Abstract
Background:
Nigella sativa has been a nutritional flavoring factor and natural treatment for many ailments for so many years in medical science. Earlier studies have been reported that thymoquinone (TQ), an active compound of its seed, contains anticancer properties. Previous studies have shown that TQ induces apoptosis in breast cancer cells but it is unclear the role of P53 in the apoptotic pathway. Hereby, this study reports the potency of TQ on expression of tumor suppressor gene P53 and apoptosis induction in breast cancer cell line Michigan Cancer Foundation-7 (MCF-7).
Methods:
MCF-7 cell line was cultured and treated with TQ, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was carried out for evaluating the half-maximal inhibitory concentration (IC50) values after 24 h of treatment. The percentage of apoptotic cells was measured by flow cytometry. Real-time polymerase chain reaction (PCR) was performed to estimate the messenger RNA expression of P53 in MCF-7 cell line at different times.
Results:
The IC50 value for the TQ in MCF-7 cells was 25 μM that determined using MTT assay. The flow cytometry and real-time PCR results showed that TQ could induce apoptosis in MCF-7 cells, and the P53 gene expression was dramatically up-regulated by ascending time, respectively. Hence, there was significant difference in 48 and 72 h.
Conclusions:
Our results demonstrated that TQ could induce apoptosis in MCF-7 cells through up-regulation of P53 expression in breast cancer cell line (MCF-7) by time-dependent manner.
Keywords: Apoptosis, genes P53, Michigan C]ancer Foundation-7 cells, thymoquinone
INTRODUCTION
Breast cancer is the most common cancer among women worldwide and it has been reported that each year about 502,000 die from the disease.[1,2] Breast cancer originates in the breast but metastasizes to other distant organs such as bone, lungs, and brain.[3] Global statistics showed that by the year 2020, there will be twenty million new cancer patients every year.[4] Herbal medications are the source of many chemotherapeutic agents and it has also been demonstrated to have anti-neoplastic potential (Nigella sativa, paclitaxel, vincristine, etc.).[5] Thymoquinone (TQ) is as a potential chemo preventive and chemotherapeutic compound of oil of the black seed.[4] Many investigations have shown that TQ has anti-inflammation, anti-hypertensive, anti-asthmatic, anti-microbial, and anti-oxidant effects.[5,6,7] In addition, anticancer properties of TQ have also been reviewed and has been found that TQ applies anticancer effects (both in vitro and in vivo) in various patterns of carcinogenesis.[8,9] In earlier works, it was found that TQ prevents the growth of different types of cancer, including breast adenocarcinoma (multi-drug-resistant Michigan Cancer Foundation-7 [MCF-7]/TOPO, MCF-7, MDA-MB-231, and BT-474),[8,10] lung cancer (NCI-H460 and A549),[11] pancreatic cancer (MIA PaCa-2, HPAC, and BxPC-3),[11] and prostate cancer (LNCaP, C4-2B, and DU145).[12,13,14] Several lines of studies have determined that TQ exhibits antimetastatic effects, and it can inhibit angiogenesis, in a way that, this compound was found to prevent C26 colon cancer cell invasion.[9] Moreover, some other studies have reported that TQ was able to suppress tumor growth in PC-3 prostate cancer cells, probably through angiogenesis inhibition and it can cause to significant reduction in the number of blood vessels in the tumor.[15] TQ has shown little effect on noncancerous cells such as mouse fibroblasts,[16] prostate epithelial cells,[12] human normal intestinal cells,[17] and human normal lung fibroblasts.[18] This result shows that TQ may have beneficial effects in different types of malignancy while has little effect on normal human cells. Shoieb et al. found that TQ induces apoptosis in osteosarcoma cells and also decrease the number of cells in S-phase. Hence, it induces the cell cycle arrest at G1-phase.[19] Other investigations have demonstrated that TQ can induce apoptosis via p53-dependent/independent pathways.[20,21,22,23] In spite of TQ good anti-neoplastic activities, the molecular mechanism of its pharmacologic effects is not fully understood. Gali-Muhtasib et al. investigated the effects of TQ against HCT-116 human colon cancer cells. They found that TQ induced apoptosis by up-regulating p53 and the downstream p53 target gene, p21WAF1.[20] Roepke et al. have shown that TQ induce apoptosis by p53-independent pathway.[23] Regarding increasing incidence of breast cancer in developing countries like Iran, many investigations have done on different cell line of breast cancer and many different ways have supposed for TQ induced apoptosis. Hence, in the present study, we hypothesized that TQ may up-regulate activity of P53 gene in breast cancer cell line MCF-7 that has a wild-type P53, and inhibit tumor cell growth.
METHODS
Cell lines, drug, culture, and treatment
Human breast cancer cell line (MCF-7) was purchased from the national cell bank of Iran-Pasteur Institute. TQ was purchased from Sigma (C6499, USA). Cell line used in the present study was cultured in Dulbecco's modified Eagle medium-F12 medium (Sigma) supplemented with 10% fetal bovine serum (Sigma) and 1% penicillin-streptomycin (Sigma), and incubated at 37°C and in humidified atmosphere containing 5% CO2. TQ was dissolved in minimum volume of dimethyl sulfoxide (DMSO) (2%) as working solution were prepared by adding of adequate water to reach the appropriate concentrations according to reported procedures.[24,25] After the cells were >80% confluent and growing exponentially in flask 75, 105 cells (MCF-7) were counted and plated in flask 25 and kept in culture medium for 24 h which were then incubated with certain concentrations of TQ, based on half-maximal inhibitory concentration (IC50) index, at different times (24, 48, and 72 h).
Half-maximal inhibitory concentration assay
The IC50 values for the TQ in MCF-7 cells were acquired after 24 h of treatment. Briefly, 104 cells (MCF-7) were counted and placed into each well of a 12well microplate and were treated with various drug concentrations (0, 10, 20, 30, 40, 50, 60, 70, and 80 μM doses) for 24 h, and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) survival assay was done according to the protocol, for evaluating the cell viability in all cells.[26,27] A graph of viability versus drug concentration was used to calculate IC50 values for MCF-7 cell line.[28]
Flow-cytometric analysis
Percentage of apoptotic cells was measured by flow cytometry following Annexin V flow cytometry 1-histogram (FL1-H) and propidium iodide (FL2-H) labeling. A minimum of 5 × 105 cells/ml were analyzed for each sample. Cells were treated with TQ (25 μmol/L) for 24, 48, and 72 h and so washed in phosphate-buffered saline and resuspended in binding buffer (10X; 5 μl). Annexin V-FITC was added to 195 μl cell suspensions, and so analysis was done according to the manufacturer's protocol (BMS500F1/100CE Annexin V-FITC Apoptosis Detection Kit - eBioscience, USA). Eventually, the apoptotic cells were counted by FACScan flow cytometry (Becton Dickinson, Heidelberg, Germany). These experiments were done in triplicate and were, independently, repeated at least 3 times.[25,26]
Real-time polymerase chain reaction analysis
Real-time polymerase chain reaction (PCR) was performed to quantitatively assessment the messenger RNA (mRNA) expression of P53 gene in MCF-7 cells and after treatment with TQ at different times. Total RNA was isolated by RNeasy mini kit treated by RNase-Free DNase set (Qiagen) to remove the genomic DNA. The RNA concentration was defined using a BioPhotometer (Eppendorf BioPhotometer® D30). Total RNA (100 ng) was reverse-transcribed to complementary DNA (cDNA) using the RevertAid™ First Strand cDNA Synthesis Kit (Fermentas) according to the manufacturer's protocol. The maxima SYBR Green ROX qPCR Master Mix kit (Fermentas) was used for real-time PCR. Real-time PCR reactions were performed with using Step One Plus (Step One™ Real-Time PCR Systems - Applied Biosystems). The PCR amplification conditions consisted of 10 min at 95°C followed by 40 cycles of denaturation step at 95°C for 15 s and annealing and extension for 1 min at 60°C. This data were analyzed using the comparative Ct (ΔΔCt) method. The relative expression level of P53 was calculated by determining a ratio between the amount of P53 and that of endogenous control (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]). Melting curve analysis (60°C → 95°C increment of 0.3°C) was used to determine the melting temperature of special amplification products and primer dimers. These experiments were done in triplicate and were independently repeated at least 3 times.[27] Primer sequences for P53 and GAPDH were as follow:[29]
PF GAPDH: CACCACCATGGAGAAGGCTGG
PR GAPDH: CCAAAGTTGTCATGGATGACC
PF P53: CGAGTCCCGCGGTAATTCTT
PR P53: TGCAGAAGAGGTGCAAGACC.
Statistical analysis
All the quantitative data were presented as the mean ± standard deviation. Repeated measure analysis of variance with post hoc test was performed to determine the statistical significance among different groups using the SPSS software package 20.0 IBM modeler (2009). Significance was accepted at a level of P < 0.05.
RESULTS
Half-maximal inhibitory concentration analysis
After the treatment of MCF-7 cells with MTT solution in this study, the dark blue formazan crystals were seen in cells, which indicated their metabolic activity. The reduction in the number of cells was dependent on the cell type as shown by the IC50 index. The IC50 value for the TQ was established. The results showed that the essential TQ concentration to achieve the IC50 in MCF-7 cells at 24 h was 25 μmol/L [Figure 1].
Figure 1.
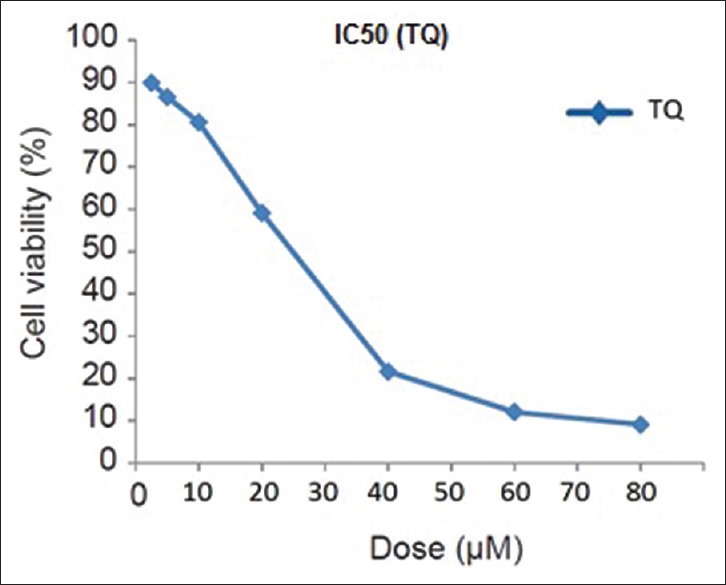
Half-maximal inhibitory concentration assay for half-maximal inhibitory concentration analysis of thymoquinone in Michigan Cancer Foundation-7 cancer cell lines after 24 h of treatment. Cells were incubated with or without the thymoquinone using 25 μM dose. The relative amount of viable cells was estimated by measuring the absorbance of the cell suspension after incubation with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. A graph of viability versus drug concentration was used to calculate half-maximal inhibitory concentration values for Michigan Cancer Foundation-7 cell line
Flow cytometry
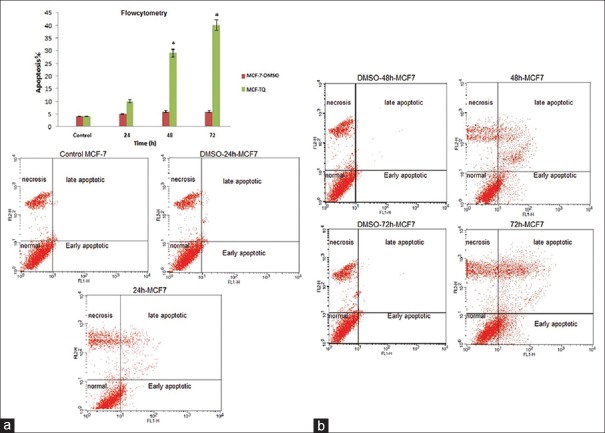
To establish the anti-apoptosis potential of the TQ, we first investigated the effects of TQ on the proliferation in MCF-7 cells. The flow cytometry results showed that TQ at different time points (24, 48 and 72 h) could induce apoptosis in MCF-7 cells, and it was increased with ascending time. TQ arrested MCF-7 cells proliferation (40% of inhibition) in 72 h and (approximately 30% of inhibition) in 48 h [Figure 2a and b, P < 0.05]. DMSO was used in the control sample (vehicle drugs). Using Dunnett test, average of apoptotic cells at different times were compared with the control group. Groups 48 h and 72 h had significant difference to the control group (*P < 0.05).
Figure 2.
(a) Relative levels of apoptotic cells in Michigan Cancer Foundation-7 treated with thymoquinone for different times. Cells incubated with the vehicle dimethyl sulfoxide were used as a control. The percentage of apoptotic cells was measured using the Annexin V-FITC flow cytometry 1-histogram and propidium iodide flow cytometry 2-histogram. Dunnett's test was used for considered comparisons (*P < 0.05) (in any figure, right upper quarter show late apoptotic cells, left upper quarter show necrosis cells, right lower quarter show early apoptotic cells and left lower quarter show normal cells). (b) Cells that are Annexin V-positive and propidium iodide negative are in early apoptosis as phosphatidyl serine translocation has occurred; although, the plasma membrane remains intact. Cells that are positive for both Annexin V and propidium iodide either are in the late stages of apoptosis or are already dead as phosphatidyl serine translocation has occurred and the loss of plasma membrane integrity is visible. *P < 0.05 compared to controls. Dunnett's test was used for considered comparisons (*P < 0.05)
Real-time polymerase chain reaction
It was suggested that apoptotic induction in cancer cells by TQ requires the activation of P53 gene expression. To examine this hypothesis, we used MCF-7 cell line, as cancerous cell line. We examined the inhibitory effects of 25 μmol/L TQ (based on IC50 index) at different times on the mRNA expression of P53 in MCF-7 cell line. The P53 gene expression was dramatically up-regulated by ascending time, in particular, at 72 h treatment its expression was increased significantly [Figure 3, P < 0.05]. Using Dunnett test, average of P53 gene expression at different times was compared with the control group. Groups 48 h and 72 h had significant difference to the control group (*P < 0.05).
Figure 3.
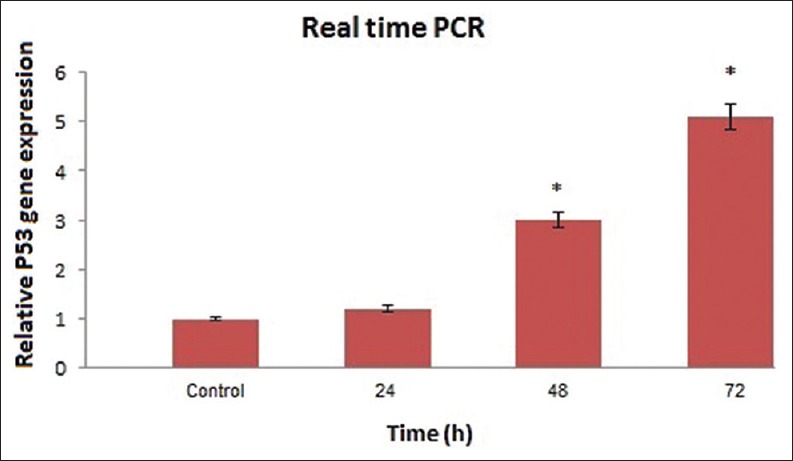
Results of real-time quantitative polymerase chain reaction before and after thymoquinone treatment at different times on the P53 messenger RNA expression in Michigan Cancer Foundation-7 cell. Relative expression levels were obtained using the comparative Ct (ΔΔCt) method (*P < 0.05)
DISCUSSION
Breast cancer is the most common cancer among women worldwide and it is one of the most common cancers that lead to death.[30,31] Therefore, many continuous searches have done for usage of new chemotherapeutic drugs in this type of cancer.[32] Over the centuries, different plant species have been used for chemotherapy.[33,34] Several lines of studies have shown cytotoxicity effects of N. sativa against various carcinomas.[35,36,37] TQ as an active ingredient isolated from N. sativa has tested for its efficacy against cancer.[19] To date, many studies have demonstrated that this compound can inhibit the growth of many different types of cancer, including glioma/glioblastoma (U87 MG),[18,38] breast adenocarcinoma (multi-drug-resistant MCF-7/TOPO, MCF-7),[8,10] leukemia (HL-60 and Junket),[8] lung cancer (NCI-H460),[11] colorectal carcinoma (HT-29),[11,17] and pancreatic cancer (HPAC and BxPC-3).[11] In the present study, we investigated the effect of TQ on P53 gene expression and consequence apoptosis in breast cancer cell line. TQ molecular mechanisms are not fully understood. However, the assumptions indicated that the anti-tumor effect of TQ may happen by one or more of the following mechanisms, antioxidant activity and cytotoxicity.[39] The results of the present study showed that TQ inhibits the viability and proliferation of breast cancer cell line by a time-dependent manner. Many studies demonstrated that TQ induces apoptosis in p53-dependent or p53-independent pathway.[20,21,22,23] Mansour and Tornhamre and Badary et al. have shown TQ property as a radical scavenger with a potential role in the prevention and treatment of oxidative stress.[40,41] A number of studies have shown that TQ could prevent from chemical effects of cancer and inhibit tumorigenesis through different molecular mechanisms.[42] Apoptosis is one form of physiological or a key pathway for regulating homeostasis and morphogenesis of cells and is related to cancer.[43] Numerous in vitro and in vivo studies have indicated that TQ has anti-proliferative effects and can induce cell cycle arrest and also induces pro-apoptotic effects in cancer cells.[23,44] Woo CC et al. have reported that the apoptotic effect of TQ is p53-independent in p53-null myeloblastic leukemia HL-60 cells.[10] They also confirmed that the apoptotic effect of TQ in leukemia cells is caspase-dependent. In some other studies, TQ induces apoptosis through the expression of p53 gene in HCT116 human colorectal carcinoma cells.[20] Thus, the results of different studies show that the anticancer activity of TQ is dependent on cell type. The potencies and functional mechanisms of the TQ were studied at the concentration that was confirmed by IC50 on the MCF-7 as breast cancer cell line. In recent years, a few researchers have described the therapeutic effect of TQ by p53 up-regulation in different cell line;[45] here, we report for the first time that TQ was able to up-regulate the p53 expression in MCF-7 cell line. To understand the inhibitory effect of TQ in breast cancer cells, we used different molecular techniques. In the present study, we have shown that TQ induce apoptosis in MCF-7 cells via time-dependent manner. In addition, real-time PCR results on MCF-7 cells, showed apoptotic induction in cancer cells by TQ requires the activation of P53 gene expression as it was dramatically up-regulated by ascending time, in particular, at 72 h treatment its expression was increased significantly (P < 0.001). In this study, treatment by TQ at various time showed a time-dependent increase in apoptotic cell count of the cancerous cells, as measured by flow-cytometric assay. We have found that TQ caused a significant up-regulation p53 in a time-dependent manner. Our results were supported by the findings of Gali-Muhtasib et al., which indicated that TQ is anti-neoplastic and pro-apoptotic against colon cancer cell line HCT116, and the apoptotic effects of TQ are dependent on p53.[20] Our results were matched with the findings of Woo et al. which stated that TQ exerted strong anti-proliferative effect in breast cancer cells.[10] In addition, they found that TQ activated caspases 8, 9, and 7 in a dose-dependent manner (possible involvement of peroxisome proliferator activated receptor-γ [PPAR-γ] pathway). Besides, El-Mahdy et al. have reported that TQ exhibits antiproliferative effect, induces apoptosis and triggers the activation of caspases 8, 9, and 3 inmyeloblastic leukemia HL-60 cells (p53-independent).[10]
Taken together, our results indicated that TQ inhibited breast cancer cell proliferation in a time-dependent manner. Also, for the first time, we have shown that TQ induce apoptosis through activation of the P53 pathway in MCF-7 cell line. Further, it is required to evaluate the effect of TQ on prevention and treatment of breast cancer.
CONCLUSIONS
The results showed that the drug's effect was significant at 48 and 72 hours. Finally, TQ treatment for 72 hours would have to make the most of apoptosis by expression of P53 gene and it's Resumption of activities apoptotic. Considering the valuable properties of the drug, can be hope to the emergence of a new strategy in the fight against cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Autier P, Boniol M, Gavin A, Vatten LJ. Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: Trend analysis of WHO mortality database. BMJ. 2011;343:d4411. doi: 10.1136/bmj.d4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montazeri A, Vahdaninia M, Harirchi I, Harirchi AM, Sajadian A, Khaleghi F, et al. Breast cancer in Iran: Need for greater women awareness of warning signs and effective screening methods. Asia Pac Fam Med. 2008;7:6. doi: 10.1186/1447-056X-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coley HM. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat Rev. 2008;34:378–90. doi: 10.1016/j.ctrv.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–8. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Ravindran J, Nair HB, Sung B, Prasad S, Tekmal RR, Aggarwal BB. Thymoquinone poly (lactide-co-glycolide) nanoparticles exhibit enhanced anti-proliferative, anti-inflammatory, and chemosensitization potential. Biochem Pharmacol. 2010;79:1640–7. doi: 10.1016/j.bcp.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Kruk I, Michalska T, Lichszteld K, Kladna A, Aboul-Enein HY. The effect of thymol and its derivatives on reactions generating reactive oxygen species. Chemosphere. 2000;41:1059–64. doi: 10.1016/s0045-6535(99)00454-3. [DOI] [PubMed] [Google Scholar]
- 7.El Gazzar M, El Mezayen R, Marecki JC, Nicolls MR, Canastar A, Dreskin SC. Anti-inflammatory effect of thymoquinone in a mouse model of allergic lung inflammation. Int Immunopharmacol. 2006;6:1135–42. doi: 10.1016/j.intimp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Effenberger-Neidnicht K, Schobert R. Combinatorial effects of thymoquinone on the anti-cancer activity of doxorubicin. Cancer Chemother Pharmacol. 2011;67:867–74. doi: 10.1007/s00280-010-1386-x. [DOI] [PubMed] [Google Scholar]
- 9.Gali-Muhtasib H, Ocker M, Kuester D, Krueger S, El-Hajj Z, Diestel A, et al. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J Cell Mol Med. 2008;12:330–42. doi: 10.1111/j.1582-4934.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo CC, Loo SY, Gee V, Yap CW, Sethi G, Kumar AP, et al. Anticancer activity of thymoquinone in breast cancer cells: Possible involvement of PPAR-g pathway. Biochem Pharmacol. 2011;82:464–75. doi: 10.1016/j.bcp.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 11.Rooney S, Ryan MF. Effects of alpha-hederin and thymoquinone, constituents of Nigella sativa, on human cancer cell lines. Anticancer Res. 2005;25:2199–204. [PubMed] [Google Scholar]
- 12.Kaseb AO, Chinnakannu K, Chen D, Sivanandam A, Tejwani S, Menon M, et al. Androgen receptor and E2F-1 targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer Res. 2007;67:7782–8. doi: 10.1158/0008-5472.CAN-07-1483. [DOI] [PubMed] [Google Scholar]
- 13.Koka PS, Mondal D, Schultz M, Abdel-Mageed AB, Agrawal KC. Studies on molecular mechanisms of growth inhibitory effects of thymoquinone against prostate cancer cells: Role of reactive oxygen species. Exp Biol Med (Maywood) 2010;235:751–60. doi: 10.1258/ebm.2010.009369. [DOI] [PubMed] [Google Scholar]
- 14.Richards LR, Jones P, Hughes J, Benghuzzi H, Tucci M. The physiological effect of conventional treatment with epigallocatechin-3-gallate, thymoquinone, and tannic acid on the LNCaP cell line. Biomed Sci Instrum. 2006;42:357–62. [PubMed] [Google Scholar]
- 15.Woo CC, Kumar AP, Sethi G, Tan KH. Thymoquinone: Potential cure for inflammatory disorders and cancer. Biochem Pharmacol. 2012;83:443–51. doi: 10.1016/j.bcp.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Ivankovic S, Stojkovic R, Jukic M, Milos M, Milos M, Jurin M. The antitumor activity of thymoquinone and thymohydroquinone in vitro and in vivo. Exp Oncol. 2006;28:220–4. [PubMed] [Google Scholar]
- 17.El-Najjar N, Chatila M, Moukadem H, Vuorela H, Ocker M, Gandesiri M, et al. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010;15:183–95. doi: 10.1007/s10495-009-0421-z. [DOI] [PubMed] [Google Scholar]
- 18.Gurung RL, Lim SN, Khaw AK, Soon JF, Shenoy K, Mohamed Ali S, et al. Thymoquinone induces telomere shortening, DNA damage and apoptosis in human glioblastoma cells. PLoS One. 2010;5:e12124. doi: 10.1371/journal.pone.0012124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoieb AM, Elgayyar M, Dudrick PS, Bell JL, Tithof PK. In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone. Int J Oncol. 2003;22:107–13. [PubMed] [Google Scholar]
- 20.Gali-Muhtasib H, Diab-Assaf M, Boltze C, Al-Hmaira J, Hartig R, Roessner A, et al. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int J Oncol. 2004;25:857–66. [PubMed] [Google Scholar]
- 21.Gali-Muhtasib H, Roessner A, Schneider-Stock R. Thymoquinone: A promising anti-cancer drug from natural sources. Int J Biochem Cell Biol. 2006;38:1249–53. doi: 10.1016/j.biocel.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB (Oxford) 2009;11:373–81. doi: 10.1111/j.1477-2574.2009.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roepke M, Diestel A, Bajbouj K, Walluscheck D, Schonfeld P, Roessner A, et al. Lack of p53 augments thymoquinone-induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol Ther. 2007;6:160–9. doi: 10.4161/cbt.6.2.3575. [DOI] [PubMed] [Google Scholar]
- 24.Liu G, Chen X. Regulation of the p53 transcriptional activity. J Cell Biochem. 2006;97:448–58. doi: 10.1002/jcb.20700. [DOI] [PubMed] [Google Scholar]
- 25.Dastjerdi MN, Kavoosi F, Valiani A, Esfandiari E, Sanaei M, Sobhanian S, et al. Inhibitory effect of genistein on PLC/PRF5 hepatocellular carcinoma cell line. Int J Prev Med. 2015;6:54. doi: 10.4103/2008-7802.158914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: Functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Yang T, Fu M, Pestell R, Sauve AA. SIRT1 and endocrine signaling. Trends Endocrinol Metab. 2006;17:186–91. doi: 10.1016/j.tem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Motaghed M, Al-Hassan FM, Hamid SS. Cellular responses with thymoquinone treatment in human breast cancer cell line MCF-7. Pharmacognosy Res. 2013;5:200–6. doi: 10.4103/0974-8490.112428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crijns AP, Gerbens F, Plantinga AE, Meersma GJ, de Jong S, Hofstra RM, et al. A biological question and a balanced (orthogonal) design: The ingredients to efficiently analyze two-color microarrays with confirmatory factor analysis. BMC Genomics. 2006;7:232. doi: 10.1186/1471-2164-7-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–43. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 31.Velho-Pereira R, Kumar A, Pandey BN, Jagtap AG, Mishra KP. Radiosensitization in human breast carcinoma cells by thymoquinone: Role of cell cycle and apoptosis. Cell Biol Int. 2011;35:1025–9. doi: 10.1042/CBI20100701. [DOI] [PubMed] [Google Scholar]
- 32.Mukherjee AK, Basu S, Sarkar N, Ghosh AC. Advances in cancer therapy with plant based natural products. Curr Med Chem. 2001;8:1467–86. doi: 10.2174/0929867013372094. [DOI] [PubMed] [Google Scholar]
- 33.Schuppan D, Jia JD, Brinkhaus B, Hahn EG. Herbal products for liver diseases: A therapeutic challenge for the new millennium. Hepatology. 1999;30:1099–104. doi: 10.1002/hep.510300437. [DOI] [PubMed] [Google Scholar]
- 34.Rajput S, Mandal M. Antitumor promoting potential of selected phytochemicals derived from spices: A review. Eur J Cancer Prev. 2012;21:205–15. doi: 10.1097/CEJ.0b013e32834a7f0c. [DOI] [PubMed] [Google Scholar]
- 35.Randhawa MA, Alghamdi MS. Anticancer activity of Nigella sativa (black seed) – A review. Am J Chin Med. 2011;39:1075–91. doi: 10.1142/S0192415X1100941X. [DOI] [PubMed] [Google Scholar]
- 36.Torres MP, Ponnusamy MP, Chakraborty S, Smith LM, Das S, Arafat HA, et al. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: Implications for the development of novel cancer therapies. Mol Cancer Ther. 2010;9:1419–31. doi: 10.1158/1535-7163.MCT-10-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–70. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Cecarini V, Quassinti L, Di Blasio A, Bonfili L, Bramucci M, Lupidi G, et al. Effects of thymoquinone on isolated and cellular proteasomes. FEBS J. 2010;277:2128–41. doi: 10.1111/j.1742-4658.2010.07629.x. [DOI] [PubMed] [Google Scholar]
- 39.Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317–22. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 40.Badary OA, Abdel-Naim AB, Abdel-Wahab MH, Hamada FM. The influence of thymoquinone on doxorubicin-induced hyperlipidemic nephropathy in rats. Toxicology. 2000;143:219–26. doi: 10.1016/s0300-483x(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 41.Mansour M, Tornhamre S. Inhibition of 5-lipoxygenase and leukotriene C4 synthase in human blood cells by thymoquinone. J Enzyme Inhib Med Chem. 2004;19:431–6. doi: 10.1080/14756360400002072. [DOI] [PubMed] [Google Scholar]
- 42.Salim EI. Cancer chemopreventive potential of volatile oil from black cumin seeds, Nigella sativa L. in a rat multi-organ carcinogenesis bioassay. Oncol Lett. 2010;1:913–24. doi: 10.3892/ol_00000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nobel CS, Burgess DH, Zhivotovsky B, Burkitt MJ, Orrenius S, Slater AF. Mechanism of dithiocarbamate inhibition of apoptosis: Thiol oxidation by dithiocarbamate disulfides directly inhibits processing of the caspase-3 proenzyme. Chem Res Toxicol. 1997;10:636–43. doi: 10.1021/tx970006a. [DOI] [PubMed] [Google Scholar]
- 44.Gali-Muhtasib HU, Abou Kheir WG, Kheir LA, Darwiche N, Crooks PA. Molecular pathway for thymoquinone-induced cell-cycle arrest and apoptosis in neoplastic keratinocytes. Anticancer Drugs. 2004;15:389–99. doi: 10.1097/00001813-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Duan S, Tsai Y, Keng P, Chen Y, Lee SO, Chen Y. IL-6 signaling contributes to cisplatin resistance in non-small cell lung cancer via the up-regulation of anti-apoptotic and DNA repair associated molecules. Oncotarget. 2015;6:27651–60. doi: 10.18632/oncotarget.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]