Abstract
Recently, the fingerprint approach using chromatography has become one of the most effective tools for quality assessment of herbal medicines and food supplements: due to the complexity of the chromatographic fingerprint and the irreproducibility of chromatographic instruments and experimental conditions, chemometric approach is employed to deal with the chromatographic fingerprint. The study was aimed at developing new analytical methods for the multivariate phytochemical fingerprinting of bioactive compounds in eight tree-species bud-preparations, commonly used in phytotherapy. Methods was used to identify and quantify the main bioactive compounds (polyphenols, organic acids and vitamins), and obtain a specific botanical profile in order to assess the contribution of each single bioactive class to the total bud preparation phytocomplex. A chemometric approach was used to distinguish among different genotypes assuring the identity, safety and quality of the botanical raw materials. The established protocol was simple, sensitive and reliable and it could be used for the evaluation and quality control of bud-extracts and natural food supplements: the proposed method was successfully applied to the characterization of commercial bud-preparations, demonstrating to be an effective tool for the fingerprinting of this plant material. The new approach developed in this study represents a good alternative for improving the classification results of herbal materials with complex chromatograms. It should be necessary to develop a “multivariate chromatographic fingerprint”, in order to differentiate the herbal preparations according to their genotype, avoiding substitutions, changes or adulterations with other species or synthetic drugs.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-015-2115-6) contains supplementary material, which is available to authorized users.
Keywords: Multivariate fingerprinting, Natural food-product, Raw material control, HPLC
Introduction
Herbal medicines (HM) and their preparations have been widely used for hundreds of years all over the world. They are all presented either as single herbs or as a combination of several herbs in composite formulae, and historically extracted with boiling water during the decoction process (Gad et al. 2013). Phytotherapy is the study of natural extracts used as health-promoting agents for medical care; herbal products are often called botanical food supplements, and there are different forms, depending on the used plant parts; this research focuses on bud-preparations, derived from meristematic fresh plant tissues (buds, sprouts) (Donno et al. 2012). Herbals are as old as human civilization and they have provided a complete storehouse of remedies to cure acute and chronic diseases. The knowledge of herbals has accumulated over thousands of years so that today there are many effective means of ensuring health care. Different bioactive compounds (botanicals) are present in medicinal herbs and derived-products as key components (Prabu et al. 2012): phenolic compounds, terpenoids, sulfur compounds, vitamins, pigments, and other natural antioxidants have been associated with protection from and/or treatment of conditions as cardiovascular disease and cancer (Canterino et al. 2012; Donno et al. 2014b). All these secondary metabolites are known to be quite variable in the plant material, according to intra-specific chemodiversity, different collection stages or cultivation zone, and post-harvest handling (Prencipe et al. 2014).
As pointed out in the ‘General Guidelines for Methodologies on Research and Evaluation of Traditional Medicines’, that despite the existence and continued use of HM over many centuries, and its popularity and extensive use during the past decade, traditional medicine has not been officially recognized in most countries, but it shows an increasing acceptance by consumers and medical professionals that pushed world demand for herbal extracts up to 7.5 % annually to US $ 1.95 billion in 2012 (Prabu et al. 2012). The more detailed regulations on botanicals, phytotherapy, or nutritional therapy are being worked out through consultations with expert panels that can provide descriptions of regulatory hurdles for these products and practices, Good Manufacturing Practice (GMP) compliance, generally recognized as safe (GRAS) status, analytical methods and protocol validation (Silano et al. 2011).
Nowadays, medicinal herbs have gained popularity in many countries: a great deal of current research is focused on traditional herbal extracts. Investigators are examining claims linking these extracts with health enhancement and prevention of chronic diseases. At least in part, these studies represent an effort to legitimize homeopathic remedies and Eastern medicine. Additionally, it seeks to provide patients and physicians with much-needed safety and efficacy data (Prabu et al. 2012). This major expansion in the use of herbal medicines raises many concerns about quality control of plant material and derived-preparations. Unfortunately, education, training and research in this area have not been accorded due attention and support: the data reported on traditional medicine are far from sufficient to meet the criteria needed to support its use worldwide (Donno et al. 2013b). The lack of quality control is a major area of concern for botanicals: the quality of plant material and manufacturing processes used for phytochemicals are regulated by food laws, which lack the specificity required for botanical drugs (Silano et al. 2011). It could have serious consequences: contamination, for instance, with toxins after fungal infection of raw plant material or with other ingredients has been repeatedly reported and can have potential fatal consequences (Halt 1998). Adulterations and different other kinds of impurity of different compounds conceivably remain undetected simply because there is an almost total absence of specific quality control. Absence of quality control not only increases the risk to the consumer, it also results in a total lack of impetus to conduct adequate research that demonstrates the potential benefits of botanicals or ensures their safety (Ernst 2001).
The reasons for the lack of research data are not only national health care policies, but also a lack of adequate or accepted research methodology for evaluating traditional medicine (Liang et al. 2004). However, assuring the quality of a herbal preparation is one of the main challenge in the phyto-pharmaceutical and food industries, because the herb chemical content ranges greatly according to a wide range of factors as species variation (genotype), growth location, climate, harvesting season, storage conditions and processing (Donno et al. 2014a). A growing consumer market for botanical supplements has surpassed the availability of reliable analytical methods to verify botanical identify, purity and strength. The lack of publicly available validated methods makes it difficult to assess product quality, both composition and stability, and has stymied scientific research on these products (Brown and Lister 2014). This need for validated methods is further driven by laws that require publicly available methods to enforce legal action against food supplements: initiatives have been taken in response to the need for validated analytical methods for botanical supplements. These actions involve collaborations between government, industry and private scientific organizations where scientists and industry members have been working to develop and validate standard analytical methods for dietary supplements. In order to deal with the problems related to the herbal extract chemical complexity, the researchers adopted the herbal fingerprinting approach: the chromatographic fingerprint of a herbal material is a feature obtained by a defined procedure, separating as many compounds as possible to construct a specific pattern of recognition (Fan et al. 2006). Since the entire pattern of compounds (phytocomplex) characterizes the chemical composition of the herbal drug, the chromatographic fingerprint represents a comprehensive qualitative methodology, in which the entire chromatograms were evaluated during data analysis to discriminate among different genotypes (Feng et al. 2014). Furthermore, updated regulations on herbal medicines from the World Health Organization (WHO), the US Food and Drug Administration (FDA), the State Food and Drug Administration (SFDA) of China, and European Medicines Agency (EMA), refer to the use of fingerprints (Kong et al. 2009). As the overall herbal preparation quality and properties derive from the synergistic and simultaneous intervention of the whole phytocomplex, a comprehensive and accurate fingerprinting is valuable to properly evaluate raw material for both commercial (quality grading), technological (evaluation of their presence during industrial processing), agronomic (breeding of enhanced cultivars) and ecological reasons (evaluation of wild genotypes) (Prencipe et al. 2014): as opposed to the present research, many of the existing methods applied to plant material are focused on a single class of compounds and do not provide at the same time a rapid, validated and complete fingerprinting method for medicinal plants and derived-products.
There are many reports about fingerprint techniques to address the identity and quality of botanicals, which are mainly chromatographic analysis, including high performance liquid chromatography (HPLC) (Donno et al. 2013c), gas chromatography (GC) (Pan et al. 2011), ultra performance liquid chromatography (UPLC) (Dan et al. 2009), and capillary electrophoresis (CE) (Zhang and Cheung 2011). Spectroscopy methods are also applied to gain fingerprints (Boggia et al. 2013); however, the acquisition of a fingerprint and quantitative analysis by these methods is a rigorous operation as it generally needed about one or more hour for a single run (Kong et al. 2009). Among the analytical methods for herbal drug fingerprinting, high performance liquid chromatography, hyphenated to UV – visible diode array detector (HPLC-DAD), is still the most popular to be applied in order to ensure product quality and for discriminating related genotypes or adulterated samples (Donno et al. 2015).
These techniques are generally powerful and can provide a wealth of information on complex samples. However, in some cases, the limited information provided by conventional fingerprint may not be enough to reveal the quality characteristics of some extremely complex herbal products, comprehensively (Peng et al. 2011): although it is possible to visually differentiate the different chromatograms, however, the process is subjective and not quantitative. In addition, the fingerprint chromatograms are complex multivariate data sets due to the complexity of herbal medicines, so minor differences between very similar chromatograms might be missed (Zhu et al. 2014). When the compositions of the herbal medicine are too complex, e.g. multi-herb botanical drug products, simple HPLC fingerprint is inadequate to represent all chemical patterns or characteristics. At the same time, data multivariate processing techniques are applied in order to eliminate or reduce unwanted sources of variations due to different variables or instrumental responses from modern analytical techniques and to obtain more effective data from which meaningful information can be extracted (Gad et al. 2013). For this reason, analytical fingerprint should be coupled to a multivariate analysis. Principal Component Analysis (PCA), an unsupervised pattern recognition technique, for data exploration, is able to extract and to rationalize the analytical information of the considered herbal preparations (Wang et al. 2014): in this way, chromatographic fingerprinting shows potential to determine the identity, authenticity, and lot-to-lot consistency of herbal medicines. Recently developed chromatographic instruments and chemometric resolution methods provide powerful tools to resolve the overlapping peaks of a complex system (Hakimzadeh et al. 2014).
In the light of all the above, this study was aimed at optimizing and validating specific HPLC-DAD methods, for the fingerprinting of eight tree-species bud-preparations, commonly used in phytotherapy. Methods were used to identify and quantify the main bioactive compounds (polyphenols, organic acids and vitamins), and obtain a specific phytochemical profile in order to assess the contribution of each single bioactive class to the total bud preparation phytocomplex. In order to compare the HPLC fingerprints among different species, PCA was applied to classify the samples according to their genotype. The fingerprint – chemometrics system was also successfully applied to the characterization of commercial bud-preparations from three different companies: the combination of enhanced fingerprinting analysis and chemometric methods could provide a powerful and meaningful tool to comprehensively conduct the quality control for herbal preparations in the future.
Materials and methods
Plant material
University lab preparations and commercial preparations were evaluated. All the buds for the University lab extracts were picked and collected in 2014 (March–May period) at bud breaking in three germplasm repositories in Turin Province (Italy). The following species were analyzed: Castanea sativa Mill., Corylus avellana L., Tilia x vulgaris Hayne, Juglans regia L., Salix caprea L., Ribes nigrum L., Rubus idaeus L. e Rubus ulmifolius L. Per sample and replication 50 buds were collected. The collected buds were used fresh to manually produce herbal preparations in the lab and these extracts were labelled with a code (Table 1).
Table 1.
Genotype, provenience and identification code of the analyzed bud-preparations
| Species | Provenience | Sample name |
|---|---|---|
| Tilia x vulgaris Hayne | University | Linden |
| Corylus avellana L. | University | Hazelnut |
| Juglans regia L. | University | Walnut |
| Castanea sativa Mill. | University | Chestnut |
| Ribes nigrum L. | University | Blackcurrant |
| Rubus ulmifolius L. | University | Blackberry |
| Rubus idaeus L. | University | Raspberry |
| Salix caprea L. | University | Willow |
| Tilia x vulgaris Hayne | Company 1 | Linden_C1 |
| Tilia x vulgaris Hayne | Company 2 | Linden_C2 |
| Tilia x vulgaris Hayne | Company 3 | Linden_C3 |
| Corylus avellana L. | Company 1 | Hazelnut_C1 |
| Corylus avellana L. | Company 2 | Hazelnut_C2 |
| Corylus avellana L. | Company 3 | Hazelnut_C3 |
| Juglans regia L. | Company 1 | Walnut_C1 |
| Juglans regia L. | Company 2 | Walnut_C2 |
| Juglans regia L. | Company 3 | Walnut_C3 |
| Castanea sativa Mill. | Company 1 | Chestnut_C1 |
| Castanea sativa Mill. | Company 2 | Chestnut_C2 |
| Castanea sativa Mill. | Company 3 | Chestnut_C3 |
| Ribes nigrum L. | Company 1 | Blackcurrant_C1 |
| Ribes nigrum L. | Company 2 | Blackcurrant_C2 |
| Ribes nigrum L. | Company 3 | Blackcurrant_C3 |
Commercial products (Castanea sativa Mill., Corylus avellana L., Tilia x vulgaris Hayne, Juglans regia L., Ribes nigrum L.) from three different Italian herbal companies were also considered: the companies are located in San Gregorio di Catania (Catania Province, Company 1), Predappio (Forlì-Cesena Province, Company 2), and Binasco (Milano Province, Company 3). Commercial preparations were also labeled with a code (Table 1).
Solvents and chemicals
Ethanol, hydrochloric acid, formic acid and all the standards of organic acids were purchased from Fluka Biochemika (Buchs, Switzerland). Analytic HPLC grade acetonitrile, methanol, glycerol, all the polyphenolic standards, potassium dihydrogen phosphate, 1,2-phenylenediamine dihydrochloride (OPDA) and phosphoric acid were purchased from Sigma Aldrich. Milli – Q ultrapure water was produced by using Sartorius Stedium Biotech mod. Arium (Sartorius, Goettingen, Germany).
Cetyltrimethylammonium bromide (cetrimide), ascorbic and dehydroascorbic acids were purchased from Extrasynthése (Genay, France).
Sample preparation protocols
The extraction solution was prepared based on the protocol of bud-preparations detailed in the monograph “Homeopathic preparations”, quoted in the French Pharmacopoeia, 8th edition, 1965 (Pharmaciens 1965). The mother bud extracts were prepared using one part of the fresh material (calculated as dried weight) in 20 parts of glycerol-ethanol solution (1:1 ratio).
Bioactive compounds were extracted through a cold maceration process for 21 days, in a solution of ethanol (95 %) and glycerol, followed by a first filtration (Whatman Filter Paper, Hardened Ashless Circles, 185 mm Ø), a manual pressing and, after 2 days of decanting, a second filtration (Whatman Filter Paper, Hardened Ashless Circles, 185 mm Ø). Macerated samples were prepared in the analytical laboratory of the University of Turin (DISAFA) in Italy.
Macerated preparations were filtered with circular pre-injection filters (0.45 μm, polytetrafluoroethylene membrane, PTFE) and then stored for a few days at N.A., 4 °C and 95 % R.H until analysis. All samples were analyzed as such without dilution. For vitamin C analysis, 250 μl of OPDA solution (18.8 mmol/L) was added to 750 μl of extracted samples for dehydroascorbic acid derivatization into the fluorophore 3-(1,2-dihydroxyethyl)furo(3,4-b)quinoxalina-1-one (DFQ). After 37 min in the dark the samples were analyzed with a High Performance Liquid Chromatograph (HPLC) coupled to a diode array detector (DAD) (Donno et al. 2013a).
Standard preparation
The stock standard solution of each compound was prepared as follows: an accurately weighed amount of reference standard was placed into a volumetric flask; then solvent was added and the solution was diluted to volume.
Stock solutions of ascorbic and dehydroascorbic acids, cinnamic acids, and flavonols with a concentration of 1.0 mg·mL−1 were prepared in methanol: four calibration standards were prepared by dilution with methanol, while stock solutions of benzoic acids and catechins with a concentration of 1.0 mg·mL−1 were prepared in 95 % methanol and 5 % water for a better solubility. In this case, four calibration standards were prepared by dilution with 50 % methanol–water.
Stock solutions of organic acids with a concentration of 1.0 mg·mL−1 were prepared in ultrapure water; from these solutions, four calibration standards were prepared by dilution with water.
Apparatus and chromatographic conditions
An Agilent 1200 High Performance Liquid Chromatograph, equipped with a G1311A quaternary pump, a manual injection valve, and a 20 μL sample loop, coupled to an Agilent GI315D UV–vis diode array detector (Agilent Technologies, Santa Clara, CA, USA), was used for the analysis.
Four different chromatographic methods were used to analyze the samples, two for polyphenols and one for organic acids, and vitamins, respectively. In this study, effective HPLC–DAD methods were used for fingerprint analysis and phytochemical identification of different tree-species bud-preparations.
In all of the used methods, bioactive compound separation was achieved on a KINETEX – C18 column (4.6 × 150 mm, 5 μm, Phenomenex, Torrance, CA, USA).
Different mobile phases were used: a solution of 10 mM potassium dihydrogen phosphate in water (pH 2.8, KH2PO4+H3PO4) and acetonitrile with a flow rate of 1.5 mL·min−1 (method A, 20 + 2 min of conditioning time, gradient analysis of cinnamic acids and flavonols), a solution of methanol/water/formic acid (5/95/0.1 v/v) and methanol/formic acid (100/0.1 v/v) with a flow rate of 0.6 mL·min−1 (method B, 23 + 2 min of conditioning time, gradient analysis of benzoic acids and catechins), a solution of 10 mM potassium dihydrogen phosphate in water (pH 2.8, KH2PO4+H3PO4) and acetonitrile with a flow rate of 0.6 mL·min−1 (method C, 13 + 2 min of conditioning time, gradient analysis of organic acids), and methanol – water (5:95, v/v) containing 5 mM cetrimide and 50 mM potassium dihydrogen phosphate with a flow rate of 0.9 mL·min−1 (method D, 10+5 min of conditioning time, isocratic analysis of ascorbic and dehydroascorbic acids).
UV spectra were recorded at 330 nm (A); 280 nm (B); 214 nm (C); 261, and 348 nm (D).
Identification and quantification of bioactive compounds
All the single compounds were identified in samples by comparison and combination of their retention times and UV spectra with those of authentic standards in the same chromatographic conditions. The external standard method was used for quantitative determinations. The external standard calibration curves were generated using four data points. Twenty μL aliquots of each standard solution were used for HPLC analysis and injections were performed in triplicate for each concentration level. The calibration curves were obtained by plotting the peak area (y) of the compound at each level versus the concentration of the sample (x). For reference compounds, the limit of detection (LOD) and the limit of quantification (LOQ) were experimentally determined by HPLC analysis of serial dilutions of a standard solution to reach a signal-to-noise (S/N) ratio of 3 and 10, respectively. The main analytical method validation data are summarized in Table 2.
Table 2.
Identification standard codes, standard retention time (tR), calibration curve equations, R2, calibration curve ranges, LOD, and LOQ of the used chromatographic methods for each calibration standard
| Class | Standard | Identification code | Retention time (tR) (min) | Method | Calibration curve equation | R2 | Calibration curve range | LOD (mg L−1) | LOQ (mg L−1) |
|---|---|---|---|---|---|---|---|---|---|
| Cinnamic acids | Caffeic acid | 1 | 4.54 | A | y = 59.046x + 200.6 | 0.996 | 111–500 | 0.305 | 1.016 |
| Chlorogenic acid | 2 | 3.89 | A | y = 13.583x + 760.05 | 0.984 | 111–500 | 0.940 | 3.134 | |
| Coumaric acid | 3 | 6.74 | A | y = 8.9342x + 217.4 | 0.997 | 111–500 | 2.907 | 9.690 | |
| Ferulic acid | 4 | 7.99 | A | y = 3.3963x + 4.9524 | 1.000 | 111–500 | 1.245 | 4.150 | |
| Flavonols | Hyperoside | 5 | 10.89 | A | y = 7.1322x − 4.583 | 0.999 | 111–500 | 3.372 | 11.241 |
| Isoquercitrin | 6 | 11.24 | A | y = 8.3078x + 26.621 | 0.999 | 111–500 | 0.252 | 0.840 | |
| Quercetin | 7 | 17.67 | A | y = 3.4095x − 98.307 | 0.998 | 111–500 | 4.055 | 13.518 | |
| Quercitrin | 8 | 13.28 | A | y = 2.7413x + 5.6367 | 0.998 | 111–500 | 5.456 | 18.187 | |
| Rutin | 9 | 12.95 | A | y = 6.5808x + 30.831 | 0.999 | 111–500 | 2.937 | 9.790 | |
| Benzoic | Ellagic acid | 10 | 18.65 | B | y = 29.954x + 184.52 | 0.998 | 62.5–250 | 0.611 | 2.035 |
| Gallic acid | 11 | 4.26 | B | y = 44.996x + 261.86 | 0.999 | 62.5–205 | 0.435 | 1.451 | |
| Catechins | Catechin | 12 | 10.31 | B | y = 8.9197x + 66.952 | 1.000 | 62.5–250 | 2.343 | 7.809 |
| Epicatechin | 13 | 14.30 | B | y = 12.88x − 43.816 | 0.999 | 62.5–250 | 0.763 | 2.543 | |
| Organic acids | Citric acid | 14 | 5.30 | C | y = 1.0603x − 22.092 | 1.000 | 167–1000 | 18.805 | 62.682 |
| Malic acid | 15 | 4.03 | C | y = 1.415x − 80.254 | 0.996 | 167–1000 | 15.721 | 52.404 | |
| Oxalic acid | 16 | 7.85 | C | y = 6.4502x + 6.1503 | 0.998 | 167–1000 | 0.550 | 1.835 | |
| Quinic acid | 17 | 3.21 | C | y = 0.8087x − 38.021 | 0.998 | 167–1000 | 26.106 | 87.021 | |
| Succinic acid | 18 | 3.46 | C | y = 0.9236x + 8.0823 | 0.995 | 167–1000 | 7.135 | 23.783 | |
| Tartanic acid | 19 | 5.69 | C | y = 1.8427x + 15.796 | 1.000 | 167–1000 | 8.520 | 28.401 | |
| Vitamins | Ascorbic acid | 20 | 4.14 | D | y = 42.71x + 27.969 | 0.999 | 100–1000 | 0.836 | 2.786 |
| Dehydroascorbic acid | 21 | 3.41 | D | y = 4.1628x + 140.01 | 0.999 | 30–300 | 1.095 | 3.649 |
All samples were analyzed in triplicate, and standard deviations are given in order to assess the repeatability of the used methods. Accuracy was checked using the recovery test by spiking samples with a solution containing each bioactive compound (10 mg·mL−1) to reach 100 % of the test concentration.
According to “multi-marker approach” (Mok and Chau 2006), total bioactive compound content (TBCC) was determined as the sum of the most important classes of bioactive compounds present in the samples. Bioactive markers were selected comparing bud-preparation health-promoting properties and the most important compounds in literature with an important role in the positive effects on human organism. Four polyphenolic classes were considered: benzoic acids (ellagic and gallic acids), catechins (catechin and epicatechin), cinnamic acids (caffeic, chlorogenic, coumaric, and ferulic acids), and flavonols (hyperoside, isoquercitrin, quercetin, quercitrin, and rutin). Organic acids (citric, malic, oxalic, quinic, succinic, and tartaric acids) and vitamin C (ascorbic and dehydroascorbic acids) were also considered to obtain a complete analytical fingerprint. All results were expressed as mg per 100 g of fresh weight (FW).
Statistical Analysis
Results were subjected to analysis of variance (ANOVA) test for mean comparison (SPSS 22.0 Software) and HSD Tukey multiple range test (P < 0.05). Principal component analysis (PCA) was also performed on the phytochemical class data using the R-based chemometric software of the Italian chemometrics society and the V-PARVUS 2008 software (Forina et al. 2008).
Results
Reference species
Total bioactive compound content (TBCC) and fingerprint profile
ANOVA test showed statistically significant differences among the considered genotypes both on the single bioactive compound concentrations and the total bioactive compound content. Three repetitions from three plants for each species (N = 9) were considered.
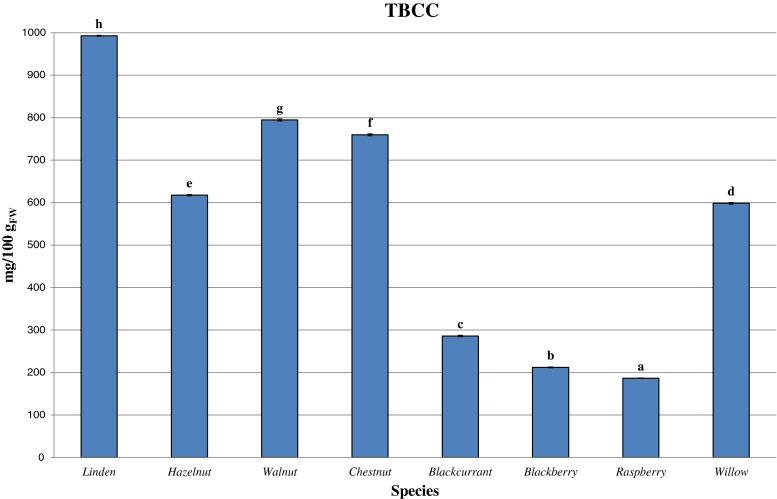
The content of total bioactive compounds in the evaluated extracts is reported in Fig. 1. Statistically significant differences were observed among the analyzed samples, with a lower TBCC value of 186.56 ± 2.14 mg 100 g−1FW for raspberry and an higher value of 992.79 ± 1.19 mg 100 g−1FW for linden, followed by walnut and chestnut.
Fig. 1.
TBCC of bud-extract samples of the different reference species. Different letters for each sample indicate the significant differences at P < 0.05
All chemical composition data are reported in Table 3: for each bioactive compound, content mean value, standard deviation (SD), as indication of the data variability, and Tukey test results were reported. Some peaks remained unidentified: they probably represent other bioactive markers with less therapeutically important effects on human health, according to other studies (Dillard and German 2000). Statistically significant differences were observed among the different genotypes for all single bioactive compounds: the most important differences were observed in the concentration of flavonols, vitamin C, ellagic acid, catechins, and some organic acids, as malic, oxalic, and quinic acids.
Table 3.
Single compound profile of analyzed bud-preparations for reference species. Different letters for each sample indicate the significant differences at P < 0.05
| Sample | Cinnamic acids | |||||||||||||||||
| Caffeic acid (mg/100 g FW) | Chologenic acid (mg/100 g FW) | Coumaric acid (mg/100 g FW) | Ferulic acid (mg/100 g FW) | |||||||||||||||
| Mean value | SD | Turkey test | Mean value | SD | Turkey test | Mean value | SD | Turkey test | Mean value | SD | Turkey test | |||||||
| Linden | 1.515 | 0.005 | a | n.q. | / | / | n.q. | / | / | 3.961 | 0.029 | a | ||||||
| Hazelnut | n.q. | / | / | n.q. | / | / | n.q. | / | / | n.d. | / | |||||||
| Walnut | 7.527 | 0.009 | c | n.q. | / | / | n.q. | / | / | n.d. | / | |||||||
| Chestnut | n.q. | / | / | n.q. | / | / | n.q. | / | / | n.d. | / | |||||||
| Blackcurrant | n.q. | / | / | n.q. | / | / | n.q. | / | / | 10.088 | 0.085 | b | ||||||
| Blackberry | 6.855 | 0.004 | b | n.q. | / | / | n.q. | / | / | n.d. | / | |||||||
| Raspberry | n.q. | / | / | n.q. | / | / | n.q. | / | / | n.d. | / | |||||||
| Willow | n.q. | / | / | n.q. | / | / | n.q. | / | / | n.d. | / | |||||||
| Sample | Flavonols | |||||||||||||||||
| Hyperoside (mg/100 g FW) | Isoquercitrin (mg/100 g FW) | Quercetin (mg/100 g FW) | Quercitrin (mg/100 g FW) | Rutin (mg/100 g FW) | ||||||||||||||
| Mean value | SD | Turkey test | Mean value | SD | Turkey test | Mean value | SD | Turkey test | Mean value | SD | Turkey test | Mean value | SD | Turkey test | ||||
| Linden | 24.922 | 0.084 | e | 8.106 | 0.053 | b | 27.513 | 0.200 | b | 452.304 | 0.173 | g | 68.272 | 0.028 | g | |||
| Hazelnut | 30.111 | 0.065 | f | 48.217 | 0.033 | f | 45.747 | 0.180 | d | 50.299 | 0.214 | d | 23.409 | 0.068 | e | |||
| Walnut | 21.002 | 0.072 | d | 38.673 | 0.033 | e | n.d. | / | / | 308.180 | 0.216 | f | 12.128 | 0.060 | c | |||
| Chestnut | n.d. | / | / | 8.168 | 0.207 | b | 68.626 | 0.383 | f | 8.225 | 0.331 | c | n.d. | / | / | |||
| Blackcurrant | 16.035 | 0.094 | c | 11.253 | 0.078 | d | 36.295 | 0.218 | c | 6.583 | 0.228 | b | 5.514 | 0.125 | b | |||
| Blackberry | n.d. | / | / | 1.119 | 0.018 | a | 19.660 | 0.063 | a | n.d. | / | / | 18.249 | 0.044 | d | |||
| Raspberry | 7.949 | 0.006 | b | 10.314 | 0.015 | c | n.d. | / | / | 5.086 | 0.071 | a | 4.148 | 0.005 | a | |||
| Willow | 3.578 | 0.072 | a | 66.189 | 0.037 | g | 54.758 | 0.062 | e | 62.564 | 0.198 | e | 36.522 | 0.078 | f | |||
| Sample | Benzoic acids | Catechins | Vitamins | |||||||||||||||
| Ellagic acid (mg/100 g FW) | Gallic acid (mg/100 g FW) | Catechin (mg/100 g FW) | Epicatechin (mg/100 g FW) | Ascorbic (mg/100 g FW) | Dehydroascorbic (mg/100 g FW) | |||||||||||||
| Mean value | SD | Turkey test | Mean value | SD | Turkey test | Mean value | SD | Turkey test | Mean value | SD | Turkey test | Mean value | SD | Turkey test | Mean value | SD | Turkey test | |
| Linden | 112.759 | 0.010 | g | n.d. | / | / | n.d. | / | / | 217.276 | 0.029 | f | 4.760 | 0.007 | d | 14.485 | 0.136 | c |
| Hazelnut | 42.264 | 0.005 | e | n.q. | / | / | 18.562 | 0.054 | b | 20.308 | 0.055 | a | 5.595 | 0.016 | e | 17.011 | 0.145 | d |
| Walnut | 154.250 | 0.021 | h | 3.393 | 0.023 | a | 34.729 | 0.042 | c | n.d | / | / | 8.587 | 0.016 | f | 23.318 | 0.170 | g |
| Chestnut | 65.039 | 0.055 | f | 14.441 | 0.029 | b | 151.750 | 0.122 | d | 63.742 | 0.140 | b | 12.159 | 0.021 | g | 54.925 | 0.115 | h |
| Blackcurrant | 24.973 | 0.015 | c | n.d. | / | / | 8.944 | 0.075 | a | n.d. | / | / | 16.343 | 0.009 | h | 17.660 | 0.048 | e |
| Blackberry | 6.721 | 0.012 | a | n.d. | / | / | n.d. | / | / | 79.685 | 0.035 | c | 4.582 | 0.006 | c | 5.617 | 0.045 | a |
| Raspberry | 10.932 | 0.003 | b | n.d. | / | / | n.d. | / | / | 97.666 | 0.015 | d | 1.201 | 0.004 | a | 11.630 | 0.044 | b |
| Willow | 36.225 | 0.008 | d | n.d. | / | / | n.d. | / | / | 156.007 | 0.025 | e | 2.784 | 0.010 | b | 19.490 | 0.008 | f |
| Sample | Organic acids | |||||||||||||||||
| Citric acid (mg/100 g FW) | Malic acid (mg/100 g FW) | Oxalic acid (mg/100 g FW) | Quinic acid (mg/100 g FW) | Succinic acid (mg/100 g FW) | Tartaric acid (mg/100 g FW) | |||||||||||||
| Mean value | SD | Turkey test | Mean value | SD | Turkey test | Mean value | SD | Turkey test | Mean value | SD | Turkey test | Mean value | SD | Turkey test | Mean value | SD | Turkey test | |
| Linden | n.d. | / | / | n.d. | / | / | 1.990 | 0.031 | d | 54.929 | 0.399 | d | n.d. | / | / | n.d. | / | / |
| Hazelnut | 31.511 | 0.092 | a | 54.858 | 0.210 | c | 1.530 | 0.070 | c | 98.294 | 0.099 | f | 17.204 | 0.238 | c | 112.710 | 0.259 | c |
| Walnut | n.d. | / | / | 81.050 | 0.500 | d | n.d. | / | / | 78.270 | 0.873 | e | 23.690 | 0.619 | d | n.d. | / | / |
| Chestnut | n.d. | / | / | 151.466 | 0.314 | e | 2.637 | 0.095 | e | 158.613 | 0.639 | g | n.d. | / | / | n.d. | / | / |
| Blackcurrant | n.d. | / | / | n.d. | / | / | 2.811 | 0.129 | e | 78.608 | 0.505 | e | n.d. | / | / | 50.842 | 0.297 | a |
| Blackberry | n.d. | / | / | 31.428 | 0.081 | b | 0.878 | 0.041 | b | 32.517 | 0.401 | b | 4.746 | 0.250 | a | n.d. | / | / |
| Raspberry | n.d. | / | / | 19.095 | 0.043 | a | 0.229 | 0.017 | a | 14.377 | 0.274 | a | 3.929 | 0.186 | a | n.d. | / | / |
| Willow | 34.056 | 0.176 | b | n.d. | / | / | n.q. | / | / | 38.349 | 0.529 | c | 11.758 | 0.573 | b | 76.153 | 0.362 | b |
an.d. = not detected
bn.q. = not quantified
Phytocomplex
Bud-preparation phytochemical fingerprint of reference species was reported: in total, 21 botanicals were evaluated by HPLC/DAD. By single bioactive compound profile, phytochemicals were grouped into single bioactive classes to evaluate the contribution of each class to total phytocomplex composition.
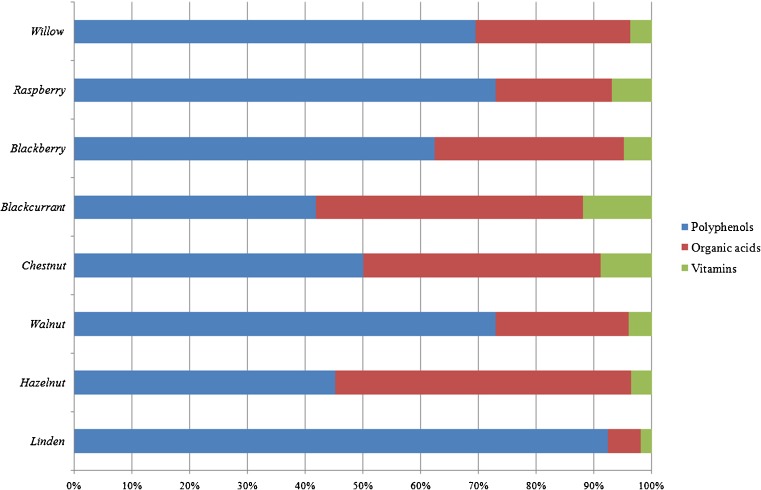
Depending on genotype, fingerprint profile showed the prevalence of different bioactive classes in chemical composition of all the analyzed preparations: considering the mean value of all the species, the most quantitatively important classes were flavonols in linden (58.53 %) and walnut (47.81 %), catechins in raspberry (52.35 %), blackberry (37.58 %) and chestnut (28.36 %), and organic acids in hazelnut (51.18 %) and blackcurrant (46.25 %). Cinnamic and benzoic acids are poorly represented in the samples, except for benzoic acids in walnut (19.83 %), while vitamin C is mainly present in blackcurrant and chestnut bud-preparations (11.89 and 8.83 %, respectively).
For each species, the percentage ratio between single bioactive class content (polyphenols, organic acids and vitamins) and TBCC were reported (Fig. 2): in particular, regarding the polyphenolic compounds, linden, walnut and raspberry are the most important species (92.33, 72.96, and 72.95 %, respectively).
Fig. 2.
Phytocomplex representation of all the considered University natural food supplements
Multivariate analysis
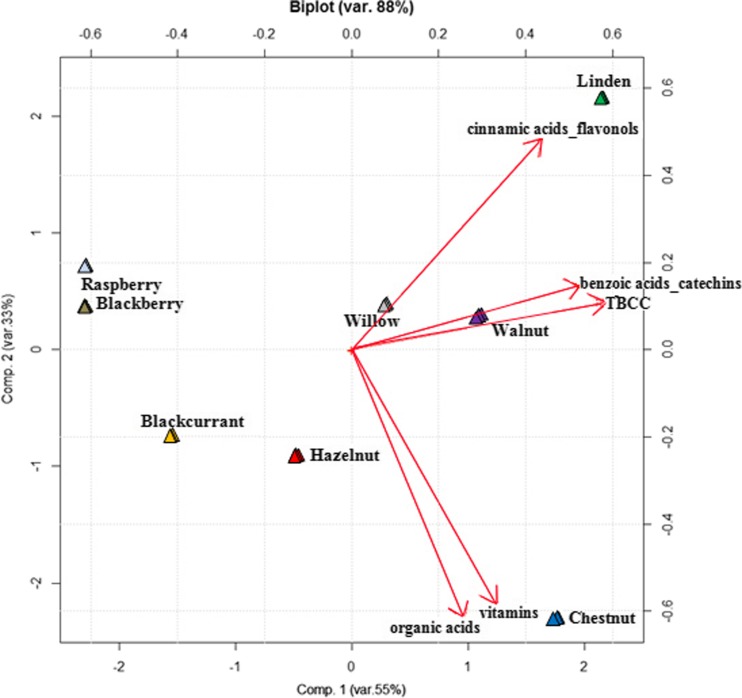
Principal Component Analysis was performed on all the University samples as unpattern recognition technique in order to rationalize the data information. This data set was structured in a data matrix named U24,5 whose rows are the 24 university samples and columns are the 4 bioactive classes and their sum (cinnamic acids + flavonols, benzoic acids + catechins, organic acids, vitamins, TBCC), respectively. PCA was performed on the autoscaled data and the corresponding biplot (score and loading plot) on the first two PCs, accounting for 88 % of the total variance, was shown in Fig. 3.
Fig. 3.
The PC1-PC2 scores and loadings plot (biplot) of the U24,5 data matrix (autoscaled data)
As far as score plot is concerned PC1 divides bud-extract of berries from the other tree-species while PC2 discriminates among the different kind of trees. PCA loading plot showed that the sum of all the bioactive components and in particular benzoic acids and catechins mainly composed PC1 whose amounts are different in berries and other species. The remain bioactive compounds seem to be more important in discriminating among the different tree-species.
Commercial bud-preparations
Total bioactive compound content (TBCC), fingerprint profile and phytocomplex
All phytochemical class composition and TBCC data are reported in Table 4: blackcurrant showed the lowest TBCC value (287.24 ± 6.74 mg 100 g−1FW), while linden the highest value (972.67 ± 6.25 mg 100 g−1FW), followed by walnut and chestnut.
Table 4.
Phytochemical composition in the analyzed commercial bud-preparations
| mg/100 gFW | Cinnamic acids | Flavonols | Benzoic acids | Catechins | Organic acids | Vitamins | TBCC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Mean value | SD | Mean value | SD | Mean value | SD | Mean value | SD | Mean value | SD | Mean value | SD | Mean value | SD |
| Linden_C1 | 6.547 | 0.873 | 573.025 | 56.279 | 109.589 | 1.019 | 210.301 | 1.865 | 60.506 | 8.224 | 19.005 | 1.236 | 978.972 | 69.496 |
| Linden_C2 | 6.204 | 0.556 | 570.432 | 55.680 | 109.391 | 1.257 | 209.945 | 1.567 | 59.704 | 8.253 | 17.559 | 1.308 | 972.569 | 69.015 |
| Linden_C3 | 5.667 | 0.578 | 568.433 | 56.205 | 108.765 | 1.286 | 209.249 | 1.690 | 58.669 | 8.350 | 16.467 | 1.866 | 966.474 | 70.415 |
| Hazelnut_C1 | n.d. | / | 195.401 | 8.019 | 40.217 | 0.614 | 37.040 | 1.638 | 305.797 | 26.397 | 22.796 | 0.570 | 601.252 | 37.239 |
| Hazelnut_C2 | n.d. | / | 197.905 | 7.611 | 40.612 | 0.803 | 38.297 | 1.555 | 308.525 | 26.076 | 23.739 | 1.082 | 609.078 | 37.127 |
| Hazelnut_C3 | n.d. | / | 200.427 | 8.499 | 41.194 | 0.941 | 39.094 | 1.247 | 311.918 | 26.654 | 24.629 | 1.266 | 617.262 | 38.606 |
| Walnut_C1 | 5.952 | 0.102 | 363.412 | 16.358 | 123.664 | 0.847 | 28.121 | 0.909 | 180.492 | 13.466 | 24.781 | 1.123 | 726.422 | 32.806 |
| Walnut_C2 | 5.583 | 0.153 | 368.048 | 10.596 | 122.848 | 1.358 | 27.472 | 1.087 | 175.563 | 17.566 | 23.900 | 1.384 | 723.414 | 32.143 |
| Walnut_C3 | 5.137 | 0.458 | 362.152 | 12.359 | 122.059 | 1.289 | 26.881 | 1.228 | 180.984 | 14.709 | 23.008 | 1.092 | 720.222 | 31.131 |
| Chesnut_C1 | n.d. | / | 76.872 | 3.300 | 73.382 | 1.512 | 230.235 | 13.699 | 344.321 | 23.679 | 57.542 | 0.666 | 782.351 | 42.856 |
| Chesnut_C2 | n.d. | / | 78.395 | 3.649 | 74.459 | 0.785 | 230.574 | 13.857 | 348.954 | 16.732 | 58.669 | 1.278 | 791.050 | 36.301 |
| Chesnut_C3 | n.d. | / | 80.020 | 11.136 | 75.219 | 1.005 | 234.751 | 12.535 | 336.958 | 23.203 | 59.554 | 1.322 | 786.502 | 49.201 |
| Blackcurrant_C1 | 11.246 | 0.623 | 78.244 | 3.952 | 25.331 | 0.270 | 9.485 | 1.000 | 135.635 | 10.769 | 34.019 | 1.578 | 293.960 | 18.201 |
| Blackcurrant_C1 | 10.817 | 0.273 | 75.604 | 3.448 | 24.938 | 0.261 | 8.999 | 1.149 | 133.921 | 11.280 | 32.992 | 2.213 | 287.270 | 18.624 |
| Blackcurrant_C1 | 10.301 | 0.460 | 72.295 | 3.587 | 24.309 | 0.415 | 8.756 | 0.982 | 132.877 | 11.641 | 31.948 | 2.098 | 280.486 | 19.182 |
For each bioactive class, content mean value and standard deviation (SD), as variability indication data, were reported. For each species, commercial preparations showed different single class content: in terms of absolute amounts of compounds, blackcurrant extracts were the best food supplements for cinnamic acids, linden for flavonols, walnut for benzoic acids, chestnut for catechins, organic acids, and vitamins.
Commercial preparations of the same species from different companies showed similar phytocomplex, while the differences among species are confirmed according to the previous results obtained on reference species; moreover, the percentage ratio between bioactive class content (polyphenols, organic acids and vitamins) and TBCC confirmed this hypothesis.
Multivariate analysis
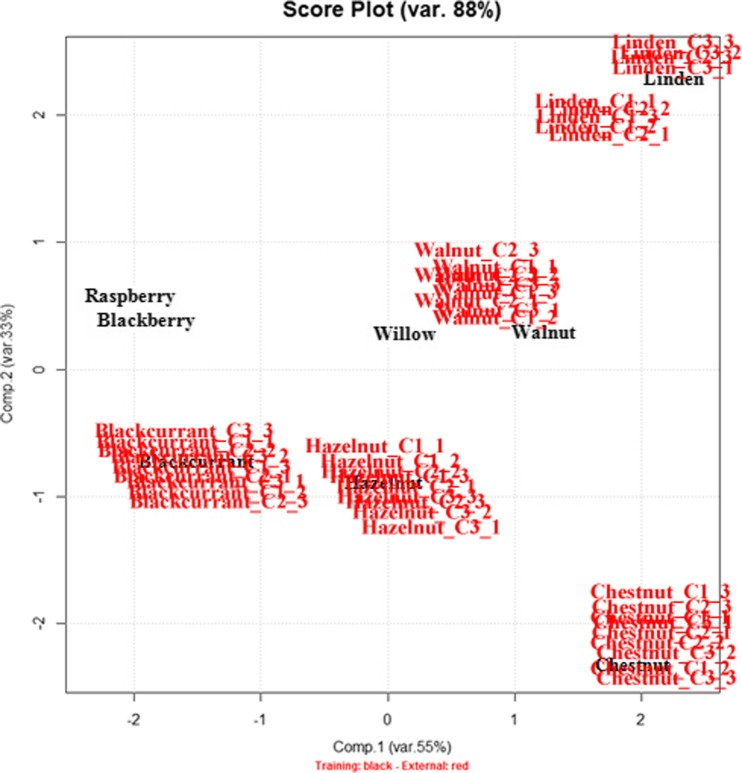
In order to test the effective performance in differentiating bud-extracts from different genotypes by the fingerprint - multivariate analysis system, PCA was performed on all the commercial samples. In details, the 45 commercial samples were structured in a data matrix called C45,5 whose rows are the 45 samples and columns the five already mentioned variables, respectively.
C45,5 data matrix was considered as an external test set and the data were projected on the PC1/PC2 score plot already calculated on the “authentic” university samples, considered as training set (see Fig. 4). The results are shown in Fig. 4 where the external test set samples are highlighted in red. Since the test set samples which are not considered in the PCA calculation are positioned very close to the corresponding authentic samples (training set), it is evident that the five variables are reliable in discriminating the different botanical species and they can be used as fingerprint of them.
Fig. 4.
The PC1-PC2 scores plot resulting from the 45 commercial samples of the test set (C45,5 – red) projected onto the PCA hyperplane calculated for the training set (U24,5 - black)
More in details, all the herbal-extracts of the test set have been assigned to the correct statistical group according to the genotype.
PCA loading plot confirmed the correlation between the initial variables and PCs, highlighted by previous PCA on the reference bud-preparations.
Discussion
Quality assurance (QA) practice is now regarded as a core requirement for international trade, especially for food, food supplements, botanicals and pharmaceuticals. In general, QA is applied for processes and products, which are well documented and characterized, and it is quite an exacting and involved task. However, there are many examples where popular products, as herbal preparations, are not fully or exactly specified and QA becomes an even more challenging undertaking (Feng et al. 2014).
The traditional quality control technique of herbal preparations or food supplements encounters more and more challenges because one or two biological active markers, employed for evaluating the herbal medicine quality and authenticity, cannot give a complete picture of the herbal products. Fast screening techniques and simple sample preparation are growing priorities intended to make methods more convenient and cost-effective: chromatographic fingerprint, a more significant formulation for controlling the quality of herbal medicines and their products, has been accepted by many countries and organizations. To provide a fingerprint, the entire chromatogram may be used as a chemical profile, or a chromatographic discriminating region or a selection of significant chemical markers: in this study, all the chromatographic pattern was used for fingerprint evaluation, according to other similar studies (Bian et al. 2013).
HPLC – DAD was chosen for quantitative analysis of the bioactive metabolites in bud-extracts, because of the wider availability and use of this equipment in the phytochemical analysis and quality control of natural products (Valls et al. 2009). In this research, new analytical methods were developed for phytochemical fingerprinting of secondary metabolites in tree-species bud-preparations in order to meet the need for a high throughput procedure for routine quantification of bioactive compounds; the validation of the HPLC – DAD methods was performed in agreement with international guidelines for analytical techniques for the quality control of biopharmaceuticals (ICH guidelines) (Kamboj 2012).
The chromatographic conditions were set to obtain an analytical fingerprint containing complete information of chemical composition with a good resolution and a reasonable analysis time. Over the tested concentration range, good linearity was observed for reference standard compounds used in this study; moreover, LOD and LOQ values indicated that the proposed HPLC – DAD methods showed a suitable sensitivity for the analysis of the selected biomarkers. The validation data highlighted the suitability of the proposed methods for the quali- and quantitative analysis of bioactive compounds in bud-extracts. Different linear gradients in different slopes were used for optimizing the analyte separation: indeed, some compounds were similar in structure with each other in the same chemical class. The presence of formic and phosphoric acids in the mobile phases was highly recommended, because a low pH value provides a better peak shape and improves the resolution, especially for acidic compounds, according to other studies (Nikolić and van Breemen 2013). The wavelength selection was an important step for developing a reliable fingerprint; only selected wavelengths were suitable to achieve more specific peaks as well as a smooth baseline after a full-scan on the chromatogram from 190 to 400 nm, according to other similar research (Canterino et al. 2012).
The methods developed in this study were applied to quantitative analysis of phytochemicals in University lab bud-preparations (eight tree-species, used as reference species), and in commercial products from three different companies (five tree-species, selected for multivariate system validation).
Chromatographic fingerprint of linden bud-extracts showed the highest value of TBCC, followed by walnut and chestnut: in particular, Castanea sativa bud-preparation is among the most commonly used herbal medicines and it is popularized for its effects on stagnant and vascular fluids or against recurrent cystitis and for its curative and restorative properties (Donno et al. 2014a), despite a high content of organic acids. Ribes nigrum extracts, the best preparations among the berry species, showed a TBCC value lower than the other tree-species because of their low content of organic acids (the same consideration is true for raspberry and blackberry): in any case, blackcurrant bud-preparations remain the most popular extracts in the herbal preparation market thanks to their high content of flavonols and vitamins, according to the results of other previous researches (Donno et al. 2013c).
The main fingerprint method of herbal product characterization is through comparison of HPLC chromatograms. However, since these chromatograms are highly complex and contain many classes of compounds, the comparison, often highly qualitative, can lead to missed features or unnecessarily tight requirements. The use of chemometric techniques to analyze the HPLC data could provide a higher level of assurance that important characteristics are not overlooked, and provide consistency in the final botanical drug products (Zhu et al. 2014). For this reason, in this study, the quantitative data obtained by HPLC were further processed by PCA; the score plot shows the distribution of the samples along the PCs, while the loading plot shows the contribution of each variable to the PCs, which is known to be influenced by the angle between them: if the angle of the variable with a PC is closer to 0, the contribution of the variable to this component is strong. In this research, PCA divided berry species from the other tree-species bud-preparations: in particular, confirming the ANOVA test results, the PCA score plot of reference species highlighted that raspberry and blackberry belong to the same statistical group. This result confirmed the hypothesis that species belonging to the same genus present similar chromatographic fingerprint and phytocomplex, as shown in previous studies (Donno et al. 2014a). Moreover, the analysis on commercial preparations showed that unsupervised PCA technique, using the data obtained from the selected bioactive classes, discriminated the samples according to the different genotypes, and it was able to show which component had an important influence on the sample discrimination. The results of PCA could provide more references for the quality evaluation and differentiation of herbal preparations: the samples grouped together were associated for similar chemical composition and properties.
The new approach developed in this study represents a good alternative for improving the classification results of herbal materials with complex chromatograms. It could be necessary to develop a “multivariate chromatographic fingerprint”, which represents the whole chemical characteristics of the complex herbal products, in order to differentiate the herbal preparations according to their genotype, avoiding substitutions, changes or adulterations with other species or synthetic drugs, which explain their (unexpected) power but they are also responsible for side effects of “unknown” reason. In a regulatory setting, having a simple methodology to ensure the identity of the raw material will help to ensure the quality of botanical drug products, showing evidence that approved products will provide similar safety and efficacy as the clinical trial supplies. The determination of common peaks/regions in a set of chromatographic fingerprints could provide useful qualitative and quantitative information on the characteristic components of investigated herbal medicines; on the other hand, whether the real samples were identified as the herbs with the same quality grade, it could be successfully determined comparing the chromatographic fingerprints. Moreover, pattern recognition could be used to discriminate different kinds of samples of investigated herbal medicines.
The fields in which this method can be applied range from routine quality control and standardization of bud-extracts, to germplasm evaluation and selecting of new cultivars with high content of bioactive compounds, up to the phytochemical fingerprinting of the plant material to be used in pharmaceutical and nutraceutical investigations, in particular when the effects of crude extracts are evaluated. In the future, these techniques could be used not only for control of approved products, but also to monitor the quality and identity of other herbal preparations, as dietary supplements, which are available in the marketplace and are not under the same rigorous control as approved pharmaceuticals.
Conclusions
The increasing consumer use of botanical supplements has led to studies and support for validating analytical methods to verify their identity, purity and strength. Through government, academic and industry initiatives, validated analytical methods and reliable reference material are becoming more prevalent and available to the public.
In this research, new methods were developed for the comprehensive multi-component analysis of several tree-species bud-preparations: multivariate chromatographic fingerprinting, which consists of more than one chromatographic fingerprint and represent the whole chemical characteristics of the samples, is proposed as a strategy for phytochemical characterization and quality control of complex herbal medicines instead of reported single chromatographic fingerprinting. The protocol established in this study was simple, sensitive and reliable and could be used for the evaluation and quality control of bud-extracts and natural food supplements: the proposed method was successfully applied to the characterization of commercial bud-preparations, demonstrating to be an effective tool for the fingerprinting of this plant material. It also provided an important reference for the establishment of the method for pattern recognition and quality control of different herbal products.
Botanicals will continue to appeal because they are convenient for today’s lifestyle and they are destined to play an important role in future therapeutic development, but their success will be governed by control of purity, safety and efficacy without inhibiting innovation. A place for phytochemicals in clinical practice is emerging, but, in any case, important pharmaceutical and clinical issues need to be addressed by further research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 12 kb)
(DOCX 12 kb)
(DOCX 403 kb)
(DOCX 204 kb)
References
- Bian Q, Yang H, Chan CO, Jin D, Mok DK, Chen S. Fingerprint analysis and simultaneous determination of phenolic compounds in extracts of curculiginis rhizoma by HPLC-diode array detector. Chem Pharm Bull. 2013;61:802–808. doi: 10.1248/cpb.c12-01058. [DOI] [PubMed] [Google Scholar]
- Boggia R, Casolino MC, Hysenaj V, Oliveri P, Zunin P. A screening method based on UV–visible spectroscopy and multivariate analysis to assess addition of filler juices and water to pomegranate juices. Food Chem. 2013;140:735–741. doi: 10.1016/j.foodchem.2012.11.020. [DOI] [PubMed] [Google Scholar]
- Brown PN, Lister P. Current initiatives for the validation of analytical methods for botanicals. Curr Opin Biotechnol. 2014;25:124–128. doi: 10.1016/j.copbio.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Canterino S, Donno D, Mellano MG, Beccaro GL, Bounous G. Nutritional and sensory survey of Citrus sinensis (L.) cultivars grown at the most Northern limit of the Mediterranean latitude. J Food Qual. 2012;35:108–118. doi: 10.1111/j.1745-4557.2012.00435.x. [DOI] [Google Scholar]
- Dan M, et al. A rapid ultra-performance liquid chromatography–electrospray Ionisation mass spectrometric method for the analysis of saponins in the adventitious roots of Panax notoginseng. Phytochem Anal. 2009;20:68–76. doi: 10.1002/pca.1099. [DOI] [PubMed] [Google Scholar]
- Dillard CJ, German JB. Phytochemicals: nutraceuticals and human health. J Sci Food Agric. 2000;80:1744–1756. doi: 10.1002/1097-0010(20000915)80:12<1744::AID-JSFA725>3.0.CO;2-W. [DOI] [Google Scholar]
- Donno D, Beccaro GL, Mellano GM, Cerutti AK, Canterino S, Bounous G. Effect of agronomic and environmental conditions on chemical composition of tree-species buds used for herbal preparations. Int J Plant Res (VEGETOS) 2012;25:21–29. [Google Scholar]
- Donno D, Beccaro GL, Mellano MG, Canterino S, Cerutti AK, Bounous G. Improving the nutritional value of kiwifruit with the application of agroindustry waste extracts. J Appl Bot Food Qual. 2013;86:11–15. [Google Scholar]
- Donno D, Beccaro GL, Mellano MG, Cerutti AK, Bounous G. Medicinal plants, chemical composition and quality: may blackcurrant buds and blackberry sprouts be a new polyphenol source for herbal preparations? J Appl Bot Food Qual. 2013;86:79–89. [Google Scholar]
- Donno D, Beccaro GL, Mellano MG, Cerutti AK, Marconi V, Bounous G. Botanicals in ribes nigrum bud-preparations: an analytical fingerprinting to evaluate the bioactive contribution to total phytocomplex. Pharm Biol. 2013;51:1282–1292. doi: 10.3109/13880209.2013.786101. [DOI] [PubMed] [Google Scholar]
- Donno D, Beccaro GL, Mellano MG, Bonvegna L, Bounous G. Castanea spp. buds as a phytochemical source for herbal preparations: botanical fingerprint for nutraceutical identification and functional food standardisation. J Sci Food Agric. 2014;94:2863–2873. doi: 10.1002/jsfa.6627. [DOI] [PubMed] [Google Scholar]
- Donno D, Beccaro GL, Mellano MG, Cerutti AK, Bounous G. Goji berry fruit (Lycium spp.): antioxidant compound fingerprint and bioactivity evaluation. J Funct Foods. 2014 [Google Scholar]
- Donno D, Cerutti AK, Prgomet I, Mellano MG, Beccaro GL. Foodomics for mulberry fruit (Morus spp.): analytical fingerprint as antioxidants’ and health properties’ determination tool. Food Res Int. 2015;69:179–188. doi: 10.1016/j.foodres.2014.12.020. [DOI] [Google Scholar]
- Ernst E. Functional foods, neutraceuticals, designer foods: innocent fad or counterproductive marketing ploy? Eur J Clin Pharmacol. 2001;57:353–355. doi: 10.1007/s002280100327. [DOI] [PubMed] [Google Scholar]
- Fan X-H, Cheng Y-Y, Ye Z-L, Lin R-C, Qian Z-Z. Multiple chromatographic fingerprinting and its application to the quality control of herbal medicines. Anal Chim Acta. 2006;555:217–224. doi: 10.1016/j.aca.2005.09.037. [DOI] [Google Scholar]
- Feng X, Kong W, Wei J, Ou-Yang Z, Yang M. HPLC fingerprint analysis combined with chemometrics for pattern recognition of ginger. Pharm Biol. 2014;52:362–367. doi: 10.3109/13880209.2013.837493. [DOI] [PubMed] [Google Scholar]
- Forina M, Lanteri S, Armanino C, Casolino C, Casale M, Oliveri P (2008) V-PARVUS 2008 vol Available (free, with manual and examples) from authors or at <http://www.parvus.unige.it. Dip. Chimica e Tecnologie Farmaceutiche e Alimentari. University of Genova
- Gad HA, El-Ahmady SH, Abou-Shoer MI, Al-Azizi MM. Application of chemometrics in authentication of herbal medicines: a review. Phytochem Anal. 2013;24:1–24. doi: 10.1002/pca.2378. [DOI] [PubMed] [Google Scholar]
- Hakimzadeh N, Parastar H, Fattahi M. Combination of multivariate curve resolution and multivariate classification techniques for comprehensive high-performance liquid chromatography-diode array absorbance detection fingerprints analysis of Salvia reuterana extracts. J Chromatogr A. 2014;1326:63–72. doi: 10.1016/j.chroma.2013.12.045. [DOI] [PubMed] [Google Scholar]
- Halt M. Moulds and mycotoxins in herb tea and medicinal plants. Eur J Epidemiol. 1998;14:269–274. doi: 10.1023/A:1007498613538. [DOI] [PubMed] [Google Scholar]
- Kamboj A (2012) Analytical evaluation of herbal drugs. INTECH Open Access Publisher
- Kong WJ, Zhao YL, Xiao XH, Jin C, Li ZL. Quantitative and chemical fingerprint analysis for quality control of Rhizoma Coptidischinensis based on UPLC-PAD combined with chemometrics methods. Phytomedicine. 2009;16:950–959. doi: 10.1016/j.phymed.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Liang Y-Z, Xie P, Chan K. Quality control of herbal medicines. J Chromatogr B. 2004;812:53–70. doi: 10.1016/S1570-0232(04)00676-2. [DOI] [PubMed] [Google Scholar]
- Mok DKW, Chau FT. Chemical information of Chinese medicines: a challenge to chemist. Chemom Intell Lab Syst. 2006;82:210–217. doi: 10.1016/j.chemolab.2005.05.006. [DOI] [Google Scholar]
- Nikolić D, van Breemen RB. Analytical methods for quantitation of prenylated flavonoids from hops. Curr Anal Chem. 2013;9:71–85. doi: 10.2174/157341113804486554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan R, Guo F, Lu H, W-W F. Development of the chromatographic fingerprint of Scutellaria barbata D. Don by GC–MS combined with Chemometrics methods. J Pharm Biomed Anal. 2011;55:391–396. doi: 10.1016/j.jpba.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Peng L, Wang YZ, Zhu HB, Chen QM. Fingerprint profile of active components for Artemisia selengensis Turcz by HPLC-PAD combined with chemometrics. Food Chem. 2011;125:1064–1071. doi: 10.1016/j.foodchem.2010.09.079. [DOI] [Google Scholar]
- Pharmaciens OND (1965) Pharmacopée Française, Codex Medicamentarius Gallicus, Codex Français: Monographie, Préparations Homéopathiques, VIII edn., Paris
- Prabu SL, Suriyaprakash T, Dinesh K, Suresh K, Ragavendran T. Nutraceuticals: a review. Elixir Pharm. 2012;46:8372–8377. [Google Scholar]
- Prencipe FP, et al. Development of a new high-performance liquid chromatography method with diode array and electrospray ionization-mass spectrometry detection for the metabolite fingerprinting of bioactive compounds in Humulus lupulus L. J Chromatogr A. 2014;1349:50–59. doi: 10.1016/j.chroma.2014.04.097. [DOI] [PubMed] [Google Scholar]
- Silano V, Coppens P, Larranaga-Guetaria A, Minghetti P, Roth-Ehrang R. Regulations applicable to plant food supplements and related products in the European Union. Food Funct. 2011;2:710–719. doi: 10.1039/c1fo10105f. [DOI] [PubMed] [Google Scholar]
- Valls J, Millán S, Martí MP, Borràs E, Arola L. Advanced separation methods of food anthocyanins, isoflavones and flavanols. J Chromatogr A. 2009;1216:7143–7172. doi: 10.1016/j.chroma.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li BH, Ni YN, Kokot S. Multi-wavelength high-performance liquid chromatography: an improved method for analysis of complex substances such as Radix Paeoniae herbs. Chemom Intell Lab Syst. 2014;130:159–165. doi: 10.1016/j.chemolab.2013.11.002. [DOI] [Google Scholar]
- Zhang QF, Cheung HY. Development of capillary electrophoresis fingerprint for quality control of Rhizoma Smilacis Glabrae. Phytochem Anal. 2011;22:18–25. doi: 10.1002/pca.1245. [DOI] [PubMed] [Google Scholar]
- Zhu JQ, Fan XH, Cheng YY, Agarwal R, Moore CMV, Chen ST, Tong WD (2014) Chemometric analysis for identification of botanical raw materials for pharmaceutical use: a case study using panax notoginseng. PLoS One 9 doi:10.1371/journal.pone.0087462 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 12 kb)
(DOCX 12 kb)
(DOCX 403 kb)
(DOCX 204 kb)