Abstract
Background:
Altered immunoresponse is associated with tumorigenesis and cancer progression. This study assessed the levels of tumor-infiltrating CD3+ or CD8+ T lymphocytes and interleukin-2 (IL-2) protein in radically resected non-small cell lung cancer (NSCLC) tissues to predict overall survival (OS) of the patients.
Methods:
Paraffin-embedded tissue specimens from 129 NSCLC patients were retrospectively collected for immunostaining of CD8+, CD3+, and IL-2 expression. Clinicopathological and survival data were collected and analyzed using the Chi-squared test, Kaplan–Meier curves, and the log-rank test or the Cox regression model.
Results:
The data showed a significant inverse association between CD8+ T lymphocyte levels and IL-2 expression (r = −0.927; P = 0.000) and between the levels of CD8+ and CD3+ T lymphocytes (r = −0.722; P = 0.000), but a positive association between CD3+ T lymphocyte levels and IL-2 expression (r = 0.781; P = 0.000) in NSCLC tissues. Furthermore, the levels of CD3+ and CD8+ T lymphocytes and IL-2 expression were associated with tumor stage (P = 0.023, 0.006, and 0.031, respectively) and the level of CD8+ T lymphocytes was associated with the patient gender (P = 0.024). In addition, the levels of CD8+ T lymphocytes were associated with an unfavorable 5-year OS, whereas patients with high levels of CD3+ T lymphocytes in tumor lesions and IL-2-expressing tumors had significantly better 5-year OS rates than patients with low levels.
Conclusions:
The levels of CD8+ T cells in tumor lesions and IL-2 expression were both independent predictors of OS for these NSCLC patients. Thus, the detection of tumor-infiltrating CD3+ or CD8+ T lymphocytes and IL-2 expression could be useful to predict the prognosis of radically resected NSCLC patients.
Keywords: Immunohistochemistry, Immunological Parameters, Non-small Cell Lung Cancer, Prognostic Factors
INTRODUCTION
Lung cancer is the leading cause of cancer-related mortality in the world for both men and women.[1] Histologically, non-small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancer cases; and to date, most NSCLC patients are still diagnosed at an advanced stage of disease, leading to limited treatment options and a poor 5-year overall survival (OS).[1] Currently, the tumor, lymph node, metastasis (TNM) staging system is one of the best means to predict survival of NSCLC patients.[2] Molecularly, different biological factors are involved in NSCLC development and progression; thus, they may serve as biomarkers for prediction of prognosis or treatment responses.
Altered inflammatory and immune responses play a key role in tumor development and progression.[3] Tumor-associated immune responses are more likely to contribute to tumor progression and immunosuppression than effective antitumor responses, depending on the tissue context and cellular stimuli.[4] For example, tumor-infiltrating lymphocytes (TILs) are found in a variety of cancer tissues and consist of a significantly higher population of CD3+ and CD8+ T cells. CD3 is the surface marker of mature T cells and is used to detect T helper and cytotoxic T cells. CD3+ T cells have antitumor activity[5] and TILs and macrophages have a potential dual role in lung cancer by supporting both host-defense and tumor progression. In contrast, CD8+ T lymphocytes have cytotoxic activity against cancer cells, and these T cells could play an important role in antitumor immunity. Overall, these immune cells produce and release cytokines that modulate the balance between humoral and cell-based immune responses and regulate pro-or antitumor activity in the body. For example, interleukin-2 (IL-2), produced by T cells during an immune response, is necessary for growth, proliferation, and differentiation of T lymphocytes to become effector (such as CD8+) or regulatory T cells (T-regs). In this regard, detection of CD3+ and CD8+ T lymphocytes as well as IL-2 expression in a tumor lesion may help us to predict tumor progression or even the prognosis of patients. In addition, tumor-bearing hosts are frequently in an immunosuppressive state and the function of TILs is impaired due to the unavailability of IL-2 and/or its suppression due to lung cancer cell-derived factors.[6] IL-2 is mainly secreted by Th1 cells and can induce natural killer (NK) cell proliferation and activity.[7] A high-dose IL-2 treatment can cause mononuclear macrophage proliferation and differentiation as well as induces mononuclear macrophages to kill tumor cells. IL-2 can also stimulate B cell proliferation and activation. With a high affinity IL-2 receptor expressed in the human body, IL-2 possesses antitumor and antimicrobial activity as well as causes graft rejection and autoimmune responses. For example, Wakabayashi et al.[8] have reported that tumor-infiltrating CD8+ T lymphocytes were associated with a shorter OS of NSCLC. Another study[9] reported that higher numbers of CD8+ T cells were associated with better survival of patients with Stage IV NSCLC. In contrast, there was no correlation between CD8+ TIL infiltration and the outcome of patients with NSCLC.[10] Therefore, in this study, we further analyzed the levels of TILs (CD3+ or CD8+) and IL-2 expression in NSCLC tissues for association with clinicopathological parameters and patient survival.
METHODS
Study population
This study was approved by the Institutional Review Board of The First Affiliated Hospital, Zhengzhou University. We collected tissue specimens from 129 patients who received lung cancer resections between January 2004 and January 2007 in the Department of Thoracic and Cardiovascular Surgery, The First Affiliated Hospital, Zhengzhou University. Patients who received adjuvant radiotherapy or chemotherapy and immunotherapy before surgery were excluded. Patients with squamous cell carcinoma (SCC), adenocarcinoma (AC), adenosquamous carcinoma (ASC), or large cell carcinoma were eligible; but patients with small cell lung cancer, sarcoma, or carcinoid tumors were excluded. All patients were followed up until March 2013 through an outpatient clinic visit and/or telephone interview with family members. The median follow-up time was 36.0 months (ranged between 6 and 109 months). The formalin-fixed, paraffin-embedded tissue blocks were retrieved from the Pathology Department and sectioned for immunohistochemistry. Clinicopathological data were also collected from patient medical records [Table 1] according to the TNM criteria of the Union for International Cancer Control.[11] The histopathological diagnosis was established for each patient according to the World Health Organization guidelines.[12]
Table 1.
Association of CD3+ and CD8+ T cells and IL-2 expression with clinicopathological features of NSCLC
| Variable | CD3+ | CD8+ | IL-2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| High | Low | P | High | Low | P | High | Low | P | |
| Age (years) | |||||||||
| <60 | 16 | 34 | 0.914 | 21 | 29 | 0.691 | 30 | 20 | 0.707 |
| ≥60 | 26 | 53 | 36 | 43 | 50 | 29 | |||
| Gender | |||||||||
| Male | 29 | 62 | 0.095 | 46 | 45 | 0.024 | 54 | 37 | 0.333 |
| Female | 18 | 20 | 11 | 27 | 26 | 12 | |||
| pT stage | |||||||||
| pT1 | 12 | 8 | 0.023 | 3 | 17 | 0.006 | 18 | 2 | 0.031 |
| pT2 | 26 | 40 | 28 | 38 | 40 | 26 | |||
| pT3 | 4 | 15 | 10 | 9 | 10 | 9 | |||
| pT4 | 5 | 19 | 16 | 8 | 12 | 12 | |||
| pN stage | |||||||||
| pN0 | 27 | 40 | 0.476 | 28 | 39 | 0.188 | 46 | 21 | 0.431 |
| pN1 | 9 | 12 | 6 | 15 | 12 | 9 | |||
| pN2 | 8 | 23 | 18 | 13 | 17 | 14 | |||
| pN3 | 3 | 7 | 5 | 5 | 5 | 5 | |||
| pTNM stage | |||||||||
| I | 23 | 25 | 0.058 | 17 | 31 | 0.169 | 34 | 14 | 0.136 |
| II | 11 | 18 | 12 | 17 | 19 | 10 | |||
| III | 13 | 39 | 28 | 24 | 27 | 25 | |||
| Histology | |||||||||
| SCC | 20 | 41 | 0.626 | 32 | 29 | 0.136 | 33 | 28 | 0.174 |
| AC | 20 | 28 | 16 | 32 | 32 | 16 | |||
| Others | 7 | 13 | 9 | 11 | 15 | 5 | |||
| Smoking status | |||||||||
| Never | 17 | 31 | 0.853 | 17 | 31 | 0.123 | 32 | 16 | 0.402 |
| Smoker | 30 | 51 | 40 | 41 | 48 | 33 | |||
NSCLC: Nonsmall cell lung cancer; SCC: Squamous cell carcinoma; AC: Adenocarcinoma; TNM: Tumor, lymph node, metastasis; IL-2: Interleukin-2.
Immunohistochemistry
Tissue sections were deparaffinized in xylene and rehydrated in a series of ethanol solutions and then subjected to antigen retrieval in a microwave using a middle-to-high power setting for 8 minutes, followed by a low-to-high temperature for 5 minutes, and cooled down to room temperature. A rabbit monoclonal anti-human CD3 or anti-human CD8 (recognizing cytotoxic T cells) antibody, a rabbit polyclonal anti-human IL-2 antibody, and a streptavidin-peroxidase-conjugated secondary antibody were obtained from Zhongshan Goldenbridge Biotechnology Co., Ltd. (Beijing, China). Immunostaining was performed according to the manufacturer's instructions. The tissue sections were then briefly counterstained with hematoxylin and mounted with a coverslip and Permount (Zhongshan Goldenbridge Biotechnology Co., Ltd.). Tissue sections with known positivity from previous experiments were used as a positive control, whereas tissue sections incubated with phosphate-buffered saline to replace the first antibody served as a negative control. The immunostained sections were then assessed by two experienced pathologists (HSM and DHC) without knowledge of patient identification.
To score the immunostaining results, we randomly selected five representative high-power microscopic fields (×400 magnification) of the tumor nest and stroma per section, counted the numbers of positively stained cells, and photographed the sections with a digital camera (Nikon Eclipse 80i; Tokyo, Japan). The mean percentages of stained cells were counted as 0 (negative), 1 (≤10%), 2 (11–50%), 3 (51–80%), and 4 (>80%). Each tissue section was scored semi-quantitatively as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong) staining intensities. Next, we multiplied them to form an immunohistochemical score (H-score) according to a previous study.[13] An H-score of 0–4 was considered as low expression, while a score of 5–12 was considered as a high expression.
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS, version 12.0; SPSS Inc., Chicago, IL, USA). The OS was calculated from the dates of surgery and death from any cause as the endpoint. For each clinicopathological variable and biological marker, the OS and 95% confidence intervals were calculated using the Kaplan-Meier method and statistically analyzed using the log-rank test. Multivariate analysis of OS was performed using Cox's proportional hazard model. A P < 0.05 was considered statistically significant.
RESULTS
Characteristics of patients
These 129 NSCLC patients included 91 males and 38 females with a median age of 61 years old (ranged between 32 and 83 years old). The histology showed that 47.29% of patients had SCC, 37.21% had AC, and 15.5% had others (ASC and large cell carcinoma); while 37.2% of patients had Stage I cancer, 22.4% had Stage II, and 40.3% had Stage III. Eighty-one patients (62.79%) had a history of tobacco smoking, and 92 patients (71.32%) had received adjuvant chemotherapy. The median survival of these patients was 34.0 months, and the 5-year OS rate was 36.4% (i.e. 34.1% for males and 42.1% for females) [Table 1].
Levels of CD3+ and CD8+ T lymphocytes and interleukin-2 expression in non-small cell lung cancer tissue specimens
Interleukin-2 protein and CD3+ and CD8+ T cells were present in the cancer stroma and cancer cell nests [Figure 1]. Specifically, 91 of these 129 tumor tissue specimens had CD8+ T cells in the cancer nests, with a mean number of 4.65 ± 4.25 CD8+ T cells; while 126 of these 129 specimens showed CD8+ T cells in the cancer stromal tissues, with a mean number of 57.63 ± 23.71. Moreover, 88 of these 129 cases had CD3+ T cells in the cancer nests, with a mean number of 4.95 ± 10.46 CD3+ T cells; and 117 of these 129 cases also showed CD3+ T cells in the cancer stromal tissues, with a mean number of 23.06 ± 21.38. In addition, IL-2 protein was detected in the cancer cells of 122 NSCLC cases and the cancer stromal cells of 79 cases, with mean numbers of 26.08 ± 21.00 and 2.00 ± 2.04, respectively.
Figure 1.
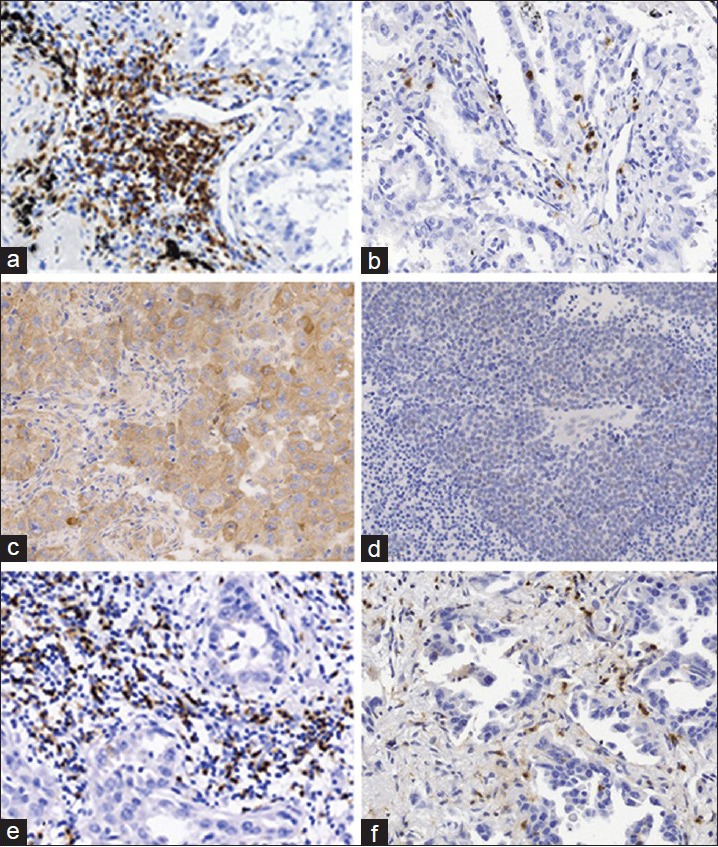
Representative images of immunohistochemically stained non-small cell lung cancer tissue specimens. (a) High levels of CD3+ T cell infiltration in the tumor stroma (adenocarcinoma [AC]). (b) Low level of CD3+ T cell infiltration in the cancer cell nest and stroma (AC). (c) High level of interleukin-2 (IL-2) expression in the cancer nest (squamous cell carcinoma [SCC]). (d) Low level of IL-2 expression in the cancer nest (SCC). (e) High level of CD8+ T cell infiltration within the cancer stroma (AC). (f) Low level of CD8+ T cell infiltration within the cancer stroma (AC) (×200).
The number of infiltrating CD3+ and CD8+ T cells in the cancer stroma was clearly higher than that in the cancer nests (P = 0.000), whereas IL-2 protein expression was higher in the cancer nests than in the cancer stroma (P = 0.000). There was a significant inverse association (r = −0.927; P = 0.000) between the number of CD8+ T cells and IL-2 protein expression in NSCLC tissues and between the numbers of CD8+ T cells and CD3+ T cells (r = −0.722; P = 0.000), whereas there was a positive association between the number of CD3+ T cells and IL-2 protein expression (r = 0.781; P = 0.000) in NSCLC tissue specimens.
Association of CD3+ and CD8+ T cell levels and interleukin-2 expression with clinicopathological variables from non-small cell lung cancer patients
Next, we associated these parameters with the clinicopathological data and found a significant association between the numbers of CD3+ and CD8+ T cells and the level of IL-2 expression with tumor stage (P = 0.023, 0.006, and 0.031, respectively). There was a significant association between the number of CD8+ T cells and the patient gender (P = 0.024). However, there was no association between the numbers of CD3+ and CD8+ T cells or the level of IL-2 expression and gender, age, tumor stage, lymph node or distant metastasis, tobacco smoking, or tumor histology [Table 1].
Association of CD3+ and CD8+ T cell levels and interleukin-2 expression with survival of these non-small cell lung cancer patients
The survival data from each patient were collected and stratified based on the CD3+ and CD8+ T cell numbers and IL-2 expression levels [Table 2]. We found that tumor histology was a prognostic factor for these patients (the 5-year OS rates of patients with SCC, AC, or other histological subtypes were 34.4%, 45.8%, and 20.0%, respectively; P = 0.009). The same was true for the tumor pathological stage (P = 0.00001). However, the patient gender, age, and tobacco smoking status as well as adjuvant chemotherapy treatment had no statistically significant impact on the OS (P > 0.05).
Table 2.
Univariate and multivariate analyses
| Characteristic | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Number of patients | 5-year OS (%) | P | HR | 95% CI | P | |
| Histology | - | - | 0.009 | - | - | 0.000 |
| SCC | 61 | 34.4 | - | 1 (Ref) | - | - |
| AC | 48 | 45.8 | - | 1.275 | 0.743 – 2.186 | 0.378 |
| Others | 20 | 20.0 | - | 4.201 | 2.024 – 8.721 | 0.000 |
| pT classification | - | - | 0.000 | - | - | 0.000 |
| T1 | 20 | 70.0 | - | 1 (Ref) | - | - |
| T2 | 66 | 45.5 | - | 1.201 | 0.338 – 2.393 | 0.833 |
| T3 | 19 | 10.5 | - | 2.280 | 0.634 – 8.197 | 0.207 |
| T4 | 24 | 4.2 | - | 4.974 | 1.451 – 17.052 | 0.011 |
| pN classification | - | - | 0.000 | - | - | 0.042 |
| N0 | 67 | 53.7 | - | 1 (Ref) | - | - |
| N1 | 21 | 33.3 | - | 1.381 | 0.608 – 3.137 | 0.440 |
| N2 | 31 | 12.9 | - | 4.160 | 1.341 – 12.905 | 0.014 |
| N3 | 10 | 0 | - | 4.508 | 1.397 – 14.548 | 0.012 |
| pTNM stage | - | - | 0.000 | - | - | 0.125 |
| I | 48 | 70.8 | - | 1 (Ref) | - | - |
| II | 29 | 24.1 | - | 1.216 | 0.327 – 4.519 | 0.770 |
| III | 52 | 11.5 | - | 2.158 | 0.859 – 5.418 | 0.102 |
| CD3 | - | - | 0.002 | - | - | - |
| Low | 82 | 25.6 | - | 1 (Ref) | - | - |
| High | 47 | 55.3 | 1.205 | 0.642 – 2.264 | 0.562 | |
| CD8 | - | - | 0.000 | - | - | - |
| Low | 72 | 48.6 | - | 1 (Ref) | - | - |
| High | 57 | 21.1 | 2.154 | 1.323 – 3.509 | 0.002 | |
| IL-2 | - | - | 0.000 | - | - | - |
| Low | 49 | 18.4 | - | 1 (Ref) | - | - |
| High | 80 | 47.5 | - | 1.940 | 1.104 – 3.408 | 0.021 |
OS: Overall survival; HR: Hazard ratio; P: Significance level for log-rank test; 95% CI: 95% confidence interval; Ref.: Reference group; SCC: Squamous cell carcinoma; AC: Adenocarcinoma; TNM: Tumor; lymph node, metastasis; IL-2: Interleukin-2.
Furthermore, the number of CD8+ T cells in the tumor lesion appeared to be an unfavorable prognostic factor (5-year OS of 21.1% vs. 48.6% for high vs. low levels; P = 0.000, Table 2 and Figure 2b); Table 2 and Figure 2b Patients with high levels of CD3+ T cells in the tumor lesions had a significantly better 5-year OS than patients with low levels of CD3+ T cells in the tumor lesions (55.3% vs. 25.6%; P = 0.002; Table 2 and Figure 2a). Similarly, patients with IL-2-expressing tumors had a better 5-year OS rate than those patients with a low IL-2-expressing tumor (47.5% vs. 18.4%; P = 0.000; Table 2 and Figure 2c).
Figure 2.
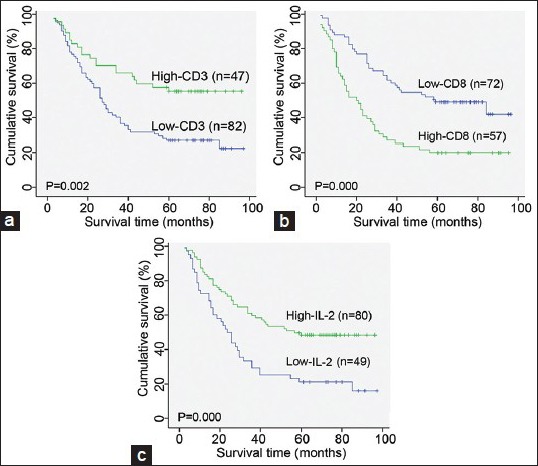
Kaplan–Meier analysis of OS of non-small cell lung cancer patients according to the level of infiltration of CD3+ T cells (a) and CD8+ T cells (b) as well as interleukin-2 expression (c).
The multivariate analyses showed that tumor histology (P = 0.000), tumor stage (P = 0.000), TNM (P = 0.042), number of CD8+ T cells in the tumor lesions (P = 0.002), and IL-2 expression levels (P = 0.021) were all independent predictors of OS [Table 2].
DISCUSSION
A previous study has demonstrated that TILs contain significantly higher levels of CD8+ and CD3+ T cells compared to those of peripheral blood.[14] In this study, we found that IL-2 protein was expressed in tumor and cancer stromal cells and that CD3+ T cells and CD8+ T cells were present in cancer stromal tissue and in the cancer nest. The number of CD3+ and CD8+ T cells and IL-2 expression were associated with the NSCLC stage, and the patients with high levels of CD3+ T cells in the tumor lesion or with an IL-2-expressing tumor had a significantly better 5-year OS. In contrast, high levels of CD8+ T cells were associated with an unfavorable prognosis.
CD8+ T cells can recognize tumor-associated antigens as major histocompatibility complex (MHC) class I molecules on the cancer cell surface and can directly lyse cancer cells. Thus, the presence of tumor-infiltrating CD8+ T cells is considered as a host immunoreaction against a tumor and is associated with a better prognosis in a variety of cancers.[15] However, our current study showed that high levels of CD8+ T cells in the tumor lesion were associated with a poor prognosis. These data contradict our current knowledge, and the reason is unclear. One possible explanation may be that some immune cells can induce immune tolerance and even promote tumor growth and metastasis, i.e. CD8+ T cells per se might have diverse functions in a tumor microenvironment;[16] these cells could lose their antitumor activity after interacting with other B or T lymphocytes through several mechanisms, e.g. escape of immune surveillance due to secretion of immunosuppressive factors (IL-10 and transforming growth factor-β), lack of adequate T-cell costimulation, or downregulation of cell-surface MHC Class II protein expression (immunoediting).[17] A previous study has shown that nonclassical HLA-G is involved in immune escape mechanisms and could be one of the most powerful molecules for suppression of the innate and/or adaptive immune response in lung and other cancers.[18] Another possibility may be that CD8+ T cells in the tumor nest are associated with survival,[19] whereas CD8+ T cells in the tumor stroma are inversely associated with survival. In this study, the number of CD8+ T cells was significantly higher in the stroma than inside the cancer nest. An additional possibility may be that tumor-infiltrating CD8+ T cells contain high levels of T-regs in addition to cytotoxic T cells. However, further studies are needed to clarify and confirm our current data.
Furthermore, our current study demonstrated that the levels of CD8+ T cells in the tumor microenvironment were associated with the pT stage, suggesting that CD8+ T cells are more abundant in the tumor stroma with high cellular growth rates and malignancy potential. These CD8+ T cells are anergic but cannot lyse tumor cells. Trojan et al.[20] found that CD8+ T cells in the tumor cell nest were inadequately activated and incapable of mounting an antitumor immune effect. Tumor immunology is a very complicated field of research, and a great number of factors affect, interrupt, or interact with the favorable immune activity against tumor cells.
A high number of CD3+ T cells have been associated with increased apoptosis in patients with NSCLC.[21] Al-Shibli et al.[22] also have reported that an increasing number of stromal and cancerous CD3+ T cells were associated with a better disease-specific survival and that a high stromal density of CD3+ T cells was an independent indicator for survival in patients with NSCLC. Our current univariate analysis showed a significant correlation of CD3+ T cell levels with better OS of NSCLC patients, but the Cox multivariate model did not confirm the data. We speculate that mature T cells in the tumor microenvironment have an important role in tumor recurrence but might be affected by the ratio and composition of different T cell subtypes. Similar to CD8+ T cells, our current data showed that the presence of CD3+ T cells within the tumor microenvironment was positively associated with the pT stage; however, some CD4+ CD25+ Treg cells are included in these TILs,[23] which could suppress immune function. CD3 staining alone cannot identify these T-regs. This may contribute to a lack of association of CD3+ T cell levels in a tumor tissue with prognosis.
In addition, IL-2 can activate T cells, NK cells, mononuclear macrophages, and marrow B cells to participate in killing tumor cells. However, activated T cells express the IL-2 receptor for IL-2 binding, leading to cell proliferation. A previous study has shown that IL-2 protein is expressed and detected in all types of lung tumor cells,[24] including atypical carcinoids, and is inversely associated with the proliferative activity of these tumor cells. An impaired immune defense or suppression of cytokine secretion capacity in cancer patients may have clinical relevance and influence patient survival. In addition, suppression of IL-2 secretion has been significantly associated with reduced survival of NSCLC.[25] The expression of different cytokines in tumor lesions may be a better predictor for prognosis and reflect antitumor immunity. Indeed, our current study showed that IL-2 was an independent prognostic parameter. An inverse correlation of CD8+ T cells with IL-2 expression and CD3+ T cells as well as the association of CD3+ T cells and IL-2 expression showed the double-edged sword nature of immune factors as well as the complex relationship between them.
However, our current study was just a proof-of-principle study, and much more research is needed because antitumor immunology is very complex and a great number of factors and cells are involved. Future studies will precisely identify the subtypes of lymphocytes in tumor lesions and assess the expression of cytokines and chemokines in NSCLC tissues to better understand their role in NSCLC and develop some of them as biomarkers to predict the prognosis or treatment responses.
ACKNOWLEDGMENTS
We would like to thank Prof. Shunchang Jiao of the General Hospital of Chinese People's Liberation Army for technical assistance.
Footnotes
Edited by: Limin Chen
Source of Support: This study was supported by a grant from the Eleventh Five Years Plan of Emphasis Topic Fund of People's Liberation Army (No. 06G106).
Conflict of Interest: None declared.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Groome PA, Bolejack V, Crowley JJ, Kennedy C, Krasnik M, Sobin LH, et al. The IASLC lung cancer staging project: Validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 3.Dalgleish AG, O’Byrne K. Inflammation and cancer: The role of the immune response and angiogenesis. Cancer Treat Res. 2006;130:1–38. [PubMed] [Google Scholar]
- 4.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 5.Ortegel JW, Staren ED, Faber LP, Warren WH, Braun DP. Modulation of tumor-infiltrating lymphocyte cytolytic activity against human non-small cell lung cancer. Lung Cancer. 2002;36:17–25. doi: 10.1016/s0169-5002(01)00472-x. [DOI] [PubMed] [Google Scholar]
- 6.Yoshino I, Yano T, Murata M, Ishida T, Sugimachi K, Kimura G, et al. Tumor-reactive T-cells accumulate in lung cancer tissues but fail to respond due to tumor cell-derived factor. Cancer Res. 1992;52:775–81. [PubMed] [Google Scholar]
- 7.Gong F. Beijing: Science Publishing Company; 2008. Medical Immunology; pp. 102–19. [Google Scholar]
- 8.Wakabayashi O, Yamazaki K, Oizumi S, Hommura F, Kinoshita I, Ogura S, et al. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94:1003–9. doi: 10.1111/j.1349-7006.2003.tb01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T, et al. Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer. 2008;113:1387–95. doi: 10.1002/cncr.23712. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi E, Yamazaki K, Torigoe T, Cho Y, Miyamoto M, Oizumi S, et al. HLA class I antigen expression is associated with a favorable prognosis in early stage non-small cell lung cancer. Cancer Sci. 2007;98:1424–30. doi: 10.1111/j.1349-7006.2007.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UICC. 5th ed. New York: Wiley; 1997. TNM Classification of Malignant Tumors; pp. 93–7. [Google Scholar]
- 12.Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E. 3rd ed. Berlin, Heidelberg, New York: Springer; 1999. Histological Typing of Lung and Pleural Tumour, International Histological Classification of Tumours. [Google Scholar]
- 13.Friedrichs K, Gluba S, Eidtmann H, Jonat W. Overexpression of p53 and prognosis in breast cancer. Cancer. 1993;72:3641–7. doi: 10.1002/1097-0142(19931215)72:12<3641::aid-cncr2820721215>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Kuo SH, Chang DB, Lee YC, Lee YT, Luh KT. Tumour-infiltrating lymphocytes in non-small cell lung cancer are activated T lymphocytes. Respirology. 1998;3:55–9. doi: 10.1046/j.1440-1843.1998.d01-9.x. [DOI] [PubMed] [Google Scholar]
- 15.Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol Oncol. 2012;124:192–8. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kataki A, Scheid P, Piet M, Marie B, Martinet N, Martinet Y, et al. Tumor infiltrating lymphocytes and macrophages have a potential dual role in lung cancer by supporting both host-defense and tumor progression. J Lab Clin Med. 2002;140:320–8. doi: 10.1067/mlc.2002.128317. [DOI] [PubMed] [Google Scholar]
- 17.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: From immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 18.Yie SM, Yang H, Ye SR, Li K, Dong DD, Lin XM. Expression of human leucocyte antigen G (HLA-G) is associated with prognosis in non-small cell lung cancer. Lung Cancer. 2007;58:267–74. doi: 10.1016/j.lungcan.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Ruffini E, Asioli S, Filosso PL, Lyberis P, Bruna MC, Macrì L, et al. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. 2009;87:365–71. doi: 10.1016/j.athoracsur.2008.10.067. [DOI] [PubMed] [Google Scholar]
- 20.Trojan A, Urosevic M, Dummer R, Giger R, Weder W, Stahel RA. Immune activation status of CD8+ T cells infiltrating non-small cell lung cancer. Lung Cancer. 2004;44:143–7. doi: 10.1016/j.lungcan.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Törmänen-Näpänkangas U, Soini Y, Pääkkö P. High number of tumour-infiltrating lymphocytes is associated with apoptosis in non-small cell lung carcinoma. APMIS. 2001;109:525–32. [PubMed] [Google Scholar]
- 22.Al-Shibli K, Al-Saad S, Andersen S, Donnem T, Bremnes RM, Busund LT. The prognostic value of intraepithelial and stromal CD3-, CD117- and CD138-positive cells in non-small cell lung carcinoma. APMIS. 2010;118:371–82. doi: 10.1111/j.1600-0463.2010.02609.x. [DOI] [PubMed] [Google Scholar]
- 23.Karagöz B, Bilgi O, Gümüs M, Erikçi AA, Sayan O, Türken O, et al. CD8+ CD28- cells and CD4+ CD25+ regulatory T cells in the peripheral blood of advanced stage lung cancer patients. Med Oncol. 2010;27:29–33. doi: 10.1007/s12032-008-9165-9. [DOI] [PubMed] [Google Scholar]
- 24.Kasprzak A, Olejniczak K, Przybyszewska W, Zabel M. Cellular expression of interleukin 2 (IL-2) and its receptor (IL-2R, CD25) in lung tumours. Folia Morphol (Warsz) 2007;66:159–66. [PubMed] [Google Scholar]
- 25.Neuner A, Schindel M, Wildenberg U, Muley T, Lahm H, Fischer JR. Prognostic significance of cytokine modulation in non-small cell lung cancer. Int J Cancer. 2002;101:287–92. doi: 10.1002/ijc.10604. [DOI] [PubMed] [Google Scholar]


