Abstract
Artemisia selengensis Turcz (AST) is a perennial herb with therapeutic and economic applications in China. The effects of ultrasound-assisted extraction (UAE) parameters upon extraction yield (EY%), antioxidant and antitumor activities of the polysaccharides extracts were studied by using a factorial design and response surface methodology. The optimal conditions determined were as: ultrasonic power 146 W, extraction time 14.5 min. and extraction temperature 60 °C. The average molecular weights of two homogeneous polysaccharides (APS1 and APS2) purified by DEAE cellulose-52 and Sephadex G-100 column chromatography were 125.4 and 184.1 kDa, respectively. Monosaccharide analysis showed that APS1 and APS2 were composed of five common monomers i.e., galactose, mannose, arabinose, xylose and rhamnose and one different monomer glucose and galacturonic acid respectively, with a most abundant part in molar % of APS1 and APS2 were glucose (83.01 %) and galacturonic acid (48.87 %) while least were xylose (0.80 %) and mannose (1.73 %) respectively. The antioxidant properties were determined by evaluating DPPH, hydroxyl radical scavenging activity and reducing power which indicated both APS1 and APS2 showed strong scavenging activities and anticancer activities on HT-29, BGC823 and antitumor activity on HepG-2. As UAE improved the polysaccharides yield than CSE, meanwhile, no significant difference of polysaccharides chemical compositions. Therefore, the present study suggests that the consumption of AST leaves may beneficial for the treatment of many diseases.
Keywords: Ultrasound-assisted extraction, Artemisia selengensis Turcz, Polysaccharides, Antioxidant activity, Antitumor activity
Introduction
Artemisia selengensis Turcz (AST) is a succulent perennial herb, which is distributed in low-lying damp area, marshes, and freshwater lake (Peng et al. 2010). AST is often called “Li hao”, “Lou hao” or “Lu hao” in China (Shi et al. 2010) It has been used for thousands of years in China as vegetable to produce beverages, tea, fermented yoghurt and functional shampoo (Peng et al. 2011). AST can also be used as medicine to diminish inflammation, stop bleeding, relieve a cough, flavor enhancer and act as appetizers (Fang et al. 2014; Peng et al. 2011). As a group of biologically active compositions, polysaccharides are well-known for their health-beneficial functions attributed to their anti-oxidant and anti-cancer activities (Li et al. 2014a, b, c; Wu et al. 2013). Although it has been reported that bioactive compositions from water extraction of AST contained abundant of polysaccharides compounds (Zhang et al. 2014), and polysaccharides isolated from AST were also previously demonstrated to have immunomodulatory and antitumor activities (Koo et al. 1994), there are very limited researches about extraction of polysaccharides from AST, as well as the components and bioactivity of extracts.
Ultrasonic-assisted extraction (UAE) is a rapid, effective extraction technique that uses ultrasonic waves to generate a cavitation in the solvent, which allows higher penetration of solvent into the raw plant materials. Compared with other modern extraction techniques such as microwave assisted extraction, supercritical fluid extraction, and ion-pair extraction, UAE is more secure, economic, environmentally friendly, and easier to use. Mode of heat transfer in UAE is from outside to inner side as compare to the CSE. So it can enter to the molecules and can provide more efficient extraction than CSE. Comparing with other extraction methods, ultrasonic-assisted extraction (UAE) can accelerate the extraction process at low temperatures, causing less damage to the structural and molecular properties of compounds in plant materials. Ultra sonication has received more and more attention in the isolation of polysaccharides from plants. Compared with conventional reference extraction methods, the present approach provides higher extraction efficiency (Fan et al. 2015). When a new UAE method is developed, optimization of extraction conditions, such as extraction temperature, extraction time and sample-solvent ratio, is indispensable for the best extraction effect within the shortest time. Extraction processes are usually optimized by employing one-factor-at-a-time approach which is often expensive and time consuming. Therefore, in order to maximize extraction yield, extraction conditions should be studied.
To the best of our knowledge, there is no report available on the application of UAE for the extraction of polysaccharides from AST leaves. Therefore, in the present study the conditions for polysaccharides extraction from AST leaves, such as different ultrasonic power, extraction time and temperature and raw material to water ratio, were optimized by applying RSM approach. Additionally, purification, characterization, in vitro antioxidant and antitumor activities of polysaccharides were also assessed.
Materials and methods
Procurement of raw materials and other reagents
Leaves of AST were collected from Hongze Lake Beach, Jiangsu Province, China. Fresh samples were cleaned with water and then crushed into powder by versatile plant pulverizer after being dried at 50 °C. Milled samples were kept in a drying oven to prevent moisture gain.
Different standards regents were procured like Diethylaminoethyl cellulose-52 (DEAE-cellulose-52) and Sephadex G-100 from Whatman Co. (Maidstone, Kent, UK). D-Glucose and standard dextran (molecular weight cut-off 1.26 × 104–2.89 × 105 Da) were purchased from Chinese Institute of Mertology (Beijing, China). Others Seven standards of monosaccharide’s (galactose, mannose, glucose, arabinose, xylose, rhamnose, galacturonic acid), and 1,1-Diphenyl-2-picryl-hydrazyl (DPPH), dihydromi-cotineamidadenine dinucleotide (NADH), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylteteazolium bromide (MTT) and 5-fluorouracil (5-Fu) were all purchased from Sigma Chemical Co. (St. Louis, MO, USA). And all other reagents used were of analytical grade.
Extraction of polysaccharides using ultrasound-assisted extraction (UAE) technique
The powder of dried AST leaves (5.0 g) was immersed in distilled water in a round bottomed flask, then put it into a KQ-500E ultrasonic cleaner bath (Dimension: 24 × 30.5 × 18 cm, Kun Shan Ultrasound Instrument Co., Jiangsu, China). Experiment conditions are shown in Table 1. Debris fragments of the polysaccharides extracts were removed by centrifugation. The solution of polysaccharide was concentrated with a rotary evaporator and precipitated with four volumes of 95 % ethanol (V/V) for 24 h at 4 °C. The precipitates were collected by centrifugation (10,000 g/min, 15 min), and associated proteins were removed by using trichloroacetic acid (TCA) (Lau et al. 1985). And then crude polysaccharides were lyophilized by using a freeze drier.
Table 1.
Central composite rotatory design (CCRD) and responsea of UAE
| Run | Independent variables | Response (%) Yb |
||
|---|---|---|---|---|
| Ultrasonic power (W) A |
Extraction time (min) B |
Extraction temperature (°C) C |
||
| 1 | 150 | 15 | 50 | 8.69 |
| 2 | 150 | 15 | 50 | 8.52 |
| 3 | 150 | 15 | 50 | 8.36 |
| 4 | 100 | 20 | 50 | 5.87 |
| 5 | 150 | 15 | 50 | 8.58 |
| 6 | 150 | 20 | 60 | 7.48 |
| 7 | 150 | 15 | 50 | 8.65 |
| 8 | 200 | 15 | 60 | 6.97 |
| 9 | 200 | 15 | 40 | 5.57 |
| 10 | 200 | 20 | 50 | 5.01 |
| 11 | 100 | 15 | 60 | 7.68 |
| 12 | 200 | 10 | 50 | 5.63 |
| 13 | 150 | 10 | 60 | 8.11 |
| 14 | 150 | 10 | 40 | 5.98 |
| 15 | 150 | 20 | 40 | 6.13 |
| 16 | 100 | 15 | 40 | 6.55 |
| 17 | 100 | 10 | 50 | 5.68 |
a Non-randomised
b Y = polysaccharides weight / AST leaves powder weight × 100
Chemical analysis
The total contents of carbohydrate were determined by the phenol-sulfuric acid colorimetric method by using D-glucose as the standard (Dubois et al. 1956). The contents of uronic acid, protein and sulfate group were measured as described by the reported methods (Bradford. 1976; Cheng et al. 2013.).
Isolation and purification of polysaccharides
The crude polysaccharides were applied to a column of DEAE cellulose-52 (2.6 cm × 30 cm) The samples were eluted with de-ionized water, 0.1, 0.3 and 0.5 M NaCl at a flow rate of 1 mL/min. Every 8 mL of elution was collected automatically and the polysaccharides contents were determined by the phenol-sulfuric acid colorimetric method. The obtained fractions were further purified by a Sephadex G-100 column (1.6 × 60 cm) and eluted with de-ionized water at a flow rate of 0.2 mL/min. As a result, two fractions were collected and named as APS1 and APS2, respectively.
Homogeneity and Mw
The homogeneity and molecular weight of purified polysaccharides were determined by high performance liquid chromatography (HPLC, Dionex UltiMate 3000, USA) equipped with a TSK-Gel G4000SWxl column (300 × 7.8 mm, Tosoh Co., Tokyo, Japan). The eluent was de-ionized water and flow rate was 0.6 mL/min. The elution was monitored by a parallax detector. Standard dextran (1.26 × 104–2.89 × 105 KDa) was used to determine the Mw of purified polysaccharides.
Qualitative and quantitative analysis of monosaccharide
The monosaccharide composition of different purified AST fractions was quantitatively analyzed by HPLC after hydrolysis and derivatization (Stepan and Staudacher 2011). Fifty microgram of sample was dissolved in 300 μL of 4 M trifluoroacetic acid (TFA) and hydrolyzed at 115 °C for 2 h. Then the samples were dried under reduced pressure prior to further 3-fold re-evaporation with 500 μL of 30 % (v/v) methanol. Dry samples were dissolved in sodium acetate trihydrate solution by mixing vigorously with a vortex mixer. Then anthranilic acid reagent solution was added and incubated at 80 °C for 1 h. An Agilent 1200 series HPLC with a reversed phase C18 column (250 × 4.6 mm) was used to analyze the monosaccharide composition. Analysis was carried out with a RF-20A xs fluorescence detector at 360 nm excitation and 425 nm emissions. The flow rate was 1 mL/min. For quantitative analysis, samples in the range from 0.5 to 350 nM of monosaccharide (Gal, Man, Glc, GalA, Ara, Xyl and Rha) were derivatized according to the standard protocol.
Antioxidant activity in vitro
DPPH free radical scavenging activity
The DPPH free radical scavenging activity was determined according to the reported method with minor modifications,ascorbic acid (Vc) was used as a reference standard for comparison (Li et al. 2014a, b, c). DPPH radical scavenging activity measures the antioxidant capacity of donating hydrogen atom to eliminate the DPPH radical (Suttirak and Manurakchinakorn 2014). The sample of the polysaccharide was dissolved in de-ionized water to get a series of solutions with different concentrations (0.125–4 mg/mL). About 0.2 mL of ethanolic DPPH radical solution (0.4 mM), 1.0 mL of the polysaccharide sample and 2.0 mL of deionized water were mixed vigorously and incubated at room temperature for 30 min in the dark. Absorbance of the mixture was measured at 517 nm using UV-2600 spectrophotometer (Shimadzu Co., Kyoto, Japan). The scavenging activity (%) was then calculated by using the following equation:
Where A0 was the absorbance of the system under identical conditions as Asample with deionized water instead of DPPH. Deionized water was used as the blank.
Hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity was measured by the modified reported method using Vc as positive control (Li et al. 2014a, b, c). The system was performed by adding 1 mL of 1,10-phenanthroline (0.75 mM), 1 mL of FeSO4 (0.75 mM), 1 mL of H2O2 (0.01 %, v/v), 1.5 mL of sodium phosphate bufer (pH 7.4, 0.15 M) and 1 mL of sample solutions with different concentrations (0.125–4 mg/mL) in sequence. The reaction was conducted at 37 °C for 1 h. Then the absorbance of the mixture was determined at 536 nm. The scavenging activity (%) was calculated by using the following formula:
Where A0 was the absorbance of the system with deionized water instead of H2O2 and sample. Deionized water was used as the blank.
Reducing power
The reducing power was measured by the method of Wang et al. (2014a, b) using Vc as positive control (Wang et al. 2014a, b). The assay was performed by adding 2.5 mL of the sample with various concentrations (10–100 μg/mL), 2.5 mL of 0.2 M phosphate buffer (pH 6.6), 2.5 mL of potassium ferricyanide (1 %, w/v). The reaction was then conducted at 50 °C for 20 min, and stopped by adding 2.5 mL of trichloroacetic acid (10 %, w/v). The mixture was centrifuged at 5000 rpm for 10 min. About 5 mL of the supernatant was mixed with 0.5 mL of ferric chloride (0.1 %, w/v). The absorbance was measured at 700 nm after 10 min.
Antitumor activity in vitro
The antitumor activities of APS1 and APS2 were evaluated by MTT colorimetry (Wang et al. 2014a, b). In the present study, three human tumor cells were tested: HT-29 (colon cancer), BGC-823 (gastric cancer) and HepG-2 (hepatocellular cancer). The fluorouracil (5-FU) was used as positive control. These cells were inoculated in 2-diethylaminoethanol (DEME) medium containing 10 % fetal bovine serum (FBS), streptomycin (100 μg/mL) and penicillin (100 U/mL) in a humidified air incubator of 5 % CO2 at 37 °C. The 96-well plate was used to incubate cell suspension with concentration of 1 × 104 cells/mL. After culture for 24 h, samples with various concentrations (50, 100, 200, 400 and 600 μg/mL) and 5-FU (50 μg/mL) were added to each well (100 μl/well), and then cells were incubated for 24, 48 and 72 h, respectively. Finally, 150 μl of DMSO was added to the well. The absorbance was measured at 570 nm by enzyme-linked immunosorbent analyzer (BioTek Instruments, Inc., Burlinton, VT). The inhibition ratio of cell growth was expressed according to the following formula:
Where Acontrol was the absorbance of the system without the addition of APS1 and APS2, while Ablank was the absorbance of the system without the addition of cells.
Statistical analysis
All the data of experimental result were analyzed by analysis of variance (ANOVA) of Tukey method (spss 16.0). P values < 0.05 were considered to be significantly different. In all cases, there were three replicates.
Results and discussions
Effects of UAE extraction parameters and yield of polysaccharides
Studied were conducted on following extraction parameters of UAE to examine the effects on yield of polysaccharides.
Extraction power
Extraction temperature
Extraction time
Effects of extraction power on polysaccharides yield
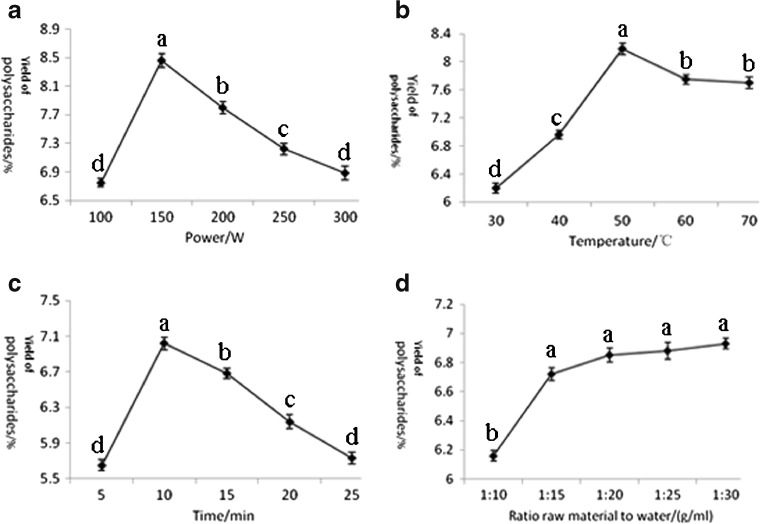
To investigate the influence of extraction power on the yield of polysaccharides, ultrasonic power was set at 100, 150, 200, 250 and 300 W with the other extraction conditions were set as follows: extraction temperature 50 °C, extraction time 10 min, raw material to water ratio 1:30. Figure 1a showed that the yield of polysaccharides was influenced by the ultrasonic power greatly and increased with the increase of ultrasonic power till 150 W. And when the power was over 150 W, the yield decreased. It is well known that the ultrasonic could destroy cell walls of plants, and the yield of anthocyanin from fresh fruits was enhanced by the increasing power in the early period (Chen et al. 2007). However, the extracted polysaccharides may be degraded with the higher ultrasonic power and the yield of polysaccharides decreased (Hromádková et al. 2002). Therefore, 150 W was chosen as the optimum ultrasonic power in the following experiments.
Fig. 1.
Effects of extraction power (a), extraction temperature (b), extraction time (c) and raw material to water ratio (d) on the yield of polysaccharides. Data are expressed as the mean ± SE of triplicate assays. Vertical bars represent the standard errors of the means. Data points carrying different letters indicate statistically significant differences (P < 0.05)
Effects of extraction temperature on polysaccharides yield
Different temperature was set at 30, 40, 50, 60 and 70 °C to investigate the influence of extraction temperature on the yield of polysaccharides and the other extraction conditions were set as follows: extraction power 150 W, extraction time 10 min, raw material to water ratio 1:30. Figure 1b showed that the yield of polysaccharides increased significantly with increasing extraction temperature, and then decreased gradually when the temperature was over 50 °C. The higher temperature could improve the leaching rate of extracted polysaccharides (Koo et al. 1994). However, with the synergistic effect of high temperature and ultrasonic, the polysaccharide structure was easier to be destroyed. Thus, the optimum temperature was 50 °C.
Effects of extraction time on polysaccharides yield
The effect of different extraction time (5, 10, 15, 20 and 25 min) on the yield of polysaccharides was shown in Fig. 1c, and other three factors were as follows: extraction power 150 W, extraction temperature 50 °C, raw material to water ratio 1:30. The results showed that the yield of polysaccharides increased in the first 10 min, and then decreased with the increase of extraction time. However, longer extraction time induced the degradation of polysaccharides (Hromádková et al. 1999). Thus, the optimum time was chosen as 10 min.
Effects of raw material to water ratio of UAE on the yield
The effect of raw material to water ratio (1:10, 1:15, 1:20, 1:25 and 1:30) on the yield of polysaccharides was shown in Fig. 1d, and other three factors were as follows: extraction power 150 W, extraction temperature 50 °C, extraction time 10 min. Results indicated that the yield of polysaccharides was enhanced with the larger raw material to water ratio, and the yield increased slowly when the raw material to water ratio was over 1:15. The higher raw material to water ratio may improve the solubility of extracted polysaccharides. Therefore, 1:30 was chosen as the optimum raw material to water ratio.
Optimization of UAE condition of polysaccharides
Response surface methodology (RSM) is a commonly statistical method in analyzing quantitative experimental data resulting in the optimization of processes (Selvamuthukumaran and Khanum 2014). Based on the results of single factor experiment, the face centered cubic design (FCD) was employed to estimate the pivotal and interaction effects of factors, including ultrasonic power (A), extraction time (B) and extraction temperature (C) on the yield of polysaccharide. Table 1 showed the experimental parameters of FCD, whole design consisted of 17 experimental treatments for optimizing these three factors. The maximum yield of the polysaccharide was 8.69 %, obtained at extraction power 150 W, extraction time 15 min and extraction temperature 50 °C. The mathematical model representing the yield of polysaccharide as a function of the independent variables within region, was expressed by the following equation:
Where Y was the yield of polysaccharides, whereas A, B and C were coded variables for ultrasonic power, extraction time and extraction temperature, respectively.
The model F-value of 81.35 implied model terms were significant. The adjusted coefficient of determination (Adj R-Squared) of the model was 0.9784, and the ‘Pred R-Squared’ of 0.8842 was in reasonable agreement with the “Adj R-Squared”. The “Lack of Fit F-value” of 3.76 implied the “Lack of Fit” was not significant relative to the pure error. The results indicated that the model exhibited a good fit, which could analysis and predict extraction factors of polysaccharides by UAE (Song et al. 2009). The regression coefficient calculated for significance is shown in Table 2. The P-value was considered as a testing tool to inspect the significance of each coefficient between the variables (Liyana and Shahidi 2005). Values of “Prob>F” less than 0.05 indicated model term was significant, and values greater than 0.1000 indicated model term was not significant (Song et al. 2011). In this case, there were significant model terms of A, C, A2, B2, C2.
Table 2.
Test of significance for regression coefficient
| Model term | The yield of polysaccharide (Y) | |||||
|---|---|---|---|---|---|---|
| Coefficient estimate | DF | Standard error | 95 % CI low | 95 % CI high | Prob>F | |
| Intercept | 8.56 | 1 | 0.086 | 8.36 | 8.76 | |
| A | −0.32 | 1 | 0.068 | −0.48 | −0.17 | 0.0020 |
| B | −0.11 | 1 | 0.068 | −0.27 | 0.046 | 0.1365 |
| C | 0.75 | 1 | 0.068 | 0.59 | 0.91 | <0.0001 |
| A2 | −1.62 | 1 | 0.093 | −1.84 | −1.40 | <0.0001 |
| B2 | −1.39 | 1 | 0.093 | −1.61 | −1.17 | <0.0001 |
| C2 | −0.24 | 1 | 0.093 | −0.47 | −0.025 | 0.0340 |
| AB | −0.20 | 1 | 0.096 | −0.43 | 0.024 | 0.0720 |
| AC | 0.067 | 1 | 0.096 | −0.16 | 0.29 | 0.5032 |
| BC | −0.19 | 1 | 0.096 | −0.42 | 0.031 | 0.0809 |
By prediction of Design Expert, the optimal conditions to obtain the highest yield of polysaccharide with UAE were determined as follows: ultrasonic power 146 W, extraction time 14.5 min and extraction temperature 60 °C. Under optimal conditions, the yield of polysaccharide from dried leaves of AST was 8.98 %, but it was not significantly different to predicted value 9.09 % within 95 % confidence interval.
Comparison between UAE and CSE
Based upon above experiments and statistical analysis, we can conclude that the UAE had the advantage of shortening extraction time, saving energy and increasing the yield of polysaccharide compared to the CSE. The UAE could greatly decrease the extraction time and the extraction temperature of the UAE was lower than the temperature of the CSE. The yield of polysaccharides was increased with UAE. The higher efficiency of UAE was in connection with the broken cells of AST leaves by ultrasound, therefore polysaccharides of AST dissolved more easily in the solvent. There were some differences in the chemical components among the crude polysaccharides extracted by two methods. As shown in Table 3, crude polysaccharides isolated by UAE were shown to contain more contents of total carbohydrate and uronic acid and sulfate in addition to protein, while the crude extracts by using the CSE contained more protein composition. Therefore, it is strongly recommended by the comprehensive results of both extraction methods that UAE was more efficient technique than CSE.
Table 3.
Comparison between conventional solvent extraction and ultrasound-assisted extraction
| Extraction method | Extraction temperature (°C) | Extraction time (min) | raw material to water ratio (g/mL) | The yield of polysaccharide (%) | Carbohydrate of crude polysaccharide (%) | Protein of crude polysaccharide (%) | Uronic acid of crude polysaccharide (%) | Sulfate of crude polysaccharide (%) |
|---|---|---|---|---|---|---|---|---|
| CSE | 80 | 120 | 1:30 | 6.25 | 72.68 | 1.96 | 14.22 | 1.26 |
| UAE | 60 | 14.5 | 1:25 | 8.86 | 73.85 | 1.75 | 14.30 | 1.68 |
Isolation, purification and molecular weights of APS1 and APS2
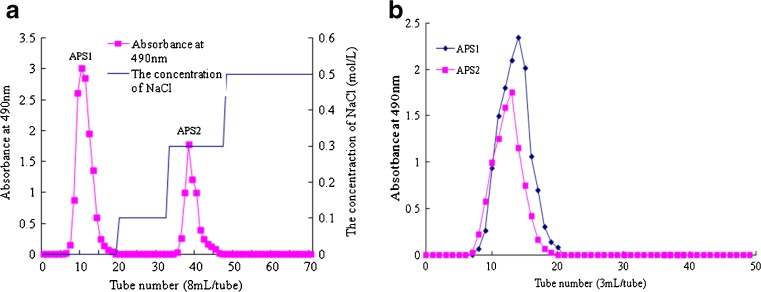
Two homogeneous polysaccharides, named APS1 and APS2 respectively, were obtained from crude polysaccharides of AST after further purification on DEAE cellulose-52 anion exchange column and Sephadex G-100 column chromatography. As showed in Fig. 2a, 5–12 tubes collected was APS1 and 36–42 tubes collected was APS2 by using DEAE cellulose-52 anion exchange column, and when the Sephadex G-100 column was used, APS1 was collected at 8–21 tubes, and APS2 was collected at 8–19 tubes respectively (Fig. 2b). The APS1 was known as a neutral polysaccharide, while the APS2 was considered as the acidic fraction. They were determined by HPLC, and both appeared as symmetrical sharp peaks (data not shown). The recovery ratios of two purified polysaccharides were 38.5 and 20.8 %, respectively. The HPLC was also used to determine the molecular weights (Mw) of homogeneous polysaccharides by using dextran as standards. The average molecular weight values of APS1 and APS2 were estimated to be 125.4 and 184.1 kDa, respectively. It has been reported that the molecular weight of the one polysaccharide from Artemisiae species was 2500 Da (Koo et al. 1994). However, our results were quite different from the previous study, which indicated the larger molecular weights of APS1 and APS2. The molecular weights and structures of polysaccharides extracted from distinct Artemisiae species may be very different (Koo et al. 1994). The higher Mw value of the extracted polysaccharide would be associated with higher activities in some biological systems (Paulsen 2001).
Fig. 2.
DEAE cellulose-52 anion exchange chromatograms (a) and Sephadex G-100 chromatograms (b) of APS1 and APS2
Compositional analysis of monosaccharide eluted from APS1 and APS2
Monosaccharide components of APS1 and APS2 were determined by HPLC analysis, and Table 4 showed that APS1 was composed of galactose, mannose, glucose, arabinose, xylose and rhamnose, with molar percentages of 7.40: 3.11: 83.01: 4.60: 0.80: 1.08. APS2 was consisted of galactose, mannose, galacturonic acid, arabinose, xylose and rhamnose, with the molar percentages of 12.38: 1.73: 48.87: 23.62: 4.50: 8.90. The result indicated APS1 was mainly composed of glucose (83.01 %), while the APS2 was mostly consisted of galacturonic acid (48.87 %). Moreover, the APS2 contained considerable amounts of arabinose (23.62 %) compared to the APS1 (4.60 %). It has reported that the polysaccharides purified from leaves of Artemisiae species plants were composed of mainly glucose and uronic acids (Koo et al. 1994). Our results are in accordance with the previous studies. Almost all monosaccharides eluted from APS1 were similar to those of APS2 expect to glucose and galacturonic acid. No galacturonic acid was detected in APS1. Total carbohydrate contents of APS1 and APS2 were more than 90 %. Additionally, protein was not detected in both APS1 and APS2. The biological activities of polysaccharides are closely related to monosaccharide components (Paulsen 2001). Polysaccharides containing complicated components have certain biological activities as described in previous research (Ovodova et al. 2009). The antioxidant activity of polysaccharide with much higher uronic acid content would be stronger and vice versa.
Table 4.
The monosaccharide compositions and chemical analysis for APS1 and APS2
| Sample | Sugar component (mol %) | Carbohydrate (%) | Sulfate (%) | Protein (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gal | Man | Glc | GalA | Ara | Xyl | Rha | ||||
| APS1 | 7.40 | 3.11 | 83.01 | –n.d | 4.60 | 0.80 | 1.08 | 97.25 | 0.28 | – |
| APS2 | 12.38 | 1.73 | – | 48.87 | 23.62 | 4.50 | 8.90 | 93.68 | 0.45 | – |
n.d Not detected
Determination of antioxidant properties in APS1 and APS2
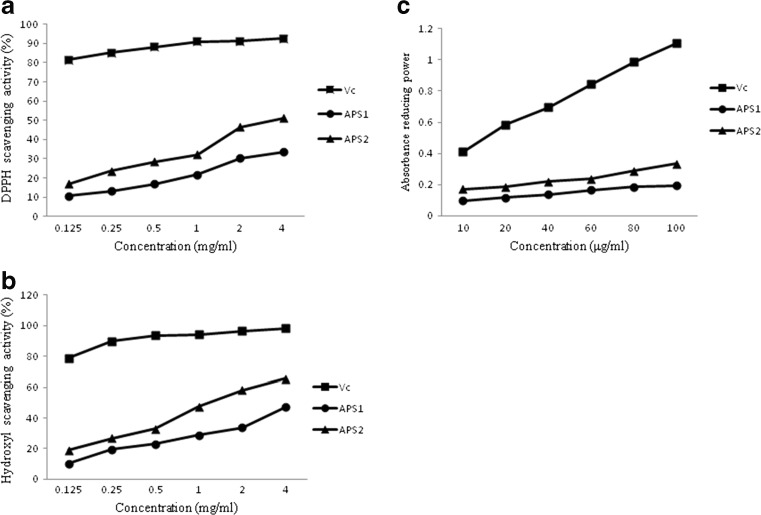
The antioxidant activities of APS1 and APS2 were assessed with DPPH and hydroxyl scavenging and reducing power taking Vc as reference standard for comparison. The result showed a steady increase in antioxidant activities of APS1 and APS2 with the increase in sample concentration, however, the standard Vc showed significantly higher antioxidant activity than APS1 and APS2. Moreover, the antioxidant properties of APS2 were stronger than that of APS1 in the same concentration. At 4 mg/mL, the APS1 and APS2 both showed good DPPH scavenging activity (38.5 and 46.2 %, Fig. 3a and hydroxyl scavenging activity (47.4 and 65.5 %, Fig. 3b. The higher absorbance of the reaction mixture indicated higher reducing power (Malsawmtluangi et al. 2014). At the highest concentration (100 μg/mL), absorbances of reducing power were (0.195 and 0.285) for APS1 and APS2, respectively Fig. 3c. The differences of the antioxidant property of two purified polysaccharides may be due to their structural characterization discrepancy. APS2 contained uronic acid showed stronger antioxidant activity than APS1 without uronic acid. In addition, it has been reported that there is some relation between the reducing power and the free radical scavenging activity (Wang et al. 2014a, b). In our study, the correlation between radical scavenging activity and reducing power in APS1 and APS2 is also demonstrated.
Fig. 3.
Scavenging activities of APS1 and APS2 on DPPH radical (a), hydroxyl radical (b) and reducing power (c)
Antitumor activity
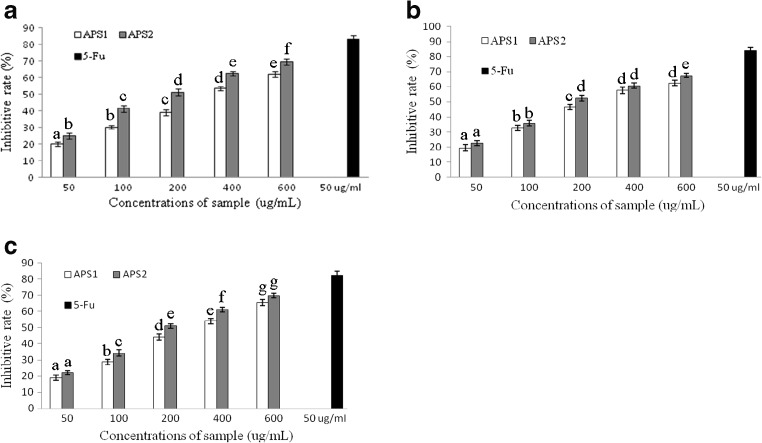
The inhibition ratios of APS1 and APS2 at various concentrations (50–600 μg/mL) for 72 h against BGC-823, HepG-2 and HT-29 cells were exhibited in Fig. 4a–c. The results indicated that there were significant inhibition effects on three human cancer cells with the increasing concentrations of APS1 and APS2. While for the HT-29 colon carcinoma, the inhibitory activity of the samples was the most effective, And highest inhibition ratios of APS1 and APS2 against HT-29 were 65.36 ± 1.77 and 69.84 ± 1.29 %, respectively. Overall, the APS2 seemed to be good against BGC-823, HepG-2 and HT-29 cancer cell lines as compared to the APS1. It has been reported that higher antitumor activity was related to greater molecular mass of polysaccharides (Pei et al. 2015), and it is in accordance with our results. These results showed that the inhibition ratios of APS2 with Mw of 184.1 kDa were observably higher than that of APS1 with Mw of 125.4 kDa at the same concentration. Although the mechanism behind the biological activity of polysaccharides is not fully known, the monosaccharide composition also has an important influence on the antitumor activity of polysaccharides (Inngjerdingen et al. 2013; Li et al. 2014a, b, c; Suresh et al. 2013). Bioactive polysaccharides containing abundant uronic acid contents have been isolated from several plants and algaes, including Cochlospermum tinctorium A. Rich, A. membranaceus and Sargassum plagiophyllum (Inngjerdingen et al. 2013; Li et al. 2014a, b, c; Suresh et al. 2013). These results showed that the antitumor activity of APS2 with 48.87 % galacturonic acid was stronger than APS1 without galacturonic acid, and it is in accordance with previous results.
Fig. 4.
Antitumor activities of APS1 and APS2 on BGC-823 (a), HepG-2 (b) and HT-29 (c) cells. Data are expressed as the mean ± SE of triplicate assays. Vertical bars represent the standard errors of the means. Data points carrying different letters indicate statistically significant differences (P < 0.05)
Conclusions
In the present work, the mathematical model employed to optimize ultrasound assistant extraction factors of polysaccharides was studied, and the optimal conditions to obtain the highest extraction efficiency of polysaccharides from AST leaves were set as follows: ultrasonic power 146 W, extraction time 14.5 min and extraction temperature 60 °C. Compared with the CSE, the UAE seemed more suitable to extract crude polysaccharides from dried leaves of AST. The results showed that the independent variables (ultrasonic power, extraction temperature and extraction time), had significant effects on the yield of polysaccharides. In addition, the purification, characterization, antioxidant and anticancer activities in vitro of two purified fractions (named APS1 and APS2) were investigated. Results from these tests showed that the APS1 and APS2 were found to be consisting of glucose (83.01 %) and galacturonic acid (48.87 %), and the APS2 had stronger antioxidant and anticancer activities. Therefore, the present study suggests that the consumption of AST leaves may beneficial for the treatment of many diseases.
Acknowledgments
All authors are grateful to Jinhu Qinrong Tea factory, for providing fresh experimental materials. We are also thankful to Instrumental Analysis of CPU Center of China Pharmaceutical University for technical assistance.
Footnotes
Juan Wang and He Dong Lu contributed equally to this work.
Research highlights
1. UAE could efficiently improved the polysaccharides yield from AST
2. Two polysaccharides (APS1 and APS2) were separated from AST
3. APS1 and APS2 both showed strong antioxidant properties
4. Both APS1 and APS2 showed strong anticancer activities
Contributor Information
Juan Wang, Email: 726412046@qq.com.
He Dong Lu, Email: luhd100@163.com.
Umair Muḥammad, Email: 2012208009@njau.edu.cn.
Jin Zhi Han, Email: 2391142192@qq.com.
Zhao Hui Wei, Email: luhd163@163.com.
Zhao Xin Lu, Email: 1649597029@qq.com.
Xiao Mei Bie, Email: 415248004@qq.com.
Feng Xia Lu, Phone: 0086-25-84395963, Email: lufengxia@njau.edu.cn.
References
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J] Anal Biochem. 1976;72(1):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen F, Sun YZ, Zhao GH, Liao XJ, Hu XS, Wu JH. Optimisation of ultrasound-assisted extraction of anthocyanins in red raspberries and identification of anthocyanins in extract using high-performance liquid chromatography–mass spectrometry. Ultrason Sonochem. 2007;14:767–778. doi: 10.1016/j.ultsonch.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Cheng H, Feng S, Jia X, et al. Structural characterization and antioxidant activities of polysaccharides extracted from Epimedium acuminatum[J] Carbohydr Polym. 2013;92(1):63–68. doi: 10.1016/j.carbpol.2012.09.051. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Fan T, Hu J, Fu L, et al. Optimization of enzymolysis-ultrasonic assisted extraction of polysaccharides from Momordica charabtia L. by response surface methodology[J] Carbohydr Polym. 2015;115:701–706. doi: 10.1016/j.carbpol.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Fang X, Wang J, Wang Y, et al. Optimization of ultrasonic-assisted extraction of wedelolactone and antioxidant polyphenols from Eclipta prostrate L using response surface methodology[J] Sep Purif Technol. 2014;138:55–64. doi: 10.1016/j.seppur.2014.10.007. [DOI] [Google Scholar]
- Hromádková Z, Ebringerová A, Valachovic P. Comparison of classical and ultrasound-assisted extraction of polysaccharides from Salvia officinalis L. Ultrason Sonochem. 1999;5:163–168. doi: 10.1016/S1350-4177(98)00046-7. [DOI] [PubMed] [Google Scholar]
- Hromádková Z, Ebringerová A, Valachovic P. Ultrasound-assisted extraction of water-soluble polysaccharides from the roots of valerian (Valeriana officinalis L.) Ultrason Sonochem. 2002;9:37–42. doi: 10.1016/S1350-4177(01)00093-1. [DOI] [PubMed] [Google Scholar]
- Inngjerdingen KT, Ballo N, Zhang BZ, Malterud KE, Michaelsen TE, Diallo D, Paulsen BS. A comparison of bioactive aqueous extracts and polysaccharide fractions from roots of wild and cultivated Cochlospermum tinctorium A. Rich. Phytochemistry. 2013;93:136–143. doi: 10.1016/j.phytochem.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Koo KA, Kwak JH, Lee KR, Zee OP, Woo ER, Park HK, Youn HJ. Antitumor and immunomodulating activities of the polysaccharide fractions from Artemisia selengensis and Artemisia iwayomogi. Arch Pharm Res. 1994;5:371–374. doi: 10.1007/BF02974179. [DOI] [Google Scholar]
- Lau JM, McNeil M, Darvill AG, Albersheim P. Structure of the backbone of rhamnogalacturonan I, a pectic polysaccharide in the primary walls of plants. Carbohydr Res. 1985;137:111–125. doi: 10.1016/0008-6215(85)85153-3. [DOI] [Google Scholar]
- Li JE, Nie SP, Xie MY, Li C. Isolation and partial characterization of a neutral polysaccharide from Mosla chinensis Maxim. Cv. Jiangxiangru and its antioxidant and immunomodulatory activities. J Funct Foods. 2014;6:410–418. doi: 10.1016/j.jff.2013.11.007. [DOI] [Google Scholar]
- Li S, Bian F, Yue L, Jin H, Hong ZG, Shu GW. Selenium-dependent antitumor immunomodulating activity of polysaccharides from roots of A. Membranaceus. Int J Biol Macromol. 2014;69:64–72. doi: 10.1016/j.ijbiomac.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Li W, Ji J, Chen XH, Jiang M, Rui X, Dong MS. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr Polym. 2014;102:351–359. doi: 10.1016/j.carbpol.2013.11.053. [DOI] [PubMed] [Google Scholar]
- Liyana PC, Shahidi F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005;93:47–56. doi: 10.1016/j.foodchem.2004.08.050. [DOI] [Google Scholar]
- Malsawmtluangi C, Thanzami K, Lalhlenmawia H, Selvan V, Palanisamy S, Kandasamy R, Pachuau L. Physicochemical characteristics and antioxidant activity of prunus cerasoides D. Don gum exudates. Int J Biol Macromol. 2014;69:192–199. doi: 10.1016/j.ijbiomac.2014.05.050. [DOI] [PubMed] [Google Scholar]
- Ovodova RG, Golovchenko VV, Popov SV, Popova FY. Chemical composition and anti-inflammatory activity of pectic polysaccharide isolated from celery stalks. Food Chem. 2009;114:610–615. doi: 10.1016/j.foodchem.2008.09.094. [DOI] [Google Scholar]
- Paulsen BS. Plant polysaccharides with immunostimulatory activities. Curr Org Chem. 2001;5:939–950. doi: 10.2174/1385272013374987. [DOI] [Google Scholar]
- Pei JJ, Wang ZB, Ma HL, Yan JK. Structural features and antitumor activity of a novel polysaccharide from alkaline extract of Phellinus linteus mycelia. Carbohydr Polym. 2015;115:472–477. doi: 10.1016/j.carbpol.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Peng L, Jia XP, Wang YZ, Zhu HB, Chen QM. Ultrasonically assisted extraction of rutin from Artemisia selengensis Turcz: comparison with conventional extraction techniques. Food Anal Methods. 2010;3:261–268. doi: 10.1007/s12161-009-9113-0. [DOI] [Google Scholar]
- Peng L, Wang YZ, Zhu HB, Chen QM. Fingerprint profile of active components for Artemisia selengensis Turcz by HPLC-PAD combined with chemometrics. Food Chem. 2011;125:1064–1071. doi: 10.1016/j.foodchem.2010.09.079. [DOI] [Google Scholar]
- Selvamuthukumaran M, Khanum F. Optimization of spray drying process for developing seabuckthorn fruit juice powder using response surface methodology. J Food Sci Technol. 2014;51(12):3731–3739. doi: 10.1007/s13197-012-0901-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Jia XB, Zhao CL, Chen Y. Antioxidant activities of various extracts from Artemisia selengensis Turcz (LuHao) Molecules. 2010;15:4934–4946. doi: 10.3390/molecules15074934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JF, Li DJ, Liu CQ. Response surface analysis of microwave-assisted extraction of polysaccharides from cultured Cordyceps militaris. J Chem Technol Biotechnol. 2009;84:1669–1673. doi: 10.1002/jctb.2227. [DOI] [Google Scholar]
- Song JF, Li DJ, Liu CQ, Zhang Y. Optimized microwave-assisted extraction of total phenolics (TP) from Ipomoea batatas leaves and its antioxidant activity. Innovative Food Sci Emerg Technol. 2011;12:282–287. doi: 10.1016/j.ifset.2011.03.001. [DOI] [Google Scholar]
- Stepan H, Staudacher E. Optimization of monosaccharide determination using anthranilic acid and 1-phenyl-3-methyl-5-pyrazolone for gastropod analysis. Anal Biochem. 2011;418:24–29. doi: 10.1016/j.ab.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh V, Senthilkumar N, Thangam R, Rajkumar M, Anbazhagan C, Rengasamy R, Gunasekaran P, Kannan S, Palani P. Separation, purification and preliminary characterization of sulfated polysaccharides from Sargassum plagiophyllum and its in vitro anticancer and antioxidant acitivity. Process Biochem. 2013;48:364–373. doi: 10.1016/j.procbio.2012.12.014. [DOI] [Google Scholar]
- Suttirak W, Manurakchinakorn S. In vitro antioxidant properties of mangosteen peel extract. J Food Sci Technol. 2014;51(12):3546–3558. doi: 10.1007/s13197-012-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li W, Rui X, Chen XH, Jiang M, Dong MS. Structual characterization and bioactivity of released exopolysaccharides from Lactobacillus plantarum 70810. Int J Biol Macromol. 2014;67:71–78. doi: 10.1016/j.ijbiomac.2014.02.056. [DOI] [PubMed] [Google Scholar]
- Wang K, Li W, Rui X, Chen XH, Jiang M, Dong MS. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int J Biol Macromol. 2014;63:133–139. doi: 10.1016/j.ijbiomac.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Wu SJ, Liaw CC, Pan SZ, Yang HC, Ng LT. Phellinus linteus polysaccharides and their immunomodulatory properties in human monocytic cells. J Funct Foods. 2013;5:679–688. doi: 10.1016/j.jff.2013.01.011. [DOI] [Google Scholar]
- Zhang L, Tu ZC, Yuan T, Wang H, Fu ZF, Wen QH. Solvent optimization, antioxidant activity, and chemical characterization of extracts from Artemisia selengensis Turcz. Ind Crop Prod. 2014;56:223–230. doi: 10.1016/j.indcrop.2014.03.003. [DOI] [Google Scholar]