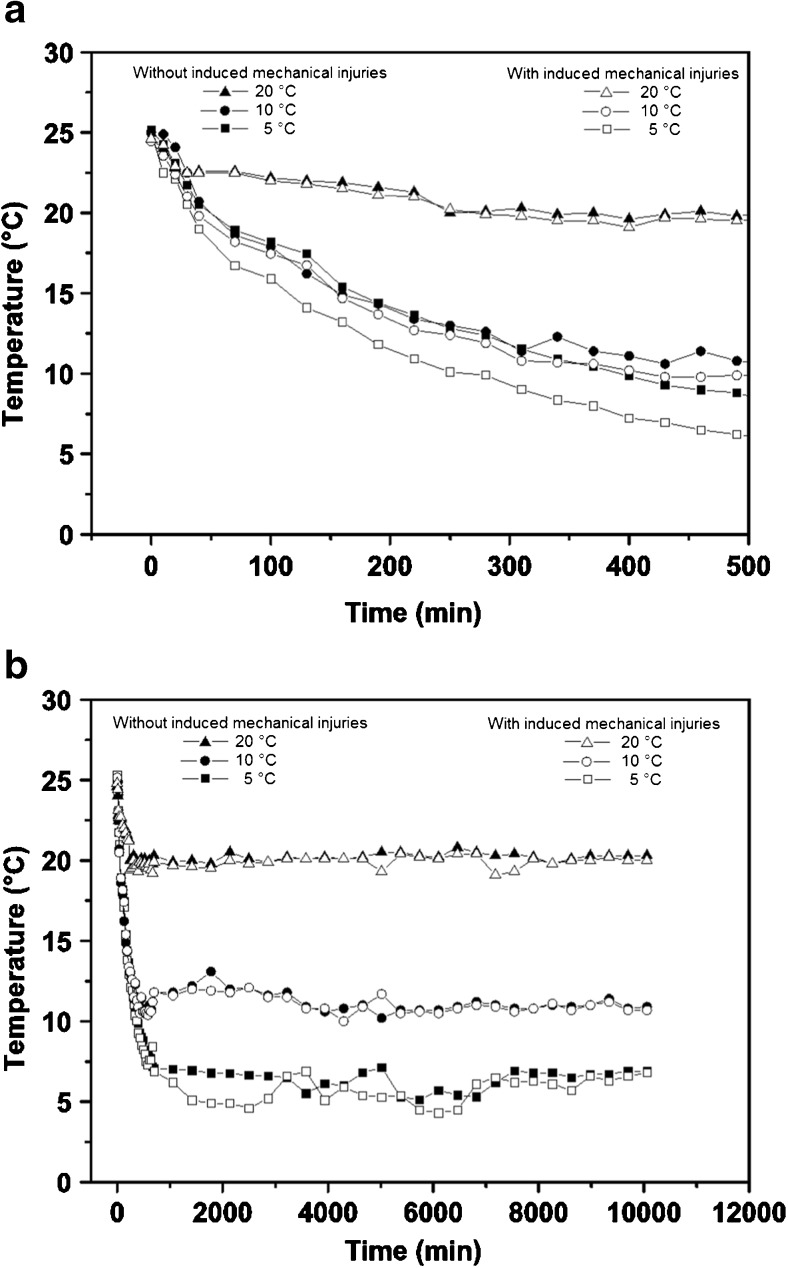
Figure 2 shows the surface temperature for the three studied processes obtained using IR camera during the cooling (Fig. 2a) and the time that the temperature is constant (Fig. 2b). Figure 2 shows that the surface of guavas were quickly cooled and the differences in temperature between bruised and sound tissues were detected in the first hours (500 min) of cooling for treatments with 5 and 10 °C. For times where the temperature is constant, the highest temperature oscillations observed in treatments of 5 and 10 °C are justified by on-off control of the coolers (Fig. 2b) or by error sources that limit the accuracy of the temperature determination. These errors sources can be the emissivity of guava, presence and position of the inspection window and diffuse radiation. According to Hellebrand et al. (2000), the share of direct radiation of water, the main component of fruits and vegetables, depends on the thickness of the water layer. Only those areas of fruits will be measured correctly, which are parallel oriented to the sensor plane of the camera. This effect is visible in thermal images of spherical shaped fruits. The temperature at the outer circumference seems to be lower than from the center of the fruit.
Fig. 2.
Cooling curves for the 3 configurations obtained from IR thermal images analyses. a In the first hours of guava cooling, and b during all the cooling time and guava storage
In this work, the measurements of temperatures were taken in center of guava and parallel with the camera sensor. The imaging parameters were as follows: 0.95 emissivity, 0.50 m distance, 90 % relative humidity, and atmospheric temperature of 25 °C. The emissivity was chosen based on average values found in the literature of work performing measurements on fruit surfaces. The spectral emissivity as measured by Veraverbeke et al. (2006) was 0.96 for both the apple cultivars Jonagored and Elshof. The emissivity of 0.95 was measured for citrus fruit by Fito et al. 2004.
Veraverbeke et al. (2006) showed the potential of thermal imaging for surface quality analysis of apples. They monitored the cooling rate and surface temperature of apples in relation to the surface quality and wax layer structure before and during storage. They detected a significant difference in the cooling rate between apple cultivars (Elshof and Jonagored), in relation to wax structure. The lower surface temperature of Elshof was explained by the higher moisture losses and transpiration rates at the surface of Elshof apples due to their more cracked wax layer and higher amount of lenticels. In this work for treatments at 5, 10 and 20 °C, the differences in the temperature response between bruised and sound tissue observed in the cooling step were attributed to thermal property differences caused by mechanical damage and cold-induced injuries.
Veraverbeke et al. (2006) did not detect differences during apple storage. In this work in experiments with 20 and 10 ° C, the temperature differences between bruised and sound tissues were not detected during storage. At 5 °C the differences were detected and justified as due cold injury development.
Surface temperature analysis by IR thermography
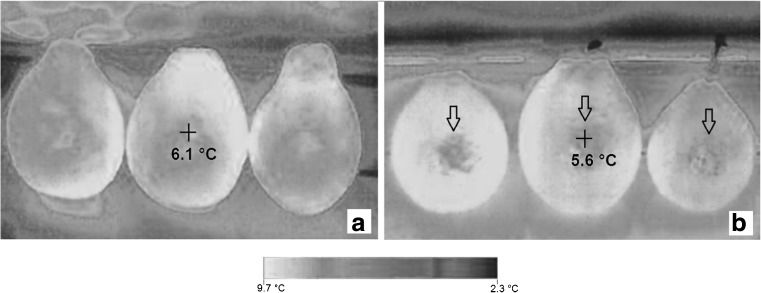
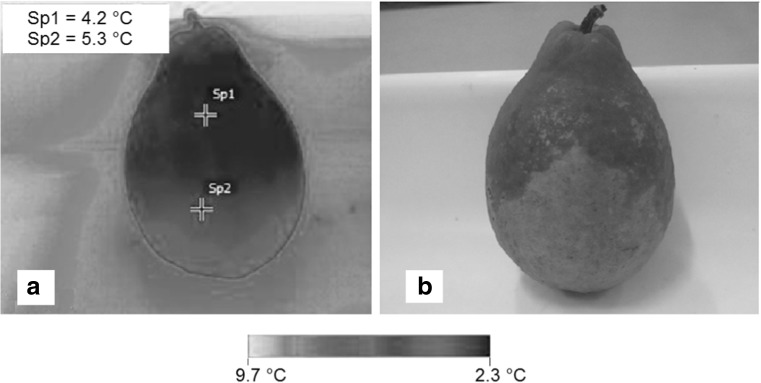
Figures 3 and 4 display the thermograms generated by infrared thermography in guavas with and without injuries and stored at 5 and 20 °C (determined by the cooling method) taken on the fifth day of storage. Figure 5 displays the thermograms generated by infrared thermography in guavas without injuries and stored at 5 °C taken on the fifth day of storage. The Figs. 3 and 5 suggest that the storage of guava fruits at 5 °C in static air resulted in cold-induced injury, while storage at 20 °C (Fig. 4) resulted in an altered activity pattern with quality loss and fruit maturation. In thermograms generated by the infrared thermal imager, the arrows in Figs. 3 and 4 indicate regions on the guavas with mechanical injury.
Fig. 3.
Thermal images for guava fruits stored at 5 °C for 5 days; without induced mechanical injuries (a) and with induced mechanical injuries (b)
Fig. 4.
Thermal images for guava fruits with (delineated) and without induced mechanical injuries and stored at 20 °C for 5 days
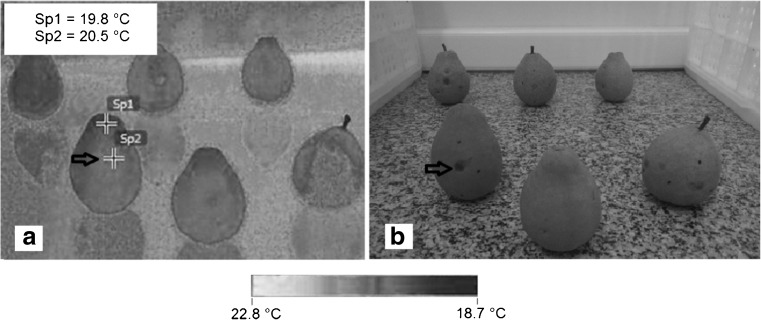
Fig. 5.
Cold-induced injury identified by infrared thermography in guava fruits stored at 5 °C. Sp1 and Sp2 are the temperatures of the injured tissue and unaffected tissue, respectively
Figure 2 shows that at 10 °C, the surface of guavas were quickly cooled and the differences in temperature between bruised and sound tissues were lower when compared with 5 °C. For experiments that consisted of guavas with and without mechanical damage and stored at 10 °C, the average air velocity in cross section of the chamber was 0.893 m/s. The expectation is that at 10 °C (optimum temperature for guava storage) the rapid cooling preserves the post-harvest quality and avoids the cold-induced injuries.
Figures 3 and 5 prove that thermography can be used to distinguish injured tissues from unaffected tissues at temperature of 5 °C. Figure 5 contains a thermal image that was obtained for guavas with cold-induced injury when stored in a refrigerator at 5 °C (Fig. 5a). In addition, Fig. 5 contains an image that was obtained with a digital camera with visually delineated cold-induced injuries (Fig. 5b). The temperature differences between the intact tissues, mechanically injured tissues and tissues with cold-induced injuries ranged from 0.5 to 3.0 °C during cooling and storage. These temperature differences were similar to the values observed by Baranowski and Mazurek (2009) in apples, which ranged from 0.5 to 1.5 °C. The value of 4.2 ° C obtained by the thermal imager in Fig. 5a, which is below the set-point value can be justified by temperature fluctuations caused by on-off controller that has a permanent deviation of 3 ° C for this treatment.
Figure 4 exhibits a thermal image that was obtained for guava fruits with a mechanical injury when stored in a refrigerator at 20 °C (Fig. 4a). In addition, Fig. 4 contains an image that was obtained with a digital camera where mechanical injury can be observed (Fig. 4b) and the arrow indicates the guava with mechanical injury. Figure 4 shows opposite trends, where guava bruises were 0.7 °C warmer than the sound tissue. We assigned this phenomenon to guava senescence and rot after 9 days stored at 20 °C. Varith et al. (2003) cooled apples with forced convection in ambient air at 50 % RH, 26 °C after heating in 40 °C water for 2–3 min. They verified that the temperatures of bruises in most apples were 1–2 °C cooler than those of sound tissues, which contradicted the results in heating treatments. It also appeared that some red Delicious apple bruises were 2–3 °C warmer than the sound tissue under this treatment. This treatment involved briefly heating and then cooling the outside of apples whose centers remained cold; the result was a combined transient heating and cooling that was too complex to draw a conclusive pattern of temperature differences (Varith et al. 2003).
According to Figs. 4 and 5, infrared thermography can potentially be used to assess and monitor the quality of guava fruits because it detects the appearance of mechanical and cold-induced injuries and fruit senescence. According to Hobson (1987), lower temperatures correspond with a longer storage life and greater incidence of cold-induced injuries, which result in guava quality loss and reduced shelf life. Figure 5 displays the temperature variations in the intact and cold-injured tissues with a coloration gradient in the thermal images. Injuries were observed at lower temperatures, potentially due to cell breakage and cellular fluid leakage. Water is more available and has potential heat conduction activity. According to Pereira et al. (2013), water is with the food component with the highest thermal conductivity and diffusivity. Thus, water contributes to the quicker temperature decrease that occurred on guava surfaces stored at 5 °C. At 10 °C, significant difference also was observed between the mechanically injured and intact tissues, but with lesser intensity. Varith et al. (2003) obtained similar results when applying thermography to detect injuries in apples and observed different temperature responses between injured and intact tissues. These differences were attributed to thermal property differences. Damaged tissues exhibited a lower temperature than the intact tissues under low cooling temperatures and a greater temperature under high cooling temperatures. The authors concluded that the detection of bruises mainly resulted from variations in thermal diffusivity between bruised and sound tissues and not due to thermal emissivity. With the higher thermal diffusity, bruises can transfer heat from fruit exterior into the sound interior tissue faster than can the surrounding sound tissue, resulting in lower surface temperature in bruised than in sound tissue (Gowen et al. 2010).
Physical and chemical analysis
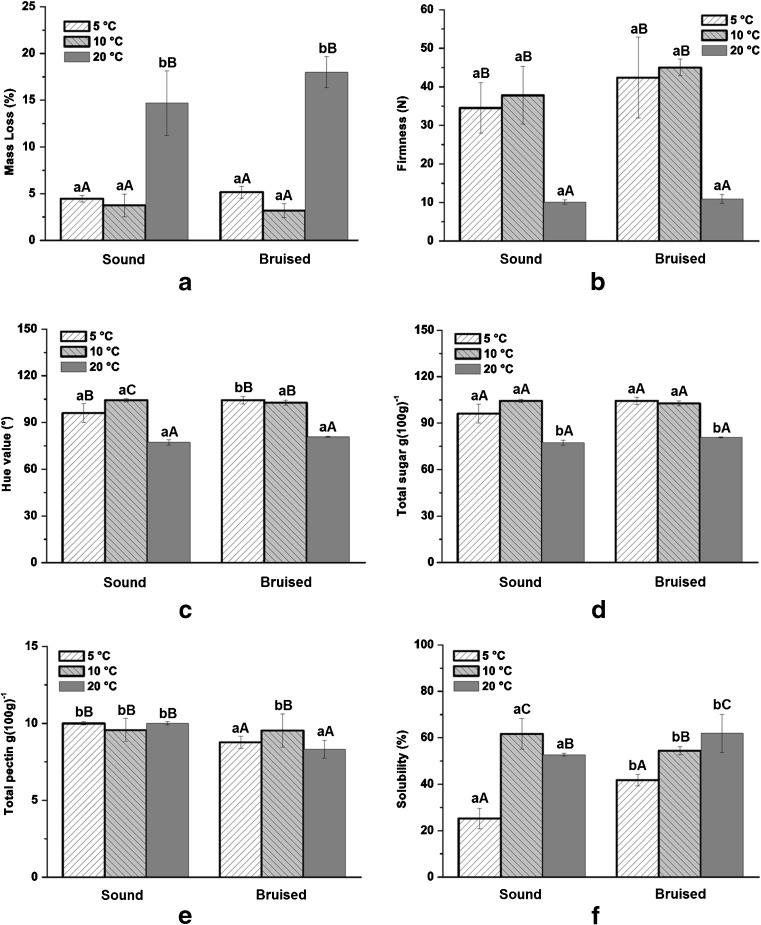
Trying to explain the temperature difference obtained from IR thermography and to verify the type of injury or physiological disorders during the cold-storage in different configurations, we performed some physical and chemical analyses of guava after 9 days of storage (Fig. 6). The results presented in Fig. 6a suggest that the mass loss in guavas increased during storage and mass loss are related to temperature. This increase was significant at 20 °C with a mass loss of 18.0 % relative to the initial mass for control. Figure 6 shows that, at 20 °C, there was not significant difference between the guavas with and without induced injury. Mass loss was lower during the forced-air cooling and storage at 5 and 10 °C. When stored at 20 °C, mass loss resulted from the transpiration process and caused tissue softening, which makes the fruits more susceptible to deterioration (Azzolini et al. 2005).
Fig. 6.
Results of physical and chemical analyses in cold-stored guavas. a Mass Loss (%), b firmness (N), c Hue value (°), d total sugar (g(100 g)−1), e total pectin (g(100 g)−1), and f solubility (%). Values with the same capital letters in the same bar group and lowercase letters between sound and bruised treatments at the same temperature do not differ by the Scott-Knott (P < 0.05) test
The fruits exhibited an overall decrease in pulp firmness throughout the 9 days of storage (Fig. 6b). On the first day of storage, the firmness of the guava fruits was 49.28 N. The decrease in firmness during storage was more pronounced at 20 °C, which indicated a lower resistance at this temperature. No significant difference was found between the control and induced injury treatments. Small variations in the firmness of the guava fruits occurred at 5 and 10 °C. The h value (Hue) indicates the ripening or the color changes between physiologically mature and mature fruits. Changes in variable were observed throughout the experiment and the most intense reductions in h were observed in induced injury fruits at 20 °C (Fig. 6c). The initial total sugar concentration was 5.68 g (100 g)−1. A significant difference was observed for guava fruits with or without impact-induced mechanical injury at 20 °C (Fig. 6d). The greater total sugar content was most likely caused by the greater mass loss, which increased the total soluble solid concentrations in the fruits (Jacomino et al. 2003). In addition, the fruits exhibited signs of senescence during this period. Furthermore, Sharaf and El-Saadany (1986) observed lower sugar contents in guava fruits during their maturation from green to fully mature.
Significant differences occurred for total pectin contents between the analyzed temperatures and injury induced treatments (only at 10 °C) (Fig. 6e). In this case, the mechanically injured guava fruits exhibited higher total pectin concentrations. After 9 days of storage, the guava fruits had higher mean soluble pectin contents relative to their initial value of 70.75 g (100 g)−1. This increase suggests that the fruit tissues became less stable, resistant, and durable (Mowlah and Itoo 1983). The percentage of soluble pectin that was evaluated at the beginning of the storage period was 23.45 %. In addition, increasing solubility can be observed in Fig. 6 for all treatments (Fig. 6f). The increase of the soluble pectin levels is an excellent index indicative of the tissue softening and the evolution of the maturation (Carvalho et al. 2001). Differences in tissue temperatures without and with injuries were detected in the thermography images shown in Figs. 3, 4 and 5. These differences were not significant for bruised and sound tissues of guavas at 10 °C, which is considered optimal for guava storage.
Considering the IR thermographic results, the temperature differences between the samples with and without injuries are evident at 5 and 20 °C. These effects were not observed at 10 °C, which is indicated as the optimum temperature of guava storage where the physiological disorders are reduced. According to these results, the internal defects and physiological disorders of guava fruits alter their characteristics and affect the thermal properties of the tissue (as evaluated by the thermograms). Defects caused by disease, mechanical damage, and physiological disorders manifest themselves as changes in the thermodynamic properties of the affected tissues (Baranowski and Mazurek 2009).
Conclusions
Infrared thermography proved to be a useful tool for evaluating the sound and bruised tissues of guavas stored at different temperatures. Differences in guava temperature were observed in the form of color gradients by using thermograms. In addition, thermography was effective for relating physiological disorders validating storage temperatures without directly contacting the guavas.
The temperature differences between the sound and bruised tissues can be quantified. The internal defects and physiological disorders of guava fruits alter their characteristics and affect the thermal properties of the tissue. With thermography, it was possible to distinguish between sound and bruised tissues at 5 and 20 °C. The parameters assessed by chemical analyses supported the results that were observed in the thermographic analysis, including the presence of cold-induced and mechanical injuries and quality loss when stored at 5 and 20 °C. However, the opposite trends observed in temperature results not allowed a correlation between the infrared thermography and the variation of physical and chemical parameters which were measured only at the start and at the end of cold storage.
Refrigerated storage at 10 °C for guava fruits with or without induced injuries is important for maintaining fruit quality. This finding demonstrates that the ideal storage conditions for fruit favors the characteristics that are observed by consumers at the time of purchase. Thus, storage under ideal conditions allows the fruits to have a longer commercialization period than fruits stored at room temperature. The guavas stored at 5 and 20 °C lost their acceptable characteristics for consumption.
Acknowledgments
The authors wish to thank the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG- Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - Brazil), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES - Brazil) for financial support for this research.
References
- Azzolini M, Jacomino AP, Bron IU, Kluge RA, Schiavinato MA. Ripening of “Pedro Sato” guava: study on its climacteric or non-climacteric nature. Braz J Plant Physiol. 2005;17(3):299–306. doi: 10.1590/S1677-04202005000300004. [DOI] [Google Scholar]
- Baranowski P, Mazurek W. Detection of physicological disorders and mechanical defects in apples using thermography. Int Agrophys. 2009;23:9–17. [Google Scholar]
- Bitter T, Muir HMA. Modified uronic acid carbazole reaction. Anal Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Carvalho HA, Chitarra MIF, Chitarra AB, Carvalho HS. Efeito da atmosfera modificada sobre componentes da parede celular da goiaba. Cienc Agrotecnol. 2001;25(3):605–615. [Google Scholar]
- Dische Z. General color reactions. In: Whistler RL, Wolfram ML, editors. Carbohydrate chemistry. New York: Academic; 1962. pp. 477–512. [Google Scholar]
- Fito PJ, Ortola MD, De Los Reyes R, Fito P, De Los Reyes E. Control of citrus surface drying by image analysis of infrared thermography. J Food Eng. 2004;61(3):287–290. doi: 10.1016/S0260-8774(03)00120-1. [DOI] [Google Scholar]
- Gan-Mor S, Regev R, Levi A, Eshel D. Adapted thermal imaging for the development of postharvest precision steam-disinfection technology for carrots. Postharvest Biol Technol. 2011;59:265–271. doi: 10.1016/j.postharvbio.2010.10.003. [DOI] [Google Scholar]
- Gowen AA, Tiwari BK, Cullen PJ, McDonnell K, O’Donnell CP. Applications of thermal imaging in food quality and safety assessment. Trends Food Sci Technol. 2010;21(4):190–200. doi: 10.1016/j.tifs.2009.12.002. [DOI] [Google Scholar]
- Hellebrand H, Linke M, Beuche H, Herold B, Geyer M. Horticultural products evaluated by thermography. Warwick: AgEng; 2000. [Google Scholar]
- Hobson GE. Low-temperature injury and the storage of ripening tomatoes. HortSci. 1987;62:55–61. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística. Produção agrícola Municipal 2011. Disponível em: <http://www.sidra.ibge.gov.br/>. Acesso em: 10 jan. 2014
- Jacomino AP, Ojeda RM, Kluge RA, Scarpare Filho JA. Conservação de goiabas tratadas com emulsões de cera de carnaúba. Rev Bras Frutic. 2003;25(3):401–405. doi: 10.1590/S0100-29452003000300010. [DOI] [Google Scholar]
- Mattiuz B, Biscegli CI, Durigan JF. Aplicação da tomografia de ressonância magnética nuclear como método não-destrutivo para avaliar os efeitos de injurias mecânicas em goiabas ‘Paluma’ e ‘Pedro Sato’. Rev Bras Frutic. 2002;24(3):641–643. doi: 10.1590/S0100-29452002000300016. [DOI] [Google Scholar]
- McCready RM, McComb EA. Extraction and determination of total pectin materials in fruit. Anal Chem. 1952;24(12):1586–1588. doi: 10.1021/ac60072a033. [DOI] [Google Scholar]
- Mowlah G, Itoo S. Changes in pectic components, ascorbic acid, pectic enzymes and cellulase activity in ripening and stored guava (Psidium guajava L.) Nippon Shokuhin Kogyo Gakkaishi. 1983;30(8):454–461. doi: 10.3136/nskkk1962.30.454. [DOI] [Google Scholar]
- Neves LC, Benedette RM, Silva VX, Prill MAS, Roberto SR, Vieites RL. Qualidade pós-colheita de mangas, não refrigeradas, e submetidas ao controle da ação do etileno. Rev Bras Frutic. 2009;30(1):94–100. doi: 10.1590/S0100-29452008000100018. [DOI] [Google Scholar]
- Pereira CG, Resende JV, Pereira GG, Giarola TMO, Prado MET. Thermal conductivity measurements and predictive models for frozen guava and passion fruit pulps. Int J Food Prop. 2013;16(4):778–789. doi: 10.1080/10942912.2011.566030. [DOI] [Google Scholar]
- Sharaf A, El-Saadany SS. Biochemical studies on guava fruits during different maturity stages. Ann Agric Sci. 1986;24(2):975–984. [Google Scholar]
- Vadivambal R, Jayas DS, Chelladurai V, White NDG (2007) Temperature distribution studies in microwave-heated grains using a thermal camera. North Dakota: ASABE, 2007. (ASABE Annual Meeting. Paper, RRV- 07100)
- Varith J, Hyde GM, Baritelle AL, Fellman JK, Sattabongkot T. Noncontact bruise detection in apple by thermal imaging. Innovative Food Sci Emerg Technol. 2003;4:211–218. doi: 10.1016/S1466-8564(03)00021-3. [DOI] [Google Scholar]
- Veraverbeke EA, Verboven P, Lammertyn J, Cronje P, De Baerdemaeker J, Nicolaï BM. Thermographic surface quality evaluation of apple. J Food Eng. 2006;77:162–168. doi: 10.1016/j.jfoodeng.2005.06.059. [DOI] [Google Scholar]