Abstract
Plant derived pharmacologically active compounds have gained importance in food and pharmaceutical industries. The aim of the present study is to identify and study the antioxidant, antimicrobial properties of the phytochemicals present in the crude extract of Eugenia caryophyllus flower buds. The antioxidant activity of the methanol, acetone and chloroform extract was evaluated by 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay. The methanol extract showed better radical scavenging activity than other selected solvents. Preliminary screening of phytochemicals was carried out in methanol extract and total phenol content was found high. Antibacterial activity was determined by well diffusion assay and methanol extract was found effective against Klebsiella pneumonia. FTIR and GC-MS results indicate the presence of aromatic compounds and major constituents were found to be eugenol and eugenyl acetate. Results of this study implied that Eugenia caryophyllus flower bud extract could be considered as health nutriments in food and pharmaceutical industries.
Keywords: Eugenia caryophyllus, Phytochemicals, Antibacterial, Antioxidant activity, Eugenol
Introduction
Spices are playing an important role in the human diet for improving flavor and aroma of food stuffs. It is also used in traditional medicine for several years in many countries. Besides using as flavoring agents in food, it also acts as an antioxidative and antimicrobial agent. Eugenia caryophyllus is commonly known as clove plant which belongs to Myrtaceae family. Clove bud essential oil possess biological activities such as antibacterial, antifungal, insecticidal, antioxidant properties and used traditionally as flavoring and antimicrobial agents in food products (Jasna et al. 2013; Nazrul et al. 2010). Strong antibacterial activity of the clove oil is mainly due to the presence of eugenol in high levels. Clove oil also has several therapeutic effects including anti-phlogistic, anti-vomiting, analgesic, antispasmodic, anti-carminative and antiseptic (Mahmoud et al. 2011).
Free radicals or reactive oxygen species (ROS) produced from living systems which can able to initiate the disease by damaging the biomolecules (Gulcin et al. 2012; Edziri et al. 2011). Antioxidants can help to protect cell damage causing by free radicals via inhibiting or slow down the oxidizing reactions occurring in the cells (Halliwell and Gutteridge 2007). Synthetic antioxidant such as butylated hydroxytoluene (BHT) is utilized effectively in industrial applications but, it may cause health hazards to humans (Boulekbache-Makhlouf et al. 2013; Barlow 1990). Plant based natural antioxidants gain much interest in recent days and it is employed as alternate antioxidant substance (Skerget et al. 2005). Plants possess several antioxidative chemicals such as tannins, phenolics, carotenes, vitamins etc. (Govardhan Singh et al. 2013; Gian et al. 2012). Among them, plant phenolics have great antioxidant potential because of high redox activity (Lu and Foo 2002; Peter and Wong 2006). Mihara and Shibamoto (1982) reported that eugenol in clove oil posess high antioxidant activity and phototoxicity by actively involved in photochemical reactions. It also reported that hydroxyl radicals generation can be inhibited by eugenol and found that clove oil exhibits similar antioxidant effect at low concentration as compared to BHT (Jirovetz et al. 2006). Previous studies on antibacterial activity of E. caryophyllus extracts have been reported against several pathogenic bacteria such as E.coli, Salmonella, Listeria, and Staphylococcus (Burt and Reinders 2003; Feres et al. 2005; Mytle et al. 2006; Ogunwande et al. 2005). Previous literature showed that clove essential oil is extensively studied for antioxidant and antimicrobial activities. In this study, clove powder is used for investigating antioxidant and antimicrobial potential. The aim of the present work was to investigate the efficiency of different solvents for the phytochemical extraction from E. caryophyllus and to evaluate the antioxidant and antibacterial efficacy of E. caryophyllus extract. Further, secondary metabolites and functional groups of the phytochemicals were analyzed by GC-MS and FTIR respectively.
Materials and methods
Clove bud powder preparation
The flower buds of fresh E. caryophyllus used in this study were acquired at the local market in Chengalpet. Clove buds were carefully air-dried for one week and ground to fine powder. Clove bud powder was stored in air tight container at room temperature.
Preparation of E. caryophyllus extract
The crude extract of clove bud was obtained by direct extraction with chloroform, acetone and methanol (Bishnu et al. 2011). In brief, 10 g of finely ground bud powder was extracted with 100 mL of chloroform, acetone and methanol in separate conical flasks in shaking condition. This process was repeated thrice with the same material but using fresh solvent and each time the extract was decanted into pre-weighed glass vials. The solvent in the extract was removed by condensation. The extracted residues were weighed and re-dissolved in solvents to yield 10 mg/mL solutions for further analysis.
Phytochemical analysis
The phytochemicals present in the crude extract were screened by the qualitative assays for plant secondary metabolites. Qualitative phytochemical tests for alkaloids, phenols, glycosides, saponins, flavonoids, tannins, reducing sugars were performed (Harborne 1973; Evans 1997). The colour intensity was used as response for these tests.
Estimation of total phenolic content
Total phenolics present in the methanolic extract of E. caryophyllus were determined using the Folin-Ciocalteau reagent (Mc Donald et al. 2001). In brief, 0.5 mL of extract along with 0.1 mL of 0.5 N Folin-Ciocalteu’s reagent was incubated at room temperature for 15 min. Then 2.5 mL saturated sodium carbonate solution was added and further incubated for 30 min at room temperature. The absorbance was measured at 760 nm in UV-Vis spectrophotometer. Gallic acid was used as a positive control. Total phenol values were expressed in terms of gallic acid equivalent (mg/g of extracted compounds).The assay was carried out in triplicates and expressed as mean ± SD.
Estimation of total flavonoid content
Total flavonoids in the methanol extract of E. caryophyllus flower buds was determined by aluminium chloride colorimetric method (Chang et al. 2002). The reaction mixture 3 mL consisting of 1 mL of sample (1 mg/mL), 0.5 mL of (1.2 %) aluminium chloride and 0.5 mL (120 mM) potassium acetate was incubated at room temperature for 30 min. The absorbance of the sample was measured at 415 nm and Quercetin was used as positive control. Flavonoid content was expressed in terms of quercetin equivalent (mg/g of extracted compound). The assay was carried out in triplicates and expressed as mean ± SD.
Evaluation of antioxidant potential of E. caryophyllus extract by DPPH assay
Radical scavenging capacity of E. caryophyllus crude extract prepared by three solvents was determined and compared with α-tocopherol by using DPPH (Molyneux 2004).The ability to scavenge DPPH radical was calculated by the following formula.
where,
- Acontrol
is the absorbance of DPPH radical and ethanol.
- Asample
is the absorbance of DPPH radical and extract.
Measurements were done in triplicate and the values were corrected for radical decay using blank solutions.
Thin layer chromatography
Thin layer chromatography (TLC) is used to separate the compounds present in the crude extract of E. caryophyllus (Abu et al. 2011). Methanol and chloroform (1:9) was used as mobile phase. The sample with the concentration of about (1 mg/mL) was spotted on the TLC plates and dried. The spots were identified in long UV, short UV and also in the iodine chamber. Rƒ value was calculated to find the active metabolites. Rƒ value is distance travelled by the solute to the distance traveled by the solvent.
Bioautography
Bioautography was performed to isolate the anti-oxidant compounds and fractions (Subramanion et al. 2011). Developed standardized chromatography plates of E. caryophyllus crude extract were sprayed with DPPH (dissolved in DMSO) and the zone of inhibition was observed. The specific compounds (band) which possess antioxidative properties shows clear zone.
Gas chromatography-mass spectrometry (GC-MS) analysis
The methanol extract of E. caryophyllus was analyzed using GC-MS (SHIMADZU QP2010). The GC specifications were as follows: column oven temperature: 70 °C, injector temperature: 200 °C, injection mode: Split, Split Ratio: 40, Flow control mode: Linear velocity, Column Flow: 1.51 mL/min, Carrier Gas: Helium 99.99 % purity. Column oven temperature program: rate temperature (°C) hold time (min) -70 2, (35.0 min).column: VF-5 ms: length: 30.0 m, diameter: 0.25 mm. The MS specifications were as follows: Ion source temp: 200 °C, interface temp: 240 °C, scan range: 40–1000 m/z, event time: 0.5 s, solvent cut time: 5 min, start time: 5 min, end time: 35 min, ionization: EI (−70ev) Ayoola et al. (2008).
Fourier transform infra-red (FTIR) spectroscopy
2 mg of the methanol extract of E. caryophyllus were mixed with 200 mg KBr (FT-IR grade) and made into a pellet in a hydraulic press by applying 500 kg/m3 pressure. The pellet was immediately put into the sample holder and FT-IR spectra were recorded in the range 450–4000 cm−1 using Bruker 55 model FT-IR spectrometer.
Antimicrobial activity of E. caryophyllus extract
The antimicrobial activity of E. caryophyllus extract against different bacterial pathogens was evaluated using well diffusion assay (Anja et al. 2010). Bacterial strains were maintained in nutrient agar plates and subcultured at regular intervals. For evaluating antibacterial efficacy of E. caryophyllus extract, two Gram-positive bacteria (Bacillus subtilis, Staphyloccocus aureus) and two Gram-negative bacteria (Klebsiella pneumonia, Vibrio cholerae) were tested in well diffusion assay. The wells (8 mm diameter) were made in the nutrient agar plate using a cork borer. Stock solution of each plant extract was prepared at a concentration of 1 mg/mL in different plant extract. About 100 μL of different concentrations of plant solvent extracts were added with sterile syringe into the wells and allowed to diffuse at room temperature for 2 h. Control experiments comprising inoculums without plant extract were set up. The plates were incubated at 37 °C for 18–24 h and the diameter of the inhibition zone (mm) was measured.
Results and discussion
Phytochemicals extraction and analysis
In the present study, three solvents (acetone, chloroform and methanol) were used for extraction of phytochemicals and their extractive values were determined. Extractions with methanol yield 3.7 g whereas acetone and chloroform yielded 3.0 g and 2.4 g respectively. Methanol showed better extractive efficiency than other solvents and this result was found to be similar with the existing literature (Nazrul et al. 2010; Edziri et al. 2011). E. caryophyllus extract was subjected to phytochemical analysis by both qualitative and quantitative methods. In qualitative analysis, various tests for phytochemicals were carried out and the results confirmed the presence of alkaloid, phenols, flavonoids, tannins and saponins in the crude extract. The phytoconstituents detected in the extract could be responsible for the antioxidant and antimicrobial activity (Peter and Wong 2006). In quantitative analysis, the phytochemicals evaluated in the methanol extract of E. caryophyllus were in the order of total phenolics > flavonoids with the respective values of 596.6 > 62.6 mg/g of extract. Presence of high level of phenolic compounds revealed that E. caryophyllus extract can have good antioxidant capacity because of their ability to scavenge free radicals and active oxygen species (Miliauskas et al. 2004).
Antioxidant activity assay
Radical scavenging activity of acetone, chloroform and methanol extract of E. caryophyllus was performed by DPPH assay using α-tocopherol as standard. All the solvent extracts showed antioxidant activity in a concentration range of 20–200 μg/mL (Table 1). Among the three extracts studied, methanol showed maximum antioxidant capacity of 93.2 % whereas the antioxidant activity exhibited by acetone and chloroform were of 85.4 and 81.4 % respectively. This result revealed that methanol has more extractive efficiency than other two solvents (Tepe 2008).
Table 1.
Antioxidant activity of acetone, chloroform and methanolic extract of E. caryophyllus flower buds
| Concentration (μg/ml) | Radical scavenging activity (%) | |||
|---|---|---|---|---|
| α-tocopherol | Acetone extract | Chloroform extract | Methanol extract | |
| 20 | 17.16 ± 0.76 | 75.20 ± 0.20* | 65.56 ± 0.51* | 81.8 ± 0.72* |
| 40 | 34.73 ± 0.25 | 76.10 ± 0.15* | 71.06 ± 1.0* | 82.8 ± 0.80* |
| 60 | 46.50 ± 0.70 | 77.00 ± 0.20* | 71.5 ± 0.51* | 83.8 ± 0.72* |
| 80 | 49.30 ± 1.12 | 78.20 ± 0.20* | 72.6 ± 0.62* | 85.3 ± 0.30* |
| 100 | 54.33 ± 0.61 | 79.50 ± 0.26* | 73.8 ± 0.76* | 86.4 ± 0.51* |
| 120 | 58.63 ± 0.40 | 80.06 ± 0.30* | 76.6 ± 0.52* | 87.5 ± 0.51* |
| 140 | 65.80 ± 0.34 | 81.50 ± 1.00* | 77.0 ± 0.60* | 88.9 ± 0.36* |
| 160 | 72.80 ± 0.72 | 82.20 ± 0.25* | 78.7 ± 0.25* | 90.0 ± 0.50* |
| 180 | 73.70 ± 0.30 | 84.10 ± 0.23* | 79.9 ± 1.05* | 91.6 ± 0.57* |
| 200 | 84.10 ± 0.17 | 85.30 ± 0.15* | 81.4 ± 0.45* | 92.2 ± 0.64* |
*Significant difference at 5 % level; Student t-test analysis of radical scavenging activity in different concentration of standard (α-Tocopherol) and test sample (solvent extract of E. caryophyllus shows statistically different at p < 0.05 level)
Antimicrobial activity
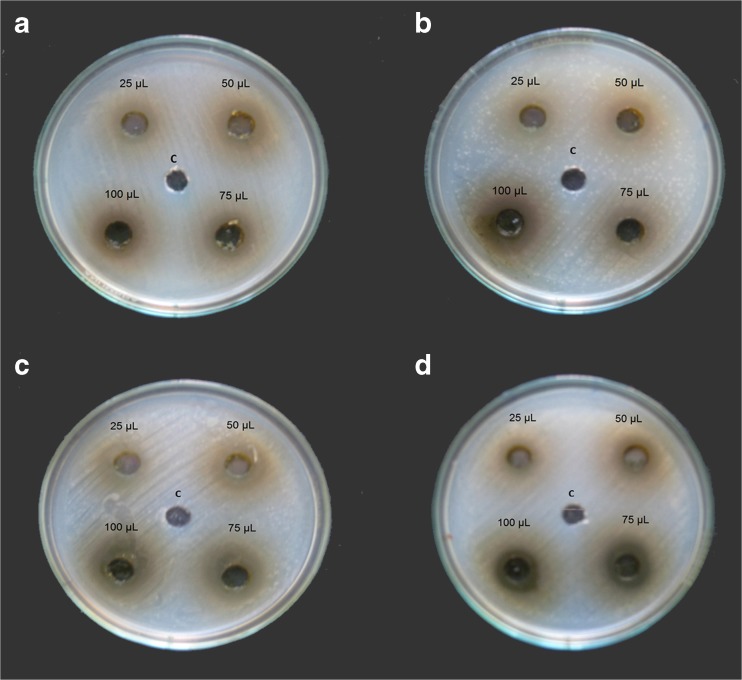
Agar well-diffusion method was followed to determine the antimicrobial activity. Antimicrobial activities of E. caryophyllus flower bud extracts were evaluated against four bacterial (two Gram-positive, two Gram-negative) strains. The methanolic solutions of the extracts were found to have potent antimicrobial activity against all the Gram-positive and Gram-negative bacteria tested by well diffusion assay as shown in Fig.1. Clove extract exhibited a broad spectrum antimicrobial activity with a minimum zone diameter of 17 mm for Vibrio cholerae and Klebsiella pneumonia with a maximum zone diameter of 26 mm. Methanol extract showed moderate activity against Vibrio cholerae, Bacillus subtilis, Staphylococcus aureus (Table 2 and Fig. 1). The diameter of the inhibition zone (mm) was measured and the activity index was also calculated. Recently it was reported that ethanolic extract of clove possess antimicrobial effect (Nazrul et al. 2010). Siddiqua et al. (2015) currently reported that combination of clove oil with cinnamaldehyde showed better better antibacterial activiy aganist food brone pathogens.
Fig. 1.
Antibacterial activity of E. caryophyllus extract on a Bacillus subtilis, b Staphylococcus aureus c Klebsiella pneumonia and d Vibrio cholerae
Table 2.
Effect of E. caryophyllus methanol extract against different pathogens
| Bacteria | Zone of inhibition (mm) | Positive control (taxim) | Negative control (methanol) | |||
|---|---|---|---|---|---|---|
| 25 μL | 50 μL | 75 μL | 100 μL | |||
| Bacillus subtillus | 18.3 ± 0.57 a | 21.3 ± 0.57b | 23 ± 1.00 bc | 24.3 ± 0.57c | 17 ± 1.00a | - |
| Staphylococcus aerus | 18 ± 1.00a | 22 ± 1.00b | 22 ± 0.57b | 25 ± 1.00c | 16 ± 1.00a | - |
| Klebsiella pneumonia | 19.33 ± 0.57b | 22.6 ± 0.57c | 24.33 ± 1.52cd | 26 ± 1.00d | 15.6 ± 1.15a | - |
| Vibrio chlorea | 17.33 ± 0.57a | 18.0 ± 1.00a | 21.0 ± 1.00b | 24 ± 1.00c | 17 ± 1.00a | - |
Different superscripts in the same row shows significant different at p < 0.05 level
- denotes no inhibition
Thin layer chromatography
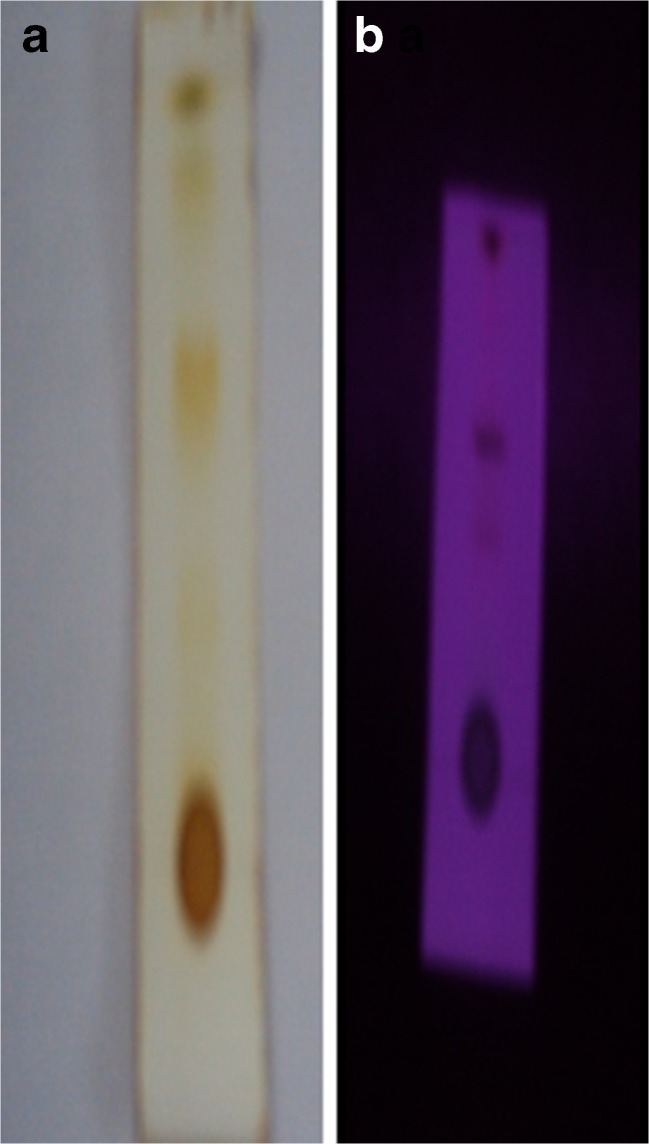
The methanol extract with a concentration of 1 mg/mL reported the presence of four major compounds with Rf values of 0.48 0.62, 0.71 and 0.8 as visualized under iodine chamber and UV light and it is represented in Fig. 2a, and b. The larger Rf value of a compound, the larger the distance it travels on the TLC plate.
Fig. 2.
TLC plate showing the phytochemicals separation in a iodine chamber and b under UV light
Bioautography
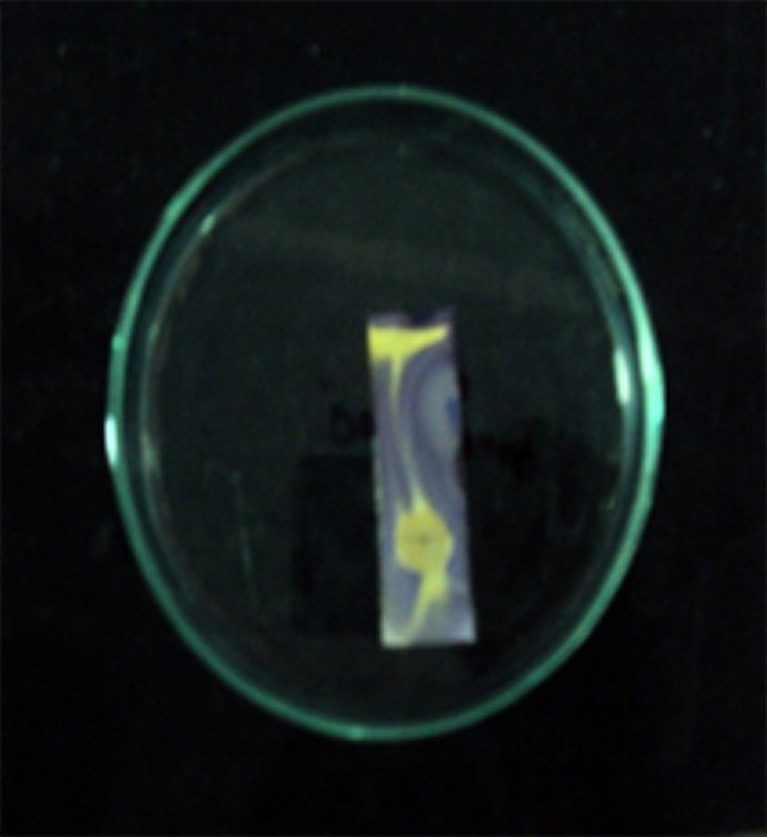
The methanol in chloroform (1:9) ratio was subjected to run the TLC for detecting antioxidant activity, chromatograms were sprayed with 0.2 % 2,2-diphenyl-2-picrylhydrazyl (DPPH) methanol, as an indicator. The presence of antioxidant compounds were detected by yellow spots against a purple background on TLC plates sprayed with 0.2 % DPPH. Developed TLC plates were carefully dried for complete removal of the solvents. The areas of inhibition (colored yellow) were compared with the Rf value of the related spots on the reference TLC plate. Figure 3 showed the presence of clear bands on the plates against a purple background indicated the growth inhibition (Iqbal and Arina 2001).The partially purified compound showed the inhibitory activity which has an Rf value of 0.48 against the free radical as assessed by DPPH.
Fig. 3.
Bioautograph showing the presence of antioxidant compounds (yellow spots) on TLC plate
Gas chromatography-mass spectrometry (GC-MS)
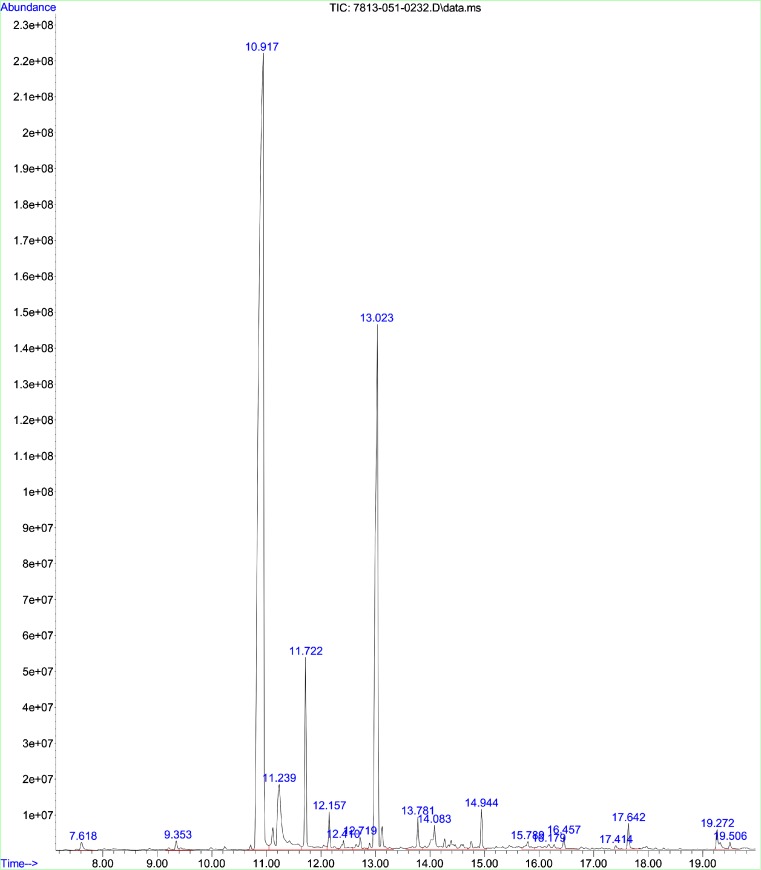
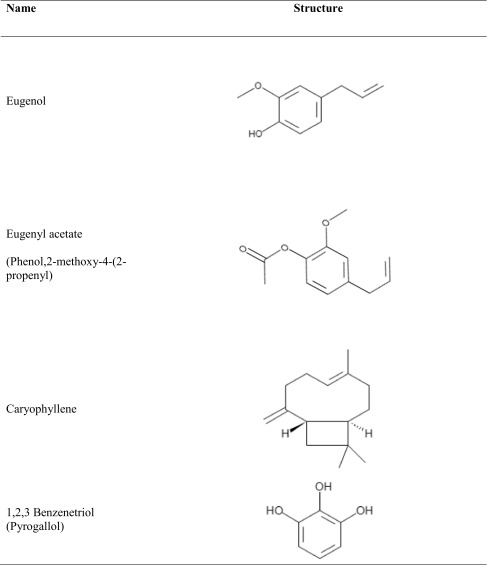
The GC and MS running time for the methanol extract of E. caryophyllus were 36 min and spectrum is shown in Fig. 4. Interpretation of mass spectrum (GC-MS) was done by using data base of National Institute Standard and Technology (NIST). The spectrum of the unknown component was compared with the spectrum of known components stored in the NIST library. GC-MS result analysis includes the active principles with their retention time, molecular formula, molecular weight and composition in the methanol extracts of E. caryophyllus. List of the identified compounds in the extract and percentage composition are shown in Table 3. Results showed that the extracts were complex mixture of numerous compounds; many of which were present in trace amounts. Eugenol (54.88 Phenol,2-methoxy-4-(2-propenyl) (19.46 %), 1,2,3-Benzenetriol (5.48 %) and caryophyllene (4.48) were observed as the versatile common components present in the clove extract and their structure were shown in Table 4. Besides the presence of other phytochemicals such as eugenyl acetate, caryophyllene and 1,2,3 benzenetriol, eugenol plays a vital role for antioxidant and antibacterial activities. Eugenol is the major components in clove extract and had good agreement with the results reported by Jasna et al. (2013). The high concentration of eugenol in clove extract makes it potential for antibacterial, and antioxidant properties. Studies on antibacterial action of eugenol represented that it disrupts the cell membrane and enhances its non-specific permeability (Gill and Holly 2006). Also, lipophilicity of eugenol enables it to disrupt the cell structure by incorporating with the lipopolysaccharide layer of bacterial cell membrane and results in the intracellular components leakage which leads to death (Burt 2004; Pandima Devi et al. 2010). Burt (2004) studied effect of eugenol on Enterobacter aerogenes and reported that eugenol possess enzyme inhibitory action due to the binding of OH group of eugenol to the proteins. Besides antibacterial effect, eugenol is also as potential antioxidant by donating a hydrogen atom and stabilizes the phenoxyl radicals to inhibit the oxidation. Conjugation of carbon chain with aromatic ring of eugenol involved in the phenoxyl radical stabilization process by resonance (Diego et al. 2014). Eugenol has the capability to reduce two or more DPPH radicals by forming dimers. Pyrogallol or 1,2,3 benezenetriol also shown antibacterial activities. It is a hydroxylated phenol, having three OH groups attached to the phenol ring which is responsible for increased toxicity against microorganisms.
Fig. 4.
GC-MS chromatograph for methanolic extract of E. caryophyllus powder
Table 3.
GC-MS analysis report for the methanol extract of E. caryophyllus
| Retention time | Name | Molecular formula | Molecular weight | Area (%) |
|---|---|---|---|---|
| 7.618 | 4 H-Pyran-4-one,2,3-dichloro-3,5-dihydroxyl-6-methyl | C9H15Cl6O4P | 430.91 | 0.33 |
| 9.353 | Benzaldehyde,4-ethyl | C9H10O | 134.18 | 0.48 |
| 10.91 | Eugenol | C10H12O2 | 164.2 | 54.88 |
| 11.239 | 1,2,3-Benzenetriol | C6H6O3 | 126.11 | 5.48 |
| 11.722 | Caryophyllene | C15H24 | 204.35 | 4.48 |
| 12.157 | 1,4,7-Cycloundecatriene,1,5,9,9-tetramethyl-z,z,z | C15H24 | 204.35 | 0.88 |
| 12.410 | Gamma-muurolene | C15H24 | 204.35 | 0.49 |
| 12.719 | Alpha-farnesene | C15H24 | 204.35 | 0.60 |
| 13.023 | Phenol,2-methoxy-4-(2-propenyl) | C12H14O3 | 206.23 | 19.46 |
| 13.781 | Caryophyllene oxide | C15H24O | 220.35 | 0.94 |
| 14.083 | Asarone | C12H16O3 | 208.25 | 2.53 |
| 14.944 | 2′,3′,4’Trimethoxyacetophenone | C11H14O4 | 210.23 | 1.15 |
| 15.780 | Benzeneacetamide,N-(aminocarbonyl)-4-hydroxy-3-methoxy | C19H29NO3 | 319.44 | 0.46 |
| 16.179 | Phenol,2-methoxy-4-(methoxymethyl) | C9H12O3 | 168.19 | 0.75 |
| 16.457 | Farnesol,acetate | C17H28O2 | 264.41 | 0.39 |
| 17.414 | 2,4,4-Trimethyl-3-(3-methylbutyl)cyclohex-2-enone | C14H24O | 208.33 | 0.12 |
| 17.642 | n-Hexadecanoic acid | C16H32O2 | 256.42 | 0.57 |
| 19.272 | 9,12-octadecadienoic acid(z,z) | C18H32O2 | 280.45 | 0.77 |
| 19.506 | Octadecanoic acid | C18H32O2 | 284.48 | 0.21 |
Table 4.
Major phytochemical constituents present in the tested sample of E. caryophyllus
Fourier transform infra-red spectroscopy
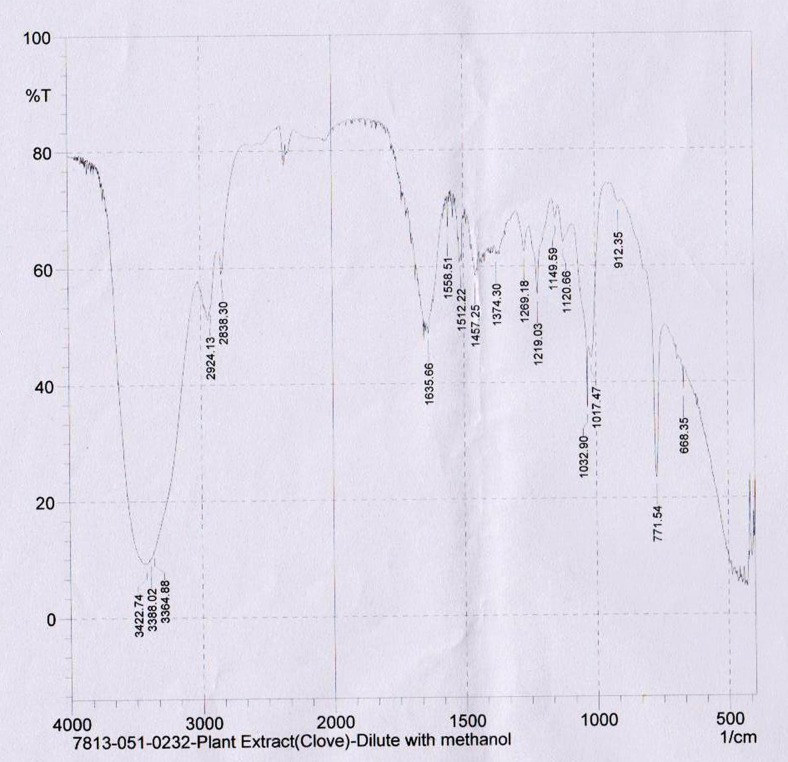
Interpretation of infrared spectra involves the correlation of absorption bands in the spectrum of an unknown compound with the known absorption frequencies for types of bonds. Significant for the identification of the source of an absorption band are intensity (weak, medium or strong), shape (broad or sharp), and position (cm−1) in the spectrum. The methanol extract was analyzed by FTIR to identify the functional groups in the bioactive components based on its peak ratio and electron transition of compounds. FTIR spectrum for the methanol extract of E. caryophyllus with its peak values were obtained at 668.35, 771.54, 912.35,1017.47, 1032.9, 1120.66, 1149.59, 1219.03, 1269.18, 1374.3, 1457.25, 1512.22, 1558.51, 1635.66, 2838.3, 2924.13, 3364.88, 3388.02, 3422.74 and confirmed the presence of the carboxylic acids, amines, esters, aldehydes, aromatic ring, alkanes and alkenes (Fig.5).
Fig. 5.
FTIR spectra of E. caryophyllus methanolic extract
Conclusions
In summary, methanol was found to be best solvent for phytochemicals extraction from E. caryophyllus. The best results of radical scavenging activity of methanol were obtained with a maximum of (92.2 ± 0.64) sample which was nearly equal to the radical scavenging activity of the standard α-tocopherol (84.06 ± 0.901).The methanol extract showed maximum number of bioactive compounds in preliminary phytochemical analysis and good amount of total phenolics in the antioxidant activity. From antibacterial assay results E. caryophyllus extract was very effective against K. pneumonia than other test bacterial strains. Bioautography analysis showed that the whole extract had the free radical scavenging potential. GC-MS and FTIR analysis revealed the presence of good number of bioactive metabolites such as eugenol, caryophyllene and Phenol,2-methoxy-4-(2-propenyl) in the extract. The results of this study implied that E. caryophyllus have shown better antioxidant and antibacterial activities which could be used in food and therapeutic applications.
Acknowledgments
We would like to express my sincere gratitude to Director, Principal and staff of Department of Biotechnology, Karpaga Vinayaga College of Engineering and Technology for providing facilities to carry out this study. We also thank the Journal Editor and Reviewers for their valuable suggestions to improve our article and publication in JFST.
References
- Abu OM, Abdul AM, Rowshanul HM, Rezaul KM. Antimicrobial investigation on Manilkara zapota (L.) P. Royen. Int J Drug Develop Res. 2011;3:185–190. [Google Scholar]
- Anja K, Sasa P, Barbara J, Sonja SM. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J Microbiol Method. 2010;8:121–126. doi: 10.1016/j.mimet.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Ayoola GA, Lawore FM, Adelowotan T, Aibinu IE, Adenipekun E, Coker HAB, et al. Chemical analysis and antimicrobial activity of the essential oil of syzigium aromaticum (clove) Afr J Microbiol Res. 2008;2:162–166. [Google Scholar]
- Barlow SM. Toxicological aspects of antioxidants used as food additives. In: Hudson BJ F, editor. Food antioxidants. New York: Elsevier; 1990. [Google Scholar]
- Bishnu J, Govind B, Raj M, Dinita S, Krishna S. Phytochemical extraction and antimicrobial properties of different medicinal plants: Ocimum sanctum (tulsi), Eugenia caryophyllata (clove), achyranthes bidentata (datiwan) and Azadirachta indica (neem) J Microbiol Antimicrob. 2011;3:1–7. [Google Scholar]
- Boulekbache-Makhlouf L, Slimani S, Madani K. Total phenolic content, antioxidant and antibacterial activities of fruits of Eucalyptus globulus cultivated in Algeria. Ind Crop Prod. 2013;41:85–89. doi: 10.1016/j.indcrop.2012.04.019. [DOI] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods-a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Burt SA, Reinders RD. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett Appl Microbiol. 2003;36:162–167. doi: 10.1046/j.1472-765X.2003.01285.x. [DOI] [PubMed] [Google Scholar]
- Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Diego FC-R, Claudia RF, Wanderley PO. Clove (Syzygium aromaticum): a precious spice. Asia Pac J Trop Biomed. 2014;4:90–96. doi: 10.1016/S2221-1691(14)60215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edziri HL, Smach MA, Ammar S, Mahjoub MA, Mighri Z, Aouni M, et al. Antioxidant, antibacterial, and antiviral effects of lactuca sativa extracts. Ind Crop Prod. 2011;34:1182–1185. doi: 10.1016/j.indcrop.2011.04.003. [DOI] [Google Scholar]
- Evans WC. Trease and Evans pharmacognosy. 14th. Singapore: Harcourt Brace; 1997. [Google Scholar]
- Feres M, Figueiredo LC, Barreto IM, Coelho MN, Araujo MW, Cortelli SC. In vitro antimicrobial activity of plant extracts and propolis in saliva samples of healthy and periodontally-involved subjects. J Int Acad Periodontol. 2005;7:90–96. [PubMed] [Google Scholar]
- Gian CT, Ettore N, Adriana B. Nutraceutical potential and antioxidant benefits of red pitaya (hylocereus polyrhizus) extracts. J Funct Food. 2012;4:129–136. doi: 10.1016/j.jff.2011.09.003. [DOI] [Google Scholar]
- Gill AO, Holly RA. Disruption of Escherichia coli, Listeria monocytogenes and lactobacillus sakei cellular membranes by plant oil aromatics. Int J Food Microbiol. 2006;108:1–9. doi: 10.1016/j.ijfoodmicro.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Govardhan Singh RS, Pradeep SN, Radha C. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of moringa oleifera seed flour. J Funct Food. 2013;5:1883–1891. doi: 10.1016/j.jff.2013.09.009. [DOI] [Google Scholar]
- Gulcin I, Elmastas M, Aboul-Enein HY. Antioxidant activity of clove oil – a powerful antioxidant source. Arab J Chem. 2012;5:489–499. doi: 10.1016/j.arabjc.2010.09.016. [DOI] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 4th. Oxford: Oxford University Press; 2007. [Google Scholar]
- Harborne JB. Phytochemical methods. London: Chapman and Hall; 1973. [Google Scholar]
- Iqbal A, Arina ZB. Antimicrobial and phytochemical studies on 45 India medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol. 2001;74:113–123. doi: 10.1016/S0378-8741(00)00335-4. [DOI] [PubMed] [Google Scholar]
- Jasna I, Suzana D-B, Dusan M, Mihailo R, Irena Z. Evaluation and improvement of antioxidant and antibacterial activities of supercritical extracts from clove buds. J Funct Food. 2013;5:416–423. doi: 10.1016/j.jff.2012.11.014. [DOI] [Google Scholar]
- Jirovetz L, Buchbauer G, Stoilova I, Stoyanova A, Krastanov A, Schmidt E. Chemical composition and antioxidant properties of Clove leaf essential oil. J Agric Food Chem. 2006;54:6303–6307. doi: 10.1021/jf060608c. [DOI] [PubMed] [Google Scholar]
- Lu Y, Foo YL. Polyphenolics of salvia—a review. Phytochem. 2002;75:197–202. doi: 10.1016/s0031-9422(01)00415-0. [DOI] [PubMed] [Google Scholar]
- Mahmoud H, Mina KA, Hassan R. Analgesic effect of clove essential oil in mice. Avi J Phytomed. 2011;1:1–6. [Google Scholar]
- Mc Donald S, Prenzler PD, Antolovich M, Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73:73–84. doi: 10.1016/S0308-8146(00)00288-0. [DOI] [Google Scholar]
- Mihara S, Shibamoto T. Photochemical reactions of eugenol and related compounds: synthesis of new flavor chemicals. J Agric Food Chem. 1982;30:1215–1218. doi: 10.1021/jf00114a053. [DOI] [Google Scholar]
- Miliauskas G, Venskutonis PR, van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–237. doi: 10.1016/j.foodchem.2003.05.007. [DOI] [Google Scholar]
- Molyneux P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004;26:211–219. [Google Scholar]
- Mytle N, Anderson GL, Doyle MP, Smith MA. Antimicrobial activity of clove (syzgium aromaticum) oil in inhibiting Listeria monocytogenes on chicken frankfurters. Food Control. 2006;17:102–107. doi: 10.1016/j.foodcont.2004.09.008. [DOI] [Google Scholar]
- Nazrul IB, Jaripa MD, Nemai CN, Farhana A. Constituents of the essential oil from leaves and buds of clove (syzigium caryophyllatum L. Alston) African J Plant Sci. 2010;4:451–454. [Google Scholar]
- Ogunwande IA, Olawore NO, Ekundayo O, Walker TM, Schmidt JM, Setzer WN. Studies on the essential oils composition, antibacterial and cytotoxicity of Eugenia uniflora L. Int J Aromather. 2005;15:147–152. doi: 10.1016/j.ijat.2005.07.004. [DOI] [Google Scholar]
- Pandima Devi K, Arif Nisha S, Sakthivel R, Karutha Pandian S. Eugenol (an essential oil of clove) acts as an antibacterial agent against salmonella typhi by disrupting the cellular membrane. J Ethnopharmacol. 2010;130:107–115. doi: 10.1016/j.jep.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Peter YY, Wong DD. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006;97:505–515. doi: 10.1016/j.foodchem.2005.05.031. [DOI] [Google Scholar]
- Siddiqua S, Anusha BA, Ashwini LS, Negi PS. Antibacterial activity of cinnamaldehyde and clove oil: effect on selected foodborne pathogens in model food systems and watermelon juice. J Food Sci Technol. 2015;52:5834–5841. doi: 10.1007/s13197-014-1642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerget M, Kotnik P, Hadolin M, Hras AR, Simonic M, Knez Z. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89:191–198. doi: 10.1016/j.foodchem.2004.02.025. [DOI] [Google Scholar]
- Subramanion JL, Zakaria Z, Sreenivasan S. Phytochemicals screening, DPPH free radical scavenging and xanthine oxidase inhibitiory activities of Cassia fistula seeds extract. J Med Plant Res. 2011;5:941–947. [Google Scholar]
- Tepe B. Antioxidant potentials and rosmarinic acid levels of the methanolic extracts of salvia virgata (Jacq), salvia staminea (montbret and aucher ex Bentham) and salvia verbenaca (L.) from Turkey. Bioresour Technol. 2008;99:1584–1588. doi: 10.1016/j.biortech.2007.04.008. [DOI] [PubMed] [Google Scholar]