Abstract
This manuscript is an adaptation of the closing keynote presentation of the Digital Pathology Association Pathology Visions Conference 2015 in Boston, MA, USA. In this presentation, analogies are drawn between the adoption of whole slide imaging (WSI) and other mainstream digital technologies, including digital music and books. In doing so, it is revealed that the adoption of seemingly similar digital technologies does not follow the same adoption profiles and that understanding the unique aspects of value for each customer segment is critical. Finally, a call to action is given to academia and industry to study the value that WSI brings to the global healthcare community.
Keywords: Adoption, digital pathology, industry, surgical pathology, whole slide imaging
INTRODUCTION
The World Health Organization cites that there were 14 million new cases of cancer in 2012, making it among the leading causes of death globally.[1] The number of new cancer cases is expected to grow by 70% over the next two decades, further stressing the global healthcare systems.[2] Among those who will first feel the impact of this crisis will be providers who are making the initial determination of cancer, namely, anatomic pathologists.
Despite being a critical member of cancer care team (i.e., surgeons, oncologists, and pathologists), a pathologist has historically been underrepresented in the minds of patients as they go from diagnosis to treatment. Fortunately, this is beginning to change. In 2015, three mainstream media stories put the surgical pathologist directly in the spotlight. In March, television and print media reported on a landmark study published in JAMA citing the complexity associated with differentiating ductal carcinoma in situ from atypical ductal hyperplasia of breast biopsies, even among experts.[3] The New York Times, citing an editorial, stated, “the study should be a call to action for pathologists and breast cancer scientists to improve the accuracy of biopsy readings, by consulting with one another more often on challenging cases.”[4] One month later, actress Rita Wilson went public with her breast cancer fight, speaking about the importance of the pathologist and second opinions in her diagnostic journey.[5] Finally, the Institute of Medicine released a report on diagnostic error stating that “every person will experience at least one significant diagnostic error in their lifetime.”[6] Collectively, these stories suggest that the national attention to diagnostic error in cancer care is growing.
INNOVATION BEYOND PROOF-OF-CONCEPT
Despite the increasing demands on surgical pathology and growing public pressure to improve diagnostic accuracy and turnaround times, the technology available to the surgical pathologist has not changed substantially for hundreds of years. This is simply not sustainable. The need for 21st century tools to improve efficiencies and diagnostic accuracy is clearer than ever.
Industry plays a critical role in delivering on the promise of all relevant medical technologies, including digital pathology. It is the collective industry's responsibility to take “proof-of-concept inventions” and develop them to be safe, effective, reliable, affordable, practical, and most importantly, useful. This is no small task, and there is deep and broad innovation in all aspects of device development. In addition, industry must adhere to strict compliance directives throughout the process while at the same time paying close attention to the value equation so that the products are ultimately adopted by intended users. Above all, in the early stages of innovation, industry must invest on mere faith that a market will develop. If a market fails, industry will bear the majority of sunk costs. If a market succeeds, the benefits extend far beyond the company to the healthcare providers and ultimately the patients they serve. This is why the risk is so worth it for many of us who chose industry over academia and government sectors. Success or failure of all medical breakthroughs lies squarely at the feet of industry. It is a responsibility we should not take lightly.
Given the extraordinary role that industry plays in the realization of novel technologies, it is reasonable to ask how long will adoption take. This is probably the most frequently asked question by investors. It seems intuitively obvious that in an era of digital “everything” digital pathology adoption is a no brainer. Indeed this is what i thought when i publicly stated that adoption would be fast at the 2007 College or American Pathology Foundation Futurescape meeting. However, now, after being in digital pathology for over 10 years, I wonder if I need to reassess this thesis.
ADOPTION OF DIGITAL TECHNOLOGIES IN OTHER MARKETS
Before examining digital pathology, perhaps we should examine other markets to glean insights about the adoption of innovative digital technologies. First, not all investments in digital technology are success stories. How many of us are zipping around town on Segways, navigating with Google Glasses, or talking into our iWatches? Clearly heavy investment in digital technology is not a predictor of mainstream adoption. The most notable dichotomy of digital adoption is that of digital music and digital books. At face value, digital books and digital music share much in common. They both target consumers and face minimal regulatory constraints. From a technology perspective, the associated devices are handheld and can link to online stores to access vast amounts of content. Content is fairly inexpensive and can be downloaded very quickly. The value propositions for digital music and digital books are very similar as well. First and foremost is the simple portability of both types of digital media. You can have your entire music library in your pocket as easily as you can have magazines, novels, and textbooks all in your purse or briefcase at once. Workflow and usability of these two media are almost identical.
Despite similarities in the technology and value propositions of digital books and digital music, their rates of adoption are very different. The first release of digital music players (i.e., the MP3 player) was in 1998 and the first iPod and iTunes were in 2001. iTunes was launched the same year. In 2005, the adoption of digital music in the United States was 10% and reached 40% by 2008.[7,8,9] Thus, depending on when we consider the start of true adoption, the MP3 player, or the iPod/iTunes, the adoption is about 10–40% at 7 years postlaunch. Since most people would agree that the adoption of digital music was very fast, we can use this as a reference point for what we consider “fast adoption.”
The first digital book was launched in 1998 with the release of the Rocket Book.[10] The Kindle was released in 2007. Twelve years after the release of the Rocket Book, the adoption of digital books was only 1.3%. In 2014, 7 years after the Kindle launch, the adoption was <5%.[11] The adoption numbers are normalized to the total recorded music or printed books, thus the relative size of each market is not a factor. Put simply, there is nearly an order of magnitude difference in the adoption rates of these two seemingly similar technologies.
What we can learn from this is that digital for digital's sake is not a value proposition. Likewise, “workflow” and “efficiency” are not, in and of themselves, value propositions. They are more accurately “categories” of value propositions. True value propositions must be thought of at a much more granular level, taking into consideration the psychology of the user and absolute true problem being solved as well as the quantitative outcomes. What worked for one digital modality may not hold for another seemingly similar use case. While the reasons for the slower adoption of digital books versus digital music are debatable, we have to ask how much value do we gain by having print media in a digital format versus music media in a digital format? The granular use cases for music are different than print media. A person can listen to ten songs in 30 min, but can only read a fraction of a book in the same time. Thus, do we really need to carry all of our books and printed magazines around with us all the time? Put simply, the value may not be there for the portability of printed material in the same way it exists for music. This is not to say that there is no value. Certainly there are use cases for digital print media and adoption is occurring, just not at the rate one might expect if based on digital music. Perhaps there is different value for books which has not been tapped into yet or perhaps the value will never be there in the same way as music. More important to consider is whether there are new ancillary components that can be added to digital print media such as annotating or sharing excerpts to social media sites that will increase the value of print media being digital.
ADOPTION OF DIGITAL PATHOLOGY
Moreover, it goes for digital pathology. We have been guilty of comparing the potential adoption of digital pathology to that of digital radiology.[12,13] We should not do this. The value of digital pathology is unique and lies in the granularity of the unique problems pathologists, and healthcare institutions are facing in relation to pathology services. As with digital books, adoption is certainly occurring. Long gone are the days of having to convince the pathology community that reading digital images is going to be a reality.[14] There is too much demand for services and not enough supply to keep up – across all global geographies. Diagnosis is getting more complex and sharing cases is more important than ever. The public is watching and learning. Hence, it is not “if” but “when”… When, when, when. Seriously … when?!
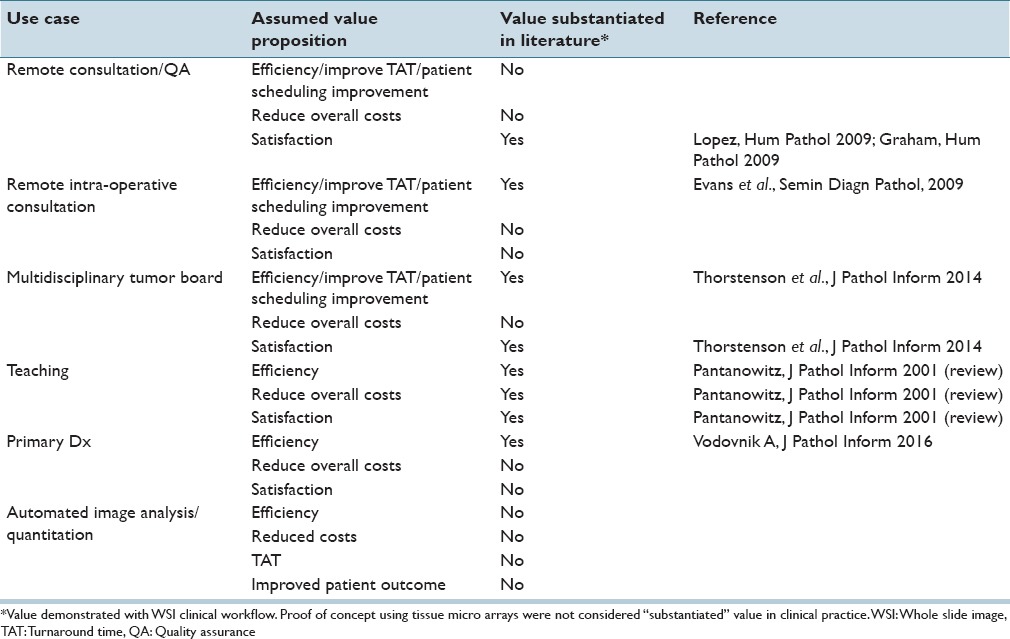
Industry members who have been in the space long enough can feel adoption happening. Yet, it likely feels sluggish. If we map this feeling to the Gartner–Hype Cycle, we could be somewhere along the continuum of “peak of inflated expectations” and the “trough of disillusionment.”[15] This is because we are seeing some success stories, for example, in teaching, remote consultations, and tumor boards [Table 1]. However, we also know that some value has not yet been fully substantiated in clinical practice settings [Table 1]. To be fair, there are reasons beyond the value equation for the slow growth, namely, laboratory reimbursement cuts and the Food and Drug Administration's (FDA's) decision to classify whole slide image (WSI)-based primary diagnosis as a high-risk Class III device.[16] Interestingly in countries outside the US, where these factors are not as relevant, adoption has also not been fast for high volume use cases such as primary diagnosis. It is possible that the FDA's classification has also cast a shadow on adoption outside the US. It is worth mentioning that recent and significant progress has been made by the FDA toward potential down classification in the US.[17] Nonetheless, demonstrating value for using digital pathology for primary diagnosis has been difficult to achieve and user sentiments seem to agree.[18] However, fully implemented WSI at an enterprise level is expected to give great value in efficiency, cost savings, and patient outcomes.[19] Such studies are likely underway.
Table 1.
Literature substantiating whole slide imaging value
COST AND THE VALUE EQUATION
Before delving into how we can prove value, we must consider the cost side of the equation. Value is generally defined as the perceived benefit minus the cost. Thus, cost is a critical component of value. Unlike radiology, which benefited from the removal of film costs to help offset the cost of adopting digital radiography systems, digital pathology cannot dispense with the glass slide.[20] Further, there are several sources of cost to implement digital pathology including the WSI systems (scanners and software), staff to operate the systems, IT resources for installation and maintenance, as well as on-going service costs. Since there is currently no additional reimbursement for WSI, beyond minimal remuneration for image analysis of breast markers (CPT 88361), there is no doubt that the cost to implement WSI is substantial. Thus, the benefits must be substantiated in a meaningful way.
PROVING VALUE WITH UTILITY STUDIES
The good news is that our next phase is going to be the “slope of enlightenment,” where we learn, reinvest, and value crystallizes. In this phase, we begin to see large-scale enterprise enrollments with much-anticipated case studies. During this critical phase, we must look deeply at the benefits side of the value equation and ask if we are designing studies to prove we are hitting the mark.
Unfortunately, value studies in digital pathology have been elusive. On the other hand, there are many validation studies in the literature.[21,22] Validation studies are important to demonstrate safety and effectiveness, and those studies that are truly novel and advance the science beyond our current understanding should continue to be published. For example, large multisite studies or deep examinations of unstudied tissue types, stain types or complex diagnoses are still worthy of scientific study. The numbers of such studies continue to grow. In contrast, we have not seen many, if any, utility studies in the literature.
Utility studies demonstrate value in a controlled and quantitative manner. The endpoints in such studies could be turnaround time, cost, time saved, physician and patient satisfaction, time to recurrence, stratification, and response to treatment, accuracy, and survival. There are some studies in the literature that anecdotally (not quantitatively) show value for teaching, remote consultation, and tumor boards [Table 1]. However, there are almost no studies demonstrating the utility of primary diagnosis or of image-based automated algorithms. The latter of which has been touted as having the most potential value in digital pathology. One study has demonstrated the value of Her2 automated image analysis (Her2-Connect, Visiopharm, Denmark) as reducing the need for FISH testing by 68%.[23] This is an excellent example of a study that measured quantitative value. Recently Vodovnik demonstrated a time savings using WSI for routine primary reads.[24] In addition, this study demonstrated qualitative improvements in ergonomics, and physical slide handling. It should be noted that a fully integrated laboratory information management system (LIS) was available for this study, which may have contributed to their results. Nonetheless, reports such as these are beginning to quantify true value, which is required to offset the costs of implementation.
Industry and academia need to collaborate on more value studies where utility endpoints are presented. We see such utility studies in radiology. One study, the National Lung Screening Trial, went beyond validation to associate the low-dose computed tomography to a 20% reduction in lung-cancer mortality.[25] This method is an example of not only innovative preventative care, but also one that is cost effective and worth continued research efforts. This must be done in our industry now.
CONCLUSION
Industry has an important role to play in bringing novel innovations to the public. Although the timing of adoption of digital pathology is nearly impossible to predict, there are signs that this technology is on track for true and sustainable growth. Value studies are a key catalyst in igniting the next phase of adoption.
Finally, we cannot forget about the important role of investors, boards of directors, and senior management of many digital pathology companies. They have taken the risk to invest in new technologies that will improve global healthcare, and they have kept the faith that the delivery of 21st century tools into pathology will improve patients’ lives. As Thomas Edison said, “If we did all the things we are capable of, we would literally astound ourselves.” We have an obligation to demonstrate to the greater world beyond our own audience what we all know to be true: There is great value in digital pathology. We just need to prove it.
Financial Support and Sponsorship
The author is an employee of Omnyx. General Electric Healthcare and UPMC hold financial interests in Omnyx.
Conflicts of Interest
There are no conflicts of interest.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2016/7/1/18/180014
REFERENCES
- 1.Stewart BW, Wild BP. Lyon, France, Geneva, Switzerland: International Agency for Research on Cancer WHO Press; 2014. World Cancer Report 2014; p. 630. [Google Scholar]
- 2.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the human development index (2008-2030): A population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 3.Elmore JG, Pepe MS, Weaver DL. Discordant interpretations of breast biopsy specimens by pathologists - Reply. JAMA. 2015;314:83–4. doi: 10.1001/jama.2015.6239. [DOI] [PubMed] [Google Scholar]
- 4.Grady D. Breast Biopsies Leave Room for Doubt, Study Finds, in the New York Times. 2015 [Google Scholar]
- 5.CBS News, Rita Wilson's “excellent” Advice on Breast Cancer, Second Opinions. 2015. [Published on 2015 Apr 15, 03:43 pm; Last accessed on 2016 Mar 07]. Available from: http://www.cbsnews.com/news/rita.wilsoncancer-diagnosis.importance.second.opinion/
- 6.Washington, DC: The National Academies Press; 2015. National Academy of Sciences, Engineering and Medicine. Improving Diagnosis in Healthcare. [Google Scholar]
- 7.Richter F. The United States Lead the Way in Digital Music Adoption. [Published on 2012 Jun 25; Last accessed on 2016 Mar 07]. Available from: https://www.statista.com/chart/403/digital.music.sales/
- 8.Smith T. Ten Years Old: The World's First MP3 Player. [Published on 2008 Mar 10; Last accessed on 2016 Mar 07]. Available from: http://www.theregister.co.uk/2008/03/10/ft_first_mp3_player .
- 9.Apple Press Info. iPod+iTunes Timeline. [Last accessed on 2016 Mar 07]. Available from: https://www.apple.com/pr/products/ipodhistory .
- 10.Mironchuk I. E-books – How Far Have We Come? 2011. [Last cited on 2016 Mar 03]. Available from: http://www.dpci.com/blog/e-books-how-far-have-we-come .
- 11.Bonfanti G. Do Readers Dream of Electronic Books? [Published on 2010 Jun; Last accessed on 2016 Mar 07]. Available from: https://www.atkearney.com/innovation/ideas.insights/article/./asset_publisher/VHe1Q1yQRpCb/content/do-readers.dream-of-electronicbooks/10192?_101_INSTANCE_VHe1Q1yQRpCb_redirect=%2Finnovation%2Fideas.insights .
- 12.Cornish TC, Swapp RE, Kaplan KJ. Whole-slide imaging: Routine pathologic diagnosis. Adv Anat Pathol. 2012;19:152–9. doi: 10.1097/PAP.0b013e318253459e. [DOI] [PubMed] [Google Scholar]
- 13.Montalto MC. Pathology RE-imagined: The history of digital radiology and the future of anatomic pathology. Arch Pathol Lab Med. 2008;132:764–5. doi: 10.5858/2008-132-764-PRTHOD. [DOI] [PubMed] [Google Scholar]
- 14.Park S, Pantanowitz L, Parwani AV. Digital imaging in pathology. Clin Lab Med. 2012;32:557–84. doi: 10.1016/j.cll.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Gartner Inc. Gartner Hype Cycle. [Last accessed on 2016 Mar 07]. Available from: http://www.gartner.com/technology/research/methodologies/hype.cycle.jsp .
- 16.Parwani AV, Hassell L, Glassy E, Pantanowitz L. Regulatory barriers surrounding the use of whole slide imaging in the United States of America. J Pathol Inform. 2014;5:38. doi: 10.4103/2153-3539.143325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice SL. Put it on the Board. FDA Open to Whole-slide Imaging as Class II Device, in CAP TODAY. 2016 [Google Scholar]
- 18.Onega T, Weaver D, Geller B, Oster N, Tosteson AN, Carney PA, et al. Digitized whole slides for breast pathology interpretation: Current practices and perceptions. J Digit Imaging. 2014;27:642–8. doi: 10.1007/s10278-014-9683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho J, Ahlers SM, Stratman C, Aridor O, Pantanowitz L, Fine JL, et al. Can digital pathology result in cost savings? A financial projection for digital pathology implementation at a large integrated health care organization. J Pathol Inform. 2014;5:33. doi: 10.4103/2153-3539.139714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braunschweig R, Pistitsch C, Nissen-Meyer S. Digital radiography. Cost-benefit analysis. Radiologe. 1996;36:306–14. doi: 10.1007/s001170050077. [DOI] [PubMed] [Google Scholar]
- 21.Pantanowitz L, Sinard JH, Henricks WH, Fatheree LA, Carter AB, Contis L, et al. Validating whole slide imaging for diagnostic purposes in pathology: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137:1710–22. doi: 10.5858/arpa.2013-0093-CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pantanowitz L, Valenstein PN, Evans AJ, Kaplan KJ, Pfeifer JD, Wilbur DC, et al. Review of the current state of whole slide imaging in pathology. J Pathol Inform. 2011;2:36. doi: 10.4103/2153-3539.83746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holten-Rossing H, Møller Talman ML, Kristensson M, Vainer B. Optimizing HER2 assessment in breast cancer: Application of automated image analysis. Breast Cancer Res Treat. 2015;152:367–75. doi: 10.1007/s10549-015-3475-3. [DOI] [PubMed] [Google Scholar]
- 24.Vodovnik A. Diagnostic time in digital pathology: A comparative study on 400 cases. J Pathol Inform. 2016;7:4. doi: 10.4103/2153-3539.175377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Black WC, Keeler EB, Soneji SS. Cost-effectiveness of CT screening in the national lung screening trial. N Engl J Med. 2015;372:388. doi: 10.1056/NEJMc1414726. [DOI] [PubMed] [Google Scholar]


