Abstract
In this research enzymatic hydrolysis of rice bran protein concentrate (RBPC) and soybean Protein (SBP) as control were studied with 3 commercial enzymes (Alcalase®, Papain and acommercial 3-enzyme cocktail containing of 1.6 mg ml−1 Trypsin, 3.1 mg ml−1 Chymotrypsin, 1.3 mg ml−1Aminopeptidase (SIGMA P7500) and 7.95 mg ml−1pronase type XIV (SIGMA P5147) by the pH stat method. The hydrolysis was carried out at temperature of 28 C, 60 min and pH 8.00. Results were showed that RBPC, and SBP had higher Degree hydrolysis (DH %) with Alcalase® enzyme. Alcalase®had stronger capability for hydrolysis compared to the other tested enzymes. After 60 minute of hydrolysis time, the DH% of Alcalase® for RBPC and SBP was 12.69 and 12.50 %, respectively. In contrast, papain enzyme was showed lowest DH% in three substrates that 1.56 and 1.24 % were for SBP and RBPC, respectively.The hydrolysis of the protein fraction performed the three enzymes on the two substrates was followed in SDS-PAGE. RBPC and SBP showed almost complete digestion with Alcalase® enzyme after 60 minutes. 3-enzyme cocktail enzyme hydrolyzed better the RBPC than the SBP. Papain enzyme had less effect on the two substrates than other 2 enzymes. It was found that Alcalase® has highest capability for hydrolysis compared to other enzyme preparations. The high value protein hydrolysates prepared by Alcalase® can be used as value added ingredients in many food formulations. They are also suitable for a broad range of industrial food applications and also for cosmetic and personal care products.
Keywords: Enzymatic hydrolysis, Rice bran protein concentrate, Commercial enzyme, SDS-PAGE
Introduction
Plant proteins play significant roles in human nutrition (Lamsal et al. 2007). Rice (Oyaza sativa) is one of the most staple diets for human especially in Asian countries. About 610 million metric tons of rice is produced annually in recent years (Dawe et al. 2010). More than half of the production belongs to Asian countries such as China, India, Indonesia, Bangladesh, Vietnam, and Thailand. This large amount of rice production has led to accumulation of rice byproducts. One of the major by-products is rice bran which is accounted for 8 % of milled rice (Shih and Daigle 2000). Rice bran is a source of proteins, oil, nutrients, and calories (Barber and De Barber 1991). The oil in rice bran is generally extracted and used as high quality cooking oil, while the de-oiled bran, that contains high amount of protein has not been utilized to its full potential. Due to high lysine content, protein from rice bran is considered hypoallergenic (Helm and Burks 1996), and is therefore favorable for human consumption. For this reason, it is of considerable research interest to examine the ways to extract proteins from the de-oiled by-product.
Enzymatic hydrolysis has become a widely used biotechnological process to obtain plant proteins with improved functional properties and commercial protein hydrolysates. Plant protein hydrolysates are mostly used as protein ingredients or supplements in food and beverages (Panyam and Kilara 1996) or as ingredients in special formulation for clinical nutrition (Clemente 2000). Enzymatic hydrolysis could release peptides with biological activity (Aluko and Monu 2003; Korhonen 2009; Korhonen and Pihlanto 2006) or might reduce allergenic potential of some intact plant proteins (Tsumura et al. 2005). The main variables for proteolysis enzymes that produce potentially more definedmaterial are temperature, pH, substrate and reaction time (Adler-Nissen 1979; Kim et al. 1990; Tsumura et al. 2005). The most common method for the production of rice protein is by alkali hydrolysis followed by acid precipitation (Jiamyangyuen et al. 2005). This method is simple because the reagents required for the process are easily available (Jiamyangyuen et al. 2005; Tang et al. 2003). In this research we examined the effect of different enzyme to obtain highest degree hydrolysis of protein concentrate of Rice bran (Var. Hashemi) and soybean (as control).
Materials and methods
Preparation of rice bran proteinand soybean protein
50 g of Rice bran (Var. Hashemi) was dispersed in 500 g deionized water. The pH of homogenate was adjusted to pH 12 and pH 10 with 2 N NaOH, and was incubated at room temperature, centrifuged 30 min at 2300 g in a Beckman centrifuge (Model JS.5, Beckman, USA). The supernatant was adjusted to pH 4.5, and centrifuged again 20 min at 2300 g, the supernatant was discarded and the precipitate was freeze-dried and called protein concentrate (Kumagai et al. 2006). Defatted soybean protein (Sigma) with 52 % protein was used as control.
Protease selection
Commercial enzymes used in this work were Alcalase® (produced from a strain of Bacillus licheniformis, with 89.49 U/ml of activity), papain(with 34 U/mlof activity) and a 3-enzyme 3-enzyme cocktail (1.6 mg ml−1Trypsin (E.C.3.4.21.4), 3.1 mg ml−1Chymotrypsin (E.C. 3.4.21.1), 1.3 mg ml−1Aminopeptidase (E.C. 3.4.24.x, SIGMA P7500) and 7.95 mg ml−1pronase type XIV (E.C. 3.4.24.x, SIGMA P5147with activity of 53 U/ml). All of these enzymes provided from biochemical Laboratory, La Paz, Mexico.
Enzymatic hydrolysis
The degree of hydrolysis (DH%) of rice bran protein, and soybean (control) were evaluated by the pH-stat method (Adler-Nissen 1986), using 3 enzymes (Alcalase®, papain and 3-enzyme 3-enzyme cocktail). A 718 stat Titrino (metrohom Ion Analysis, Switzerland) using Metrodata Menu computer program was performed for pH-stat analysis. Enzymes containing 4 U/ml protease activities were added to substrate containing 80 mg protein.The hydrolysis was performed at 28 °C, and pH 8.0. The reaction was monitored for 1 h. Each assay was carried out in triplicate. DH% determines on the basis of the number of free titratable amino groups produced by hydrolysis of peptide bonds.
DH% was calculated using the following equation:
Where α is the degree of dissociation of α-amino groups, MP is the mass of protein (g), htot is the number of peptide bonds in the substrate (meqv/g protein).
The hydrolysis of samples under different enzymes were followed in SDS-PAGE at 5, 10, 15, 30, 45 and 60 min after adding enzyme. 200 μ from each samples were taken of each treatment and immediately transferred to −18 Ċ.
SDS-PAGE electrophoresis
Hydrolyzed samples from the different treatments were analyzed by SDS-PAGE (Laemmli 1970). Samples (containing 40 μg protein) were mixed (1:1) with loading buffer : 0.125 M Tris-HCl, 4 % SDS, 20 % v/v glycerol, 0.2 M DTT and 0.02%bromophenol blue at pH 6.8, heated 5 min in a boiling water bath. Samples were analyzed in a vertical electrophoresis unit (SE 260, Hoefer, San Francisco,CA) using a 1.5 mm polyacrylamide gel slab (17 %) for separating protein bands. Electrophoresis was conducted at a constant current (30 mA per gel). The separated protein bands were stained with a solution containing 7 % acetic acid, 0.5 % Coomassie Brilliant Blue R-250 and 40 % methanol. The excess stain was removed with a solution containing 40 % methanol and 7 % acetic acid, and then the gel recorded with an electronic scanner (Umax Power Look 2100, UMAX Technologies, Fremont, CA).
Results
The results of proximate composition of defatted rice-bran and rice bran protein concentrate are showed in Table 1.
Table 1.
Proximate compositions of defatted rice bran and rice bran protein concentrate
| Proximate composition(%DW) | Defatted rice bran | Rice bran protein concentrate |
|---|---|---|
| protein | 19.1 ± 1.1 | 75 ± 2 |
| lipid | 2.1 ± 0.3 | 7.11 ± 0.2 |
| ash | 8.2 ± 0.2 | 6 ± 0.3 |
| NFE + Crude fiber | 70.6 ± 2.5 | 11.9 ± 0.4 |
DW= dry weight
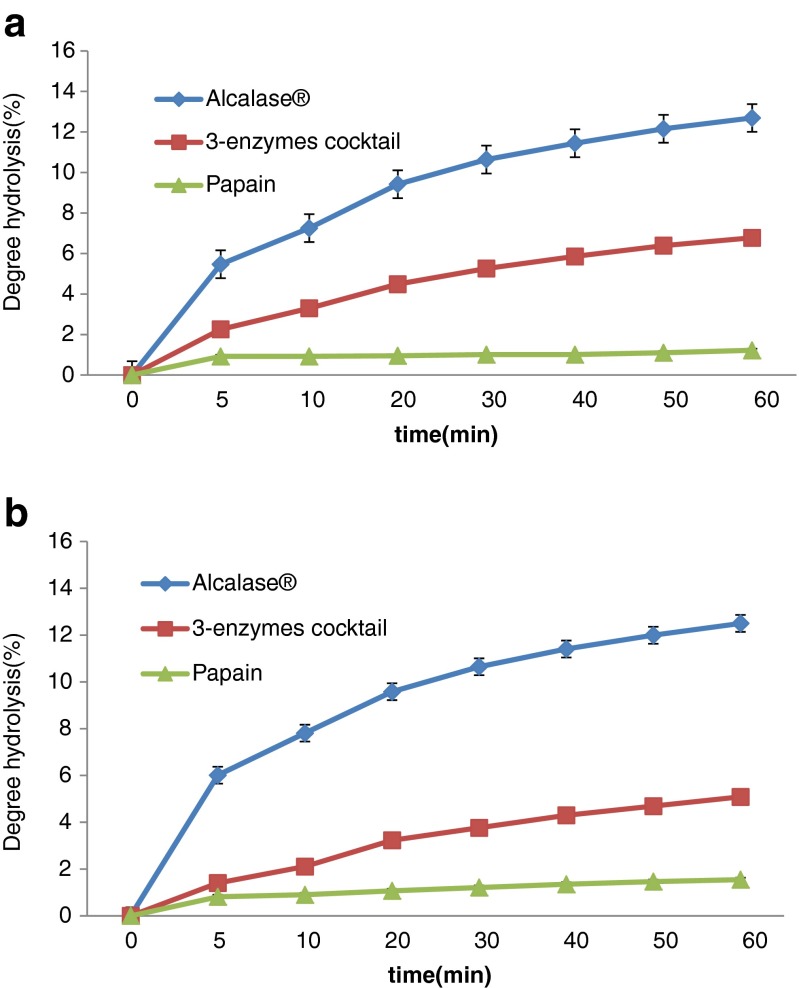
The results of the hydrolysis experiments are given in Table 2 and Fig. 1. There was significant differences between treatments (P < 0.05).The highest and lowest DH% for RBPC was 12.69 and 1.21 for Alcalase® and papain, respectively. Based on fig1 that it shows the DH% of 2 substrate with 3 commercial enzymes in over time, both substrates showed an increasing trend over time and the maximum was at 60 min.
Table 2.
Effect of different enzymes on degree hydrolysis of RBPC and SBP substrate at 60 min
| Degree hydrolysis (%) | |||
|---|---|---|---|
| Alcalase® | 3-enzyme cocktail | Papain | |
| RBPC | 12.69 ± 0.39a | 6.77 ± 0.07b | 1.21 ± 0,049c |
| SBP | 12.50 ± 0.2a | 5.08 ± 0.024b | 1.54 ± 0.05c |
RBPC Rice Bran Protein Concentrate, SBP defatted Soybean Protein
Value in the same row with a different superscript letter are significantly different(P < 0.05)
Fig. 1.
Degree of hydrolysis over time of 2 substrate with 3 commercial enzymes a rice bran protein concentrate, b defatted soybean protein
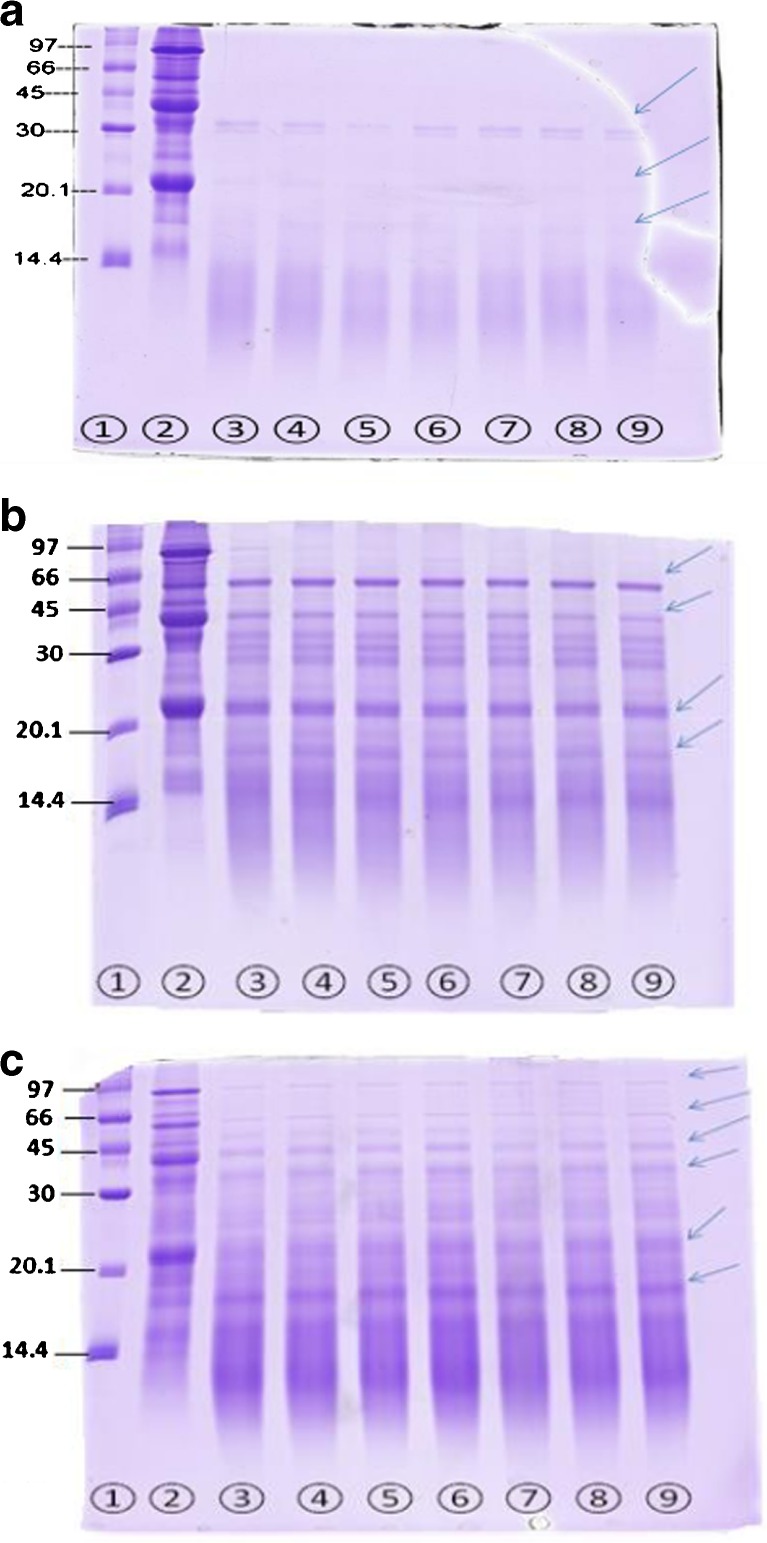
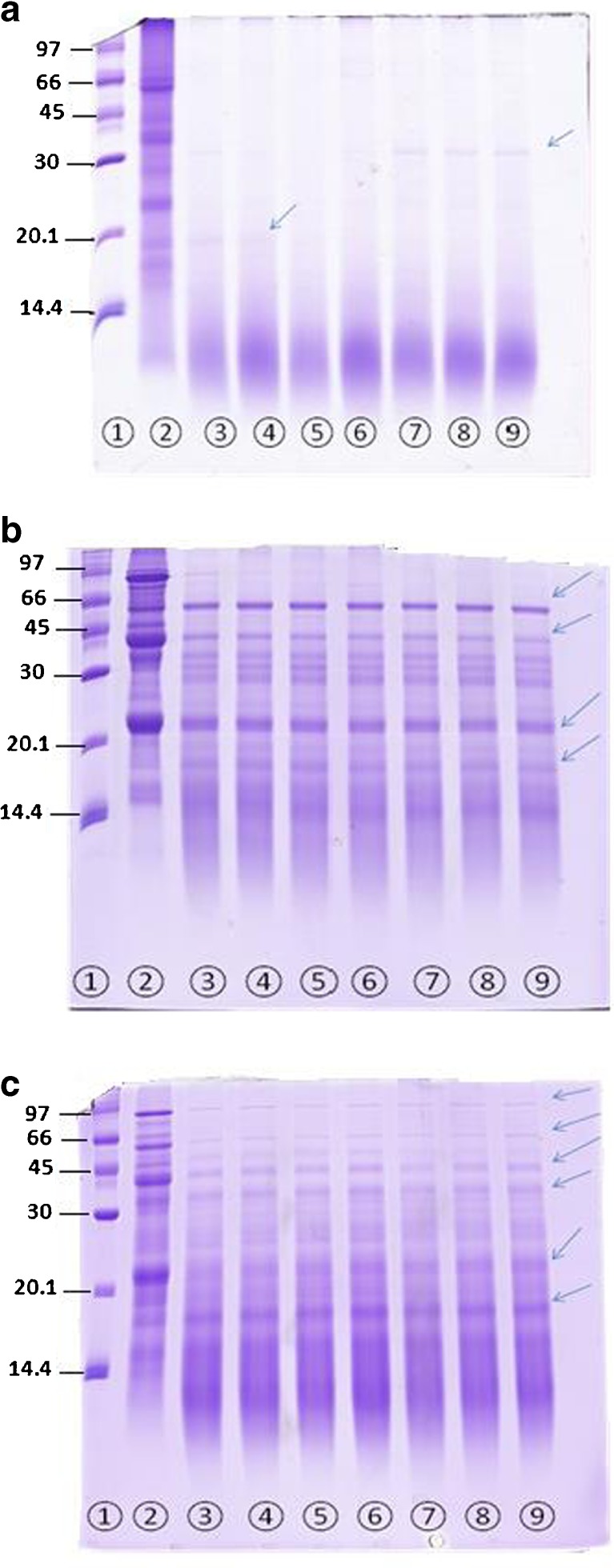
In Figs 2 and 3, the digestion of the protein fraction of the two substrates by the 3 commercial enzymes was observed over time in SDS-PAGE. Both RBPC and SBP showed almost complete hydrolysis after 60 min when Alcalase® was used. The Fig. 3 shows that the RBPC product had 11 component of protein and the molecular size of them were between 69.47 and 15.08 kDa.
Fig. 2.
SDS-PAGE showing hydrolysis over 60 min of Soybean protein with 3 commercial enzymes sampled at different times a = Soybean hydrolyzed with Alcalase, b = Soybean hydrolyzed with 3-enzymes cocktail, c = Soybean hydrolyzed with Papain,1 = low molecular weight marker, 2 = sample in zero time, 3 = sample after 5 min, 4 = sample after 10 min, 5 = sample after 15 min, 6 = sample after 30 min, 7 = sample after 40 min, 8 = sample after 50 min, 9 = sample after 60 min. SDS-PAGE electrophoresis was performed under reducing conditions. 40 μl protein of each sample was loaded at each well. The arrows indicated some protein bands in gel
Fig. 3.
SDS-PAGE showing hydrolysis over 60 min of rice bran protein with 3 commercial enzymes sampled at different times a = rice bran protein hydrolyzed with Alcalase®, b = rice bran protein hydrolyzed with 3-enzymes cocktail, c = rice bran protein hydrolyzed with Papain. 1 = low molecular weight marker, 2 = sample in zero time, 3 = sample after 5 min, 4 = sample after 10 min, 5 = sample after 15 min, 6 = sample after 30 min, 7 = sample after 40 min, 8 = sample after 50 min, 9 = sample after 60 min, SDS-PAGE electrophoresis was performed under reducing conditions. 40 μl protein of each sample was loaded at each well. The arrows indicated some protein bands in gel
Discussion
In our research Alcalase®had stronger capability for hydrolysis compared to other enzyme. Silpradit et al. (2010) obtained the 14.5 DH% for rice bran protein hydrolysates with optimum condition (60C, Enzyme/Substrate at 1 %, 340 min incubation time) (Silpradit et al. 2010). At the end of 60 min of processing time, Alcalase®hydrolyzed more than others enzymes. Based on Table2, Alcalase® and 3-enzyme cocktail effectively hydrolyzed the RBPC compared to papain.
Based on the results of degree hydrolysis of RBPC and SBP from Fig1 (a) and (b), typical hydrolysis curves were obtained in which the rate of hydrolysis decreases sensibly with time after an initial rapid phase. In both of them the degree Hydrolysis of papain after about 5 min approximately was stabled and there is no increasing until 60 min. Same results were also obtained by Demirhan et al. (2011) and Apar and Özbek (2010) for hydrolysis of sesame and corn gluten by Alcalase®, respectively (Apar and Özbek 2010; Demirhan et al. 2011). The decrease observed in the hydrolysis rate could be attributed to one of the these reason: 1) the decreases of peptide bonds that susceptible to hydrolysis by enzymes (Availability of substrate) b) Hydrolysis rates tend to constant over time c) hydrolysis products may cause inhibition of the enzyme. Based on our research RBPC product had 11 component of protein but Tang et al. (2003) reported that rice bran protein had 4 component of protein that was between 6.5 and 66.2 kDa (Tang et al. 2003). In this study it is possible that the use of alkaline method favored the hydrolysis of proteins and thus why we obtained more protein band in electrophoresis. However Wang et al. (1999) reported that rice bran protein isolate displayed more than 12 protein bands in electrophoresis (Wang et al. 1999). Based on Table 3 and Fig. 1 and Fig. 2, SBP and RBPC hydrolyzed with Alcalase® was showed only 4 and 2 bands, respectively. Most of protein fraction of RBPC hydrolyzed to smaller peptides (less than 14.4 kDa) and some may ran off the gel. In RBPC hydrolyzed with Alcalase® the 19.58 kDa band disappeared after 15 min while the 35.57 kDa band persisted during the whole experiment period (60 min). The 3-enzymes cocktail hydrolyzed the RBPC better than SBP and 4 peptides bands generated in RBPC compared to the 10 peptides bands observed in the hydrolyzed SBP. Soybean protein showed higher number of protein bands not digested by 3-enzyme cocktail compared to RBPC. In soybean ten proteins of different size were detected at time zero with 3-enzyme cocktail. The bands corresponding to non-hydrolyzed protein in soybean were persisted over 60 min of hydrolysis, but some vanished and of new ones appeared. In hydrolyzed soybean protein some proteins heavier than 66 kDa exhibited until 30 min but then disappeared. Papain enzyme had less effect on RBPC and SBP than the other 2 enzymes. Protein pattern over time in SDS-PAGE (Fig2) and in-vitro digestibility (DH %) (Fig. 1 and Table 2) reflected the affinity of RBPC and soybean protein to Alcalase® enzyme. Higher DH% of RBPC and soybean protein corresponded to important reduction in band the pattern and intensity observed in SDS-PAGE with the different enzymes. Poorly, substrate hydrolysis (low DH %) was observed when papain enzyme was used and displayed persistent protein bands in the SDS-PAGE after 60 min of hydrolysis.
Table 3.
Size of the peptides produced after the hydrolysis of RBPH and SPH with Alcalase®, 3-enzyme cocktail and papain
| Molecular weight of RBPH band (kDa) | Molecular weight of SPH band (kDa) | |||||||
|---|---|---|---|---|---|---|---|---|
| Band | Controa | Alcalase® | 3-enzyme cocktail | papain | Controlb | Alcalase® | 3-enzyme cocktail | papain |
| 1 | 69.47 | 35.57 | 44.39 | 28.93 | 74.04 | 39.67 | 61.96 | 79.51 |
| 2 | 65.16 | 19.58 | 41.73 | 20.49 | 67.82 | 36.06 | 49.29 | 63.12 |
| 3 | 60.56 | 30.94 | 16.06 | 57.75 | 22.10 | 42.71 | 56.80 | |
| 4 | 49.07 | 23.35 | 51.38 | 15.60 | 38.83 | 50.45 | ||
| 5 | 43.97 | 45.71 | 35.30 | 43.07 | ||||
| 6 | 41.24 | 39.49 | 25.28 | 25.92 | ||||
| 7 | 33.42 | 36.98 | 21.29 | 18.28 | ||||
| 8 | 26.11 | 29.70 | 18.28 | 14.80 | ||||
| 9 | 20.21 | 23.17 | 15.54 | |||||
| 10 | 17.14 | 17.17 | 12.72 | |||||
| 11 | 15.08 | |||||||
RBPC Rice Bran Protein Concentrate, SBP defatted Soybean Protein
a protein solution of RBPC without enzyme
b protein solution of SPH without enzyme
Conclusions
The pH-stat routine for determination of in vitro protein digestibility with enzymes has demonstrate adequate to check quality of feed ingredients as fish meal, soybean and plant proteinsources (Ezquerra et al. 1997; Garcia-Carreno et al. 1994; García-Carreño et al. 1997). The present degree hydrolysis results provided a ranking of enzymes (Alcalase > cocktail > papain), based on the capability to hydrolyze the protein of the assayed substrates (RBPC and SBP). Thus depending on the use of the hydrolysate the best DH% and the enzyme can be selected.
Footnotes
Research highlights
• Rice bran protein concentrate can be used as a value-added product.
• The hydrolysis was used for the study of rice bran protein concentrate.
• For enzymatic studies, pH stat method and SDS-PAGE method were used.
• The Alcalase® enzyme for substrate hydrolysis was more efficient than the other two enzymes.
• SDS-PAGE could represent protein fraction of hydrolysates.
References
- Adler-Nissen J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J Agric Food Chem. 1979;27:1256–1262. doi: 10.1021/jf60226a042. [DOI] [PubMed] [Google Scholar]
- Adler-Nissen J (1986) Enzymic hydrolysis of food proteins. Elsevier Applied Science Publishers, Barking, Essex, UK, 427 pp
- Aluko R, Monu E. Functional and bioactive properties of quinoa seed protein hydrolysates. J Food Sci. 2003;68:1254–1258. doi: 10.1111/j.1365-2621.2003.tb09635.x. [DOI] [Google Scholar]
- Apar DK, Özbek B. Corn Gluten hydrolysis by Alcalase: Kinetics of Hydrolysis. Chem Eng Commun. 2010;197:963–973. doi: 10.1080/00986440903359368. [DOI] [Google Scholar]
- Barber S, De Barber CB (1991) Rice bran: chemistry and technology Rice: Utilization 2:313
- Clemente A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci Technol. 2000;11:254–262. doi: 10.1016/S0924-2244(01)00007-3. [DOI] [Google Scholar]
- Dawe D, Pandey S, Nelson A (2010) Emerging trends and spatial patterns of rice production Rice in the Global Economy: Strategic Research and Policy Issues for Food Security Los Baños, Philippines: International Rice Research Institute (IRRI) p 15–35
- Demirhan E, Apar DK, Özbek B. Sesame cake protein hydrolysis by alcalase: effects of process parameters on hydrolysis, solubilisation, and enzyme inactivation. Korean J Chem Eng. 2011;28:195–202. doi: 10.1007/s11814-010-0316-2. [DOI] [Google Scholar]
- Ezquerra JM, García-Carreño FL, Civera R, Haard NF. pH-stat method to predict protein digestibility in white shrimp (penaeus vannamei) Aquaculture. 1997;157:251–262. doi: 10.1016/S0044-8486(97)00058-6. [DOI] [Google Scholar]
- Garcia-Carreno FL, Hernandez-Cortes MP, Haard NF. Enzymes with peptidase and proteinase activity from the digestive systems of a freshwater and a marine decapod. J Agric Food Chem. 1994;42:1456–1461. doi: 10.1021/jf00043a013. [DOI] [Google Scholar]
- García-Carreño FL, Navarrete del Toro A, Ezquerra M. Digestive shrimp proteases for evaluation of protein digestibility in vitro. I: effect of protease inhibitors in protein ingredients. J Mar Biotechnol. 1997;5:36–40.. [Google Scholar]
- Helm R, Burks A. Hypoallergenicity of rice protein. Cereal Foods World. 1996;41:839–843. [Google Scholar]
- Jiamyangyuen S, Srijesdaruk V, Harper WJ. Extraction of rice bran protein concentrate and its application in bread extraction. Songklanakarin J Sci Technol. 2005;27(1):55–64. [Google Scholar]
- Kim SY, Park PSW, Rhee KC. Functional properties of proteolytic enzyme modified soy protein isolate. J Agric Food Chem. 1990;38:651–656. doi: 10.1021/jf00093a014. [DOI] [Google Scholar]
- Korhonen H. Milk-derived bioactive peptides: from science to applications. J Funct Foods. 2009;1:177–187. doi: 10.1016/j.jff.2009.01.007. [DOI] [Google Scholar]
- Korhonen H, Pihlanto A. Bioactive peptides: production and functionality. Int Dairy J. 2006;16:945–960. doi: 10.1016/j.idairyj.2005.10.012. [DOI] [Google Scholar]
- Kumagai T, et al. Production of rice protein by alkaline extraction improves its digestibility. J Nutr Sci Vitaminol. 2006;52:467–472. doi: 10.3177/jnsv.52.467. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamsal B, Jung S, Johnson L. Rheological properties of soy protein hydrolysates obtained from limited enzymatic hydrolysis. LWT-Food Science and Technology. 2007;40:1215–1223. doi: 10.1016/j.lwt.2006.08.021. [DOI] [Google Scholar]
- Panyam D, Kilara A. Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci Technol. 1996;7:120–125. doi: 10.1016/0924-2244(96)10012-1. [DOI] [Google Scholar]
- Shih FF, Daigle KW. Preparation and characterization of rice protein isolates. J Am Oil Chem Soc. 2000;77:885–889. doi: 10.1007/s11746-000-0141-2. [DOI] [Google Scholar]
- Silpradit K, Tadakittasarn S, Rimkeeree H, Winitchai S, Haruthaithanasan V. Optimization of rice bran protein hydrolysate production usingalcalase. Asian Journal Food Agriculture-Ind. 2010;3:221–231. [Google Scholar]
- Tang S, Hettiarachchy N, Horax R, Eswaranandam S. Physicochemical properties and functionality of rice bran protein hydrolyzate prepared from heat-stabilized defatted rice bran with the aid of enzymes. J Food Sci. 2003;68:152–157. doi: 10.1111/j.1365-2621.2003.tb14132.x. [DOI] [Google Scholar]
- Tsumura K, Saito T, Tsuge K, Ashida H, Kugimiya W, Inouye K. Functional properties of soy protein hydrolysates obtained by selective proteolysis. LWT-Food Science and Technology. 2005;38:255–261. doi: 10.1016/j.lwt.2004.06.007. [DOI] [Google Scholar]
- Wang M, Hettiarachchy N, Qi M, Burks W, Siebenmorgen T. Preparation and functional properties of rice bran protein isolate. J Agric Food Chem. 1999;47:411–416. doi: 10.1021/jf9806964. [DOI] [PubMed] [Google Scholar]