Abstract
The effect of various conditions (storage temperature, exposure to light, access of oxygen) and different packaging material (amber glass, amber polyethylene terephthalate) on the nutritional value of cold-pressed rapeseed oil during 12 months of storage was investigated. Quantified quality parameters included: acidity, peroxide value, spectrophotometric indices (K232, K268), fatty acid composition, tocopherols and sterols. Storage of oil at 4 °C was found to be most appropriate for maintaining the quality of cold-pressed rapeseed oil. Exposure of oil samples stored at room temperature to light in combination with the access of oxygen caused the most pronounced losses in the total tocopherols (ca. 90–91 % of α-T, and ca. 80–81 % of γ-T), total phytosterols (ca. 15–16 %) and substantial deterioration in oil qualitative properties. Although storage at room temperature is common for use in households, storage of at low temperatures (4 °C) significantly increases the possibility of prolonged shelf life of cold-pressed rapeseed oil.
Keywords: Access of oxygen, Light exposure, Long-term storage, Nutritional value, Packaging material, Rapeseed oil, Storage temperature
Introduction
Rapeseed is the third most important source of vegetable oils in the world and the major edible oil produced and consumed in Poland. It is claimed to be one of the healthiest culinary oil of all vegetable oils due to a low saturated fatty acids (6–7 %), and high contents of monoenoic (oleic acid 58–62 %) and polyenoic fatty acids, especially α-linolenic essential fatty acid (8–12 %). Moreover, rapeseed oil contains nutritionally favourable linoleic to α-linolenic essential fatty acids ratio of 2:1, as well as it is also marked by high concentrations of tocopherols and sterols (Dubois et al. 2007; Obiedzińska and Waszkiewicz-Robak 2012). Triglycerides (or triacylglycerols) are the main constituents of vegetable oils (97–99 %), while accompanying substances like polyphenols, phytosterols, tocopherols, pigments (carotenoids and chlorophylls) as well as phospholipids, mono- and di-glycerides, and free fatty acids are present in oil in much smaller quantities (1–3 %). The amount of the unsaponifiable components (mainly tocopherols and sterols), as well as the content of unsaturated fatty acids not only determine the nutritional value of oils, but also have a significant impact on susceptibility of oil to oxidation (Choe and Min 2006; Przybylski and Eskin 2006). The rate of oxidation depends on a number of factors, the major of which are the availability of oxygen, presence of light and temperature. Different chemical mechanisms, auto-oxidation and photosensitized oxidation, are responsible for the oxidation of edible oils during processing and storage depending upon the two types of oxygen, atmospheric triplet oxygen (3O2) and singlet oxygen(1O2) (Choe and Min 2009). Auto-oxidation of oils, which proceeds in the absence of light, is accelerated by the presence of free fatty acids, mono- and diacylglycerols, metals such as iron, and thermally oxidized compounds. The rates for the formation of lipid peroxy radicals and hydroperoxides in the auto-oxidation of oils depend only on oxygen availability and temperature. Lipid hydroperoxides, the primary oxidation products, decompose at high temperature or in the presence of metals to produce off-flavour compounds of aldehydes, ketones, acids, esters, alcohols, and short-chain hydrocarbons. On the other hand, oil exposure to light accelerates photo-oxidation, in which triplet oxygen (3O2) react with natural photosensitizers in oil (i.e. chlorophyll), to form the excited state singlet oxygen. Photosensitized oxidation of edible oil follows the singlet oxygen oxidation pathway – 1O2 either reacts with other molecules or transfers its energy to them. Hydroperoxides formed by 1O2 reaction with unsaturated fatty acids are responsible for the deterioration of vegetable oil flavour (List et al. 2005; Choe and Min 2006; Przybylski and Eskin 2006).
The point for the production of a high-quality cold pressed rapeseed oil is the choice of high quality raw materials, an optimized oil pressing process, an immediate cleaning of the crude oil as well as an appropriate storage of the oil and the type of packaging material (Niewiadomski 1990). Materials used for vegetable oil packaging included glass bottles, polyethylene terephthalate (PET), polyvinyl chloride (PVC) or high density polyethylene (HDPE) plastic bottles, and tinplate cans (Robertson 2012).
One of the most important characteristics of a container used for vegetable oils packaging is related to their barrier properties against natural pro-oxidants agents, such as light and oxygen (Sacchi et al. 2008). Oxygen permeability is important property only for plastic packages, since glass containers are able to completely prevent O2 permeation, while PET is only able to slow down O2 exchange (Robertson 2012). The concentration of oxygen dissolved in oil prior to bottling is yet another factors influencing oil storage stability. Moreover, the packaging geometry and the techniques of filling and closing the containers are of importance too. On the other hand light transmission is important for both glass and plastic packages, constitute an effective barrier to light at wavelengths shorter than 340 nm (Robertson 2012). Samaniego-Sanchez et al. (2012) studied the effect of different packaging material (clear glass, clear PET, Tetra Brik®) on the olive oil quality over 9 months of storage, and found that Tetra Brik® packages were best able to preserve olive oil.
The influence of storage conditions on olive oil quality has been well investigated (Cecchi et al. 2010; Ayton et al. 2012; Samaniego-Sanchez et al. 2012). To the best of our knowledge, there have been limited number of studies considering the effect of different storage conditions on the changes in the nutritional value of cold-pressed rapeseed oil during long term storage.
The aim of this study was to explore the dependence of cold-pressed rapeseed oil quality on the different storage conditions. This was done by investigating the effect of six factors, namely storage temperature, access of light and exposure to oxygen on the nutritional value of cold-pressed rapeseed oil stored over 12 months.
Materials and methods
Rapeseed oil
The double improved winter rapeseed was provided by the HR Strzelce, IHAR group (Poland). Seeds were harvested in optimum maturity, and did not contain any impurities or broken seeds. They were stored in paper bags in atmospheric conditions at 15 ± 2 °C. Oil pressing was carried out with the use of screw press Farmer 10 (Farmet, Czech Republic), applying nozzle diameter of 8 mm. The temperature inside the press was set at 60 ± 2 °C, and the temperature of the outflowing oil was 38 ± 1 °C. Oil was collected, subjected to natural sedimentation (3 days) under refrigeration conditions (4 ± 2 °C) and decanted. Rapeseed oil sample analysed immediately after pressing (analysed on 0th day) was used as a control sample. Each oil sample was poured into 3 bottles for each the storage conditions (6 storage conditions, in triplicate). Analyses were performed immediately after oil production and after 12 months of storage. Produced cold-pressed rapeseed oil was transferred under nitrogen gas to 500 ml bottles (made of amber glass and amber PET), maintaining 5 % headspace in each bottle. Bottles were sealed with standard polypropylene threaded caps.
Amber glass and amber polyethylene terephthalate bottles specification
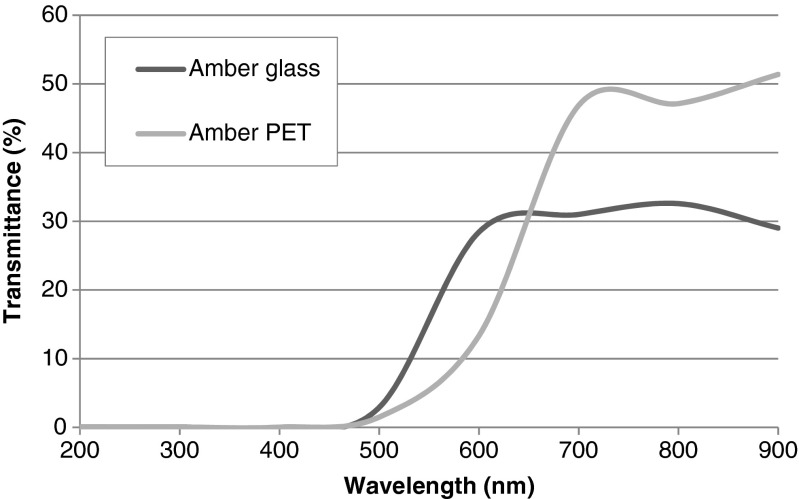
The dimensions of amber glass and amber PET bottles were: ∅ 6.20 cm × 19.5 cm height; ∅ 7.55 cm × 18.5 cm height, respectively. The oxygen transmission rate specified for PET bottles amounted to 7.8 cm3/m2/day (tested at 21 °C, at 0.21 atm driving force and 50 % relative humidity). The light transmittance (%) spectra of both packaging materials tested exhibited effective barriers to wavelengths shorter than 500 nm, followed by increase in light transmittance up to 31–33 % for 600 to 800 nm and up to 49 % for 680 to 710 nm wavelength for amber glass and amber PET, respectively (Fig. 1).
Fig. 1.
Spectra of the studied amber glass and amber polyethylene terephthalate bottles
Storage conditions
During storage oil samples were divided into two groups: (a) bottles were kept closed over entire storage period; (b) once per month (5th day of every month) bottles were opened and approximately 20 ml of oil was poured from the bottle, then bottles were re-capped and shaken to distribute the oxygen within the oil. Bottled samples were kept in three different conditions: (a) at room temperature (RT), where the average temperature during storage period was 20 °C (ranging from 18 to 22 °C), on the lab shelf exposed to daylight (day-night); (b) at room temperature (RT) in the dark; (c) and at 4 °C in a refrigerator.
Reagents
Analytical standards of γ- and α-tocopherols (purity >95 % by HPLC), a high purity standard of 5α-cholestane, and N,O-bis-(trimethylsilyl)trifluoroacetamide (BSTFA) containing 1 % trimethylchlorosilane (TMCS), were purchased from Sigma Aldrich (USA). HPLC-grade n-hexane, methanol (MeOH), acetonitrile (ACN), methyl tert-butyl ether (MtBE) and potassium hydroxide (KOH) were obtained from Polish Chemical Reagents (Poland). FeCl2 > 99 % and NH4SCN (ammonium thiocyanate) of HPLC purity were purchased from Merck Millipore (Germany), while HCl (36 %), chloroform (CHCl3), H2O2 (30 %) of analysis grade were obtained from Chempur Company (Poland). All other solvents and chemicals used in this study were of analytical grade.
Fatty acid composition
Fatty acid methyl esters (1 μl), prepared by ISO 5509 (2000) standard method, were separated on a GC–FID system (Agilent 6890 N GC, Agilent Technologies, USA) equipped with a BPX 70 capillary column (60 m length, 0.22 mm i.d., 0.25 μm film thickness). Helium was used as a carrier gas at a flow rate of 1.5 ml/min. The column temperature was programmed at 2 °C/min with initial temperature 130 °C and final temperature 235 °C. The injector was set at 230 °C with split ratio of 100:1 and the detector was set at 250 °C. The fatty acids were identified by comparing their retention times with those of the authentic fatty acid methyl esters (FAME) standards under the same conditions.
Tocopherols
Samples were prepared by dissolving 0.2 g of rapeseed oil in 5 ml of ACN/MtBE mixture (4:6 by vol.), and then analyzed by VP Shimadzu HPLC system equipped with the DAD detector (SPD-M10Avp Shimadzu), the FLD detector (RF-10ALxl Shimadzu), reversed phase octadecyl silica C18 HPLC column Gemini C18 (150 mm × 2 mm × 3 μm) (Phenomenex Torrance, CA, USA), and a suitable guard column. The isocratic mobile phase was a mixture of ACN and MtBE (4:6 v/v) at a flow rate of 0.15 ml/min, and the column oven temperature was 35 °C. Tocopherols were detected by standard UV spectrum analysis (190–370 nm). Quantification of tocopherols was conducted using data from the fluorescence detector (FLD) with excitation/emission wavelengths of 290/330 nm, respectively. All samples were analysed in triplicate and the tocopherol/oil ratio was expressed in mg/100 g.
Phytosterols
Rapeseed oil (0.2 g) was dissolved in 3 ml of hexane, and then 50 μl of 5α-cholestane (0.4 mg/ml) was added as an internal standard. The mixture was saponified with 2 M methanolic KOH at room temperature for 1 h. Then, 700 μl of unsaponified fraction was transferred into 1.5 ml vial and the solvent was evaporated to dryness under nitrogen. Dry residues were dissolved in 100 μl pyridine and silylated with 100 μl BSTFA containing 1 % TMCS. Derivatives of the sterols were separated on a GC–MS system (Agilent 6890 N GC, Agilent Technologies, USA) equipped with a DB-5MS capillary column (30 m × 0,25 mm × 0,25 μm; Phenomenex Torrance, CA, USA). Column temperature was programmed in the range from 250 to 300 °C. The temperatures of detector and injector were set at 310 and 280 °C, respectively. Helium was used as carrier gas at a flow rate of 1.5 ml/min. The split ratio was 25:1. Sterols were identified based on a laboratory sterol spectra library, as well as the on-line NIST Mass Spectra Library.
Peroxide value, acidity, specific UV extinctions (K232 and K268)
Peroxide value (PV) (meq O2/kg) and acidity (expressed in %), were determined according to the ISO standard methods 3960 (1996) and 660 (2005), respectively. The conjugated dienes and trienes, expressed by absorption coefficient at λmax 232 and 268 nm, were determined using Thermo Spectronic Helios β spectrophotometer, in accordance with ISO 3656 (2011) standard method.
Statistical analysis
All experiments were carried out in triplicate. Statistical analysis was performed using Statgraphics 4.1. software. Data were expressed as Mean ± SD or as percentage. Variables were compared using one-way ANOVA, when the variables fulfilled parametric conditions, or by the Kruskal-Wallis test when these were non-parametric. Significant differences between means were determined through Tukey’s Multiple Range Tests. Additionally, data were subjected to principal component analysis (PCA) and hierarchical cluster analysis (HCA), using Statistica v. 12 software.
Results and discussion
Fatty acid composition
The fatty acid composition of control oil sample and 12 months stored cold-pressed rapeseed oils under various conditions were presented in Table 1. No changes were found in the fatty acid composition, particularly in the composition of MUFAs and PUFAs in the oil samples stored at 4 °C and kept closed over the 12 months storage period. Moreover, packaging material also did not reflect any significant effect on the fatty acid composition of stored cold-pressed oils. As indicated by correlation analysis presented in Table 5, PUFAs and MUFAs were statistically significant (p < 0.05) reduced in stored oil samples. However, only slight alteration in the composition of fatty acids (not exceeding 0.3 % with reference to control oil sample) was found in samples exposed to oxygen and kept at RT. The loss was due to intensive oxidation, high availability of oxygen resulting from mixing, and increasing free space above oil surface in the bottle. The above results are in confirmation with the findings of Ayton et al. (2012) who reported slight increase of SFAs and MUFAs, and decrease of PUFAs up to 1.3 % after 36 months of storage of olive oil in amber glass containers, while to the contrary Méndez and Falqué (2007) found decrease in oleic fatty acid content from 76.3 to 73.3 % of extra virgin olive oil stored in clear PET packaging, whereas no alteration in the content PUFAs was reported. Furthermore, no effect of light exposure on the FA composition was found, that remains consistent with previously published data (Ayton et al. 2012; Méndez and Falqué 2007).
Table 1.
Fatty acid composition of rapeseed oil samples
| Packaging material | Storage conditions | Fatty acids (%) | PUFA n-6 |
PUFA n-3 |
n-6/n-3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature | Exposure to light | Access of oxygen | SFA | MUFA | PUFA | ||||
| Control oil | – | – | – | 7.16ab | 63.91 ± 0.11a | 28.79 ± 0.20a | 19.50 ± 0.07a | 9.22 ± 0.11b | 2.11 ± 0.06a |
| Amber glass | 20 °C | light | Closed throughout the storage period | 7.20cd | 63.75 ± 0.31b | 28.92 ± 0.18b | 19.62 ± 0.06b | 9.24 ± 0.09a | 2.12 ± 0.03ab |
| 20 °C | dark | 7.19c | 63.76 ± 0.12b | 28.91 ± 0.03b | 19.62 ± 0.13b | 9.23 ± 0.03ab | 2.13 ± 0.08b | ||
| 4 °C | dark | 7.21d | 63.88 ± 0.05a | 28.81 ± 0.07a | 19.59 ± 0.05bc | 9.16 ± 0.14c | 2.14 ± 0.10bc | ||
| Amber PET | 20 °C | light | 7.20cd | 63.75 ± 0.16b | 28.91 ± 0.15b | 19.62 ± 0.16b | 9.23 ± 0.18ab | 2.13 ± 0.11b | |
| 20 °C | dark | 7.15a | 63.86 ± 0.21a | 28.85 ± 0.13ab | 19.57 ± 0.14c | 9.22 ± 0.05b | 2.12 ± 0.04ab | ||
| 4 °C | dark | 7.21d | 63.78 ± 0.08b | 28.85 ± 0.09ab | 19.59 ± 0.16bc | 9.21 ± 0.02b | 2.13 ± 0.07b | ||
| Amber glass | 20 °C | light | Once per month opened | 7.23e | 63.96 ± 0.10a | 28.63 ± 0.11c | 19.47 ± 0.12d | 9.10 ± 0.15d | 2.14 ± 0.15bc |
| 20 °C | dark | 7.22de | 64.22 ± 0.06e | 28.38 ± 0.03e | 19.35 ± 0.07e | 8.97 ± 0.17e | 2.16 ± 0.12d | ||
| 4 °C | dark | 7.17ab | 63.89 ± 0.01a | 28.83 ± 0.06a | 19.56 ± 0.03c | 9.21 ± 0.02b | 2.12 ± 0.10ab | ||
| Amber PET | 20 °C | light | 7.19c | 63.97 ± 0.19c | 28.63 ± 0.17c | 19.47 ± 0.14d | 9.10 ± 0.15d | 2.14 ± 0.18bc | |
| 20 °C | dark | 7.19c | 64.13 ± 0.32d | 28.56 ± 0.21d | 19.45 ± 0.10d | 9.05 ± 0.12f | 2.15 ± 0.10d | ||
| 4 °C | dark | 7.17ab | 63.87 ± 0.05a | 28.83 ± 0.18a | 19.56 ± 0.15c | 9.21 ± 0.10b | 2.12 ± 0.13ab | ||
SFA Saturated fatty acids, MUFA Monounsaturated fatty acids, PUFA Polyunsaturated fatty acids
Different superscript letters within each column indicate significant differences (p < 0.05)
Table 5.
PCA factor loadings for the quality indicators of cold-pressed rapeseed oil samples stored in different type of packaging under various storage conditions
| Quality indicators | PC1 | PC2 |
|---|---|---|
| Acidity | 0.758 | 0.407 |
| PV | 0.966 | −0.039 |
| K 232 | 0.912 | −0.346 |
| K 268 | 0.923 | 0.099 |
| SFA | 0.488 | 0.192 |
| MUFA | 0.806 | −0.567 |
| PUFA | −0.895 | 0.421 |
| Total tocopherols | −0.654 | −0.507 |
| Total sterols | −0.677 | −0.534 |
Values in bold are loadings with an absolute value greater than 0.70
Tocopherol contents
The total content of tocopherols in control oil sample was 57.89 mg/100 g (23.75 mg/100 g α-T, and 34.14 mg/100 g γ-T) (Table 2). Results presented in Table 2 showed, that individual tocopherols were significantly different affected by the various storage conditions, however, in all investigated storage conditions α-T degradation proceeded more rapidly than that of γ-T. Based on the percentage of losses, the highest rate of tocopherols degradation was observed when oil was exposed to oxygen and stored at RT. Under such conditions, storage under light resulted in ca. 90–91 % loss of α-T, and ca. 80–81 % loss of γ-T. When oil samples were kept in dark, ca. 90–91 % of α-T, and ca. 77–79 % of γ-T has degraded. The substantial loss of individual tocopherols was also noted when oil samples remained closed over entire storage period. Interestingly, negligible differences in the percentage loss of tocopherols between samples kept in the dark and exposed to light, were observed. When oil samples were stored in closed containers at 20 °C under light the percentage loss of α-T was as follows: 84 % (amber glass), and 88 % (amber PET). Respective loss of γ-T, ranged from 61 % to 62 %. Storage in the dark resulted in ca. 82–84 % loss of α-T, and ca. 60–65 % loss of γ-T. The least losses in the nutritional value, and thus the highest tocopherols content was found in oil samples kept closed and stored under refrigerated temperature, where the total tocopherols content left after 12 months of storage ranged from 25.54 to 26.24 mg/100 g for oil stored in PET and glass containers, respectively. In contrast, when exposed to oxygen, the percentage loss of the total tocopherols increased to 70 % (amber glass), and 72 % (amber PET).
Table 2.
Means obtained for tocopherol contents and their respective standard deviations
| Packaging material | Storage conditions | Tocopherol contents (mg/100 g) | ||||
|---|---|---|---|---|---|---|
| Temperature | Exposure to light | Access of oxygen | γ-T | α-T | Total | |
| Control oil | – | – | – | 34.14 ± 3.66a | 23.75 ± 2.31a | 57.89 ± 2.79a |
| Amber glass | 20 °C | light | Closed throughout the storage period | 13.28 ± 1.20b | 3.84 ± 0.17b | 17.12 ± 1.38b |
| 20 °C | dark | 13.77 ± 0.62b | 3.72 ± 0.91b | 17.50 ± 1.52b | ||
| 4 °C | dark | 18.70 ± 1.52c | 7.54 ± 0.26c | 26.24 ± 1.26d | ||
| Amber PET | 20 °C | light | 13.03 ± 1.27b | 2.85 ± 0.62d | 15.88 ± 1.89b | |
| 20 °C | dark | 11.99 ± 2.11d | 4.25 ± 0.56b | 16.24 ± 1.55b | ||
| 4 °C | dark | 18.39 ± 1.28c | 7.15 ± 0.42c | 25.54 ± 1.70d | ||
| Amber glass | 20 °C | light | Once per month opened | 6.66 ± 0.14e | 2.20 ± 1.11d | 8.86 ± 0.97e |
| 20 °C | dark | 7.77 ± 0.82e | 2.22 ± 0.43d | 9.99 ± 0.39e | ||
| 4 °C | dark | 11.53 ± 0.85d | 5.76 ± 0.25e | 17.29 ± 0.60b | ||
| Amber PET | 20 °C | light | 6.62 ± 0.77e | 2.35 ± 0.73d | 8.97 ± 1.50e | |
| 20 °C | dark | 7.13 ± 0.93e | 2.41 ± 0.72d | 9.53 ± 0.21e | ||
| 4 °C | dark | 11.93 ± 0.74d | 4.48 ± 1.87e | 16.41 ± 2.61b | ||
Different superscript letters within each column indicate significant differences (p < 0.05)
The results indicating losses of tocopherols and diminished nutritional value of cold-pressed rapeseed oil were consistent with numerous studies related to extra virgin olive oil and refined oils. Fadda et al. (2012) found only ca. 13 % α-T degradation in extra virgin olive oil over 18 months of storage in the dark, while Okogeri and Tasioula-Margari (2002) reported up to 50 % loss of α-T after 12 months of storage of olive oil, whereas nearly complete loss of α-T over 12 month of storage was found by Morello et al. (2004). The substantial loss of tocopherols during entire storage period may be assigned to the fact that they act as hydrogen donor or oxygen quencher to stop the chain mechanism of auto- and photo-oxidation, respectively. Tocopherols protect lipids from the oxidation by donating their hydrogen to lipid peroxy radicals (ROO·), which leads to formation of lipid hydroperoxide (ROOH) and tocopheroxy radicals (T·) (Choe and Min 2006). These radicals were more stable than lipid peroxy radicals and thereby responsible for the slowdown in the rate of oil auto-oxidation. When oil samples were exposed to light, tocopherols prevented oil from photo-oxidation by singlet oxygen (1O2) quenching. The reaction rate of tocopherols with 1O2 was affected by their structures. α-T exhibited the highest reaction rate, followed by β-, γ-, and δ-T, which may explain greater decline of α-T than γ-T after 12 months of storage (Choe and Min 2006). The finding that α-T decomposed faster than γ-T during long-term storage of oil has also been reported previously by Mezouari and Eichner (2007) and Samaniego-Sanchez et al. (2012).
Phytosterol content
The fresh cold-pressed rapeseed oil contained a total of 700.98 mg/100 g sterols (Table 3). The following phytosterols have been determined in the analysed fresh oil sample: β-sitosterol, ca. 51 %, and campesterol, ca. 32 %, followed by brassicasterol, ca. 13 %, while the content of Δ5-avenasterol and stigmasterol was approximately 3 % of total phytosterols.
Table 3.
Means obtained for sterol contents and their respective standard deviations
| Packaging material | Storage conditions | Sterol contents (mg/100 g) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature | Exposure to light | Access of oxygen | Brassicasterol | Campesterol | Stigmasterol | β-sitosterol | Δ5-avenasterol | Total | |
| Control oil | – | – | – | 92.73 ± 0.21a | 224.56 ± 1.22a | 1.65 ± 0.13ab | 359.20 ± 13.46a | 20.28 ± 0.74a | 700.98 ± 15.83a |
| Amber glass | 20 °C | light | Closed throughout the storage period | 88.04 ± 2.14b | 207.26 ± 6.17b | 1.52 ± 0.17a | 344.38 ± 5.32a | 19.30 ± 0.58a | 662.5 ± 14.16b |
| 20 °C | dark | 91.24 ± 1.68a | 221.88 ± 3.33a | 1.77 ± 0.00b | 351.51 ± 3.56a | 20.48 ± 0.33a | 689.3 ± 8.98a | ||
| 4 °C | dark | 92.73 ± 0.21a | 225.06 ± 0.52a | 1.65 ± 0.13ab | 355.10 ± 2.00a | 20.28 ± 0.74a | 697.32 ± 3.74a | ||
| Amber PET | 20 °C | light | 89.41 ± 1.41b | 216.66 ± 2.76b | 1.56 ± 0.08a | 338.78 ± 8.05b | 19.98 ± 0.81a | 668.34 ± 11.2b | |
| 20 °C | dark | 91.71 ± 0.37a | 219.32 ± 0.73b | 1.81 ± 0.13b | 344.21 ± 2.80a | 21.01 ± 0.59a | 680.57 ± 1.56a | ||
| 4 °C | dark | 90.21 ± 1.08ab | 218.27 ± 0.62b | 1.81 ± 0.13b | 349.19 ± 1.47a | 20.96 ± 0.75a | 682.99 ± 0.75a | ||
| Amber glass | 20 °C | light | Once per month opened | 81.94 ± 2.25c | 186.07 ± 4.04c | 1.54 ± 0.16a | 298.52 ± 9.83c | 16.39 ± 0.45b | 586.37 ± 16.53c |
| 20 °C | dark | 89.62 ± 0.81b | 215.65 ± 3.44b | 1.71 ± 0.11b | 340.90 ± 2.86b | 19.83 ± 0.06a | 670.14 ± 6.99b | ||
| 4 °C | dark | 91.21 ± 0.33a | 219.32 ± 0.73b | 1.81 ± 0.13b | 347.19 ± 1.47a | 20.51 ± 0.11a | 682.49 ± 2.5a | ||
| Amber PET | 20 °C | light | 85.74 ± 3.63b | 200.70 ± 9.31c | 1.44 ± 0.08a | 289.79 ± 8.78c | 16.03 ± 0.87b | 595.34 ± 3.44c | |
| 20 °C | dark | 89.65 ± 0.86b | 212.74 ± 1.92b | 1.36 ± 0.08a | 337.46 ± 1.69b | 19.04 ± 0.21c | 662.48 ± 4.55b | ||
| 4 °C | dark | 88.35 ± 0.98b | 214.65 ± 1.86b | 1.41 ± 0.11a | 339.91 ± 3.3b | 18.92 ± 0.40c | 665.66 ± 5.73b | ||
Different superscript letters within each column indicate significant differences (p < 0.05)
As can be seen from Table 3, the highest degradation rate of total sterols was found in rapeseed oil samples exposed to oxygen and stored at RT with the access of light. Under such conditions, the percentage loss of total sterols ranged from 15.1 % (amber glass) to 16.3 % (amber PET). Respective loss of total sterols in oil samples kept closed over entire storage period was 1.7 % and 2.9 %, for glass and PET containers, respectively. Storage in the dark (at RT) resulted in similar degradation rate of total sterols, regardless of the access of oxygen. Of the total sterol content, ca. 4.7–5.5 % were lost in oil samples kept closed over entire storage period, while respective loss of sterols in samples exposed to oxygen ranged from 4.4 to 5.5 %. The type of packaging material was found to have statistically significant (p < 0.05) impact on the degradation rate of sterols in oil samples stored under refrigerated temperature. When samples were stored in amber glass containers, the percentage loss of total sterols ranged from 0.5 to 2.6 %, for containers kept closed throughout entire storage period and opened once per month, respectively. Respective loss of sterols in samples stored in amber PET containers amounted to 2.6 % and 5.0 %.
In contrary to tocopherols, sterols showed high storage stability over the entire storage period. This may be explained by the fact, that tocopherols were consumed first when protecting PUFAs from oxidation (Pekkarinen et al. 1998). Similar dependence between tocopherol and sterol stability during prolonged crude rice oil storage was observed by Mezouari and Eichner (2007), where 4.9 % loss of the total sterols content, and about 72 % decrease of the total tocopherol content was reported in oil samples kept in the dark. However, when oil samples were exposed to light, the percentage loss of the total sterols increased to 49.2 %, with practically complete loss of tocopherols after 240 days of storage.
Acidity, peroxide value, K232 and K268
After the entire storage period, the degree of hydrolysis, assessed in terms of changes in acidity, remained below the threshold limit of 0.8 %, specified for extra virgin olive oil, regardless of the type of packaging and storage conditions (Table 4). This may be linked to the fact that the FA composition showed slight alterations over the 12 months of storage, and hence, a small rise in the formation of FFA occurred.
Table 4.
Means obtained for acidity, PV, and specific UV extinctions and their respective standard deviations
| Packaging material | Storage conditions | Acidity (%) | PV (meq O2/kg) | K 232 | K 268 | ||
|---|---|---|---|---|---|---|---|
| Temperature | Exposure to light | Access of oxygen | |||||
| Control oil | – | – | – | 0.60 ± 0.03a | 2.86 ± 0.17a | 1.88 ± 0.07a | 0.11 ± 0.003a |
| Amber glass | 20 °C | light | Closed throughout the storage period | 0.70 ± 0.01c | 5.72 ± 0.26c | 2.11 ± 0.02b | 0.18 ± 0.002c |
| 20 °C | dark | 0.62 ± 0.02a | 5.96 ± 0.44d | 2.10 ± 0.01b | 0.13 ± 0.001b | ||
| 4 °C | dark | 0.59 ± 0.03a | 4.77 ± 0.13d | 1.89 ± 0.02a | 0.11 ± 0.003a | ||
| Amber PET | 20 °C | light | 0.64 ± 0.03b | 7.72 ± 0.13e | 2.05 ± 0.02b | 0.13 ± 0.010b | |
| 20 °C | dark | 0.73 ± 0.04c | 7.57 ± 0.45d | 2.26 ± 0.19b | 0.14 ± 0.001b | ||
| 4 °C | dark | 0.61 ± 0.04a | 5.96 ± 0.13e | 2.06 ± 0.02b | 0.11 ± 0.004a | ||
| Amber glass | 20 °C | light | Once per month opened | 0.74 ± 0.06c | 28.21 ± 0.72i | 3.11 ± 0.07d | 0.21 ± 0.012d |
| 20 °C | dark | 0.70 ± 0.04c | 29.60 ± 1.31hi | 5.50 ± 0.41f | 0.23 ± 0.012e | ||
| 4 °C | dark | 0.62 ± 0.05a | 11.21 ± 0.57h | 2.19 ± 0.06b | 0.10 ± 0.002a | ||
| Amber PET | 20 °C | light | 0.75 ± 0.06c | 28.25 ± 0.04i | 3.47 ± 0.02d | 0.19 ± 0.002c | |
| 20 °C | dark | 0.69 ± 0.03c | 30.26 ± 1.48i | 4.73 ± 0.08e | 0.19 ± 0.002c | ||
| 4 °C | dark | 0.60 ± 0.04a | 12.7 ± 0.11h | 2.10 ± 0.05b | 0.11 ± 0.000a | ||
Different superscript letters within each column indicate significant differences (p < 0.05)
Initial value of PV of cold-pressed rapeseed oil was 2.9 meq O2/kg (Table 4). Contrary to acidity, PV showed statistically significant (p < 0.05) variations highly correlated with the storage conditions. The PVs for samples stored at 4 °C in closed containers were as follows: 4.8 meq O2/kg, for amber glass, and 6.0 meq O2/kg for amber PET. Respective PVs in the presence of oxygen at the same temperature were 11.21 meq O2/kg, for amber glass, and 12.7 meq O2/kg, for amber PET. When oil samples were stored in closed containers at 20 °C in the dark the PVs were as follows: 6.0 meq O2/kg (amber glass), and 7.6 meq O2/kg (amber PET). Respective PVs in the presence of oxygen, for samples kept in the dark, at the same temperature were 29.6 meq O2/kg, for amber glass, and 30.3 meq O2/kg, for amber PET. When samples were kept in closed containers and stored under the same temperature in the presence of light the PVs were 5.7 meq O2/kg (amber glass), and 7.7 meq O2/kg (amber PET), and the respective PVs in the presence of oxygen were as follows: 28.2 meq O2/kg and 28.3 meq O2/kg, for amber glass and amber PET, respectively. A strong correlation has been found between peroxides formation and oil exposure to oxygen, leading to conclusion that the most critical factor affecting the rate of oxidation in the cold-pressed rapeseed oil is the oxygen concentration. Similar findings were stated by Pristouri et al. (2010) who reported higher losses in extra virgin olive oil quality stored with high oxygen headspace concentrations as compared to those stored without the access of oxygen (no headspace).
As a result of auto-oxidation and by oxygen initially dissolved in the oil, K232 increased from 1.88 to 2.10 (amber glass), and 2.26 (amber PET) after 12 months of storage in the dark at 20 °C. In contrast, when exposed to oxygen, K232 increased to 5.50 (amber glass), and 4.73 (amber PET). In the presence of light, for samples stored at 20 °C, K232 increased to 2.10 (amber glass), and 2.05 (amber PET), when exposed to oxygen, K232 increased to 3.11 (amber glass), and 3.47 (amber PET). On the other hand, storage at 4 °C resulted in the slowest dienes formation, K232 increased to 1.89 (amber glass), and 2.06 (amber PET), and to 2.19 (amber glass), and 2.10 (amber PET) in the presence of oxygen in the headspace. Similarly to Pristouri et al. (2010), the most pronounced changes in K232 were caused by the access of oxygen, followed by storage temperature and the presence of light.
The initial value for K268 was 0.11. Respective K268 for oil samples stored at 4 °C, regardless of the access of oxygen, remained practically unchanged over entire storage period (Table 4).
Similar changes in K268 were caused by the presence of light, K268 increased to 0.13–0.14, regardless of the presence of light, and to 0.19–0.23, in the presence of the oxygen in the headspace. The above results were in general in agreement with the results obtained by Guillaume and Ravetti (2012) and Méndez and Falqué (2007), who reported that amber PET bottles are able to provide similar protection of olive oil against light as amber glass bottles.
Multivariate statistical analysis
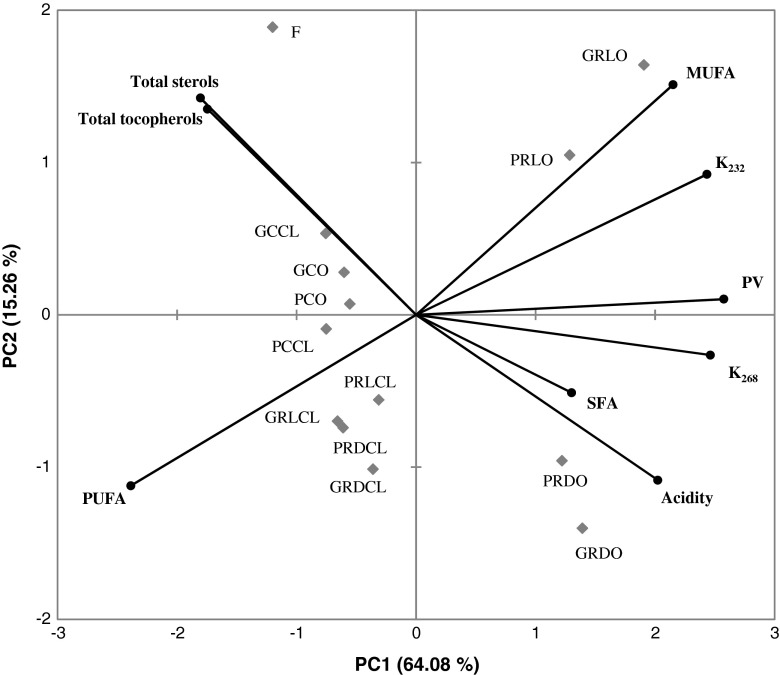
Results obtained by PCA applied to correlation matrix of the analytical data set (acidity, PV, K232, K268, total tocopherols, and total sterols), were depicted in Fig. 2. As can be seen from Table 5, the variability found in the samples was explained only by one component, which accounted for 64.08 % of variability. A significantly higher impact on the explanation of the variability was found in variables related to oil oxidative status: acidity (0.758), PV (0.966), K232 (0.912), and K268 (0.923), than in variables related to oil nutritional value, namely MUFA (0.806), and PUFA (−0.895), total tocopherols (−0.654), and total sterols content (−0.677).
Fig. 2.
Bi-plot of the first two PCs of cold-pressed rapeseed oil samples stored in different type of packaging under various storage conditions applied to the variables studied. Explanatory notes: F, freshly pressed rapeseed oil (control oil sample); C/R, storage temperature (cold/room); G/P, container (amber glass/amber PET); L/D, illumination (light/dark); CL/O, access of oxygen (closed/open)
The PCA graph revealed that samples distribution on the bi-plot was primarily determined by the storage conditions. In fact, a separation between fresh and stored under various conditions oil samples was found along PC1 – fresh rapeseed oil sample was located on the upper left zone of the plane, samples stored under refrigerated temperature, regardless of the access of oxygen, were located in the central zone of the plane, while all oil samples stored with the access of oxygen at RT, regardless of the presence of light, were placed on the lower left zone of the plane, where PC1 scores are negative. On the PC plane, samples stored with the access of oxygen, at RT, were placed on upper (samples exposed to light), and lower (samples kept in the dark) right portion of the bi-plot. In oil samples stored under mentioned above conditions the most pronounced deterioration in cold-pressed rapeseed oil quality occurred.
It is worthy to note, that oil samples distribution stored under identical conditions but kept in different containers were located in the same zones of the PC plane, indicating that both amber glass and amber PET containers are able to provide similar retention of cold-pressed rapeseed oil quality during long-term storage.
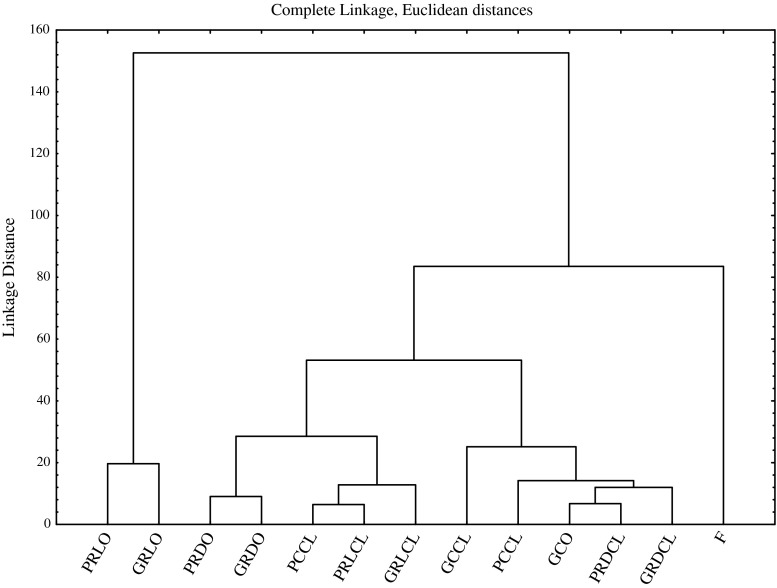
The dendrogram resulting from cluster analysis ordered storage conditions in a hierarchical manner, applying the nearest neighbour method with the Euclidean distance measure based on presence or absence of a particular component. In our study oil samples were classified by the type of packaging material, storage temperature, absence/presence of light, and absence/presence of oxygen. Figure 3 showed the resulted dendrogram obtained from HCA, where 3 clusters can be identified. Cluster 1 contains samples stored at RT, in the dark, without the access of oxygen, and samples stored at 4 °C both with and without the access of oxygen. Cluster 2 contains two distinct subclusters: first combines oil samples stored at RT, in the presence of light, without the access of oxygen, and sample stored at 4 °C with the presence of oxygen in the headspace, while second joins samples of oil stored at RT, in the dark, with the access of oxygen. Cluster 3 formed by oils stored at RT, with the presence of light and oxygen separates itself entirely from all the other, which indicated that these storage conditions are least favourable for the storage of cold-pressed rapeseed oil.
Fig. 3.
HCA dendrographic classification of rapeseed oil according to the type of packaging, storage temperature, exposure to light and access of oxygen. Explanatory notes: F, freshly pressed rapeseed oil (control oil sample); C/R, storage temperature (cold/room); G/P, container (amber glass/amber PET); L/D, illumination (light/dark); CL/O, access of oxygen (closed/open)
As discussed, samples stored under the same conditions but in different containers are classified into one clusters, with the exception of oil samples stored at 4 °C without the access of oxygen, which are separated by the type of packaging material in cluster 1 (amber glass), and 2 (amber PET), which indicates that glass containers are slightly more effective for the storage of cold-pressed rapeseed oil than amber PET containers.
Conclusions
Based on the above results the following conclusions may be drawn:
The relative contribution of storage conditions studied to the retention of rapeseed oil nutritional value can be ordered as follows: storage at 4 °C > storage at 20 °C, in the dark > storage at 20 °C, with the access of light.
Containers made of amber glass and amber PET are able to ensure comparable retention of cold-pressed rapeseed oil quality over 12 months of storage.
Rapeseed oil samples kept closed over entire storage period both in amber glass and amber PET containers under refrigeration conditions (4 °C) maintained its original profile over entire storage.
At both storage in the dark and in the presence of light at room temperature, similar loss in quality during storage was noted. This indicates the suitability of amber PET as a cheaper alternative to amber glass for cold-pressed oils packaging.
Results obtained in this study reaffirmed that the access of oxygen cause the most pronounced losses in minor components and substantial deterioration in cold-pressed rapeseed oil qualitative properties, especially in combination with light exposure.
Taking the above results into account it seems advisable, therefore, to label cold-pressed rapeseed oils with information on storage conditions recommended after their opening.
Acknowledgments
The present study was financed by the National Science Centre in Poland (project no. N N312 256740).
Abbreviations
- HCA
Hierarchical cluster analysis
- PCA
Principal component analysis
- PET
Polyethylene terephthalate
- PV
Peroxide value
- RT
Room temperature
- α-T
Alpha tocopherol
- γ-T
Gamma tocopherol
Compliance with ethical standards
Conflict of interest
The authors have declared no conflict of interest.
Footnotes
Research highlights
• Both amber glass and PET bottles ensured comparable preservation of rapeseed oil quality.
• Rapeseed oil samples kept at 4 °C maintained its original profile over 12 months of storage.
• Similar loss in oil quality occurred during samples storage under light and in the dark.
• Access of oxygen cause the most pronounced losses in minor components.
References
- Ayton J, Mailer RJ, Graham K (2012) The effect of storage conditions on extra virgin olive oil quality (accessed Apr. 2012). RIRDC Publication No. 12/024. RIRDC Project No. PRJ-002297. https://rirdc.infoservices.com.au
- Cecchi T, Passamonti P, Cecchi P. Study of the quality of extra virgin olive oil stored in PET bottles with or without an oxygen scavenger. Food Chem. 2010;120:730–735. doi: 10.1016/j.foodchem.2009.11.001. [DOI] [Google Scholar]
- Choe E, Min D. Mechanisms and factors for edible oil oxidation. Compr Rev Food Sci Food Saf. 2006;5:169–186. doi: 10.1111/j.1541-4337.2006.00009.x. [DOI] [Google Scholar]
- Choe E, Min D. Mechanisms of antioxidants in the oxidation of oils. Compr Rev Food Sci Food Saf. 2009;8:345–358. doi: 10.1111/j.1541-4337.2009.00085.x. [DOI] [Google Scholar]
- Dubois V, Breton S, Linder M, Fanni J, Parmentier M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur J Lipid Sci Tech. 2007;109:710–732. doi: 10.1002/ejlt.200700040. [DOI] [Google Scholar]
- Fadda C, Del Caro A, Sanguinetti AM, Urgeghe PP, Vacca V, Arca PP, Piga A. Changes during storage of quality parameters and in vitro antioxidant activity of extra virgin monovarietal oils obtained with two extraction technologies. Food Chem. 2012;134:1542–1548. doi: 10.1016/j.foodchem.2012.03.076. [DOI] [PubMed] [Google Scholar]
- Guillaume C, Ravetti L (2012) Evaluation of new analytical methods to detect lower quality olive oils (accessed Jun. 2012). RIRDC Publication No. 12/007, RIRDC Project No. PRJ-003349. https://rirdc.infoservices.com.au/items/12-007
- ISO (1996) Animal and vegetable fats and oils. Determination of peroxide value. International Organization for Standardization, Geneva (ISO 3960)
- ISO (2000) Animal and vegetable fats and oils. Preparation of methyl esters of fatty acids. International Organization for Standardization, Geneva (ISO 5509)
- ISO (2005) Animal and vegetable fats and oils. Determination of acid value and acidity. International Organization for Standardization, Geneva (ISO 660)
- ISO (2011) Animal and vegetable fats and oils. Determination of ultraviolet absorbance expressed as specific UV extinction. International Organization for Standardization, Geneva (ISO 3656)
- List GR, Wang T, Shukla VKS. Storage, handling and transport of oils. In: Shahidi F, editor. Bailey’s industrial oil and fat products and fats. New Jersey: Wiley; 2005. pp. 191–229. [Google Scholar]
- Méndez AI, Falqué E. Effect of storage time and container type on the quality of extra virgin olive oil. Food Control. 2007;18:521–529. doi: 10.1016/j.foodcont.2005.12.012. [DOI] [Google Scholar]
- Mezouari S, Eichner K. Comparative study on the stability of crude and refined rice bran oil during long-term storage at room temperature. Eur J Lipid Sci Tech. 2007;109:198–205. doi: 10.1002/ejlt.200600154. [DOI] [Google Scholar]
- Morello JR, Motilva MJ, Tovar MJ, Romero MP. Changes in commercial virgin olive oil (cv. Arbequina) during storage, with special emphasis on the phenolic fraction. Food Chem. 2004;85:357–364. doi: 10.1016/j.foodchem.2003.07.012. [DOI] [Google Scholar]
- Niewiadomski H. Preliminary technological operations. In: Niewiadomski H, editor. Rapeseed – chemistry and technology. Amsterdam: Elsevier; 1990. pp. 123–160. [Google Scholar]
- Obiedzińska A, Waszkiewicz-Robak B. Cold pressed oils as functional food. Food Sci Tech Quality. 2012;80:27–44. [Google Scholar]
- Okogeri O, Tasioula-Margari M. Changes occurring in phenolic compounds and alpha-tocopherol of virgin olive oil during storage. J Agric Food Chem. 2002;50:1077–1080. doi: 10.1021/jf010895e. [DOI] [PubMed] [Google Scholar]
- Pekkarinen S, Hopia A, Heinonen M. Effect of processing on the oxidative stability of low erucic acid turnip rapeseed (Brassica rapa) oil. Fett-Lipid. 1998;3:69–74. doi: 10.1002/(SICI)1521-4133(199803)100:3<69::AID-LIPI69>3.0.CO;2-H. [DOI] [Google Scholar]
- Pristouri G, Badeka A, Kontominas MG. Effect of packaging material headspace, oxygen and light transmission, temperature and storage time on quality characteristics of extra virgin olive oil. Food Control. 2010;21:412–418. doi: 10.1016/j.foodcont.2009.06.019. [DOI] [Google Scholar]
- Przybylski R, Eskin NAM. Minor components and the stability of vegetable oils. Inform. 2006;17:187–189. [Google Scholar]
- Robertson GL. Vegetable oils. In: Robertson GL, editor. Food packaging: principles and practice. New York: CRC Press; 2012. pp. 503–505. [Google Scholar]
- Sacchi R, Savarese M, Del Regno A, Paduano A, Terminiello P, Ambrosino ML. Shelf life of vegetables oils bottled in different scavenging polyethylene terephthalate (PET) containers. Packag Technol Sci. 2008;21:269–277. doi: 10.1002/pts.801. [DOI] [Google Scholar]
- Samaniego-Sanchez C, Oliveras-Lopez MJ, Quesada-Granados JJ, Villalon-Mir M, Lopez-G Serrana H. Alterations in picual extra virgin olive oils under different storage conditions. Eur J Lipid Sci Technol. 2012;114:194–204. doi: 10.1002/ejlt.201100191. [DOI] [Google Scholar]