Abstract
Theobroma cacao seeds are the main raw material for chocolate production. During their fermentation, a succession of microorganisms are responsible for the physicochemical changes occurring in the pulp and inside the beans. The aim of this study was to investigate the effects of yeast inoculation (Saccharomyces cerevisiae UFLA CA11, Pichia kluivery CCMA0237, and Hanseniaspora uvarum CCMA0236) on the profile of the volatile compounds and microbial communities in cocoa fermentation. The resulting chocolate was also evaluated by temporal dominance of sensations (TDS) analyses. The dominant microorganisms during spontaneous fermentation were S. cerevisiae, H. uvarum, H. guilliermondii, Lactobacillus fermentum, Pediococcus sp., and Acetobacter pasteurianus. Similarly, S. cerevisiae, P. kluyveri, Candida sp., Pediococcus sp., and A. pasteurianus were the predominant microorganisms assessed by Denaturing Gradient Gel Electrophoresis (DGGE) in inoculated fermentation. Sixty-seven volatile compounds were detected and quantified by gas chromatography/mass spectrometry (GC/MS) at the end of fermentation and chocolates. The main group of volatile compound found after the inoculated and spontaneous fermentations was esters (41 and 39 %, respectively). In the chocolates, the main group was acids (73 and 44 % from the inoculated and spontaneous fermentations, respectively). The TDS analyses showed a dominance of bitter and cocoa attributes in both chocolates. However, in the inoculated chocolate, lingering fruity notes were more intense, while the chocolate produced by spontaneous fermentation was more astringent. Thus, the inoculation of yeast influenced the microbial profile, which likely affected the volatile compounds that affect sensory characteristics, resulting in chocolate with dominant bitter, cocoa, and fruity attributes.
Keywords: Cocoa fermentation, Chocolate, PCR-DGGE, GC/MS, Temporal dominance of sensations
Introduction
Chocolate flavor is influenced by both the genetic characteristics of cocoa varieties and post-harvest processing practices (Afoakwa et al. 2008). The microorganisms present during cocoa fermentation are responsible for the production of metabolites and flavor precursors that affect the quality of the resulting chocolate. Cocoa fermentation can be divided into two stages: (1) the pulp is consumed by microorganisms that produce metabolites such as organic acids and ethanol; (2) subsequently, these metabolites contribute to the hydrolytic reactions inside the cotyledon. The succession of microorganisms during the fermentation process begins with yeasts, which are able to metabolize the carbohydrates in the pulp into ethanol, thus generating an anaerobic environment. Lactic acid bacteria (LAB) are then responsible for the conversion of citric acid into lactic acidic acid, which increases the pH. Finally, acetic acid bacteria (AAB) convert ethanol into acetic acid in aerobic conditions. The high temperature induced by the exothermic reaction of acetic acid and ethanol contributes to the death of the bean, which releases endogenous enzymes (Nielsen et al. 2007; Schwan et al. 1995).
The importance of yeasts in cocoa fermentation and chocolate flavor has been previously studied (Batista et al. 2015; Ho et al. 2014; Ramos et al. 2014). For example, yeast metabolism has been proven essential for the development of chocolate flavor (Ho et al. 2014). Yeasts also contribute to ethanol and pectinolytic enzyme production during cocoa fermentation and are the largest producers of esters and higher alcohols, which may contribute to the complex mixture of volatile compounds that characterizes chocolate’s aroma (Crafack et al. 2013; Owusuet al. 2012; Schwan and Wheals 2004). The mixture of volatile compounds, including the alcohols, aldehydes, ketones, acids, and pyrazines developed during the fermentation, drying, roasting, and conching steps, also characterize the flavor of chocolate (Rodriguez-Campos et al. 2012).
A previous work showed that the inoculation of Saccharomyces cerevisiae accelerated the fermentation process of fermentations using different cocoa varieties including PS1319 (Ramos et al. 2014). The cocoa variety had direct influence on the fermentation parameters (Ramos et al. 2014). However, these authors did not describe the chocolate quality. Studies of fermentation and chocolate using cocoa varieties separately and starter cultures may provide important results in order to improve the cocoa fermentation and chocolate quality.
Perception of the sensory characteristics of food products is a dynamic phenomenon and have been studied for different chocolate formulations, e.g. reduced sugar, dried fruits addition, etc. (Belščak-Cvitanović et al. 2015; Komes et al. 2013; Morais et al. 2015; Oliveira et al. 2015). The intensity of sensory attributes changed because of the different processes involved in food breakdown during consumption, including structural changes and the release of different taste and olfactory compounds (Sudre et al. 2012). For this reason, sensory data collected using static methods that rely on a single intensity measurement throughout the evaluation might miss relevant information. Temporal dominance of sensations (TDS) is a methodology that provides the sequence of sensory attributes perceived over time (Pineau et al. 2009). Tasters assessed which sensation is dominant over time until the sensation ends or another appears as dominant. TDS provide a more precise information of the temporal behavior of flavor attribute present in a chocolate.
Knowledge of the microbial communities that affect the chemical parameters of cocoa fermentation is essential for better management of cocoa beans and the production of high-quality chocolate. For this reason, the aim of this study was to investigate the effects of a mixed starter culture of yeasts (S. cerevisiae, P. kluivery, and H. uvarum) on the microbial communities and volatile compounds of cocoa fermentation. The volatile compounds and TDS of resulting chocolate were also evaluated.
Material and methods
Fermentation and sampling
The fermentation experiments were conducted at the Vale do Juliana cocoa farm in Igrapiúna, Bahia, Brazil. The ripe cocoa pods of PS1319 hybrid (Porto Seguro, Uruçuca, BA, Brazil), harvested in November 2013, were manually opened with a machete, and the beans were immediately transferred to the fermentation house. The fermentation, which was done in 0.06 m3 wooden boxes, began approximately 4 h after the pods were broken. Each fermentation batch included 100 kg of cocoa beans. Fermentations were performed both with and without inoculation (control) of a mixed yeast starter culture containing Saccharomyces cerevisiae UFLA CA11 (LNF- CA11, LNF Latino America, Bento Gonçalves, Rio Grande do Sul, Brazil), Pichia kluyveri CCMA 0237 (Moreira et al. 2013). and Hanseniaspora uvarum CCMA 0236 (Moreira et al. 2013) at the beginning of the process. The yeasts strains belong to Culture Collection of Agriculture Microbiology (CCMA) of Federal University of Lavras (Brazil). The P. kluyveri and H. uvarum species were separately grown in YPD broth (10 g/L Yeast extract [Merck]; 20 g/L Peptone [Himedia]; 20 g/L dextrose [Merck]) at 30 °C and 150 rpm, and they were replicated every 24 h. The cells were recovered by centrifugation (7000 rpm, 10 min) and re-suspended in 1 L of sterile peptone water (1 g/L Peptone [Himedia]). This solution was then spread over the cocoa beans, reaching a concentration of approximately 105 cells/g of cocoa. The lyophilized S. cerevisiae UFLA CA11 yeast was weighed (as recommended by the manufacturer’s instructions) and mixed with other yeasts in the solution to reach a population of approximately 107 cells/g of cocoa. All fermentations were evaluated over a period of 168 h, and samples of approximately 100 g each were withdrawn at 0, 24, 48, 72, 96, 120, 144, and 168 h of the process. All samples were taken approximately 40 cm from the surface of the center of the fermenting cocoa mass, placed in sterile plastic pots, and transferred to the laboratory. The samples that would be used for chemical and culture-independent analyses were stored at −20 °C. All fermentations were performed in triplicate. After fermentation, the beans were sun dried in drying greenhouses, at which point dried beans from the two different fermentation processes (control and inoculated) were sent for chocolate production at Sartori and Pedroso Alimentos Ltda. (São Roque, SP, Brazil). The chocolate contained 70 % cocoa.
DNA extraction and denaturing gradient gel electrophoresis (DGGE) analysis
The total DNA from the cocoa pulp was extracted from samples with a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions for DNA purification from tissues. The DNA from the bacterial community was amplified with the primers 338fgc (5′-CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG GAC TCC TAC GGG AGG CAG CAG-3′) (the GC clamp is underlined) and 518r (5′-ATT ACC GCG GCTGCT GG-3′), which span the V3 region of the 16S rRNA gene. A fragment of the 18S rRNA gene was amplified with the primers NS3 (5′-CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCC GGG GGG GCA AGT CTG GTG CCA GCA GCC-3′) (the GC clamp is underlined) and YM951r (5′-TTG GCA AAT GCT TTC GC-3. All reactions were performed in a 25 μL solution containing 0.125 U Taq DNA polymerase (Promega, Madison,USA), 5 μL 5 × go taq buffer, 0.2 mM dNTP, 1 μM each primer, 3.0 mM MgCl2, and 0.5 μL of extracted DNA. The amplification was performed as previously described (Ramos et al. 2010). The amplified products (2 μL) were analyzed by electrophoresis on 1 % agarose gels before DGGE analysis.
The PCR products were separated on polyacrylamide gels [8 % (w/v) acrylamide:bisacrylamide (37.5:1)] in 1 × TAE buffer with a DCode System apparatus (BioRad Universal DCode Mutation Detection System, Richmond, CA, USA), according to the procedures previously described (Ramos et al. 2010). Solutions containing 30–65 % denaturant [100 % denaturant contains 7 M urea and 40 % (v/v) formamide] were used for bacteria, and solutions containing 35–60 % denaturant were used for the yeasts. The gels were run at 60 °C for 5 h at a constant voltage of 200 V. After electrophoresis, the gels were stained with SYBR-Green I solution (Molecular Probes, Eugene, UK) (1:10,000 v/v) for 30 min, and the images were visualized and photographed with a transluminator LPix Image (LTB 20 × 20HE, LPix®, Brazil). The DGGE bands were excised from the acrylamide gels and re-amplified with the primers 338fgc and 518r for bacteria, and NS3 and YM951r for yeasts. The PCR products obtained from the bands were purified and sequenced with an ABI3730 XL automatic DNA sequencer (Advanced Genetics Technologies Center - AGTC, Kentucky, USA), and the sequences obtained were compared with those available in the GenBank database using the BLAST algorithm (National Center for Biotechnology Information, Maryland, USA).
Volatile compound extraction
Volatile compounds were extracted from the cocoa and chocolate samples (2.0 g) using the solid phase microextraction technique in the headspace (SPME-HS), as described by Rodriguez-Campos et al. (2011). with minor modifications. A 50/30 μm divinylbenzene/carboxene/polydimethylsiloxane (DVB/CAR/PDMS) fiber provided by Supelco was used to extract the volatile compounds. The fiber was balanced for 15 min at 60 °C and then exposed to the cocoa powder for 30 min at the same temperature.
Gas chromatography/mass spectrometry (GC/MS)
The volatile compounds were analyzed by GC/MS (Shimadzu, model GCMS-QP2010 SE, Tokyo, Japan), equipped with an RTX-5MS (30 m × 0.25 mm id × 0.25 μm film thickness). The oven temperature was set to 40 °C for 5 min, increased to 200 °C at a rate of 10 °C min−1, and finally maintained at 200 °C for 30 min. The carrier gas was high purity helium at 0.7 ml min−1. The splitless injection mode was at 240 °C (0.5 min). The selective mass detector was a quadrupole, with an electronic impact ionization system at 70 eV and at 260 °C. Volatile compounds were identified and peak areas were used for relative quantification using the GC/MS Solution software (Version 2.6). Linear retention indices relative to a mixture of n-alkanes were calculated according to Kovats retention index (KI). Volatile compounds were identified by probability-based matching of their mass spectra with those obtained from a database (NIST 14 GC Method/Retention Index Database) and by matching the KI of the compounds with values from the literature.
Sensory analysis
Temporal dominance of sensations (TDS) analysis was used to assess the characteristics of the chocolate produced from the fermented and dried beans of each assay (spontaneous and inoculated fermentation). Judges, who had been previously selected and trained, performed TDS analyses of the chocolates. There were two sessions. At the first session, the panelists described all the sensations perceived during chocolate tasting. The most frequently mentioned attributes were selected for TDS analysis, which were sour, bitter, nutty, sweet, astringent, coffee, cocoa, and fruity. At the second session, the software and methodology were shown to the judges. The duration of analysis was 35 s with a delay time of 2 s for each sample.
The samples of approximately 2.5 g of chocolate were presented in 50 ml plastic cups and coded with three-digit bars. Water was used to rinse the mouth between samples. The TDS curves were obtained according to the methodology proposed by Pineau et al. (2009) for SensoMaker software. The analysis was performed in triplicate.
Results
Microbial communities during cocoa fermentation
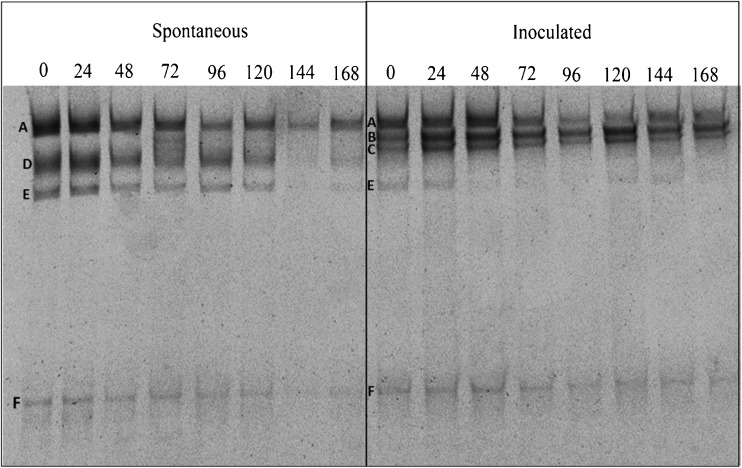
The microbial succession was observed using PCR-DGGE for yeast (Fig. 1) and bacteria (Fig. 2) communities during the spontaneous and inoculated cocoa fermentation processes. The yeast species detected were S. cerevisiae, P. kluyveri, Candida sp., H. guilliermondii, and H. uvarum. Saccharomyces cerevisiae was detected during the entire process in the inoculated and spontaneous fermentations. However, P. kluyveri and Candida sp. were only continuously detected in the inoculated fermentation. Bands corresponding to H. uvarum, and H. guilliermondii were only detected until 120 h in the spontaneous fermentation and at very low intensity bands corresponding to H. uvarum between 0 and 24 h in the inoculated fermentation. Bands corresponding to Theobroma cacao were also found in the inoculated and spontaneous fermentations.
Fig. 1.
PCR-DGGE profile of yeast community during 168 h of spontaneous and inoculated cocoa fermentations. The closest relatives of the sequenced fragments were determined via GenBank searches for sequences with over 98 % similarity: A = S. cerevisiae; B = P. kluyveri; C = Candida sp.; D = H. guilliermondii; E = H. uvarum; F = Theobroma cacao
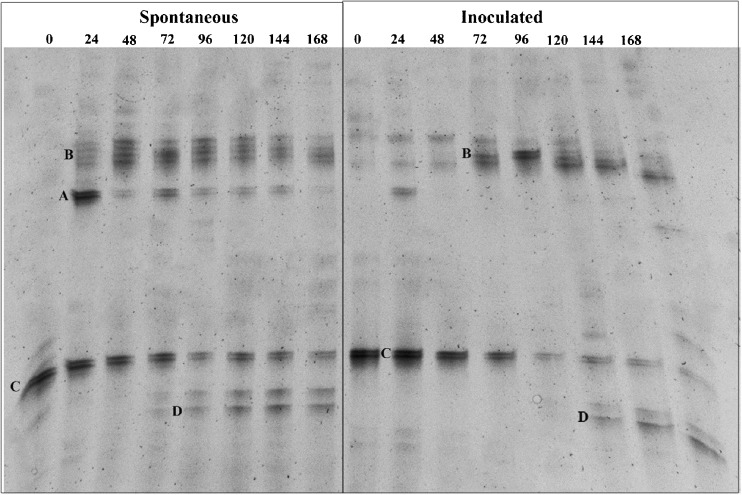
Fig. 2.
PCR-DGGE profile of bacterial community during 168 h of spontaneous and inoculated cocoa fermentations. The closest relatives of the sequenced fragments were determined via GenBank searches for sequences with over 98 % similarity A = L. fermentum; B = Pediococcus sp.; C = G. oxydans; D = A. pasteurianus
Regarding the bacterial community, the species found were Lactobacillus fermentum, Pediococcus sp., Gluconobacter oxidans, and Acetobacter pasteurianus. Bands corresponding to Lactobacillus fermentum were detected from 0 to 144 h in the spontaneous fermentation and only at 24 h in the inoculated fermentation. Pediocccus sp. were found at 24 h and 72 h in the spontaneous and inoculated fermentations, respectively. Gluconobacter oxidans were detected for the duration of both fermentations, while A. pasteurianus were found from the middle (96 h and 120 h in the spontaneous and inoculated fermentations, respectively) to the end of the processes.
Volatile compounds analysis
A total of 67 volatile compounds were identified by GC/MS at the end of the fermentation and in the chocolates produced by spontaneous and inoculated fermentations (Table 1). The volatile compounds detected were grouped into aldehydes (11), ketones (12), esters (22) acids (5), alcohols (10), pyrazines (4), furans (1), and others (2). The total volatile compounds were more pronounced in samples from the fermentation process (with area that covered more than 3 × 107) than in the chocolates (with an area that covered less than 7 × 106) (Table 1). Esters, alcohols, acids, and ketones were the main groups detected after fermentation (area higher than 4 x 106). In the chocolates, acids were more pronounced (with an area around 4 × 106).
Table 1.
Volatiles identified at end of spontaneous and inoculated fermentations and in the chocolates produced by these process. Means represent averages of GC–MS peak areas including biological and technical replicates
| Group | KI | Compound | Mean GC–MS peak area | Odora | |||
|---|---|---|---|---|---|---|---|
| Fermentation | Chocolate | ||||||
| Spontaneous | Inoculated | Spontaneous | Inoculated | ||||
| Aldehydes | 1.285 | 2-Phenyl-2-butenal | ND | ND | 8751 | 16,746 | Flowery, cocoa, roasted, rum |
| 1.504 | 5-Methyl-2-phenyl-2-hexenal | ND | ND | 1065 | 1815 | Sweet, roasted cocoa | |
| 502 | Acetaldehyde | 154,536 | ND | ND | ND | Green leaves, fruity | |
| 955 | Benzaldehyde | 83,053 | 17,322 | 88,963 | 95,355 | Nutty | |
| 1053 | Benzeneacetaldehyde | 390,316 | 359,250 | 101,012 | 110,323 | ||
| 561 | 2-Methyl-butanal | 20,517 | 18,305 | ND | ND | Malty, chocolate | |
| 554 | 3-Methyl-butanal | ND | ND | 17,852 | 16,513 | Malty, chocolate | |
| 1.209 | Decanal | ND | ND | 5074 | 4521 | Pungent, green | |
| 715 | Hexanal | ND | ND | 6690 | 6995 | Green | |
| 1.113 | Nonanal | ND | ND | 36,644 | 40,941 | Soapy–fruity | |
| 1.006 | Octanal | ND | ND | 5117 | 5419 | Orange (peel) | |
| Total | 648,422 | 394,877 | 271,168 | 298,628 | |||
| Ketones | 529 | 2,3-Butanedione | 676,517 | 580,905 | 24,400 | 21,096 | Buttery |
| 870 | 2-Acetoxy-3-butanone | 196,754 | 254,449 | ND | ND | ||
| 863 | 2-Heptanone | 285,723 | 1,219,458 | ND | ND | Fruity, flowery | |
| 1102 | 2-Nonanone | 1,509,265 | 1,306,206 | 7212 | 10,671 | Flowery, fatty | |
| 577 | 2-Pentanone | 59,818 | 160,093 | ND | ND | Fruit | |
| 1299 | 2-Undecanone | 5339 | 3126 | 2617 | 3412 | Fruity, citrus | |
| 593 | Acetoin | 1,247,111 | 1,471,769 | 133,167 | 103,088 | Butter, cream | |
| 1077 | Acetophenone | 112,709 | 138,679 | 8316 | 10,675 | Flowery, sweet | |
| 993 | 2-Octanone | ND | 36,624 | ND | ND | Bitter, green | |
| 561 | 1-Hydroxy-2-propanone | ND | ND | 10,647 | 10,700 | ||
| 1.081 | 3,5-Octadien-2-one | ND | ND | 18,757 | 12,083 | ||
| 508 | Acetone | ND | ND | 18,281 | 10,763 | ||
| Total | 4,093,236 | 5,171,309 | 223,397 | 182,488 | |||
| Esters | 844 | Isoamyl acetate | 741,420 | 1,778,463 | ND | ND | Banana |
| 912 | 3-Methyl-2-butenyl acetate | 6764 | ND | ND | ND | ||
| 1265 | 2-Phenethyl acetate | 265,949 | 440,867 | 173,104 | 137,198 | rose | |
| 1019 | Hexyl acetate | 7784 | 4291 | ND | ND | Fruit | |
| 516 | Methyl acetate | 114,257 | 78,930 | ND | ND | ||
| 899 | Amyl acetate | 5144 | ND | ND | ND | Banana | |
| 1253 | Ethyl phenylacetate | 81,801 | 68,638 | 22,975 | 42,837 | Fruity, sweet, honey-like | |
| 1179 | Ethyl benzoate | 28,783 | 37,781 | ND | ND | ||
| 1185 | Diethyl succinate | 3662 | ND | ND | ND | Fruity | |
| 1393 | Ethyl caprate | 13,465 | 50,641 | 63,890 | 25,310 | Fruity | |
| 1596 | Ethyl laurate | 4449 | 10,099 | 60,420 | 30,319 | ||
| 537 | Ethyl Acetate | 10,239,048 | 11,451,977 | ND | ND | ||
| 1.995 | Ethyl palmitate | ND | ND | 12,333 | 8373 | ||
| 1003 | Ethyl hexanoate | 10,497 | 47,898 | ND | ND | Fruity | |
| 673 | Isobutyl acetate | 162,714 | 352,913 | ND | ND | Banana | |
| 1.354 | Methyl anthranilate | ND | ND | 6928 | 5591 | ||
| 599 | Propyl acetate | 88,276 | ND | ND | ND | ||
| 1199 | Ethyl octanoate | 52,887 | 218,199 | 6030 | 3446 | Fruity | |
| 745 | Ethyl 2 hydroxypropanoate | 30,157 | ND | ND | ND | ||
| 652 | Ethyl isobutyrate | 8890 | 7828 | ND | ND | ||
| 968 | Pentyl propanoate | ND | 2756 | ND | ND | ||
| 1.795 | Tetradecanoic acid, ethyl ester | ND | ND | 16,691 | 9003 | ||
| Total | 11,865,947 | 14,551,281 | 362,371 | 262,077 | |||
| Acids | 559 | Acetic acid | 7,191,474 | 7,434,026 | 4,499,426 | 3,865,523 | |
| 1.363 | Decanoic acid | ND | ND | 6443 | 3301 | ||
| 1953 | Hexadecanoic acid | 3145 | 4817 | 6839 | 4006 | ||
| 1.177 | Octanoic acid | ND | ND | 7889 | 7395 | Sweaty | |
| 949 | 4-Methyl-pentanoic acid | ND | ND | 9537 | 9425 | ||
| Total | 7,194,619 | 7,438,843 | 4,530,134 | 3,889,650 | |||
| Alcohol | 614 | 2-Methyl-1-butanol | 35,066 | 83,248 | ND | ND | |
| 620 | 3-Methyl-1-butanol | 96,800 | 197,046 | ND | ND | ||
| 1.036 | 2-Ethyl-1-hexanol, | ND | ND | 32,185 | 23,009 | Rose, sweet, citrus, floral | |
| 879 | 2-Heptanol | 2,056,607 | 2,967,099 | 19,943 | 30,998 | ||
| 1110 | 2-Nonanol | 1,267,603 | 518,527 | 14,847 | 30,358 | Fruity | |
| 1005 | 2-Octanol | 35,023 | 24,900 | ND | ND | ||
| 585 | 2-Pentanol | 749,097 | 731,449 | ND | ND | ||
| 721 | 4-Methyl-2-pentanol | 15,442 | ND | ND | ND | ||
| 506 | Ethanol | 1,695,287 | 3,210,905 | 91,428 | 92,375 | ||
| 1126 | Phenylethyl Alcohol | 326,557 | 467,154 | 192,981 | 174,551 | ||
| Total | 6,277,482 | 8,200,328 | 351,384 | 351,291 | |||
| Pyrazines | 1.171 | 2,3,5-Trimethyl-6-ethylpyrazine | ND | ND | 8769 | 11,374 | Candy, sweet |
| 1.009 | 2,3,5-Trimethylpyrazine | ND | ND | 61,126 | 46,873 | Cocoa, roasted | |
| 910 | 2,3-Dimethylpyrazine | ND | ND | 6887 | 6594 | Caramel, cocoa | |
| 1.098 | 2,3,5,6-Tetramethyl-pyrazine | ND | ND | 167,420 | 131,938 | Roasted, chocolate | |
| Total | 0 | 0 | 244,202 | 196,779 | |||
| Furano | 813 | Furfuryl alcohol | ND | ND | 25,704 | 20,347 | Caramel-like, sweet |
| Others | 1.074 | Ethanone, 1-(1 H-pyrrol-2-yl)- | ND | ND | 105,604 | 96,154 | |
| 1035 | D-Limonene | 2632 | ND | ND | ND | ||
| Total | 2632 | 0 | 105,604 | 96,154 | |||
| Total compounds | 30,082,338 | 35,756,638 | 6,113,964 | 5,297,454 | |||
aObtained from the literature. ND = Not detected
Sensory evaluation of chocolate
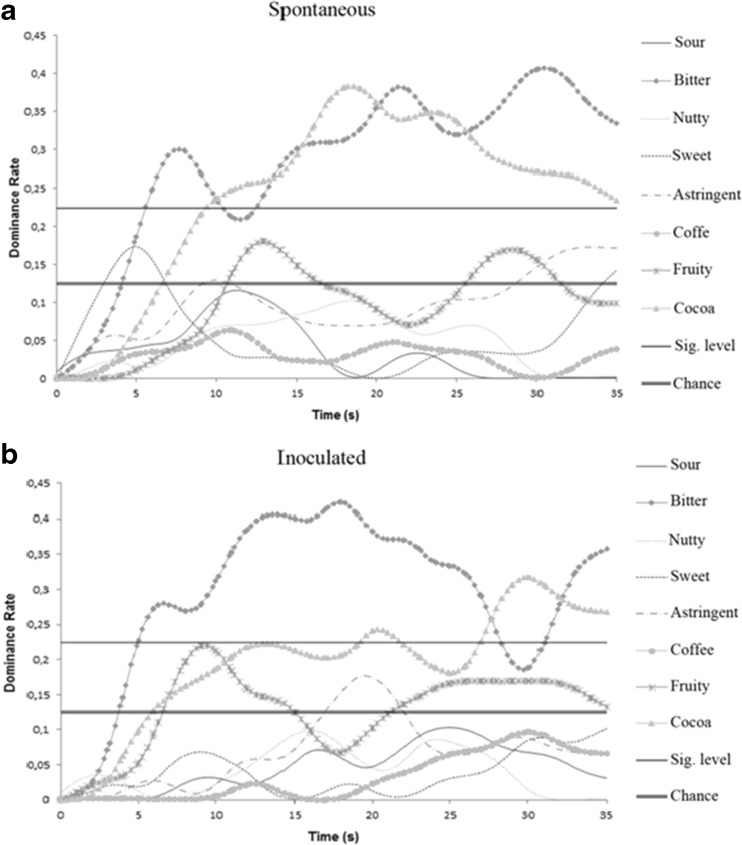
The chocolates produced by spontaneous and inoculated fermentations were subjected to TDS analysis. Figure 3 shows the sensory profiles of the acid, bitter, nutty, sweet, astringent, coffee, cocoa, and fruity attributes analyzed over 35 s of tasting.
Fig. 3.
Temporal Dominance of Sensations analysis of chocolates produced by two different fermentations: a spontaneous and b inoculated fermentations of cocoa
The cocoa and bitter attributes were equally noticeable during the 35.0 s for chocolate produced by spontaneous fermentation. The sweet attribute was perceptible at 4.9 s, followed by astringency at 9.1 and 33.6 s. The fruity attribute was perceptible at 12.9 and 28.3 s of analysis. The chocolate produced by inoculated fermentation had dominant bitterness during the 35.0 s of analysis. The cocoa attribute was noticeable after 30 s, while fruitiness was perceptible at 9.1 and 25.0 s. Astringency was also perceptible at 19.6 s.
Discussion
Cocoa fermentation is a well-defined microbial succession of yeasts, LAB and AAB (Ardhana and Fleet 2003; Garcia-Armisen et al. 2010; Lima et al. 2011; Schwan and Wheals 2004). The amount and distribution of microorganisms in the pulp determines the rate of fermentation, the quality of the fermented grains, and, consequently, the final product (Camu et al. 2008). According to previous research, yeasts are essential for the development of chocolate flavor and their inoculation may accelerate and control the fermentation process (Batista et al. 2015; Ho et al. 2014; Ramos et al. 2014). In order to better control the fermentation process and to improve the quality of chocolate, a mixture of yeasts (S. cerevsiae, H. uvarum, and P. kluyveri) was used as starter culture. One previous study (Batista et al. 2015) investigated the behavior of these three species during the fermentation process and found that the inoculation of S. cerevisiae and P. kluyveri could inhibit the H. uvarum population. In the present study, the effect of yeast inoculation on microbial diversity (fungal and bacteria) was analyzed by PCR-DGGE. The PCR-DGGE profile obtained for spontaneous and inoculated fermentations showed that inoculation affected the microbial profile. Regarding fungal communities, the three inoculated species (S. cerevsiae, H. uvarum, and P. kluyveri) and Candida sp. were all detected in the inoculated fermentation. The inoculated yeast H. uvarum was found only up until 24 h of fermentation, in concurrence with the results found by Batista et al. (2015). It seems that H. uvarum may have been inhibited by S. cerevisiae and P. kluyveri. During spontaneous fermentation, S. cerevisiae was dominant during the entire process, and H. guilliermondii and H. uvarum were found until 120 h. P. kluyveri was not detected in this process. Batista et al. (2015) also showed this species to have a similarly low population (around 3 Log cell/g) during spontaneous fermentation process. Researchers from Brazil have also found similar species in cocoa fermentation (Moreira et al. 2013; Pereira et al. 2012; Ramos et al. 2014; Schwan and Wheals 2004).
Based on the PCR-DGGE profiles obtained for the bacterial communities, yeast inoculation seemed to inhibit the LAB population. Lactobacillus fermentum was detected during the entire spontaneous fermentation process but was found only at 24 h at a low concentration (related to the low band intensity) in the inoculated fermentation. Pediococcus sp. was later found in the inoculated fermentation than in the spontaneous fermentation. LAB convert carbohydrates and citric acid into lactic acid from the second day of cocoa fermentation (Camu et al. 2007; Lefeber et al. 2010, 2012). However, recent research suggests that LAB may not be necessary for successful cocoa fermentation due beans fermented in the presence or absence of lactic acid bacteria are fully fermented, have similar shell weights and produce acceptable chocolates with no differences in sensory rankings (Ho et al. 2015).
The characteristic vinegar-like aroma of cocoa bean fermentation led early investigators to conclude that AAB were significant contributors to the process. Species belonging to the genera Acetobacter and Gluconobacter have been described in different cocoa fermentations (Ardhana and Fleet 2003; Garcia-Armisen et al. 2010; Lima et al. 2011; Moreira et al. 2013; Pereira et al. 2012; Ramos et al. 2014; Schwan and Wheals 2004). The species A. pastorianus and G. oxydans were detected by PCR-DGGE during both fermentations. A. pasteurianus is a species commonly found at the end of cocoa fermentation, capable of oxidizing both ethanol and lactic acid produced by yeast and LAB, respectively, contributing to increased acidity and temperature, and death of the bean (Moens et al. 2014). The temperature of fermentations reached 50 °C (data not shown). AAB can also metabolize sugars and organic acids to produce various aldehydic, ketogenic, and other volatile products that could affect the sensory quality of beans (Drysdale and Fleet 1998).
The metabolic activity of the yeast is essential to the success of cocoa fermentation. They perform alcoholic fermentation, thereby creating conditions (e.g., production of ethanol and its conversion to acetic acid) that contribute to the death of the beans. Bean death initiates an array of endogenous biochemical changes that are essential for the development of characteristic chocolate flavor (Hansen et al. 1998; Schwan and Wheals 2004). Furthermore, the production of secondary metabolites by yeast (organic acids, aldehydes, ketones, higher alcohols and esters) is described because of its aromatic properties that contribute to the quality of chocolate (Ardhana and Fleet 2003; Owusu et al. 2012; Schwan and Wheals 2004; Swiegers et al. 2005). Aldehydes and ketones with desired odors, such as 2-Phenyl-2-butenal, 5-Methyl-2-phenyl-2-hexenal, nonanal, octanal, and 2-Undecanone, which conferred cocoa and fruity notes, were found in both chocolates, though mainly in the inoculated samples.
The relative concentration (in area) of esters is higher after inoculated fermentation. This is probably due to the yeast metabolism during the fermentation process, which produces esters with flower, fruity, and honey flavors (Aculey et al. 2010; Frauendorfer and Schieberle 2008). Isoamyl acetate and ethyl acetate were the main esters detected (around 90 % of total esters) after inoculated fermentation; they are related to banana and pineapple flavors, respectively. In both chocolates, the esters 2-Phenethyl acetate, ethyl phenylacetate, ethyl caprate, and ethyl octanoate were identified, all of which add fruity notes. Crafack et al. (2013) reported that P. kluyveri yeast in cocoa fermentation produced aromatic volatile compounds, which positively contribute to the chocolate flavor.
Acetic acid was the main acid detected in both types of fermentation, mostly likely due to AAB metabolism. Acetic acid was also detected in the chocolates, however in lower relative concentrations (approximately 50 %) than in end of fermentations. Excess of acetic acid into the cotyledons after fermentation results in chocolate with undesirable flavor (Biehl et al. 1985; Biehl and Passern 1982; Wood and Lass 1985). Hexadecanoic acid was detected in all samples (fermentation and chocolate). This is a common fatty acid (saturated) found in animals, plants and microorganism and was detected in dark chocolate by gas chromatography-quadrupole mass spectrometry (Nolvachai et al. 2015). Although acids was the main group of volatile compounds identified in the chocolates (around 50 %), the sour attribute was not perceptible in the sensory analysis.
Higher alcohols were mainly detected at the end of the fermentations. In the chocolates, the alcohols 2-Ethyl-1-hexanol, 2-Heptanol, and 2-Nonanol were identified. Their presence is desirable because they impart sweet, fruity, and floral notes (Aculey et al. 2010; Frauendorfer and Schieberle 2008). As observed in the present study, the profile and relative concentrations of volatile compounds varied with yeast inoculation. Furthermore, the chocolate manufacturing process also affects the composition of volatile compounds. Owusu et al. (2012) reported that prolonged conching may modify the profiles of volatile compounds and thus chocolate’s sensory characteristics (Owusu et al. 2013). Variations in flavor volatile released in dark chocolate matrices varying in particle size distribution and fat content have been also described (Afoakwa et al. 2009). The chocolates from the different fermentations were prepared using the same techniques; thus, the differences were most likely related to starter culture activity and the flavor precursors produced during fermentation.
The sensory profiles of chocolates obtained by spontaneous and inoculated fermentations were evaluated. Crafack et al. (2014) found that the starter cultures affected the volatile aroma profile of chocolate; however, differences were too small for consumer perception of the chocolates as compared to a spontaneously fermented control as observed by CATA method (Check-All-That-Apply). In the present work, an in-depth sensory study (TDS analysis) was performed with a trained panel, members of whom recorded the dominant sensory characteristics of the chocolate over 35 s. This analysis concluded that cocoa and bitter attributes were predominant. However, fruity, astringent, and sweet attributes were also present. Compared to those produced by spontaneous fermentation, fruity notes were more intense at the end of the tasting period in chocolate produced by inoculated fermentation. The chocolate obtained by spontaneous fermentation was more astringent than the inoculated one, which is likely related to the more acidic nature of chocolate produced by inoculated fermentation. Other authors have also found that the presence of yeast during cocoa fermentation positively influences the sensory profile of chocolate (Ho et al. 2014).
Conclusion
The inoculation of yeasts affected the microbial profile during cocoa fermentation. S. cerevisiae was the dominant yeast during inoculated and spontaneous fermentation. H. guilliermondii was only detected in spontaneous fermentation, while Candida sp. and P. kluyveri were only found in inoculated fermentation. It seemed that the starter culture (S. cerevisiae, H. uvarum and P. kluyveri) inhibited the LAB presence. For these reasons, inoculation affected the profiles of volatile compounds and their relative concentrations, which influence the sensory characteristics of chocolate. Both chocolates showed dominant of bitter and cocoa attributes; however, chocolate produced by inoculated fermentation showed fruity notes that were more intense at the end of the taste.
Acknowledgments
The authors thank the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brasil (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support, and Fazenda Reunidas Vale do Juliana (Igrapiúna, Bahia, Brazil) where the fermentations were performed. The authors gratefully acknowledge the anonymous referees for their comments and constructive suggestions for improving the quality of this manuscript.
Footnotes
Highlights• Fermentation of cocoa varieties separately provide important data to improve fermentation and chocolate quality
• The inoculation of S. cerevisiae, H. uvarum and P. kluyveri affected the microbial profile during cocoa fermentation
• Inoculation affected the profiles of volatile compounds, which influence the sensory characteristics of chocolate
References
- Aculey PC, Snitkjaer P, Owusu M, Bassompiere M, Takrama J, Nørgaard L, Petersen MA, Nielsen DS. Ghanaian cocoa bean fermentation characterized by spectroscopic and chromatographic methods and chemometrics. J Food Sci. 2010;75:300–307. doi: 10.1111/j.1750-3841.2010.01710.x. [DOI] [PubMed] [Google Scholar]
- Afoakwa E, Paterson A, Fowler M, Ryan A. Flavor formation and character in cocoa and chocolate: a critical review. PubMed commons. Crit Rev Food Sci Nutr. 2008;48:840–857. doi: 10.1080/10408390701719272. [DOI] [PubMed] [Google Scholar]
- Afoakwa E, Paterson A, Fowler M, Ryan A. Matrix effects on flavour volatiles release in dark chocolates varying in particle size distribution and fat content using GC–mass spectrometry and GC–olfactometry. Food Chem. 2009;113:208–215. doi: 10.1016/j.foodchem.2008.07.088. [DOI] [Google Scholar]
- Ardhana M, Fleet G. The microbial ecology of cocoa bean fermentations in Indonesia. Int J Food Microbiol. 2003;86:87–99. doi: 10.1016/S0168-1605(03)00081-3. [DOI] [PubMed] [Google Scholar]
- Batista NN, Ramos CL, Ribeiro DR, Pinheiro ACM, Schwan RF. Dynamic behavior of Saccharomyces cerevisiae, Pichia kluyveri and Hanseniaspora uvarum during spontaneous and inoculated cocoa fermentations and their effect on sensory characteristics of chocolate. LWT-Food Sci Technol. 2015;63:221–227. doi: 10.1016/j.lwt.2015.03.051. [DOI] [Google Scholar]
- Belščak-Cvitanović A, Komes D, Dujmović M, Karlović S, Biškić M, Brnčić M, Ježek D. Physical, bioactive and sensory quality parameters of reduced sugar chocolates formulated with natural sweeteners as sucrose alternatives. Food Chem. 2015;167:61–70. doi: 10.1016/j.foodchem.2014.06.064. [DOI] [PubMed] [Google Scholar]
- Biehl B, Passern D. Proteolysis during fermentation- like incubation of cocoa seeds. J Sci Food Agr. 1982;33:280–290. [Google Scholar]
- Biehl B, Brunner E, Passern D, Quesnel VC, Adomako D. Acidification, proteolysis and flavour potential in fermenting cocoa beans. J Sci Food Agr. 1985;36:583–598. doi: 10.1002/jsfa.2740360710. [DOI] [Google Scholar]
- Camu N, De Winter T, Verbrugghe K, Cleenwerck I, Vandamme P, Takrama JS, Vancanneyt M, De Vuyst L. Dynamics and biodiversity of populations of lactic acid bacteria and acetic acid bacteria involved in spontaneous heap fermentation of cocoa beans in Ghana. Appl Environ Microb. 2007;73:1809–1824. doi: 10.1128/AEM.02189-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camu N, González A, De Winter T, Schoor AV, De Bruyne K, Vandamme P, Takrama JS, Addo SK, De Vuyst L. Influence of turning and environmental contamination on the dynamics of populations of lactic acid and acetic acid bacteria involved in spontaneous cocoa bean heap fermentation in Ghana. Appl Environ Microb. 2008;74:86–98. doi: 10.1128/AEM.01512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafack M, Mikkelsen MB, Saerens S, Knudsen M, Blennow A, Lowor S, Takrama J, Swiegers JH, Petersen GB, Heimdal H, Nielsen DS. Influencing cocoa flavour using Pichia kluyveri and Kluyveromyces marxianus in a defined mixed starter culture for cocoa fermentation. Int J Food Microbiol. 2013;167:103–116. doi: 10.1016/j.ijfoodmicro.2013.06.024. [DOI] [PubMed] [Google Scholar]
- Crafack M, Keul H, Eskildsen CE, Petersen MA, Saerens S, Blennow A, Skovmand-Larsen M, Swiegers JH, Petersen GB, Heimdal H, Nielsen DS. Impact of starter cultures and fermentation techniques on the volatile aroma and sensory profile of chocolate. Food Res Int. 2014;63:306–316. doi: 10.1016/j.foodres.2014.04.032. [DOI] [Google Scholar]
- Drysdale GS, Fleet GH. Acetic acid bacteria in winemaking: a review. Am J Enol Viticult. 1998;39:143–154. [Google Scholar]
- Frauendorfer F, Schieberle P. Changes in key aroma compounds of criollo cocoa beans during roasting. J Agr Food Chem. 2008;56:10244–10251. doi: 10.1021/jf802098f. [DOI] [PubMed] [Google Scholar]
- Garcia-Armisen T, Papalexandratou Z, Hendryckx H, Camu N, Vrancken G, De Vuyst L, Cornelis P. Diversity of the total bacterial community associated with Ghanaian and Brazilian cocoa bean fermentation samples as revealed by a 16 S rRNA gene clone library. Appl Microbiol Biot. 2010;87:2281–2292. doi: 10.1007/s00253-010-2698-9. [DOI] [PubMed] [Google Scholar]
- Hansen CE, Olmo M, Burri C. Enzyme activities in cocoa beans during fermentation. J Sci Food Agric. 1998;77:273–281. doi: 10.1002/(SICI)1097-0010(199806)77:2<273::AID-JSFA40>3.0.CO;2-M. [DOI] [Google Scholar]
- Ho VTT, Zhao J, Fleet G. Yeasts are essential for cocoa bean fermentation. Int J Food Microbiol. 2014;174:72–87. doi: 10.1016/j.ijfoodmicro.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Ho VT, Zhao J, Fleet G. The effect of lactic acid bacteria on cocoa bean fermentation. Int J Food Microbiol. 2015;205:54–67. doi: 10.1016/j.ijfoodmicro.2015.03.031. [DOI] [PubMed] [Google Scholar]
- Komes D, Belščak-Cvitanović A, Škrabal S, Vojvodić A, Bušić A. The influence of dried fruits enrichment on sensory properties of bitter and milk chocolates and bioactive content of their extracts affected by different solvents. LWT-Food Sci Technol. 2013;53:360–369. doi: 10.1016/j.lwt.2013.02.016. [DOI] [Google Scholar]
- Lefeber T, Janssens M, Camu N, De Vuyst L. Kinetic analysis of strains of lactic acid bacteria and acetic acid bacteria in cocoa pulp simulation media toward development of a starter culture for cocoa bean fermentation. Appl Environ Microb. 2010;76:7708–7716. doi: 10.1128/AEM.01206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefeber T, Papalexandratou Z, Gobert W, Camu N, De Vuyst L. On-farm implementation of a starter culture for improved cocoa bean fermentation and its influence on the flavour of chocolates produced thereof. Food Microbiol. 2012;30:379–392. doi: 10.1016/j.fm.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Lima LJR, Almeida MH, Nout MJR, Zwietering MH. Theobroma cacao L., “the food of the gods”: quality determinants of commercial cocoa beans, with particular reference to the impact of fermentation. Crit Rev Food Sci Nutr. 2011;51:731–761. doi: 10.1080/10408391003799913. [DOI] [PubMed] [Google Scholar]
- Moens F, Lefeber T, De Vuyst L. Oxidation of metabolites highlights the microbial interactions and role of acetobacter pasteurianus during cocoa bean fermentation. Appl Environ Microb. 2014;80:1848–1857. doi: 10.1128/AEM.03344-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais EC, Lima GC, Morais AR, Bolini HMA. Prebiotic and diet/light chocolate dairy dessert: chemical composition, sensory profiling and relationship with consumer expectation. LWT-Food Sci Technol. 2015;62:424–430. doi: 10.1016/j.lwt.2014.12.015. [DOI] [Google Scholar]
- Moreira IMV, Miguel MGCP, Duarte WF, Dias DR, Schwan RF. Microbial succession and the dynamics of metabolites and sugars during the fermentation of three different cocoa (Theobroma cacao L.) hybrids. Food Res Int. 2013;54:9–17. doi: 10.1016/j.foodres.2013.06.001. [DOI] [Google Scholar]
- Nielsen DS, Teniola OD, Ban-Koffi L, Owusu M, Andersson TS, Holzapfel WH. The microbiology of Ghanaian cocoa fermentations analysed using culture-dependent and culture-independent methods. Int J Food Microbiol. 2007;114:168–186. doi: 10.1016/j.ijfoodmicro.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Nolvachai Y, Kulsing C, Boysen RI, Matyska MT, Pesek JJ, Marriott PJ, Hearn MTW. Comparison of the performance of different silica hydride particles for the solid-phase extraction of non-volatile analytes from dark chocolate with analysis by gas chromatography–quadrupole mass spectrometry. Food Chem. 2015;174:434–439. doi: 10.1016/j.foodchem.2014.10.083. [DOI] [PubMed] [Google Scholar]
- Oliveira D, Antúnez L, Giménez A, Castura JC, Deliza R, Ares G. Sugar reduction in probiotic chocolate-flavored milk: impact on dynamic sensory profile and liking. Food Res Int. 2015;75:148–156. doi: 10.1016/j.foodres.2015.05.050. [DOI] [PubMed] [Google Scholar]
- Owusu M, Petersen MA, Heimdal H. Effect of fermentation method, roasting and conching conditions on the aroma volatiles of dark chocolate. J Food Process Pres. 2012;36:1–11. doi: 10.1111/j.1745-4549.2011.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu M, Petersen MA, Heimdal H. Relationship of sensory and instrumental aroma measurements of dark chocolate as influenced by fermentation method, roasting and conching conditions. J Food Sci Technol. 2013;50:909–917. doi: 10.1007/s13197-011-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira GVM, Miguel MGCP, Ramos CL, Schwan RF (2012) Microbiological and physicochemical characterization of small-scale cocoa fermentations and screening of yeast and bacterial strains to develop a defined starter culture. Appl Environ Microb 78:5395–5405. [DOI] [PMC free article] [PubMed]
- Pineau N, Schlich P, Cordelle S, Mathonnière C, Issanchou S, Imbert A, Rogeaux M, Etiévant P, Köster E. Temporal dominance of sensations: construction of the TDS curves and comparison with timeintensity. Food Qual Prefer. 2009;20:450–455. doi: 10.1016/j.foodqual.2009.04.005. [DOI] [Google Scholar]
- Ramos CL, Almeida EG, Pereira GVM, Cardoso PG, Dias ES, Schwan RF. Determination of dynamic characteristics of microbiota in a fermented beverage produced by Brazilian Amerindians using culture-dependent and culture-independent methods. Int J Food Microbiol. 2010;140:225–231. doi: 10.1016/j.ijfoodmicro.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Ramos CL, Dias DR, Miguel MGCP, Schwan RF. Impact of different cocoa hybrids (Theobroma cacao L.) and S. cerevisiae UFLA CA11 inoculation on microbial communities and volatile compounds of cocoa fermentation. Food Res Int. 2014;64:908–918. doi: 10.1016/j.foodres.2014.08.033. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Campos J, Escalona-Buendía HB, Orozco-Avila I, Lugo-Cervantes E, Jaramillo-Flores ME. Dynamics of volatile and non-volatile compounds in cocoa (Theobroma cacao L.) during fermentation and drying processes using principal components analysis. Food Res Int. 2011;44:250–258. doi: 10.1016/j.foodres.2010.10.028. [DOI] [Google Scholar]
- Rodriguez-Campos J, Escalona-Buendía HB, Contreras-Ramos SM, Orozco-Avila I, Jaramillo-Flores E, Lugo-Cervantes E. Effect of fermentation time and drying temperature on volatile compounds in cocoa. Food Chem. 2012;132:277–288. doi: 10.1016/j.foodchem.2011.10.078. [DOI] [PubMed] [Google Scholar]
- Schwan RF, Wheals AE. The microbiology of cocoa fermentation and its role in chocolate quality. Crit Rev Food Sci Nutr. 2004;44(4):205–221. doi: 10.1080/10408690490464104. [DOI] [PubMed] [Google Scholar]
- Schwan RF, Rose AH, Board RG. Microbial fermentation of cocoa beans, with emphasis on enzymatic degradation of the pulp. J Appl Bacteriol. 1995;79:96–107. [Google Scholar]
- Sudre J, Pineau N, Loret C, Martin N. Comparison of methods to monitor liking of food during consumption. Food Qual Prefer. 2012;24:179–189. doi: 10.1016/j.foodqual.2011.10.013. [DOI] [Google Scholar]
- Swiegers JHE, Bartowsky J, Henschke PA, Pretorius IS. Yeast and bacterial modulation of wine aroma and flavor. Aust J Grape Wine Res. 2005;11:139–173. doi: 10.1111/j.1755-0238.2005.tb00285.x. [DOI] [Google Scholar]