Abstract
Koozh is a traditional fermented millet beverage unique to south India. Analysis of six market samples of koozh for their microbial profile resulted in 69 isolates of presumptive lactic acid bacteria (LAB). They were grouped as Leuconostoc sp., Enterococcus sp., Streptococcus sp. and Lactobacillus sp. based on morphological characteristics and biochemical tests. Eight among them showed probiotic features: resistance to acid (2.5 pH for 6 h), resistance to 0.3 % ox bile, moderate hydrophobicity (40 %), antibacterial activity against 10 pathogens, susceptibility to 50 % of antibiotics tested. Sequencing of 16srDNA showed them to be five strains of Enterococcus hirae and one each of Enterococcus facecalis, Bacillus amyloliquefaciens and Lactobacillus plantarum. The probiotic isolates were encapsulated in skim milk powder by two different drying techniques: freeze drying and spray drying. The encapsulated probiotic isolates survived both in simulated gastric fluid and simulated intestinal fluid with high cell viability (98–99 %). Storage for 16 weeks at room temperature (27 °C), resulted in 2 log reduction, but better survival with only 1 log reduction was observed at 4 °C and was best at −20 °C. Survival of isolates was similar in both spray and freeze dried products.
Keywords: Lactic acid bacteria, Koozh, Fermented millet, Acid tolerance, Drying, Simulated gut tolerance
Introduction
The steadily growing research interest in probiotic microorganisms, has contributed to their recognition as health promoters that modulate the gut microbiota. The potential health benefits of probiotics include: bacteriocin production, alleviation of lactose intolerance, reduction of intestinal pathogens, hypocholesterolemic effect, stimulation of the immune system and prevention of—antibiotic associated diarrhoea, inflammatory bowel diseases and allergy (atopic eczema, food allergy).
Fermentation of millets increases bioavailability of nutrients while reducing the level of anti-nutrients through microbial activity. It decreases the carbohydrates, dietary fiber, fatty acid and increases the protein quality and B vitamins. Fermented whole grains have become the preferred choice of probiotics delivery vehicles as they offer buyers double benefits from probiotics along with whole grains. Fermented finger millet foods as probiotic carriers would enhance consumer health via the benefits from probiotics, calcium and bioactive components.
Lactic acid bacteria (LAB) are prevalent in most of the Indian cereal fermented foods and strains identified include Lactobacillus fermentum, Lactobacillus plantarum, Pediococcus pentosaceus and Leuconstoc mesnetroides (Tamang 2010). Although probiotic microbes have been isolated from many dairy products, not many Indian fermented millet foods have been explored as a potential source of probiotics.
Koozh, is a unique traditional millet food from the natural fermentation of finger or pearl millet. The fermentation is carried out on one occasion with raw millet slurry and second on the cooked porridge (Ilango and Antony 2014). The organoleptic property is similar to porridge and depending on the butter milk added, provides the sour tang flavor. Studies on koozh are very limited. Kumar et al. (2010) have studied the microflora of koozh prepared from finger millet under laboratory conditions, with fermentation for 2 days without the addition of cooked broken rice and reported the presence of Weisella paramesenteroides with probiotic properties and Lactobacillus fermentum with antibacterial activity towards Salmonella typhi, Vibrio parahaemolyticus and Listeria monocytogenes. The objective of this work was to screen, isolate and identify the lactic acid bacteria (LAB) prevalent in six market samples of koozh and to assess their probiotic characteristics in vitro. The effect of encapsulation by spray and freeze drying on the probiotic characteristics of the isolates was also studied.
Materials and methods
Sample collection
Six samples of koozh were purchased from streets and village markets of Tamil Nadu. They were collected aseptically in wide mouthed sterile bottles and transported to the laboratory in a cooling box and used immediately for the isolation of microbes.
Microbiological analysis
The non-starter LAB (NSLAB) were isolated from aliquots of koozh samples by appropriate dilution and pour-plating, in MRS agar and M17 agar (HiMedia, Mumbai, India). The plates were examined after 24 h incubation at 35 °C. The colonies obtained were examined for morphological differences (shape, size, elevation, surface characteristics and edges). All isolated Gram positive, non-motile, catalase and oxidase negative isolates were stored at −80 °C in 30 % glycerol and sub-cultured twice by quadrant streaking separately on fresh MRS and M17 media.
All the NSLAB isolates were initially screened for their ability to produce acid by pour plating on MRS and M17 agar supplemented with 1 % CaCO3 and incubated under anaerobic conditions at 35 °C for 24 h using the HiMedia’s Gas pack systems. They were grouped based on the following criteria: ammonia utilization from arginine, methylene blue reduction, gas production from glucose, ability to grow at different temperatures (15 °C, 45 °C), pH (3.9, 9.6) and in different concentrations of sodium chloride (7 %, 10 %) (Schillinger and Lücke 1987). The ability of the NSLAB isolates to ferment the following carbohydrates: adonitol, arabinose, cellobiose, dextrose, dulcitol, fructose, galactose, inositol, inulin, lactose, maltose, mannitol, mannose, melibiose, raffinose, rhamnose, salicin, sorbitol, sucrose, trehalose and xylose was determined by using carbohydrate coated paper discs according to manufacturer’s instructions (HiMedia, India).
Resistance of NSLAB to biological barriers in vitro
Five tests were conducted in vitro to assess the probiotic activity/character of the NSLAB isolates. The bacteria needs to be metabolically active and stable in the product, should survive the strong acidic conditions of upper digestive tract and alkaline conditions of small intestine of the host (Anal and Singh 2007).
The initial qualitative test for pre-selection of probiotic strains was acid tolerance (Hyronimus et al. 2000). The LAB isolates were grown in MRS and M17 broth at 37 °C in a static incubator overnight. An aliquot (0.1 mL) of the isolate was inoculated into 10 mL of MRS and M17 broth adjusted to a pH 2.5 with 3.0 M hydrochloric acid and incubated at 37 °C in aerobic static condition. One milliliter aliquots were taken at 0, 3 and 6 h intervals for identifying the cell viability by pour plating in MRS and M17 agar. The residual viable population was determined after 48 h of incubation at 37 °C. The results are expressed in logarithmic colony-forming unit per milliliter (log CFU mL −1). The survival rate of the isolates was calculated according to the formula:
Presumptive LAB that had survived after 6 h incubation with growth reduction not exceeding 2 log CFU mL −1 were considered presumptive probiotic lactic acid bacteria (PPLAB) and further screened for probiotic characteristics.
Resistance to bile was also assessed in vitro. The PPLAB isolates were grown in 3 mL MRS broth at 37 °C overnight. A 0.25 mL volume of the culture was inoculated into 25 mL of MRS broth supplemented with 0.3 % ox bile salts (test) and without (control). These were incubated at 37 °C and A600nm measured every hour for 6 h. The growth curves were plotted and time needed to attain a 0.3 A600nm was monitored for both test and control. The effect of bile inhibition “d” in terms of delay in growth of the isolate was calculated as the difference between control and test expressed in minutes (min). This difference is considered as the major factor for this arbitrary classification. According to Chateau et al. (1994), the PPLAB isolates may be classified as resistant strains (delay of growth: d is ≤15 min), tolerant isolates (d is >15 min and ≤40 min), weakly tolerant isolates (d is >40 min and ≤60 min) and sensitive (d is >60 min). Based on the isolates growth curve for 24 h, the difference in time between the control and test to reach the stationary phase, considered as Lag Time (LT) was also recorded.
The adherence of bacterial cells was evaluated using the microbial adhesion test of hyrophobicity (MATH) (Doyle and Rosenberg 1995). The PPLAB isolates were incubated in MRS broth under aerobic conditions for 15 h at 37 °C. A 3 mL aliquot was centrifuged at 6000 rpm for 10 min, the supernatant removed and the pellet washed thrice with 50 mM K2HPO4 (Merck) of pH 6.5. The absorbance of the culture (A) was adjusted to 0.7 ± 0.2 A600nm. A 3 mL aliquot of the washed culture (A) was added to 0.6 mL of n-Hexadecane and vortexed for 120 s. The tubes were left undisturbed at 37 °C for 30 min for phase separation. Without disturbing the upper phase, 0.7 mL of the lower aqueous phase was removed with a micropipette and A600nm was measured (Ao). The decreased value of Ao due to cell partitioning into the hydrocarbon and aqueous phases was noted down and percentage of hydrophobicity (H%) was calculated using the formula: H % = (A − Ao/A) × 100.
The agar-well diffusion method as described by Schillinger and Lucke (1989) was used to determine the antibacterial activities. Ten microorganisms namely Bacillus cereus ATCC 14579, Enterobacter aerogenes MTCC 111, Escherichia coli MTCC 728, Klebsiella pneumoniae subsp. ozaenae MTCC 2653, Listeria monocytogenes MTCC 1143, Micrococcus luteus MTCC 4428, Proteus vulgaris MTCC 426, Salmonella typhi MTCC734, Shigella flexneri MTCC 1457 and Staphylococcus aureus ATCC 43300 were used for assessing antimicrobial inhibitory potential. The pathogenic bacteria were grown separately in nutrient broth at 37 °C for 18 h in aerobic environment and adjusted to 1.00 A600nm before pour plating in MRS soft agar.
The cell free supernatants (CFS, 0.05 mL) of NSLAB isolates were dispensed in wells cut in the MRS agar containing the respective pathogens and incubated at 37 °C for 24 h and the zone of clearance noted. To analyze the nature of inhibition, the CFS was neutralized to pH of 6.50 ± 0.08 with 5 M NaOH and 14.4 mg of fresh catalase (HiMedia:2000–5000 unit mg −1) added. The neutralized cell free supernatants (NCFS) were dispensed into fresh wells and assessed as above. A value of 1 mm or more was considered positive inhibition.
The antibiotics used were selected based on their frequency of use in the Chennai region. The PPLAB isolates were tested for resistance to various classes of 21 regional antibiotics in ready-to-use antibiotic impregnated paper discs (HiMedia). They were ampicillin, amoxyclav, penicillin-G, cefixime, cefuroxime, vancomycin, bacitracin, amikacin, gentamicin, azithromycin, streptomycin, erythromycin, chloramphenicol, tetracycline, norfloxacin, ciprofloxacin, nalidixic acid, gatifloxacin, gemiofloxacin and levofloxacin. Initially PPLAB isolates were grown for 24 h at 37 °C in a 3 mL MRS broth and the cell density adjusted to1.00 A600nm and pour plated in 95 mm petriplates with 20 mL MRS agar. After solidification, four antibiotic discs were dispensed in equal corners of a plate according to manufacturer’s instructions and incubated at 37 °C for 24 h. The diameter of inhibition was measured and compared with interpretive chart of Charteris et al. (1998) and the isolates classified as resistant (R), susceptible (S), or intermediate (I).
Genotype of probiotic LAB
Of the 69 isolates, PPLAB isolates that showed acid tolerance, bile resistance and antibacterial activity were represented as probiotic lactic acid bacteria (PLAB) submitted to Macrogen, Korea for sequencing. These genomic DNA were obtained by InstaGene™ Matrix (BIO-RAD). The primers 27F 5′ (AGA GTT TGA TCC TGG CTC AG) 3′ and 1492R 5′ (TAC GGY TAC CTT GTT ACG ACT T) 3′ were used for executing the PCR (Lane 1991). The PCR reaction was carried out with 20 ng of DNA as the template in a 30 μL reaction mixture by using a EF-Taq (SolGent, Korea): initiation of Taq polymerase at 95 °C for 2 minutes, 35 cycles of 95 °C for 1 min, 55 °C, and each of 72 °C for 1 min, ending it with a 10-min step at 72 °C. The amplification products were purified with a multi-screen filter plate (Millipore Corp., Bedford, MA, USA). The Sequencing reaction was performed using sequencing primer 518 F 5′ (CCA GCA GCC GCG GTA ATA CG) 3′ and 800R 5′ (TAC CAG GGT ATC TAA TCC) 3′ using the cycle sequencing kit PRISM BigDye Terminator v3.1. This DNA sample containing extension products were combined with Hi-Di formamide (Applied Biosystems, Foster City, CA). The mixture was incubated at 95 °C for 5 min, then kept on ice for 5 min and analyzed in ABI Prism 3730XL DNA (Applied Biosystems, Foster City, CA). The sequence was aligned with NCBI GeneBank database using the nucleotide homology search Blast N program. Then the blast program was used to compare with other homologous sequence to identify the origin and geographical distribution of the identified PLAB with other submitted sequence around the world.
Encapsulation and effect of Storage
The PLAB isolates were grown in 3 mL MRS broth at 37 °C overnight. A 100 μL aliquot of overnight grown cultures were inoculated in 50 mL of MRS broth for 15 h in an incubator shaker. The culture was pelleted by centrifugation at 1600×g for 10 min at 4 °C. The pellet was diluted with saline to 0.93 ± 0.03 A600nm inoculated (1 % v/v) in 50 mL of 10 % skim milk powder (SMP) containing 0.5 % yeast extract (YE). Skim milk suspension (SMS) was kept in incubator shaker at 180 rpm, 37 °C for 6 h. This was then pour plated on MRS agar for cell count.
The SMS was spray dried in a bench top drier (JISL SprayMate, India) with a inlet temperature of 140 °C and outlet temperature of 40 °C at a pressure of 2 psi and feed rate of 14 rpm to obtain spray dried probiotic (SDP) powder. The SMS was freeze dried in a laboratory scale lyophilizer (SP Scientific, VirTis) at condensing temperature of −80 °C and vacuum pressure of 50 mTorr to get freeze dried probiotic (FDP) powder. The moisture of SDP and FDP (PLAB isolates encapsulated with skim milk powder) was analyzed using a moisture analyzer (Sartorius- MA35). Three batches of the encapsulated PLAB isolates were prepared.
The FDP and SDP were separately stored at temperatures of −20, 4, 27 and 37 °C in air tight containers. The survival was evaluated by pour plating samples on MRS agar every week for 1, 2, 4, 6, 8 and 16 weeks and survival percentage was calculated.
Probiotic efficacy of encapsulated probiotic isolates
The encapsulated PLAB stored at different temperatures were subjected to simulated gastric and intestinal fluid to assess their viability in such environments. Samples were rehydrated before checking viability by pour plate method in MRS agar. Samples (0.5 g) of FDP/SDP were added to test tubes containing 4.5 mL of pre-warmed (37 °C) sterile saline and incubated for 1 h at 37 °C.
The simulated gastric fluid (SGF) was primed by mixing saline with 0.32 mg mL−1 pepsin and adjusting it to pH 2 (Anal and Singh 2007); 0.45 mL of SGF was added with 0.5 g of FDP or SDP and incubated at 37 °C for 2 h. The samples were retrieved at 30, 60, 90, 120 min for pour plating on MRS agar.
The simulated intestinal fluid (SIF) was prepared by mixing pH 7.4 saline with 0.1 % pancreatin and 3.6 % w/v bile (Borza et al. 2010). As mentioned above, 0.45 mL of SIF was mixed with 0.5 g FDP or SDP and incubated for 4 h at 37 °C. These samples are again retrieved at 120, 240 min and pour plated on MRS agar to check survival of the isolates.
Statistical analysis
Each of the experiments was performed in triplicates. The difference in mean and rank correlation was evaluated by using Graph Pad 5.01 (SanDiego, Ca, USA) software.
Results and discussion
Phenotyping and characterization of NSLAB isolates
A total of 69 NSLAB were obtained from koozh samples—44 from MRS and 25 from M17 media. All isolates were Gram positive, catalase negative, non-motile and acid producing. Methylene blue reductase test proved positive for all except for nine isolates which were identified as Streptococcus sp. The morphological characteristics revealed more number of cocci (48) than rods (21). Based on these and other biochemical characteristics, the isolates were grouped as hetero-fermentative (19 of Leuconostoc sp., 5 of Lactobacillus sp.) and homo-fermentative (20 Entercoccocus sp., 9 Streptococcus sp. and 16 Lactobacillus sp.) as presented in Table 1.
Table 1.
Biochemical classification of NSLAB isolated from koozh
| Groups | 1a | 2 a | 3 a | 4 a | 5 a | 6 b | 7 b | 8 b | 9 c | 10 d | 11 d | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of Isolates | 4 | 7 | 2 | 2 | 4 | 3 | 14 | 3 | 9 | 5 | 16 | 69 |
| Cell shape | Coccus | Coccus | Coccus | Coccus | Coccus | Coccus | Coccus | Coccus | Coccus | Bacillus | Bacillus | |
| NH3 | + e | + | + | + | + | + | + | + | + | + | + | 69 |
| Gas from glucose | + | + | + | + | + | − | − | − | − | + | − | 32 |
| Methylene blue | + | + | + | + | + | + | + | + | − | + | + | 60 |
| 15 °C | + | + | + | + | − | + | + | + | + | + | + | 65 |
| 45 °C | + | + | + | + | + | + | + | + | + | + | + | 69 |
| 9.6Ph | + | + | − | + | + | + | + | − | + | + | + | 65 |
| 3.9Ph | + | − | + | − | + | + | + | + | − | – | + | 52 |
| 2.5pH at 6th hour | 4/4f | 0/7 | 0/2 | 0/2 | 2/4 | 3/3 | 06/14 | 1/3 | 2/9 | 1/5 | 07/16 | 26 |
| 7 % NaCl | ¾ | 7/7 | 2/2 | 1/1 | 4/4 | 3/3 | 14/14 | 3/3 | 7/9 | 4/5 | 15/16 | 63 |
| 10 % NaCl | 0/4 | 0/7 | 0/2 | 0/1 | 0/4 | 0/3 | 03/14 | 0/3 | 0/9 | 5/5 | 02/16 | 10 |
| Adonitol | 0/4 | 0/7 | 0/2 | 0/2 | 0/4 | 0/3 | 0/14 | 0/3 | 0/9 | 0/5 | 0/16 | 0 |
| Arabinose | 4/4 | 7/7 | 2/2 | 2/2 | 2/4 | 3/3 | 14/14 | 3/3 | 9/9 | 5/5 | 16/16 | 67 |
| Cellobiose | 4/4 | 7/7 | 2/2 | 2/2 | 4/4 | 3/3 | 14/14 | 3/3 | 9/9 | 5/5 | 16/16 | 69 |
| Dextrose | 4/4 | 7/7 | 2/2 | 2/2 | 4/4 | 3/3 | 14/14 | 3/3 | 9/9 | 5/5 | 16/16 | 69 |
| Dulcitol | 0/4 | 0/7 | 2/2 | 0/2 | 0/4 | 0/3 | 0/14 | 0/3 | 0/9 | 0/5 | 0/16 | 02 |
| Fructose | 4/4 | 7/7 | 2/2 | 0/2 | 4/4 | 3/3 | 14/14 | 3/3 | 9/9 | 5/5 | 16/16 | 69 |
| Galactose | 4/4 | 7/7 | 2/2 | 2/2 | 4/4 | 0/3 | 14/14 | 3/3 | 9/9 | 5/5 | 16/16 | 66 |
| Inositol | 0/4 | 0/7 | 0/2 | 0/2 | 0/4 | 0/3 | 0/14 | 0/3 | 0/9 | 0/5 | 0/16 | 0 |
| Inulin | 0/4 | 0/7 | 0/2 | 0/2 | 0/4 | 0/3 | 0/14 | 0/3 | 0/9 | 0/5 | 0/16 | 0 |
| Lactose | 4/4 | 7/7 | 2/2 | 2/2 | 4/4 | 3/3 | 11/14 | 1/3 | 0/9 | 5/5 | 16/16 | 60 |
| Malltose | 4/4 | 7/7 | 2/2 | 2/2 | 4/4 | 3/3 | 14/14 | 3/3 | 9/9 | 5/5 | 16/16 | 69 |
| Mannitol | 4/4 | 7/7 | 0/2 | 0/2 | 0/4 | 0/3 | 14/14 | 0/3 | 9/9 | 5/5 | 16/16 | 55 |
| Mannose | 4/4 | 7/7 | 2/2 | 2/2 | 4/4 | 3/3 | 14/14 | 3/3 | 9/9 | 5/5 | 16/16 | 69 |
| Melibiose | 4/4 | 7/7 | 2/2 | 2/2 | 4/4 | 3/3 | 14/14 | 3/3 | 9/9 | 4/5 | 16/16 | 68 |
| Raffinose | 0/4 | 0/7 | 2/2 | 2/2 | 0/4 | 0/3 | 14/14 | 0/3 | 7/9 | 5/5 | 14/16 | 44 |
| Rhamnose | 0/4 | 0/7 | 0/2 | 0/2 | 0/4 | 0/3 | 08/14 | 0/3 | 0/9 | 5/5 | 16/16 | 29 |
| Salicin | 4/4 | 7/7 | 2/2 | 2/2 | 4/4 | 3/3 | 13/14 | 3/3 | 9/9 | 5/5 | 16/16 | 68 |
| Sorbitol | 0/4 | 0/7 | 0/2 | 0/2 | 0/4 | 0/3 | 05/14 | 3/3 | 0/9 | 0/5 | 09/16 | 17 |
| Sucrose | 4/4 | 7/7 | 2/2 | 2/2 | 4/4 | 3/3 | 14/14 | 3/3 | 9/9 | 5/5 | 16/16 | 69 |
| Trehalose | 4/4 | 7/7 | 2/2 | 2/2 | 4/4 | 3/3 | 14/14 | 3/3 | 9/9 | 5/5 | 16/16 | 69 |
| Xylose | 4/4 | 5/7 | 2/2 | 2/2 | 4/4 | 3/3 | 14/14 | 3/3 | 9/9 | 0/5 | 0/16 | 46 |
| 19 | 20 | 9 | 5 | 16 |
aGroups 1 to 5 belonged to Leuconostoc sp
bGroups 6 to 8 as Enteroccocus sp
cGroup 9 as Streptococcus sp
dGroup 10 and 11 as Lactobacillus sp
e +: Positive, −: Negative
fNo of positive strains/Total no of strains
LAB have been reported in traditional fermented cereals like kisra from sorghum (Mohammed et al. 1991), pozol from maize (Ampe et al. 1999), tape from finger millet (Sujaya et al. 2001) and borde, from a mixture of maize and wheat (Abegaz 2007). LAB isolated in kodo ko jaanr made from finger millet and selroti made from rice were grouped based on biochemical tests (Yonzan and Tamang 2010). Geetha and Kalaichelvan (2013) have reported the predominance of LAB in their study where finger millet, pearl millet, sorghum and maize were made separately into koozh by fermenting for 20 h in the laboratory.
Resistance of NSLAB to biological barriers in vitro
The relationship of probiotic microorganisms to human health are well reviewed over the last decade. The required screening for effective PLAB demands that they withstand conditions of GIT (gastro intestinal tract) in vitro and in vivo.
The six koozh samples contained 26 acid tolerant isolates (growth up to 5 log CFU mL−1 after 6 h, pH 2.5) with survival of 70 % or more. Only eight of the 26 LAB survived up to 6 log CFU mL−1 after 3 h and 4 log CFU mL−1 after 6 h in the acidic condition and the decrease in growth for these isolates after 6 h was <2 log CFU mL−1. The eight isolates considered as presumptive probiotic lactic acid bacteria (PPLAB) showed survival exceeding 80 and 60 % at pH 2.5 for 3 h and 6 h respectively (Table 2).
Table 2.
Acid tolerance, bile tolerance and hydrophobicity of PPLAB isolates from koozh
| Strains | Isolate | Acid survival % at pH 2.5 | Growth delay between control and Oxbilemin | Hydrophobicity % | |||
|---|---|---|---|---|---|---|---|
| 3 h | 6 h | Resistanta | Tolerantb | Low | Medium | ||
| Enterococcus sp. | Sha1 | 95.77 ± 03.59 | 83.99 ± 05.77 | – | 22.0 | – | 41 ± 2.82 |
| Sha8 | 93.32 ± 03.36 | 69.80 ± 20.60 | 8.0 | – | – | 44 ± 2.12 | |
| Sha22 | 88.59 ± 03.05 | 66.89 ± 19.24 | 0.5 | – | 04 ± 0.70 | – | |
| Sha24 | 82.49 ± 09.34 | 71.45 ± 12.93 | 4.0 | – | 06 ± 0.70 | – | |
| Sha28 | 91.39 ± 07.33 | 61.25 ± 23.14 | 1.0 | – | 07 ± 0.70 | – | |
| Sha35 | 81.12 ± 10.05 | 73.01 ± 11.88 | 0.0 | – | 10 ± 1.41 | – | |
| Lactobacillus sp. | Sha7 | 91.01 ± 02.15 | 73.30 ± 22.90 | 2.0 | – | – | 49 ± 4.24 |
| Sha31 | 90.19 ± 02.40 | 69.17 ± 06.39 | 13.5 | – | 04 ± 0.70 | – | |
aGrowth delay ≤15 min
bGrowth delay >15 min and ≤40 min
The microbial stability of LAB may be increased in the acidic environment of koozh (pH 4.5). Further, Hung (2004) opines that rice may be considered as a good substrate for a probiotic carrier that enhances survival and stability of the LAB against gastric acid damage. Therefore, koozh made from finger millet and rice may be a unique food matrix that supports the resistance of NSLAB to biological barriers.
The acid tolerance of the eight PPLAB may be understood in terms of the individual strains (Table 2). In the presence of high amount of lactate and proton in the environment, L. plantarum maintains a proton (pH) charge gradient inside and outside the cell, tolerating low pH. The Enterococcus sp. are generally slow acid producers but certain strains of Entercoccus like E. hirae are acid tolerant through maintenance of cytoplasmic pH by amplification of the proton translocating membrane bound ATPase (Papadimitriou et al. 2007).
While seven of PPLAB, were bile resistant, one Enterococcus sp. (Sha 1) was bile tolerant (Table 2). Based on the growth curve of PPLAB in the presence of bile for 24 h, the difference in time between the control and test to reach the stationary phase, considered as Lag time (LT) was variable. An overall 6 h for Enterococcus sp. of Sha 1, Sha 8, Sha 22, Sha 24, Sha 28, Sha 35, 4 and 2 h for Lactobacillus sp., Sha 31 and Sha 7 respectively suggested varied ability to adapt to the bile environment in the intestine.
Bile is much more detrimental than low pH as it has a detergent like effect during intake, but the capacity of PPLAB to survive the bile stress (in vitro) may help them reach the intestine alive in large quantities in order to provide probiotic effect to the host.
The hydrophobicity percentages (H%) of PPLAB were classified according to Riveros et al. (2009) (Table 2). The value of hydrophobicity values provide an estimate of the hydrophobic interaction that may exist between the host intestine and the PPLAB external surface structure, providing greater ability to adhere to epithelial cells of the host.
While five Enterococcus sp. showed a low range from 4–7 %, the remaining showed an intermediate range from 45 ˗ 49 %. The Lactobacillus sp. Sha 7 isolate had comparatively greater H% and therefore might have a greater ability to adhere in the host intestine, due to the protein present for surface anchoring as observed by Kumar et al. (2011), who documented adhesion of L. plantarum strains to human intestinal HT-29 cell line.
The hydrophobic outer surface of bacteria is the first direct physical contact to the host that would provide a probable value of the bacterial adherence to the GIT. The hydrophobicity of half of the PPLAB in this study did not exceed 40 %, suggesting variability in the level of expression of cell surface proteins.
The acidity of CSF of isolates (pH of 4.49–4.58) contributed to zones of inhibition in the range of 2 to 4 mm against ten pathogenic microorganisms (Table 3). The PPLAB NCFS inhibited pathogens to a lesser extent than CFS, indicating that acidity contributed significantly to the antibacterial activity along with hydrogen peroxide and / or antimicrobial compounds produced. The PPLAB NCFS could inhibit 50˗80 % of the pathogens, while the CFS inhibited 10–90 % with three of the eight CFS showing ZI > 2 mm against 10–40 % of the pathogens. No significant difference (p < 0.05) between ZI of CFS and NCFS were noted in all pathogens except M. luteus, E coli, P. vulgaris and S. flexneri . It was interesting to note that CFS and NCFS of Enterococcus sp. (Sha 35 and Sha 26) and Lactobacillus sp. (Sha 31) showed similar antimicrobial activity in terms of number of total pathogens inhibited (Table 3). The CSF and NCSF of NSLAB efficiently inhibited E. coli. While NCFS of all PPLAB inhibited S aureus, K. pneumonia and L. monocytogenes they could not inhibit M. luteus and S. flexneri.
Table 3.
Antimicrobial activity of PPLAB strains from koozh against pathogens
| B. cereus | E. aerogenes | E. coli | K. pneumonia | L. monocytogenes | M. luteus | P. vulgaris | S.aureus | S. flexneri | S. typhi | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | Isolate | Zone of Inhibition (ZI) mm | Total Inhibition % | ||||||||||||||||||||
| CFS | NCFS | CFS | NCFS | CFS | NCFS | CFS | NCFS | CFS | NCFS | CFS | NCFS | CFS | NCFS | CFS | NCFS | CFS | NCFS | CFS | NCFS | CFS b | NCFS c | ||
| Enterococccsu sp. | Sha 1 | 0 | 10 | 0 | 0 | 32 | 15 a | 27 | 10 a | 0 | 12 | 0 | 0 | 0 | 0 | 27 | 12 a | 0 | 0 | 0 | 11 | 30 | 60 |
| Sha 8 | 22 | 0 | 0 | 10 | 23 | 15 a | 0 | 12 | 20 | 12 | 0 | 0 | 0 | 11 | 10 | 15 | 0 | 0 | 0 | 10 | 10 | 70 | |
| Sha 22 | 0 | 0 | 45 | 15 a | 32 | 15 a | 30 | 12 a | 42 | 11 a | 25 | 0 | 25 | 0 | 22 | 14 a | 22 | 0 | 22 | 0 | 90 | 50 | |
| Sha 24 | 0 | 11 | 22 | 13 a | 25 | 16 a | 0 | 12 | 0 | 11 | 20 | 0 | 0 | 0 | 0 | 13 | 25 | 0 | 61 | 11 a | 80 | 70 | |
| Sha 28 | 40 | 11 a | 50 | 19 a | 47 | 14 a | 0 | 12 | 0 | 13 | 19 | 0 | 32 | 0 | 18 | 11 | 15 | 0 | 32 | 10 | 50 | 70 | |
| Sha 35 | 45 | 12 a | 55 | 14 a | 15 | 14 a | 35 | 13 a | 53 | 19 a | 55 | 0 | 52 | 11 a | 12 | 12 | 18 | 0 | 80 | 10 | 80 | 80 | |
| Lactobacillus sp. | Sha 7 | 30 | 11 a | 0 | 20 | 11 | 15 a | 25 | 13 a | 0 | 14 | 27 | 0 | 25 | 11 a | 05 | 15 a | 0 | 0 | 0 | 15 | 40 | 80 |
| Sha 31 | 0 | 10 | 73 | 21 a | 55 | 14 a | 32 | 12 a | 32 | 13 a | 50 | 0 | 42 | 11 a | 32 | 12 a | 16 | 0 | 30 | 0 | 80 | 70 | |
aIndicate a significant difference between CFS and NCFS (p < 0.01)
bPPLAB CFS which produced ZI > 2 mm against indicator strains
cNCFS which produced ZI > 1 mm against indicator strains
The Enteroccocus sp. showed a broad antibacterial spectrum due to the presence of its natural heterologous protein. The PPLAB identified as Enterococcus sp. effectively inhibited the E. aerogenes (MTCC 111) proving the inhibitory potential against taxonomically closely related species. As in the present study, L. plantarum demonstrated antimicrobial activity in vitro and was also found in vivo systems (Kumar et al. 2011) with a wide range of inhibitory spectrum, including decreasing pathogen by trans-epithelial migration (Michail and Abernathy 2003).
The prerequisite for efficient LAB lies in their ability to counteract pathogenic organisms in the human host. The criterion for LAB to function as probiotic on ingestion is to interact with enteric pathogens and antagonise them, aiding in host defense. Lactic acid bacterial fermentation produces inhibitory compounds like organic acid, diacetyl, nitric oxide, hydrogen peroxide and antibacterial proteins. The probable mechanism for antimicrobial action may perhaps be the organic acid present in CFS that could have caused acidification of cytoplasm, collapsing the electrochemical proton gradient eventually altering the lipoteichoic acid (LTA) of the cell membrane, leading to cell disruption. Otherwise, it can also be suggested that certain LAB may be deficient in electron transport chains that cause an incomplete diminution of oxygen to hydrogen peroxide which are accumulated intracellular and released to inhibit other neighboring microbes. These synergistic inhibitory activity, are neutralized with addition of NaOH to the organic acid. Correspondingly the hydrogen peroxide after addition of catalase is converted to water. Certain NCFS had shown more antimicrobial activity than CFS, indicating they are potentially strong bacteriocin producers.
In the current antibiotic study (Table 4) intrinsic resistance to GI and G II antibiotics were identified in Enteroccocus sp. and to G III in L. plantarum, which according to Ahn et al. (1992) is due to the presence of β lactam, cat gene and tet (S) plasmid. LAB antibiotic resistance has been documented in many traditional fermented foods, similar to the commercial products (Temmerman et al. 2003). But resistance of PPLAB to antibiotics may be beneficial if the host is under antibiotic therapy, providing dual protection to the host.
Table 4.
Antibiotic susceptibility profiles of PPLAB from koozh
| Antibiotics | Enterococcus sp. | Lactobacillus sp. | ||||||
|---|---|---|---|---|---|---|---|---|
| Sha 1 | Sha 8 | Sha 22 | Sha 24 | Sha 28 | Sha 35 | Sha 7 | Sha 31 | |
| Group I | ||||||||
| Ampicllin | S | S | S | S | S | S | S | S |
| Amoxyclav | S | S | S | S | S | S | S | S |
| Pencillin G | R | R | MS | R | R | R | R | R |
| Cefuroximin | R | R | R | R | MS | R | R | R |
| Cefixime | R | R | R | R | R | R | R | R |
| Vancocmycin | S | R | R | MS | R | R | S | R |
| Bacitracin | R | MS | R | R | R | R | R | R |
| Group II | ||||||||
| Amikacin | R | R | MS | R | R | R | R | R |
| Gentamycin | R | S | MS | R | R | R | R | R |
| Streptomycin | R | R | R | R | R | R | R | R |
| Azithromycin | S | S | S | S | S | S | S | S |
| Erythromycin | R | R | R | R | R | MS | R | MS |
| Chloramphenicol | S | S | S | S | R | S | S | S |
| Tetracycline | S | S | R | S | S | MS | S | S |
| Group III | ||||||||
| Norofloaxicin | MS | R | R | MS | MS | R | R | R |
| Ciprofloaxicin | S | R | R | R | MS | R | S | R |
| Nalidixic acid | R | R | R | R | R | R | R | R |
| Levoflaxicin | S | S | R | MS | R | R | MS | MS |
| Gemoflaxicin | MS | S | MS | R | MS | R | MS | MS |
| Galtifloaxin | MS | MS | MS | R | S | MS | S | MS |
| Overall Resistance % | 45 | 50 | 55 | 60 | 55 | 65 | 50 | 55 |
R resistant, MS moderately susceptible, S susceptible
The antimicrobial activity of isolates against specific pathogens could help prevent the development of antibiotic resistance strains. The main aim of these PPLAB is to reach the distal ileum and colon in large amounts to facilitate adhesion, colonization and reduction of pathogens in vivo. As most of them have been satisfied in vitro these isolates could be considered as probiotic LAB (PLAB).
Genotyping for identification of bacteria and its geographical distribution
Molecular characterization through isolating ribosomal RNA is an essential taxonomic tool and is an unswerving method for identifying the genera of lactic acid bacteria to species, subspecies and strain levels.
All the eight PLAB strains displaying probiotic characteristics as per FAO and WHO (2006) criteria were partially sequenced for 16S ribosomal DNA and identified as E. hirae - Sha 1, Sha 8, Sha 22, Sha 24, Sha 28; E. faecalis - Sha35; B. amylolquefaciens -Sha 31 and L. plantarum - Sha 7. When compared with other similar genetic sequences, strains were from fermented foods, agriculture, livestock and feces widely distributed in Eurasia (Table 5): 8 from China and India; 4 each from Japan and Spain; 3 in Iran, Korea and Demark; 2 in Thailand; 1 in Indonesia, Taiwan, Serbia, Egypt, South Africa, France and UK.
Table 5.
Molecular identification of PLAB and their sequence analog of geographical distribution
| Isolate no. | Species Identified | Source Code | Query Length bp | Similarity % | Few e.g., of analogous gene code | Query Length bp | Source | Country |
|---|---|---|---|---|---|---|---|---|
| Sha 1 | Enteroccocus hirae | KF040093 | 970 | 98 | KC699174 | 1430 | Ewe bulk tank milk | Spain |
| LC035115 | 1459 | Wild boar- Sus scrofa | Thailand | |||||
| LC027236 | 1657 | Commercial swine feces | Thailand | |||||
| Sha 8 | Enteroccocus hirae | KF040095 | 983 | 95 | LC027236 | 1657 | Commercial swine feces | Thailand |
| LC035114 | 1461 | Wild boar - Sus scrofa | Thailand | |||||
| KF183510 | 1454 | Fish processing waste | India | |||||
| KP662076 | 1535 | Fermented Fresh Pepper | China | |||||
| EU919863 | 1487 | Cock- poultry | China | |||||
| JQ411243 | 1493 | Sicilian Pig breed- Suino Nero Dei Nebrodi | Italy | |||||
| EU722743 | 1477 | Human Feces | UK | |||||
| FN822766 | 1478 | Leaf of northern bedstraw | Finland | |||||
| KF040098 | 1421 | Ewe bulk tank milk | Spain | |||||
| Sha 22 | Enteroccocus hirae | KF040096 | 993 | 95 | KC699174 | 1430 | Ewe bulk tank milk | Spain |
| Sha24 | Enteroccocus hirae | KF040097 | 1015 | 95 | KC699174 | 1425 | Ewe bulk tank milk | Spain |
| Sha 28 | Enteroccocus hirae | KF040098 | 1001 | 94 | KC699166 | 1430 | Ewe bulk tank milk | Spain |
| Sha 35 | Enteroccocus faecalis | KF040099 | 981 | No match found at species level | ||||
| Sha 31 | Bacillus amyloliquefaciens | KF386011 | 439 | 99 | KF702296 | 885 | Corn sheath of Saffron | India |
| KF018921 | 964 | Fish | India | |||||
| KJ567097 | 1517 | Ginger rhizosphere soil | India | |||||
| KM853035 | 1429 | Tea phylloplane | India | |||||
| KM406427 | 779 | Lemon | India | |||||
| KP973969 | 1377 | Rhizosphere of Perenial herb Mimosa sp. | Indonesia | |||||
| KJ716497 | 1024 | Edible mushroom- Agaricus bisporus | China | |||||
| KP261025 | 1020 | Moldy corn | Taiwan | |||||
| KM922581 | 1359 | Fermented soybean paste | Korea | |||||
| KP273195 | 901 | Baker’s yeast | Egypt | |||||
| Sha 7 | Lactobacillus plantarum | KF040094 | 1004 | 93 | KJ802485 | 1539 | Infant stool sample | India |
| JX183220 | 1509 | Goat Milk and Cow | India | |||||
| CP004082 | 3203964 | Healthy newborn fecal | China | |||||
| KR149356 | 1489 | ZhenJiang aromatic vinegar- Fumigated vinegar | China | |||||
| KJ764641 | 1521 | Kefir | China | |||||
| KM005165 | 1489 | Silage | China | |||||
| KC166237 | 1493 | Tibetan kefir grains | China | |||||
| KF583521 | 1492 | Intestine of Rainbow trout-salmon fish | China | |||||
| LC042605 | 1492 | Pickle vegetable | Japan | |||||
| AB601179 | 1525 | Italian rye pasture | Japan | |||||
| AB713901 | 1489 | Mixed pasture of Timothy and orchard grass | Japan | |||||
| AB819501 | 1489 | Intestinal mucosal content of bivalvia | Japan | |||||
| KM670024 | 1493 | Vagina | Korea | |||||
| KM670021 | 1499 | Oyster | Korea | |||||
| KC113208 | 1472 | Traditional Salted Crab Poo-Khem | Thailand | |||||
| KM495889 | 1499 | Siahmazgi cheese | Iran | |||||
| KP090130 | 1452 | Vaginal tract of healthy woman | Iran | |||||
| KF472174 | 1527 | Traditional dairy product | Iran | |||||
| HE962114 | 1315 | Travnik young cheeses, sweet creams and sweet kajmaks | Serbia | |||||
| JX409637 | 984 | Piglet feces | Denmark | |||||
| JX409633 | 970 | Fermented liquid feed | Denmark | |||||
| JX409627 | 978 | Unsupplemented whey permeate silage | Denmark | |||||
| KC351898 | 1555 | Wine made up of grillo grapes | Italy | |||||
| JX426120 | 1502 | Natural wine | Italy | |||||
| KC416990 | 1491 | Wheat flour | Italy | |||||
| JX025073 | 1559 | Wine of the Rhone Valley | France | |||||
| JX968494 | 1548 | Barley malt | South Africa |
Characteristics and storage effects of encapsulated PLAB
The SDP and FDP containing encapsulated LAB had low moisture content of 6 ± 1 %, contributing to storage stability. The colour of SDP was found to be very similar to skim milk powder but the FDP had darkened colour due to the prolonged duration of drying. SEM analysis (Fig. 1a) indicated larger particle size in FDP compared to the SDP due to the coalescence of water and particle bridging.
Fig. 1.
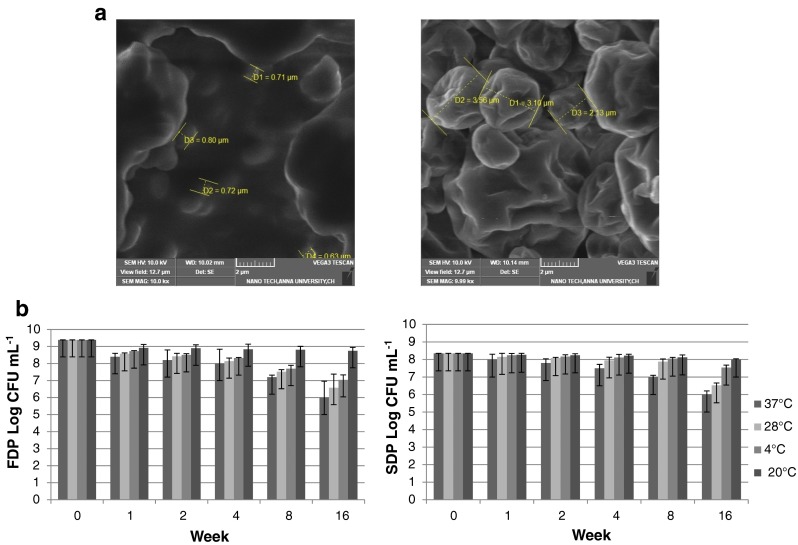
FDP and SDP of Sha 7- L. plantarum a SEM analysis and b Storage
Rehydration is an important step in dried powders for assessing the final probiotic product that also depends on temperature of the solution. Rehydration in a slow manner would decrease the osmotic shock. The main effect of spray drying is to increase the solubility of the mixture, to decrease clogging in the spray nozzle, eventually increasing the survival rate greater than control. Encapsulating materials provide resistance against damage to the PLAB, preventing significant drying effects while reducing structural and physiological damage to the cells.
Storage temperature is an important parameter for cell survival. Only a 2 log reduction was observed when spray dried L. plantarum was stored for 16 weeks at 27 °C. Storage survival of all PLAB isolates was better at 4 °C and at −20 °C at the end of 16 weeks than ambient temperatures (27 °C, 37 °C) (Fig. 1b). The decrease may be attributed to disruption membrane and cell deactivation dependent final moisture concentration.
The freeze dried L. plantarum as in the case of spray dried was stable with good survival after 16 weeks storage at low temperature (4 °C, −20 °C) compared to ambient (Fig. 1b). Survival of freeze dried PLAB isolates was poorer compared to spray dried isolates, due to the week long preparation compared to SDP. But for starter probiotic cultures freeze dried strains are preferred due to storage stability.
In our study Enterococcus sp. counts declined rapidly than the Lactobacillus sp., however Ivanova et al. (1998) observed that the inhibitory activity of Streptococcus at 4 °C was not affected for more than 2 months and in frozen state was not influenced for 6 months. Following spray drying, the lipid oxidation during storage changes lipid cell membrane composition. The limitation of drying process was the loss of viability during its storage even though no spectacular losses were seen during processing. The composition of the encapsulated powder, oxygen content and glass transition temperature have an important effect on the survival of the dried powder. It is to be noted that medium used for encapsulating probiotics for food product preparation would not provide precise protection during storage of microbial cells. The probiotic storage life could be increased by the use of better encapsulating agent, suitable packaging material and vacuum packaging.
Viability of PLAB after encapsulation
During spray drying and freeze drying (Table 6) LAB counts reduced from 109–1010 CFU mL−1 to 107–108 CFU mL−1 suggesting good retention of viability after encapsulation. The SDP resulted in 0.10 ± 0.06 log reduction with overall survival of 98 % (significant difference, p < 0.05). Similarly FDP had a log reduction of 0.9 ± 0.3 with overall survival of 91 % (significant difference, p < 0.05). Survival of strains was better (p < 0.05), after spray drying (0.10 ± 0.06 log reduction, survival of 98 %) compared to freeze drying (0.9 ± 0.3 log reduction, survival of 91 %), due to homogenous film formation, emulsification and product formation in less time. But freeze drying may help to retain biological properties of foods, e.g., fermented freeze dried soy milk had relatively lower loss of antioxidant activities than the spray dried product (Rivera-Espinoza and Gallardo-Navarro 2010). Spray-dried starter cultures cannot be used for direct inoculation in dairy fermentation due to increased lag phase before the onset of growth (Boza et al. 2004). L. plantarum showed good survival after spray drying at 160 °C, 3 bars psi (Golowczyc et al. 2011). Rice soyabean gruel fermented with L. plantarum when spray dried produced a functional starchy food (Nguyen et al. 2007). L. plantarum when lyophilized with alginate, fermented meat faster than unencapsulated cells (Kearney et al. 1990). E. faecalis demonstrated heat and oxidative tolerance during spray drying (Santivarangkna et al. 2007).
Table 6.
Viability of PLAB isolates before- after freeze drying (FDP) and spray drying (SDP)
| Strain | Freeze Drying | FDP log | Spray Drying | SDP log | |||
|---|---|---|---|---|---|---|---|
| Isolate | log CFU mL−1 | Reduction | log CFU mL−1 | Reduction | |||
| Before | After | Before | After | ||||
| E. hirae | Sha1 | 8.97 ± 0.03 | 8.58 ± 0.14 | 9.73 ± 0.20 | 9.63 ± 0.15 | 9.63 ± 0.15 | 0.10 ± 0.12 |
| Sha8 | 9.12 ± 0.20 | 8.59 ± 0.15 | 9.03 ± 0.03 | 8.85 ± 0.14 | 8.85 ± 0.14 | 0.18 ± 0.17 | |
| Sha22 | 9.34 ± 0.05 | 8.67 ± 0.10 | 9.87 ± 0.08 | 9.85 ± 0.03 | 9.85 ± 0.03 | 0.02 ± 0.10 | |
| Sha24 | 10.29 ± 0.08 | 9.30 ± 0.33 | 9.43 ± 0.03 | 9.39 ± 0.20 | 9.39 ± 0.20 | 0.04 ± 0.24 | |
| Sha28 | 10.38 ± 0.08 | 9.19 ± 0.03 | 9.36 ± 0.05 | 9.20 ± 0.10 | 9.20 ± 0.10 | 0.16 ± 0.16 | |
| E. faecalis | Sha35 | 10.41 ± 0.12 | 9.04 ± 0.05 | 9.66 ± 0.00 | 9.47 ± 0.17 | 9.47 ± 0.17 | 0.19 ± 0.17 |
| L. plantarum | Sha7 | 9.00 ± 0.00 | 8.35 ± 0.17 | 9.54 ± 0.12 | 9.39 ± 0.05 | 9.39 ± 0.05 | 0.15 ± 0.17 |
| B. amyloliquefacians | Sha31 | 10.27 ± 0.03 | 9.18 ± 0.2 | 9.30 ± 0.08 | 9.17 ± 0.33 | 9.17 ± 0.33 | 0.13 ± 0.17 |
Survival of encapsulated PLAB in simulated fluid
All freeze dried PLAB (FDP) were tolerant to gastric conditions (pH 2.0, pepsin). These isolates showed little change after 2 h in SGF, although a slight decrease was recorded in the last 30 min (Fig. 2a). Survival of encapsulated PLAB in gastric simulation was better when spray dried than freeze dried (Fig. 2a). Encapsulated L. plantarum Sha7 survived better compared to the rest of the strains.
Fig. 2.
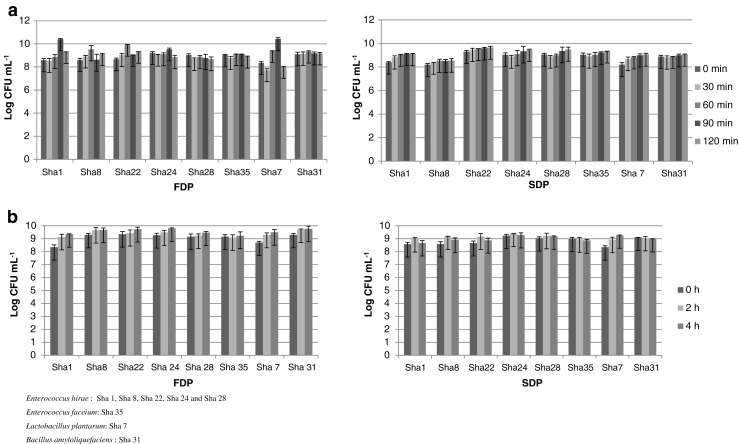
FDP and SDP survival of Sha 7- L. plantarum a SGF and b SIF
Encapsulated PLAB obtained from spray drying and freeze drying showed significant rise in cell count in SIF up to 2 h due to the protective coating of skim milk that supports growth (Fig. 2b). SDP and FDP had thrived in simulated fluids (skim milk). Our SIF results were similar to Teixeira et al. (1994) where there was no significant difference in the viability of spray dried and freeze dried isolates of L. bulgaricus.
In the GIT and food products the viability and survivability of probiotics decreases due to exposure to environmental factors: organic acids, hydrogen ions, molecular oxygen and antibacterial components (Mortazavian et al. 2007).
Spray drying also resulted in isolates with tolerance to both gastric pH and enzyme with excellent survival in the second hour. L. plantarum isolated from kefir grains and encapsulated by spray drying did not lose its viability and acidifying activity or its capacity to adhere to intestinal walls (Golowczyc et al. 2011). In all the PLAB isolates cell numbers per gram of the spray/ freeze dried powder was 108 to 109 cells, which is above the recommended probiotic dosage of 107–108 CFU g−1 laid down by FAO and WHO (2002) indicating their potential for application in food products.
Conclusion
Thus koozh a traditional millet fermented food is a source of a diverse group of NSLAB, largely strains of Leuconostoc sp., Enterococcus sp. and Lactobacillus sp. Further, eight PLAB strains among the 69 isolates, were acid and bile resistant with antimicrobial activity in vitro demonstrating their probiotic nature. As a value added product SDP performed better than FDP in simulated gastric and intestinal fluids and was stable for 16 weeks when stored at low temperature. They may be used as starter cultures in cereal based or other fermented foods and/or as a source of bacteriocins. In vivo evaluation can identify their specific application in promoting/protecting health of consumers. The outcome of this study underscores the need to modernize and commercialize traditional fermentation processes on a scientific basis. This will ensure the quality and safety of such foods and enable consumers to derive the health benefits.
Acknowledgments
This work was supported by the University Grants Commission-UGC, Rajiv Gandhi National Fellowship No.F.14-2 (SC) /2009 (SA-III) (www.ugc.ac.in/rgnf/), 2009 a doctoral studies grant, provided by the Government of India, Ministry of Social Justice and Empowerment, and Ministry of Tribal Affairs.
Contributor Information
Shankar Ilango, Email: shankilano@gmail.com.
Ruby Pandey, Email: ruby.ibt.smvdu@gmail.com.
Usha Antony, Phone: +91 44 22358379, Email: ushaantony@annauniv.edu, Email: usha.antony@gmail.com.
References
- Abegaz K. Isolation, characterization and identification of lactic acid bacteria involved in traditional fermentation of borde, an Ethiopian cereal beverage. Afr J Biotechnol. 2007;6:1469–1478. [Google Scholar]
- Ahn C, Collins-Thompson D, Duncan C, Stiles ME. Mobilization and location of the genetic determinant of chloramphenicol resistance from Lactobacillus plantarum caTC2R. Plasmid. 1992;27:169–176. doi: 10.1016/0147-619X(92)90018-6. [DOI] [PubMed] [Google Scholar]
- Ampe F, Omar NB, Moizan C, Wacher C, Guyot JP. Polyphasic study of the spatial distribution of microorganisms in Mexican pozol, a fermented maize dough, demonstrates the need for cultivation-independent methods to investigate traditional fermentations. Appl Environ Microbiol. 1999;65:5464–5473. doi: 10.1128/aem.65.12.5464-5473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anal AK, Singh H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci Technol. 2007;18:240–251. doi: 10.1016/j.tifs.2007.01.004. [DOI] [Google Scholar]
- Borza AD, Annan NT, Moreau DL, Allan-wojtas PM, Paulson AT, Hansen LT. Microencapsulation in genipin cross-linked gelatine-maltodextrin improves survival of Bifidobacterium adolescentis during exposure to in vitro gastrointestinal conditions. J Microencapsul. 2010;27:387–399. doi: 10.3109/02652040903367293. [DOI] [PubMed] [Google Scholar]
- Boza Y, Barbin D, Scamparini ARP. Effect of spray- drying on the quality of encapsulated cells of Beijerinckia sp. Process Biochem. 2004;39:1275–1284. doi: 10.1016/j.procbio.2003.06.002. [DOI] [Google Scholar]
- Charteris WP, Kelly PM, Morelli L, Collins JK. Antibiotic susceptibility of potentially probiotic Lactobacillus species. J Food Prot. 1998;61:1636–1643. doi: 10.4315/0362-028x-61.12.1636. [DOI] [PubMed] [Google Scholar]
- Chateau N, Deschamps AM, Hadj Sassi J. Heterogeneity of bile salts resistance in the Lactobacillus isolates of a probiotic consortium. Lett Appl Microbiol. 1994;18:42–44. doi: 10.1111/j.1472-765X.1994.tb00796.x. [DOI] [Google Scholar]
- Doyle RJ, Rosenberg M. Measurement of microbial adhesion to hydrophobic substrata. Methods Enzymol. 1995;253:542–550. doi: 10.1016/S0076-6879(95)53046-0. [DOI] [PubMed] [Google Scholar]
- FAO, WHO Report (2002) Guidelines for the evaluation of probiotics in food. Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food. London, Ont, Canada. ftp://ftp.fao.org/es/esn/food/wgreport2.pdf. Accessed 4 Nov 2015
- FAO, WHO Report (2006) Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. http://www.fao.org/documents/pub_dett.asp?lang=en&pub_id=61756. Accessed 4 Nov 2015
- Geetha T, Kalaichelvan GA. Study on the fermentation pattern of common millets in koozh preparation—a traditional South Indian food. Indian J Tradit Knowl. 2013;12:512–517. [Google Scholar]
- Golowczyc MA, Silva J, Teixeira P, De Antoni GL, Abraham AG. Cellular injuries of spray-dried Lactobacillus spp. isolated from kefir and their impact on probiotic properties. Int J Food Microbiol. 2011;144:556–560. doi: 10.1016/j.ijfoodmicro.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Hung CL (2004) Health food processing process using germinated rice to make health food containing natural eatable fibers, GABA, IP6, and Probiotic, US Patent: US 2004/0191392 A1
- Hyronimus B, Le Marrec C, Hadj Sassi A, Deschamps A. Acid and bile tolerance of spore-forming lactic acid bacteria. Int J Food Microbiol. 2000;61:193–197. doi: 10.1016/S0168-1605(00)00366-4. [DOI] [PubMed] [Google Scholar]
- Ilango S, Antony U. Assessment of the microbiological quality of koozh, a fermented millet beverage. Afr J Microbiol Res. 2014;8:308–312. doi: 10.5897/AJMR2013.6482. [DOI] [Google Scholar]
- Ivanova I, Miteva V, Pantev A, Budakov I, Danova S, Moncheva P, Nikolova I, Dousset X, Boyaval P. Characterization of a bacteriocin produced by Streptococcus thermophilus 81. Int J Food Microbiol. 1998;42:147–158. doi: 10.1016/S0168-1605(98)00067-1. [DOI] [PubMed] [Google Scholar]
- Kearney L, Upton M, McLoughlin A. Meat fermentations with immobilized lactic acid bacteria. App Microbiol Biotechnol. 1990;33:648–651. doi: 10.1007/BF00604931. [DOI] [Google Scholar]
- Kumar RS, Varman DR, Kanmani P, Yuvaraj N, Paari KA, Pattukumar V, Arul V. Isolation, characterization and identification of a potential probiont from south Indian fermented foods (Kallappam, Koozh and Mor Kuzhambu) and its use as biopreservative. Probiotics Antimicro Prot. 2010;2:145–151. doi: 10.1007/s12602-010-9052-5. [DOI] [PubMed] [Google Scholar]
- Kumar H, Rangrez AY, Dayananda KM, Atre AN, Patole MS, Shouche YS. Lactobacillus plantarum (VR1) isolated from an ayurvedic medicine (Kutajarista) ameliorates in vitro cellular damage caused by Aeromonas veronii. BMC Microbiol. 2011;11:152. doi: 10.1186/1471-2180-11-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. Chichester: Wiley; 1991. pp. 125–175. [Google Scholar]
- Michail S, Abernathy F. Lactobacillus plantarum inhibits the intestinal transepithelial migration of neutrophils induced by enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 2003;36:385–391. doi: 10.1097/00005176-200303000-00017. [DOI] [PubMed] [Google Scholar]
- Mohammed SI, Steenson LR, Kirleis AW. Isolation and characterization of microorganisms associated with the traditional sorghum fermentation for production of Sudanese kisra. Appl Environ Microbiol. 1991;57:2529–2533. doi: 10.1128/aem.57.9.2529-2533.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavian A, Razavi SH, Ehsani MR, Sohrabvandi S. Principles and methods of microencapsulation of probiotic microorganisms. Iran J Biotechnol. 2007;5:1–18. [Google Scholar]
- Nguyen TTT, Guyot JP, Icard-Vernière C, Rochette I, Loiseau G. Effect of high pressure homogenisation on the capacity of Lactobacillus plantarum A6 to ferment rice/soybean slurries to prepare high energy density complementary food. Food Chem. 2007;102:1288–1295. doi: 10.1016/j.foodchem.2006.07.020. [DOI] [Google Scholar]
- Papadimitriou CG, Vafopoulou-Mastrojiannaki A, Silva SV, Gomes AM, Malcata FX, Alichanidis E. Identification of peptides in traditional and probiotic sheep milk yoghurt with angiotensin I-converting enzyme (ACE)—inhibitory activity. Food Chem. 2007;105:647–656. doi: 10.1016/j.foodchem.2007.04.028. [DOI] [Google Scholar]
- Rivera-Espinoza Y, Gallardo-Navarro Y. Non-dairy probiotic products. Food Microbiol. 2010;27:1–11. doi: 10.1016/j.fm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Riveros B, Ferrer J, Borquez R. Spray drying of a vaginal probiotic strain of Lactobacillus acidophilus. Dry Technol. 2009;27:123–132. doi: 10.1080/07373930802566002. [DOI] [Google Scholar]
- Santivarangkna C, Kulozik U, Foerst P. Alternative drying processes for the industrial preservation of lactic acid starter cultures. Biotechnol Prog. 2007;23:302–315. doi: 10.1021/bp060268f. [DOI] [PubMed] [Google Scholar]
- Schillinger U, Lücke F. Identification of Lactobacilli from meat and meat products. Food Microbiol. 1987;4:199–208. doi: 10.1016/0740-0020(87)90002-5. [DOI] [Google Scholar]
- Schillinger U, Lucke F. Antibacterial activity of Lactobacillus sake isolated from meat. Appl Environ Microbiol. 1989;55:1901–1906. doi: 10.1128/aem.55.8.1901-1906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sujaya IN, Amachi S, Yokota A, Asano K, Tomita F. Identification and characterization of lactic acid bacteria in ragi tape. World J Microb Biotechnol. 2001;17:349–357. doi: 10.1023/A:1016642315022. [DOI] [Google Scholar]
- Tamang JP (2010) Himalayan fermented foods: microbiology, nutrition and ethinic values. Taylor and Francis Group, CRC Press, London
- Teixeira P, Castro H, Kirby R. Spray drying as a method for preparing concentrated cultures of Lactobacillus bulgaricus. J Appl Bacteriol. 1994;78(4):456–462. doi: 10.1111/j.1365-2672.1995.tb03433.x. [DOI] [Google Scholar]
- Temmerman R, Pot B, Huys G, Swings J. Identification and antibiotic susceptibility of bacterial isolates from probiotic products. Int J Food Microbiol. 2003;81:1–10. doi: 10.1016/S0168-1605(02)00162-9. [DOI] [PubMed] [Google Scholar]
- Yonzan H, Tamang JP. Microbiology and nutritional value of selroti, an ethnic fermented cereal food of the Himalayas. Food Biotechnol. 2010;24:227–247. doi: 10.1080/08905436.2010.507133. [DOI] [Google Scholar]


