Abstract
The Korean traditional hot sauce gochujang has been reported to have biological activities. Different kinds of gochujang products were prepared based on combinations of a fungal rice koji with two kinds of bacterial soybean mejus. Diets that included gochujang products were fed to rats and anti-obesity effects were investigated. Gochujang products reduced body weight gains, epididymal fat weights, and triglyceride levels in the serum and the liver. Effects were exerted by the diet that included the non-fermented gochujang mixture, increased using a fungal rice koji, and further enhanced using a bacterial soybean meju. Dietary effects were apparently induced via inhibition of the lipogenic enzymes fatty acid synthase, malic enzyme, and lipoprotein lipase by gochujang products in epididymal adipose tissues, and inhibition of glucose-6-phosphate dehydrogenase in the liver. High levels of capsaicin and genistein in gochujang products are considered to contribute to anti-obesity effects.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-015-2162-z) contains supplementary material, which is available to authorized users.
Keywords: Anti-obesity, Fermented gochujang, Rice koji, Soybean meju
Introduction
Gochujang is a Korean fermented food that is traditionally prepared in a paste form by mixing red pepper, grains of rice, barley, and/or wheat, and soybean meju with water (Kwon et al. 2015; Burges 2014). There are two methods for preparation of a gochujang product. These are a traditional home-based method and a commercial mass production method (Kim et al. 2010; Shin and Jeong 2015). For the traditional home-based product, after soybeans are cooked the resulting paste is molded into a rectangular shape and left to hang in the air for several months. Fungi and/or bacteria in the air naturally stick to the soybean paste and grow. The resulting fermented product is called meju. The final gochujang product is prepared by mixing rice powder, red pepper, salt, meju, and water.
On the other hand, for commercial mass production, the koji product is produced by cultivation of a fungus onto rice grains or flakes. The commercial gochujang product is made as a paste by mixing steamed rice powder, soybean flakes, red pepper, salt, meju, and the koji product with water.
Koji products contain the hydrolytic enzymes amylase, protease, and lipase that are produced by fungal and/or bacterial cells grown on soybeans or rice powder (Li et al. 2010). During fermentation, the enzymes hydrolyze starch, proteins, and lipids in rice and soybeans resulting in small molecules of sugars, amino acids, oligosaccharides, oligo-peptides, and fatty acids. The fungal and bacterial microorganisms that are used for production of koji products are species of Aspergillus, Mucor, and Rhizopus, and Bacillus species, respectively. These microorganisms produce compounds with unique flavors and tastes, and degrade unpleasant odor components.
Gochujang products are usually used as a cooking sauce. In recent years, with a focus on dietary healthy foods, anti-atherosclerotic, anti-obesity, and anti-cholesterol effects of gochujang products have been reported. Capsaicin, a key component of red pepper, has been reported to exert anti-oxidant and anti-cancer effects, to enhance lipid metabolism and immunity (Reyes-Escogido et al. 2011), and to prevent obesity and illness (Leung 2014). In this study, different koji products prepared using fermentation of rice and soybeans with a fungus and a bacterium, respectively, were combined for preparation of different gochujang products. The anti-obesity effects of diets that included gochujang products fed to rats were investigated.
Materials and methods
Materials and strains
Corn starch was obtained from CJ CheilJedang Co. (Seoul, Korea). Vitamin-mixture and Mineral-mixture diets were obtained from Dyets Inc. (Bethlehem, USA). Other chemicals were purchased from Sigma Chemical Co. (St. Louis, USA). 3H triolein and eleven other compounds for lipoprotein lipase activity analysis were purchased from Amersham (Buckinghamshire, UK). An HDL-C analysis kit was bought from Fujifilm Co. (Tokyo, Japan), and a radioimmunoassay kit was purchased from Linco Research Immnunoassay (St. Charles, USA).
The microorganisms Bacillus amyloliquefaciens CJ 3-27 (KCCM 11317P) (KCCM: Korean Culture Center of Microorganisms; Seoul, Korea), Bacillus amyloliquefaciens CJ 14-6 (KCCM 11718P), and Aspergillus oryzae CJ 1354 (KCCM 11300P) were patented strains of the CJ CheilJedang Co.
Fermentation procedures for preparation of soybean meju and rice koji
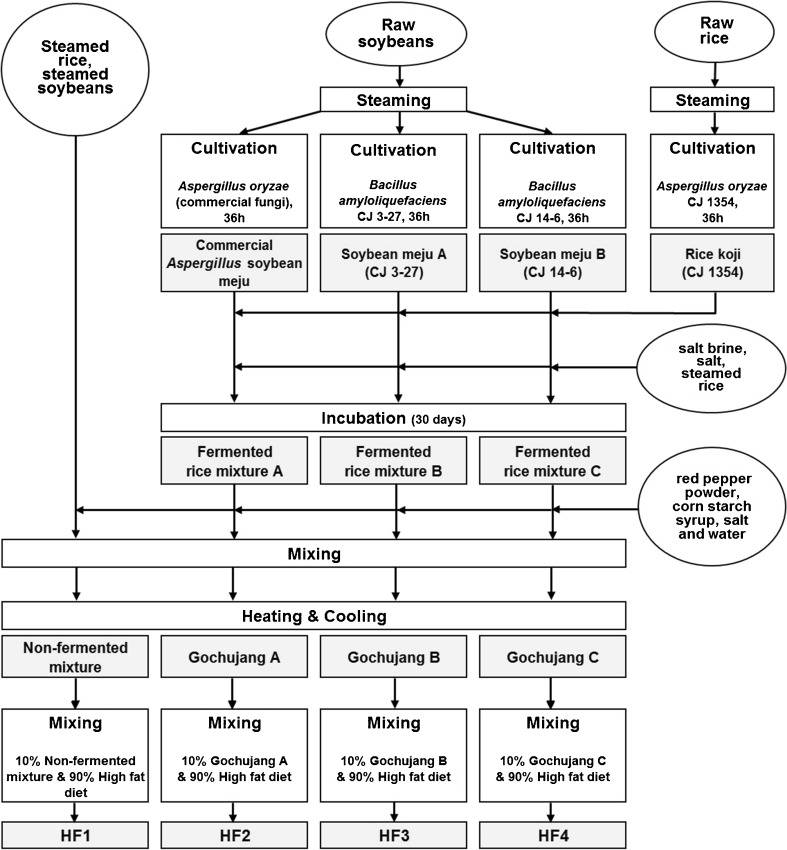
For preparation of rice koji, one kilogram of rice was immersed for 30 min in 3 L water and then water was removed. The wet rice was sterilized using an autoclave for 15 min at 1 atm, then cooled to 35 °C. The fungal strain A. oryzae CJ 1354 was inoculated onto 500 mL flasks containing 50 g of steamed rice, and cultivated for 5 days in an incubator at 35 °C. The cultured rice was added at 0.2 % (w/w) to steamed rice, followed by incubation in a chamber at 35 °C for 36 h, then drying at 35 °C for 24 h. The fermented product was called rice koji (CJ 1354) (Fig. 1).
Fig. 1.
A process scheme for preparation of diets including different types of gochujang products
For preparation of soybean meju, the bacterial strains B. amyloliquefaciens CJ 3-27 and B. amyloliquefaciens CJ 14-6 were inoculated onto 500 mL flasks including 200 mL of Nutrient Broth (BD, pH7.0), followed by incubation for 24 h on a rotary shaker at 37 °C and 200 rpm. One kilogram of soybeans was immersed for 30 min in 3 L water and then water was removed. The wet soybeans were sterilized using an autoclave for 15 min at 1 atm, then cooled to 35 °C. Cultured broths were added at 2 % (v/w) to steamed soybeans, then the mixtures were incubated for 36 h in a chamber at 37 °C. Fermented soybeans were dried for 24 h at 35 °C. Resultant products were called soybean meju A for B. amyloliquefaciens CJ 3-27 and soybean meju B for B. amyloliquefaciens CJ 14-6 (Fig. 1).
After 0.14 kg of steamed rice, 0.04 kg of salt and 0.32 kg of salt water (20 % (w/v) NaCl) were mixed with 0.35 kg of rice koji, the mixture was incubated at 30 °C for 30 days. When the product was supplemented with 0.15 kg of a commercial Aspergillus soybean meju (Doo Da Won Foodstuff Co., LTD., Dandong, China), it was called fermented rice mixture A. When the product that included rice koji was supplemented with 0.15 kg of Bacillus meju A and Bacillus meju B separately, they were called fermented rice mixture B and fermented rice mixture C, respectively.
Procedures for preparation of gochujang products
As shown in Fig. 1, different gochujang products were prepared by addition of 0.45 kg of each fermented rice mixture to a mixture consisting of 0.113 kg of red pepper powder (Capsicum annuum), 0.27 kg of corn starch syrup (CJ CheilJedang Co., Seoul, Korea), 0.025 kg of purified salt (Hanju Co., LTD., Ulsan, Korea), and 0.142 kg of tap water. Gochujang products A, B and C were named for fermented rice A, B and C, respectively. Gochujang products were heated for 15 min at 70 °C. A non-fermented mixture was prepared in the same manner as for gochujang products, except that rice and soybean were used instead of rice koji and soybean meju, respectively.
Procedures for analysis of phenolic compounds
Phenolic compounds of non-fermented mixture and gochujang products were analyzed. Samples (500 mg) were subjected to extraction using 1 mL of 80 % methanol in a 2 mL Eppendorf tube, then centrifuged at 16000 rpm for 10 min at 4 °C. The supernatant was filtered through a 0.2 μm polytetrafluoroethylene (PTFE) filter and dried in a speed vacuum concentrator (Biotron, Seoul, Korea). Dried samples were re-dissolved in 80 % methanol, then filtered.
Phenolic compounds in samples were analyzed using ultra high-performance liquid chromatography and mass spectrometry (UHPLC-LTQ-IT-MS/MS) with a LTQ XL linear ion-trap mass spectrometer (Thermo Scientific, USA) equipped with a binary solvent delivery system, an auto-sampler, and a photodiode array detector (Ultimate 3000), and Syncronis™ C18 column (i.d., 100 mm × 2.1 mm, 5 μm particle size; Thermo Scientific). A mixture of 0.1 % formic acid in water (v/v) and 0.1 % formic acid in acetonitrile (v/v) was used as the mobile phase. Solvent elution was performed in gradient mode initiated with 10 % acetonitrile for 1 min, gradually increased to 100 % acetonitrile for 14 min, remaining at 100 % acetonitrile for 3 min, and decreased to 10 % acetonitrile for 1 min. Electron spray ionization was performed in the positive and negative ion modes within an m/z range of 100 − 1000.
Rat feeding
Forty male Sprague–Dawley rats at 5 weeks of age were purchased from Central Lab Animal Inc. (Seoul, Korea). Rats had unrestricted access to rat chow and water in an animal facility for 1 week before experiments. All rats were divided into five groups, each with eight rats, and fed for 5 weeks with a high fat diet (AIN-93 diet) (Reeves et al. 1993) supplemented at 10 % (w/w) with 1) nothing (HF), 2) a non-fermented mixture (HF1), 3) gochujang A (HF2), 4) gochujang B (HF3), and 5) gochujang C (HF4) (Table 1). Rats were housed in individual stainless steel cages under controlled conditions at 21 ± 2 oC with a 12-h light/dark cycle (08:00 − 20:00 h light). Water and feed were provided ad libitum during the experimental period. Feed intake and body weight were recorded every week. The experimental protocol used in this study was approved by the Institutional Animal Care and Use Committee of Chosun University (CIACUC 2014-S0006).
Table 1.
Compositions of gochujang diets (g/kg diet)
| Diet composition | Diet groups | ||||
|---|---|---|---|---|---|
| HF | HF1 | HF2 | HF3 | HF4 | |
| Casein | 200.0 | 195.0 | 195.0 | 190.0 | 190.0 |
| DL-methionine | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Corn starch | 400.0 | 300.0 | 300.0 | 300.0 | 300.0 |
| Sucrose | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Cellulose | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Lard | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 |
| Mineral mix1) | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 |
| Vitamin mix2) | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Choline chloride | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Non-fermented mixture | – | 100.0 | – | – | – |
| Gochujang A | – | – | 100.0 | – | – |
| Gochujang B | – | – | – | 100.0 | – |
| Gochujang C | – | – | – | – | 100.0 |
Diet groups: HF = high fat diet (AIN-93 diet) (Reeves et al. 1993); HF1 = high fat diet + non-fermented mixture; HF2 = high fat diet + gochujang A; HF3 = high fat diet + gochujang B; HF4 = high fat diet + gochujang C
Preparation of rat serum samples and histology of adipose and liver tissues
At the end of the feeding period, rats were fasted for 12 h before being anesthetized using CO2. Blood samples from each rat were collected in tubes, then sera were separated from blood by centrifugation at 1,500 × g (Combi-514R, Hanil, Korea) for 20 min at 4 °C. After bleeding, liver and adipose tissues, including epididymal, mesenteric, retroperitoneal, and perirenal, were quickly excised, weighed, and frozen in liquid nitrogen.
Epididymal adipose tissues were dissected and fixed in a 3 % formaldehyde solution, then embedded in paraffin and stained with hematoxylin-eosin. Sizes of adipocyte cells were measured in randomly chosen microscopic fields at × 100 magnification under a light microscope (Olympus, Tokyo, Japan). The average adipocyte size was calculated as a division of the microscopic viewing field area by total adipocyte cell number in the field area.
Methods for measurement of lipid and leptin contents, and activities of enzymes in sera
Triglyceride, total cholesterol, and HDL-cholesterol levels in sera were measured using an auto-chemistry analyzer (Fugi Dri-Chem 3500, Fujifilm, Japan). The LDL-cholesterol level was estimated using the equation as: LDL-cholesterol = total cholesterol – (HDL-cholesterol – triglyceride/5). Values of the atherogenic index (AI) and the cardiac risk factor (CRF) were calculated as: (Rosenfeld 1989): AI = (total cholesterol – HDL-cholesterol)/HDL-cholesterol, and CRF = total cholesterol/HDL-cholesterol. Activities of alanine transaminase (ALT), asparate transaminase (AST), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) were determined using an auto-chemistry analyzer (Fugi Dri-Chem 3500, Fujifilm, Japan). The serum leptin level was determined using a radioimmuno assay kit (Linco Research Immnunoassay, St. Charles, Mo, USA) and a liquid scintillation counter (LS100C, Beckman Co., USA).
Measurement of triglyceride and total cholesterol contents in liver and adipose tissues
Lipids from liver and epididymal adipose tissues were extracted. A solution of chloroform-methanol (2:1, v/v) was added to 0.1 g of tissue and the resultant mixture was incubated at 4 °C for 3 days. After water was added to the mixture, followed by centrifugation at 3,000 rpm for 20 min (Combi-514R, Hanil, Korea), the bottom layer of lipids was recovered. The total cholesterol content was measured following the method of Zlatkis and Zak (1969) and the triglyceride content was measured following the method of Biggs et al. (1975).
Measurement of lipogenic enzyme activities in liver and adipose tissues
Activities of fatty acid synthase (FAS; EC 2.3.1.85), malic enzyme (ME; EC 1.1.1.40), and glucose-6-phosphate-dehydrogenase (G6PDH; EC 1.1.1.49) in liver and adipose tissues were determined by the procedures outlined by Linn (1981) and Zabala et al. (2004).
Measurement of HR-LPL and TE-LPL activities in adipose tissues
The heparin-releasable LPL (HR-LPL) activity was measured using the method of Nilsson-Ehle and Schotz (1976). The total-extractable LPL (TE-LPL) activity was determined (Iverius and Brunzell 1985). A 3H-triolein emulsion stabilized with glycerol was used as a substrate. One unit of LPL activity was defined as release of 1 μmol of free fatty acid in 1 h.
Statistical analysis
Data were expressed as mean ± SEM and analyzed using the SPSS software package (IBM SPSS Statistics, Version 21). Differences between means were assessed using Tuckey’s test. Significant differences were defined as p < 0.05.
Results
Diet intake rates and body weight gains of rat groups
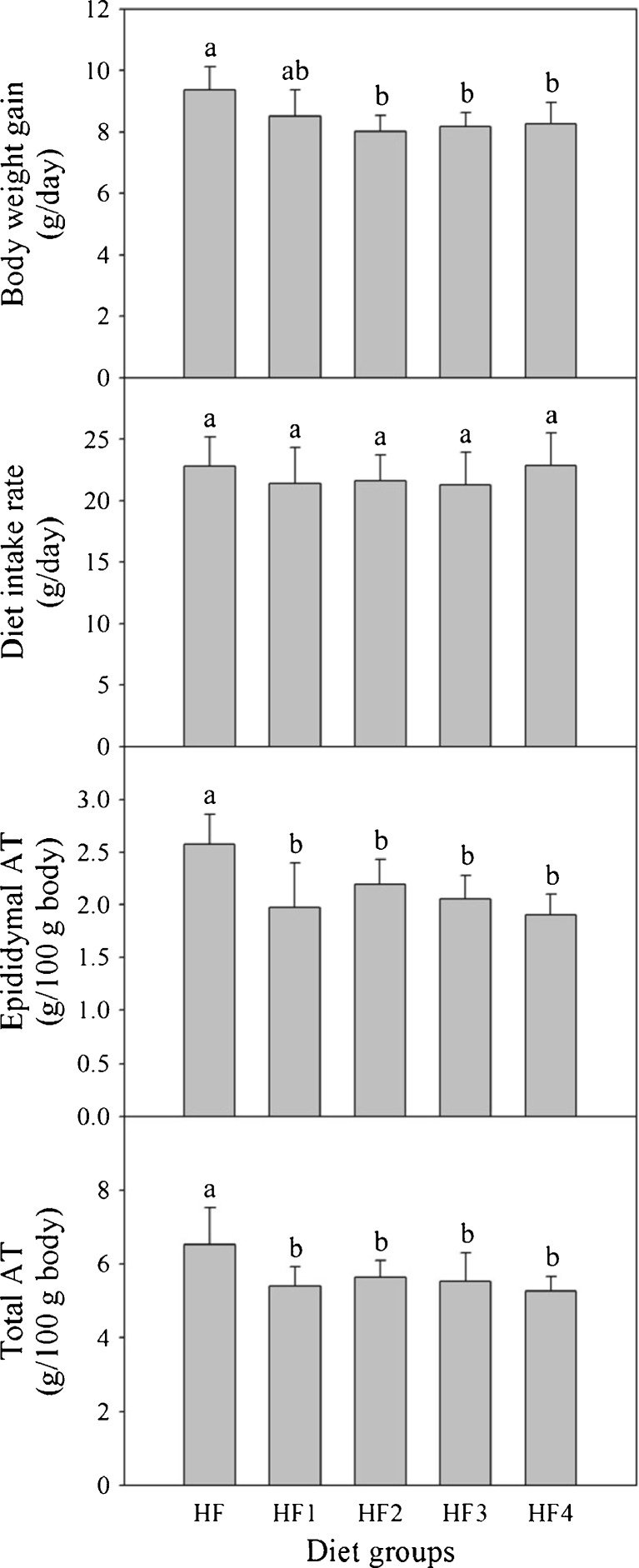
After rats of the five treatment groups were fed with diets consisting of a high fat diet and supplementation with different gochujang products, feed amounts consumed by rats and body weight gains were measured. There were no significant differences in diet intake rates among rats of the five groups (Fig. 2). Body weight gains of gochujang groups (HF2, HF3, and HF4) were 13.3 − 14.1 % less than for control group (HF) who consumed no gochujang product. There were no significant differences in body weight gain among gochujang groups. The body weight gain of HF1 group fed with a diet containing a non-fermented mixture was 9.1 % less than for control group (HF) with no statistical difference.
Fig. 2.
Body weight gains, diet intake rates, and weights of epididymal adipose tissues and total adipose tissues of rats fed different diets for 5 weeks. Values are expressed as mean ± SE (n = 8). Values with different superscript letters are significantly different at p < 0.05 based on Tukey’s test. Diet groups: HF = high fat diet; HF1 = high fat diet + non-fermented mixture; HF2 = high fat diet + gochujang A; HF3 = high fat diet + gochujang B; HF4 = high fat diet + gochujang C. AT = adipose tissue
Changes in weights of epididymal adipose tissue and liver in rats
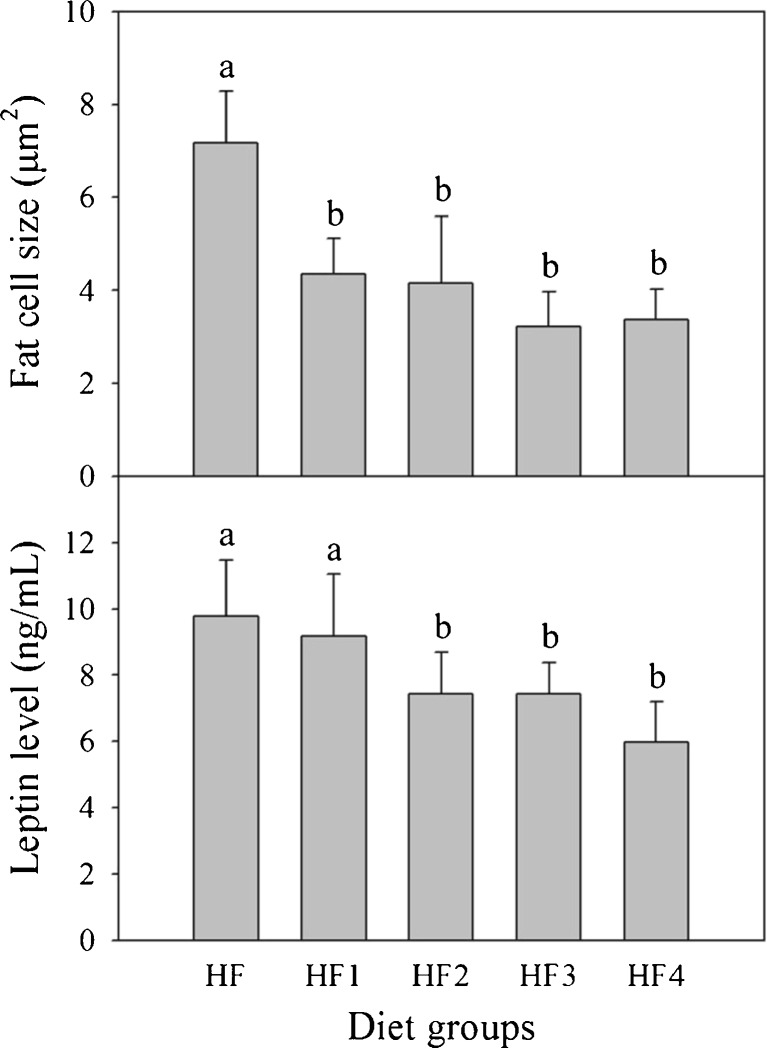
Weights of adipose tissues in rats fed different diets were measured (Fig. 2). Epididymal adipose tissue weights of non-fermented mixture group (HF1) and gochujang groups (HF2, HF3 and HF4) were 16.3 − 17.1 % less than for control group (HF). There were no significant differences among diet groups, excluding control group. The pattern for total adipose tissue weights was similar to that for epididymal adipose tissue, with 13.6 − 17.9 % reductions compared with control group (HF). The sizes of fat cells in the epididymal adipose tissues of non-fermented mixture group (HF1) and gochujang groups (HF2, HF3, and HF4) were reduced, compared with control group (Fig. 3). There were no significant differences in liver weights among the five groups (HF, HF1, HF2, HF3 and HF4) (Supplementary Fig. 1).
Fig. 3.
Fat cell sizes of epididymal adipose tissues and serum leptin levels of rats fed different diets for 5 weeks. Values are expressed as mean ± SE (n = 8). Values with different superscript letters are significantly different at p < 0.05 based on Tukey’s test. Diet groups: HF = high fat diet; HF1 = high fat diet + non-fermented mixture; HF2 = high fat diet + gochujang A; HF3 = high fat diet + gochujang B; HF4 = high fat diet + gochujang C
Effects of gochujang diets on reduction of lipids in sera
Triglyceride and cholesterol levels in rat sera were determined. Triglyceride and LDL-cholesterol levels of diet groups were in the order of HF > HF1 > HF2 > HF3 > HF4, and gochujang groups showed 27.9 − 33.9 % and 30.8 − 46.9 % reductions in triglyceride and LDL-cholesterol levels, respectively, over control group (HF) (Table 2). However, total cholesterol and HDL-cholesterol levels did not show significant differences among the five groups (HF, HF1, HF2, HF3 and HF4). The therogenic index (AI) and the cardiac risk factor (CRF) values of non-fermented mixture group (HF1) and gochujang groups (HF2, HF3, and HF4) were greatly reduced by 48.1 − 62.0 % and 22.3 − 29.6 %, respectively, over control group (HP). HF4 group showed the lowest AI and CRF values.
Table 2.
Contents of triglyceride, total cholesterol, LDL-cholesterol, and HDL-cholesterol, and AI and CRF values in the serum, liver, and epididymal adipose tissue of rats fed different diets for 5 weeks
| Groups | Serum | Liver | Epididymal AT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Triglyceride | Total cholesterol | HDL-cholesterol | LDL-cholesterol | AI | CRF | Triglyceride | Total cholesterol | Triglyceride | Total cholesterol | |
| HF | 98.00 ± 6.78a | 73.38 ± 5.04a | 43.38 ± 3.09a | 49.60 ± 6.11a | 0.79 ± 0.23a | 1.79 ± 0.23a | 13.38 ± 0.94a | 23.74 ± 1.27a | 76.45 ± 4.10a | 33.58 ± 0.93a |
| HF1 | 84.25 ± 1.51ab | 66.45 ± 2.27a | 48.75 ± 3.26a | 34.35 ± 2.19b | 0.39 ± 0.07ab | 1.39 ± 0.07ab | 8.92 ± 0.30b | 13.91 ± 0.67bc | 66.42 ± 2.84ab | 30.64 ± 0.64ab |
| HF2 | 70.70 ± 3.87bc | 68.55 ± 2.75a | 48.63 ± 2.42a | 34.06 ± 1.24b | 0.41 ± 0.05 ab | 1.37 ± 0.05 ab | 5.60 ± 0.34b | 12.47 ± 0.69c | 63.82 ± 1.58b | 26.39 ± 1.41c |
| HF3 | 68.50 ± 6.04bc | 66.13 ± 4.06a | 48.50 ± 3.17a | 31.33 ± 1.34b | 0.37 ± 0.02 ab | 1.36 ± 0.02 ab | 5.79 ± 0.48b | 12.22 ± 0.50bc | 52.57 ± 1.59c | 27.60 ± 0.56bc |
| HF4 | 64.77 ± 1.79c | 64.00 ± 3.36a | 50.63 ± 5.12a | 26.33 ± 3.51b | 0.31 ± 0.07b | 1.26 ± 0.07b | 5.16 ± 0.54b | 13.44 ± 0.66b | 56.01 ± 1.31bc | 26.01 ± 0.99ab |
Values are expressed as mean ± SE of rats (n = 8 for each group) fed different diets for 5 weeks. Values with different superscript letters are significantly different at p < 0.05 based on Tukey’s test. Diet groups: HF = high fat diet; HF1 = high fat diet + non-fermented mixture; HF2 = high fat diet + gochujang A; HF3 = high fat diet + gochujang B; HF4 = high fat diet + gochujang C. AT = adipose tissue
AI (atherogenic index) = (total cholesterol − HDL-cholesterol)/HDL-cholesterol; CRF (cardiac risk factor) = total cholesterol/HDL-cholesterol
AT = adipose tissue
In rat sera, leptin levels of gochujang groups (HF2, HF3, and HF4) were lower than for control group by 28.6 − 37.5 % (Fig. 3). The serum leptin level of non-fermented diet group (HF1) was not significantly different from control group (HF).
Effects of gochujang diets on reduction of lipid levels in liver and epididymal adipose tissues
Triglyceride and total cholesterol levels in rat liver and adipose tissues were measured (Table 2). In liver tissues, triglyceride levels of diet groups were divided into three levels in the order of HF > HF1 > HF2 ≡ HF3 ≥ HF4, and levels in gochujang groups (HF2, HF3, and HF4) were 56.7 − 61.4 % lower than for control group (HD). Total cholesterol levels were divided into two levels of control group and non-fermented mixture/gochujang groups. The latter values were 41.3 − 48.5 % lower than for control group.
Triglyceride levels in epididymal adipose tissues of diet groups were divided into three levels in the order of HF > HF1 ≡ HF2 > HF3 ≡ HF4 (Table 2). Values for HF3 and HF4 that consumed both rice koji and soybean meju were 26.7 − 31.2 % lower than for control group. On the other hand, total cholesterol levels of gochujang groups (HF2, HF3, and HF4) were significantly lower than for control group (HF) and non-fermented mixture group (HF1).
Toxicity of gochujang diets for rats
Activities of the ALT, AST, ALP, and LDH enzymes in sera after feeding of diets were measured for toxicity testing (Supplementary Table 1). ALT activities of non-fermented mixture group and gochujang groups were 19.7 − 36.1 % lower than for control group (HF). There were no significant differences in AST activities between all diet groups. ALP and LDH activities were identified in the order of HF > HF1 > HF2 > HF3 > HF4. HF4 group showed the lowest activities of ALT, AST, ALP, and LDH. Activities of ALT, ALP, and LDH in sera are used as indices for liver damage. All activities in this study were in the normal range, indicating that gochujang diets did not damage the rat liver.
Changes in activities of lipogenic enzymes
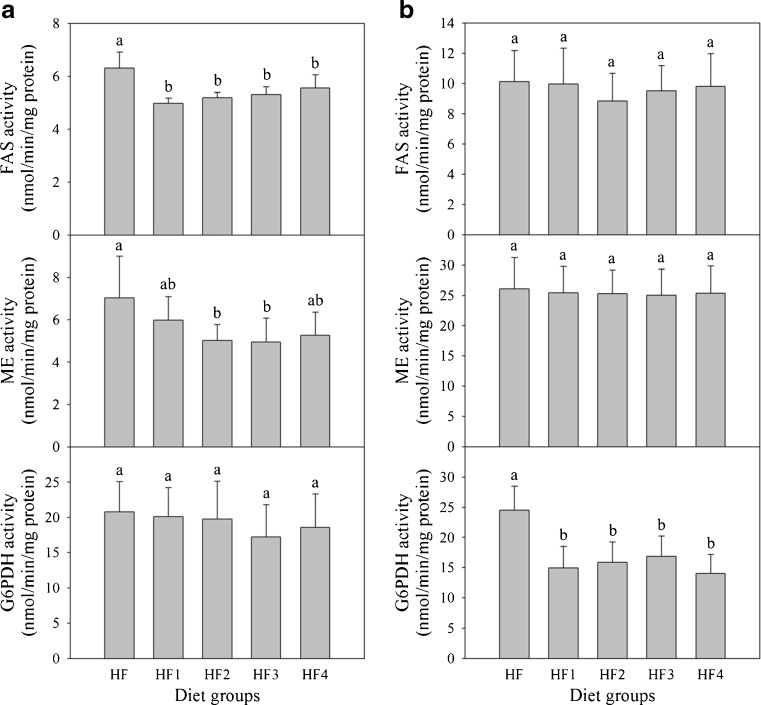
FAS activities in epididymal adipose tissues of non-fermented mixture group (HF1) and gochujang groups (HF2, HF3, and HF4) were significantly lower than for control group (HF) by 12.0 − 17.6 % (Fig. 4a). ME activities of gochujang groups (HF2, HF3, and HF4) were significantly lower than for non-fermented mixture group (HF1) and control group (HF). G6PDH activities were not significantly different among all diet groups.
Fig. 4.
Activities of FAS, ME, and G6PDH in epididymal adipose (a) and liver tissues (b) of rats fed different diets for 5 weeks. Values are expressed as mean ± SE (n = 8). Values with different superscript letters are significantly different at p < 0.05 based on Tukey’s test. Diet groups: HF = high fat diet; HF1 = high fat diet + non-fermented mixture; HF2 = high fat diet + gochujang A; HF3 = high fat diet + gochujang B; HF4 = high fat diet + gochujang C. FAS = fatty acid synthase; ME = malic enzyme; G6PDH = glucose-6-phosphate dehydrogenase
Activities of FAS, ME, and G6PDH were measured in liver tissues (Fig. 4b). Activities of FAS and ME were not significantly different among all diet groups. However, G6PDH activities of non-fermented mixture group (HF1) and gochujang diet groups (HF2, HF3, and HF4) were much lower than for control group by 31.3 − 42.8 %. There were no significant differences among gochujang groups.
LPL activities in epididymal adipose tissues of diet groups
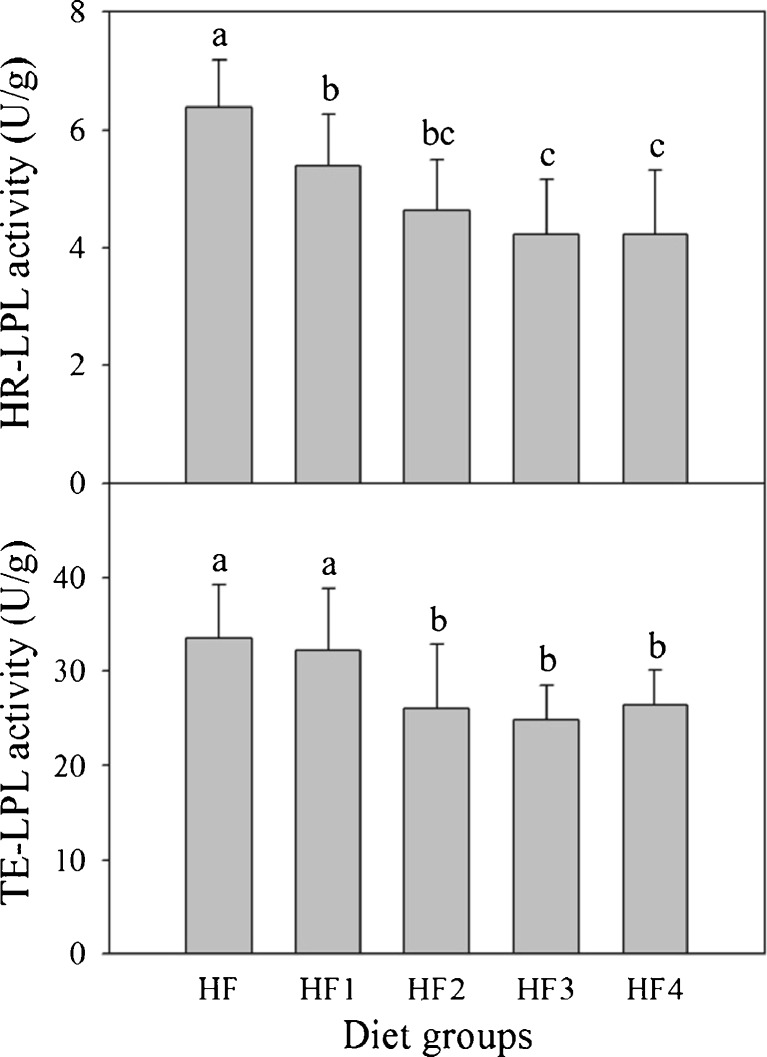
HR-LPL activities in epididymal adipose tissues were in the order of HF > HF1 > HF2 > HF3 > HF4. Values of gochujang groups (HF2, HF3, and HF4) were 26.3 − 37.3 % lower than for control group (Fig. 5). TE-LPL activities of gochujang groups (HF2, HF3, and HF4) were significantly lower than for control group. HR-LPL activities of non-fermented mixture group (HF1) were not significantly different from control group.
Fig. 5.
HR-LPL and TE-LPL activities in epididymal adipose tissues of rats fed different diets for 5 weeks. Values are expressed as mean ± SE (n = 8). Values with different superscript letters are significantly different at p < 0.05 based on Tukey’s test. Diet groups: HF = high fat diet; HF1 = high fat diet + non-fermented mixture; HF2 = high fat diet + gochujang A; HF3 = high fat diet + gochujang B; HF4 = high fat diet + gochujang C. HR-LPL = heparin-releasable lipoprotein lipase; TE-LPL = total extractable lipoprotein lipase
Phenolic compounds in non-fermented mixture and gochujang products
Isoflavones and capsaicins of non-fermented mixture and fermented gochujang products were analyzed. There was no significant difference in the genistein contents of the non-fermented mixture and gochujang A product that included rice koji (Table 3). However, genistein amounts in gochujang products B and C that included both rice koji and soybean meju A or B were 4.6 times greater than for non-fermented mixture. On the other hand, there were no significant differences in capsaicin contents between the non-fermented mixture and the three gochujang products.
Table 3.
Phenolic compounds of the non-fermented mixture and gochujang products
| Phenolic compounds | Contents (peak area, × 106) | |||
|---|---|---|---|---|
| Non-fermented mixture | Gochujang A | Gochujang B | Gochujang C | |
| Acetylgenistin | 0.713 ± 0.094 | 0.217 ± 0.012 | 0.042 ± 0.027 | 0.075 ± 0.032 |
| Daidzin | 1.167 ± 0.074 | 0.656 ± 0.048 | 0.388 ± 0.025 | 0.229 ± 0.103 |
| Luteoline-diglucosdie | 0.260 ± 0.118 | 0.989 ± 0.076 | 0.630 ± 0.022 | 0.723 ± 0.037 |
| Genistin | 2.558 ± 0.298 | 1.683 ± 0.108 | 1.680 ± 0.126 | 1.531 ± 0.121 |
| Apigenin-diglucoside | 1.445 ± 0.109 | 3.848 ± 0.268 | 2.826 ± 0.143 | 2.955 ± 0.097 |
| Apigenin-glucoside | 5.852 ± 0.334 | 6.112 ± 0.367 | 4.668 ± 0.325 | 5.077 ± 0.205 |
| Isoscoparin-glucoside/ Isovitexin-glucoside | 1.767 ± 0.139 | 2.040 ± 0.061 | 1.225 ± 0.046 | 1.496 ± 0.052 |
| Daidzein | 1.028 ± 0.085 | 7.385 ± 3.322 | 50.018 ± 3.201 | 49.167 ± 2.566 |
| Glycitein | 0.173 ± 0.026 | 3.378 ± 0.246 | 15.585 ± 0.901 | 15.072 ± 1.042 |
| Luteoline | 0.648 ± 0.034 | 0.005 ± 0.001 | 0.005 ± 0.001 | 0.005 ± 0.001 |
| Genistein | 8.434 ± 0.574 | 8.365 ± 0.500 | 39.041 ± 3.316 | 38.475 ± 1.472 |
| Hydroxydaidzein | 0.003 ± 0.001 | 0.326 ± 0.023 | 1.294 ± 0.046 | 1.360 ± 0.063 |
| Capsaicin | 79.854 ± 5.645 | 92.690 ± 5.473 | 71.198 ± 4.114 | 72.581 ± 4.725 |
| Dihydrocapsaicin | 43.396 ± 3.084 | 49.688 ± 2.935 | 36.549 ± 2.709 | 38.242 ± 2.973 |
Phenolic compounds were analyzed using UHPLC–LTQ-IT-MS/MS
Non-fermented mixture = gochujang raw materials; Gochujang A = gochujang materials with rice koji; Gochujang B = gochujang materials with rice koji and soybean meju A; Gochujang C = gochujang materials with rice koji and soybean meju B
Discussion
The non-fermented mixture (HF1) and fermented gochujang products (HF2, HF3, and HF4) were effective for reduction of body weight gain and epididymal adipose tissue weights in rats. Considering that white adipose tissues consist of epididymal and other fat types (Avram et al. 2005), a reduction in weights of epididymal adipose tissues was partly responsible for decreased body weight gains. Fat cell sizes in epididymal adipose tissues were reduced by gochujang products, probably leading to a decrease in epididymal fat weights and eventual reduction in body weight gain.
Capsaicin from red pepper and genistein from soybeans were apparently key compounds for the anti-obesity effects of gochujang products. It is known that capsaicin controls adipose tissue distribution between visceral and subcutaneous sites, prevents adipogenesis via activation of transient receptor potential vanilloid-1 channels in sensory nerves (Leung 2014), and reduces adipose tissue weights in rodents via enhancement of the energy and lipid metabolism (Reyes-Escogido et al. 2011). Since both the non-fermented mixture and gochujang products contained capsaicin, the anti-obesity effects evidenced by reductions in body weight gain and epididymal adipose tissue weights were considered to be partly induced by capsaicin contained in the diets. On the other hand, gochujang products A and B that included Bacillus soybean meju contained higher amounts of genistein than the non-fermented mixture due to bacterial fermentation. It is known that genistein inhibits adipocyte differentiation and lipid accumulation, and reduces the adipose tissue mass via stimulation of lipolysis and adipocytes apoptosis (Behloul and Wu 2013). Thus, the anti-obesity effects were apparently enhanced due to an increase in the genistein content.
The triglyceride contents in serum and epididymal adipose tissues of rats were reduced by gochujang products. Gochujang products that included both rice koji and soybean meju prepared based on cultivation of the fungus A. oryzae CJ 1354 and either of the two bacteria B. amyloliquefaciens CJ 3-27 or CJ 14-6 were more effective than a product prepared using only the fungus (currently available as a commercial product) or the non-fermented mixture. Reduction of body weight gain and epididymal fat weight exerted by gochujang products can be related to a decrease in triglyceride amounts.
On the other hand, the activities of the lipogenic enzymes G6PDH, FAS and ME and the lipoprotein lipases HR-LPL and TE-LPL were considerably decreased by both the non-fermented mixture and the gochujang products. The facts indicate that reduction in serum triglyceride levels in rats using gochujang diets was apparently caused by a combination of actions including inhibition of reactions of lipogenic enzymes and fatty acid uptake, similar to other report (Arias et al. 2014). Gochujang products probably exerted anti-obesity effects in rats via stimulation of lipid oxidation due to a reduction in the leptin level. The leptin hormone produced in adipose cells regulates the energy balance and body weight (Havel 2000; Park and Ahima 2015; Sainz et al. 2015). Genistein in gochujang products supposedly partly contributes to the anti-obesity effects by inhibition of synthesis and secretion of leptin (Phrakonkham et al. 2008; Relic et al. 2009).
In addition to triglycerides, reduction in the LDL-cholesterol level and decreases in AI and CRF values in the serum were achieved using both the non-fermented mixture and gochujang products. Arteriosclerosis and cardiovascular diseases are closely related to triglyceride levels and AI and CRF values (Kang et al. 2013). Therefore, both the non-fermented mixture and gochujang products can help to prevent arteriosclerosis and cardiovascular diseases. Among diets, the HF4 gochujang diet that included both rice koji and soybean meju (CJ 14-6) was most effective. Lowering of AI and CRF values was achieved by both a decrease in the total cholesterol level and an increase in the HDL-cholesterol level in the serum. Ingredients of the non-fermented mixture must have been responsible for reduction of the AI and CRF values, and the effects were improved using two kinds of fermentation with a fungus and a bacterium.
Conclusions
Anti-obesity effects, which were achieved by feeding of gochujang product-supplemented diets, partly originated from ingredients of the non-fermented mixture prior to gochujang production. The effect was increased by rice koji using fungal fermentation, and further enhanced by soybean meju using bacterial fermentation. The anti-obesity effects were manifested as reductions in body weight gain, epididymal adipose tissue weights, and serum triglyceride levels in rats due to inhibitory reactions against lipogenic enzymes in fat synthesis. Capsaicin and genistein, which are derived from red pepper and soybeans, respectively, might have played key roles in the observed anti-obesity effects. Gochujang products that include a fungal rice koji are currently available as a commercial product in Korea. Thus, the gochujang product that included both fungal rice koji (CJ 1354) and bacterial soybean meju (especially CJ 14-6) is expected to replace the current fungal-only commercial product in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 71 kb)
Acknowledgments
This work was supported by a grant funded by CJ CheilJedang Corp. (0F13-JF10-RD).
Footnotes
Research highlights
• Gochujang products were prepared using fungal rice koji and bacterial soybean meju.
• Anti-obesity effects of a commercial gochujang were improved by bacterial fermentation.
• Capsaicin and genistein of gochujang products were responsible for anti-obesity effects.
References
- Arias N, Macarulla MT, Aguirre L, Martinez-Castano MG, Portillo MP. Quercetin can reduce insulin resistance without decreasing adipose tissue and skeletal muscle fat accumulation. Genes Nutr. 2014;9:361. doi: 10.1007/s12263-013-0361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avram AS, Avram MN, James WD. Subcutaneous fat in normal and diseased states: 2. Anatomy and physiology of white and brown adipose tissue. J Am Acad Dermatol. 2005;53:671–683. doi: 10.1016/j.jaad.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Behloul N, Wu G. Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol. 2013;698:31–38. doi: 10.1016/j.ejphar.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Biggs HG, Erikson TM, Moorehead WR. A manual colorimetric assay of triglyceride in serum. Clin Chem. 1975;21:437–441. [PubMed] [Google Scholar]
- Burges P. Modification of a traditional Korean food product (Gochujang) to enhance its consumer acceptability as an ethnic food. J Ethn Foods. 2014;1:13–18. doi: 10.1016/j.jef.2014.11.005. [DOI] [Google Scholar]
- Havel PJ. Role of adipose tissue in body-weight regulation: mechanism regulating leptin production and energy balance. Proc Nutr Soc. 2000;59:359–371. doi: 10.1017/S0029665100000410. [DOI] [PubMed] [Google Scholar]
- Iverius PH, Brunzell JD. Human adipose tissue lipoprotein lipase: change with feeding and relation to postheparin plasma enzyme. Am J Physiol. 1985;249:E107–E114. doi: 10.1152/ajpendo.1985.249.1.E107. [DOI] [PubMed] [Google Scholar]
- Kang N-H, Lee WK, Yi B-R, Lee H-R, Park M-A, Park S-K, Park HK, Choi K-C. Risk of cardiovascular disease is suppressed by dietary supplementation with protamine and chitooligosaccharide in Sprague-Dawley rats. Mol Med Rep. 2013;7:127–133. doi: 10.3892/mmr.2012.1128. [DOI] [PubMed] [Google Scholar]
- Kim H-E, Han S-Y, Kim Y-S. Quality characteristics of gochujang meju prepared with different fermentation tools and inoculation time of Aspergillus oryzae. Food Sci Biotechnol. 2010;19:1579–1585. doi: 10.1007/s10068-010-0224-6. [DOI] [Google Scholar]
- Kwon DY, Chung KR, Yang HJ, Jang D-J. Gochujang (Korean red pepper paste): a Korean ethnic sauce, its role and history. J Ethn Foods. 2015;2:29–35. doi: 10.1016/j.jef.2015.02.006. [DOI] [Google Scholar]
- Leung FW. Capsaicin as an anti-obesity drug. Prog Drug Res. 2014;68:171–179. doi: 10.1007/978-3-0348-0828-6_7. [DOI] [PubMed] [Google Scholar]
- Li Y-Y, Yu R-C, Chou C-C. Some biochemical and physical changes during the preparation of the enzyme-ripening sufu, a fermented product of soybean cure. J Agric Food Chem. 2010;58:4888–4893. doi: 10.1021/jf904600a. [DOI] [PubMed] [Google Scholar]
- Linn TC. Purification and crystallization of rat liver fatty acid synthase. Arch Biochem Biophys. 1981;209:613–619. doi: 10.1016/0003-9861(81)90320-9. [DOI] [PubMed] [Google Scholar]
- Nilsson-Ehle P, Schotz MC. A stable radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res. 1976;17:536–541. [PubMed] [Google Scholar]
- Park H-K, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64:24–34. doi: 10.1016/j.metabol.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phrakonkham P, Viengchareun S, Belloir C, Lombes M, Artur Y, Canivenc-Lavier MC. Dietary xenoestrogens differentially impair 3T3-L1 preadipocyte differentiation and persistently affect leptin synthesis. J Steroid Biochem Mol Biol. 2008;110:95–103. doi: 10.1016/j.jsbmb.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielson FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the american institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Relic B, Zeddou M, Desoroux A, Beguin Y, Seny D, Malaise MG. Geistein induces adipogenesis but inhibits leptin induction in human synovial fibroblasts. Lab Investig. 2009;89:811–822. doi: 10.1038/labinvest.2009.41. [DOI] [PubMed] [Google Scholar]
- Reyes-Escogido CL, Gonzalez-Mondragon EG, Vazquez-Tzompantzi E. Chemical and pharmacological aspects of capsaicin. Molecules. 2011;16:1253–1270. doi: 10.3390/molecules16021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld L. Lipoprotein analysis. Arch Pathol Lab Med. 1989;113:1101–1110. [PubMed] [Google Scholar]
- Sainz N, Barrennetxe J, Moreno-Aliaga MJ, Martinez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64:35–46. doi: 10.1016/j.metabol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Shin D, Jeong D. Korean traditional fermented soybean products: Jang. J Ethn Foods. 2015;2:2–7. doi: 10.1016/j.jef.2015.02.002. [DOI] [Google Scholar]
- Zabala A, Churruca I, Macarulla MT, Rodríguez VM, Fernández-Quintela A, Martínez JA, Portillo MP. The trans-10, cis-12 isomer of conjugated linoleic acid reduces hepatic triacylglycerol content without affecting lipogenic enzymes in hamsters. Br J Nutr. 2004;92:383–389. doi: 10.1079/BJN20041220. [DOI] [PubMed] [Google Scholar]
- Zlatkis A, Zak B. Study of a new cholesterol reagent. Anal Biochem. 1969;29:143–148. doi: 10.1016/0003-2697(69)90017-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 71 kb)