Abstract
Background:
Steady-state bone marrow (SS-BM) and granulocyte colony-stimulating growth factor-primed BM/peripheral blood stem-cell (G-BM/G-PBSC) are the main stem-cell sources used in allogeneic hematopoietic stem-cell transplantation. Here, we evaluated the treatment effects of SS-BM and G-BM/G-PBSC in human leucocyte antigen (HLA)-identical sibling transplantation.
Methods:
A total of 226 patients (acute myelogenous leukemia-complete remission 1, chronic myelogenous leukemia-chronic phase 1) received SS-BM, G-BM, or G-PBSC from an HLA-identical sibling. Clinical outcomes (graft-versus-host disease [GVHD], overall survival, transplant-related mortality [TRM], and leukemia-free survival [LFS]) were analyzed.
Results:
When compared to SS-BM, G-BM gave faster recovery time to neutrophil or platelet (P < 0.05). Incidence of grade III-IV acute GVHD and extensive chronic GVHD (cGVHD) was lower than seen with SS-BM (P < 0.05) and similar to G-PBSC. Although the incidence of cGVHD in the G-BM group was similar to SS-BM, both were lower than G-PBSC (P < 0.05). G-BM and G-PBSC exhibited similar survival, LFS, and TRM, but were significantly different from SS-BM (P < 0.05). There were no significant differences in leukemia relapse rates among the groups (P > 0.05).
Conclusions:
G-CSF-primed bone marrow shared the advantages of G-PBSC and SS-BM. We conclude that G-BM is an excellent stem-cell source that may be preferable to G-PBSC or SS-BM in patients receiving HLA-identical sibling hematopoietic stem-cell transplantation.
Keywords: Bone Marrow, Granulocyte Colony-stimulating Growth Factor, Human Leucocyte Antigen-identical Sibling Hematopoietic Stem-cell Transplantation, Peripheral Blood Stem-cells
INTRODUCTION
Hematopoietic stem-cell transplantation (HSCT) is an important therapeutic option for many malignant and nonmalignant disorders. Since the first report of bone marrow (BM) transplantation, steady-state BM (SS-BM) has been the only stem-cell source for HSCT. In recent years, granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood stem-cells (G-PBSC) have become an alternative stem-cell source. G-PBSC is even preferred in adults receiving human leucocyte antigen (HLA)-identical sibling transplantation. Although G-PBSC are enriched in progenitor cells as compared to SS-BM, which allows a faster engraftment,[1] the use of G-PBSC will increase the incidence of chronic graft-versus-host disease (cGVHD).[2,3] To take advantage of G-PBSC while decrease the risk of cGVHD, G-CSF-primed BM (G-BM) has been explored. Recently, G-BM has been an attractive option for HLA-haploidentical/mismatched donor transplantation.[4,5,6] However, G-BM does not seem to be considered an alternative to SS-BM in HLA-identical HSCT due to nonsignificant improvement in survivals,[7,8,9] although its feasibleness, safety, well-engraftment and limited incidence of GVHD have been demonstrated.[7,8,9,10,11,12,13,14,15,16]
The conclusions from previous studies on HLA-identical transplantation with G-BM might be limited due to the number of patients, underlying diseases, disease status at the time of transplantation, G-CSF schedules, conditioning regimens, and GVHD prophylaxis. In this study, we conducted a retrospective nonrandomized controlled study to evaluate the clinical outcomes of G-BM, SS-BM, and G-PBSC in HLA-identical sibling HSCT in our center. The results suggested that G-BM was an excellent stem-cell source in HLA-identical sibling HSCT.
METHODS
Patients
A total of 226 early stage myeloid leukemia patients, including de novo acute myelogenous leukemia (AML) (complete remission 1 [CR1]) and chronic myelogenous leukemia (CML) (chronic phase 1 [CP1]), with HLA-identical sibling donor, were enrolled in this study (March 2004–March 2009). Patients who have received second transplants/reduced-intensity preparative regiments were excluded. The main clinical characteristics of patients at the time of HSCT were listed in Table 1. 43, 60, and 123 patients were enrolled in the G-BM, SS-BM, and G-PBSC groups, respectively.
Table 1.
Patient characteristics
| Items | G - BM | SS - BM | G - PBSC | P |
|---|---|---|---|---|
| Number of patients | 43 | 60 | 123 | |
| Sex (male/female) | ||||
| Recipients (male/female) | 29/14 | 35/25 | 80/43 | 0.576 |
| Donors (male/female) | 21/22 | 29/31 | 61/62 | 0.986 |
| Age (years) | ||||
| Recipients | 36 (12 - 52) | 28 (15 - 45) | 35 (14 - 56) | |
| Donors | 35 (14 - 60) | 28 (12 v 42) | 35 (12 - 67) | |
| Female donor/male recipient (%) | 39.5 | 33.3 | 33.3 | 0.744 |
| Diagnosis | ||||
| AML | 27 | 32 | 55 | 0.109 |
| CML | 16 | 28 | 68 | |
| Conditioning regimen | ||||
| Cy/fractionated TBI (%) | 100 | 100 | 100 |
SS-BM: Steady-state bone marrow; G-PBSC: G-peripheral blood stem cell; G-BM: G-CSF-primed bone marrow; AML: Acute myelocytic leukemia; CML: Chronic myelocytic leukemia; TBI: Total body irradiation.
This study was approved by the Ethics Committee of the affiliated hospital of Academy of Military Medical Sciences. Informed consents were obtained from all patients, donors, or their legal guardians.
Treatment
In the G-PBSC group, donors received G-CSF at 8.0 μg kg-1 d-1 divided in two subcutaneous injections for 5 consecutive days (day −3 to day +1), and PBSC was harvested on day 0 and day +1. In the G-BM group, donors received G-CSF at 5.0 μg kg-1 d-1 by a single subcutaneous injection for 3 consecutive days (day −3 to day −1), and G-BM was harvested on day 0. In the SS-BM group, BM was harvested at day 0. BMs was harvested from donors based on a target volume of 20 ml/kg. The stem-cells were infused on the same day they were collected. The median total nucleated cells infused into recipients were 4.2 × 108/kg (G-BM, range: 2.2 × 108– 8.1 × 108/kg), 7.7 × 108/kg (G-PBSC, range: 1.8 × 108– 22.7 × 108/kg), and 2.5 × 108/kg (SS-BM, range: 1.8 × 108–4.4 × 108/kg) of recipient weight, respectively. Plasma or red cell depletion of the grafts was performed if any ABO incompatibility was presented before infusions.
All recipients received the standard preparative regimen involving cyclophosphamide (60 mg∙kg-1∙d-1 × 2, day −4, day −3) and total body irradiation (TBI) (5 Gy/day × 2, day −2, day −1). The GVHD prophylaxis included cyclosporin A (CSA) and the short-term methotrexate (MTX).
Evaluations and definitions
Neutrophil recovery was defined as having occurred after the first of 3 days with an absolute neutrophil count >500/μl after the post-transplant nadir. Platelet recovery was defined as the first of 7 consecutive days with a platelet count >50,000/μl without platelet transfusions. Acute and cGVHD (aGVHD) were graded by the Seattle criteria.[17,18] Transplant-related mortality (TRM) was defined as death from any cause except relapse. Leukemia-free survival (LFS) was defined as the time interval from transplantation to the first event (either relapse or death). Overall survival (OS) was calculated from the time of transplantation to death.
Statistic analysis
The cumulative incidence curves of GVHD, TRM, LFS, and OS were plotted using the Kaplan–Meier method, and the long-rank test was used for independence comparison between variables. For all analysis, P < 0.05 was considered as statistically significant. All statistical analysis was performed with the SPSS statistics 20.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Hematopoietic reconstitution
The median time to neutrophil recovery was 15 days (range, 11–26), 17 days (range, 13–31), and 25 days (range, 15–63) in the G-PBSC group, the G-BM group, and the SS-BM group, respectively [Figure 1a]. The median time to platelet recovery were 22 days (range, 12–176), 32 days (range, 15–365), and 47 days (range, 21–200) in the G-PBSC group, the G-BM group, and the SS-BM group, respectively [Figure 1b]. The time to neutrophils and platelet engraftment was significantly different among all groups (G-PBSC vs. G-BM, G-PBSC vs. SS-BM, G-BM vs. SS-BM) (P < 0.05).
Figure 1.
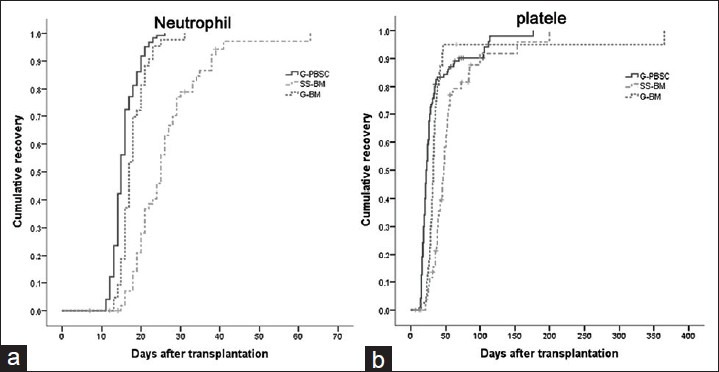
Engraftment. (a) Time to absolute neutrophil count recovery above 0.5 × 109/L. (b) Time to platelets recovery above 50 × 109/L.
G-CSF-primed bone marrow reduced the risk of graft-versus-host disease
The cumulative incidences of grade II–IV aGVHD were (30.4 ± 7.0)%, (26.7 ± 5.9)%, and (36.2 ± 4.4)% in the G-BM, SS-BM, and G-PBSC group, respectively [Figure 2a]. There was no significant difference on incidence of aGVHD among these groups (P > 0.05). The cumulative incidence of grade III–IV aGVHD in the G-BM group ([4.7 ± 3.2]%) was significantly lower than that in the SS-BM group ([17.5 ± 5.0]%, P < 0.05). There was a tendency of lower frequency of grade III–IV aGVHD in the G-PBSC group compared to SS-BM, although it was not statistically significant ([16.4 ± 3.4]% vs. [17.5 ± 5.0]%, P = 0.058) [Figure 2b].
Figure 2.
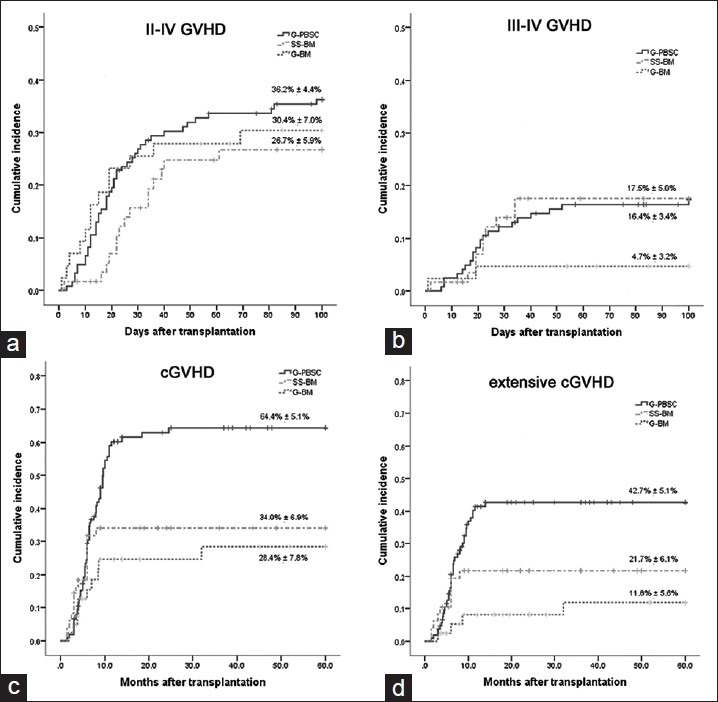
Cumulative incidence of graft-versus-host disease (GVHD). (a) Grades II–IV acute GVHD. (b) Grades III–IV acute GVHD. (c) Chronic GVHD. (d) Extensive chronic GVHD.
For cGVHD, the cumulative incidence in the G-BM group was similar to that in the SS-BM group ([28.4 ± 7.8]% vs. [34.0 ± 6.9]%, P > 0.01), while there was significant differences between the G-BM and G-PBSC group ([28.4 ± 7.80]% vs. [64.4 ± 5.1]%, P < 0.05), and between the SS-BM and G-PBSC group ([34.0 ± 6.9]% vs. [64.4 ± 5.1]%, P < 0.05) [Figure 2c]. Similar patterns were observed when the cumulative incidences of extensive cGVHD were analyzed (G-BM vs. G-PBSC: [11.8 ± 5.6]% vs. [42.7 ± 5.1]%, P < 0.05; G-BM vs. SS-BM: [11.8 ± 5.6]% vs. [21.7 ± 6.1]%, P < 0.05) [Figure 2d].
G-CSF-primed bone marrow increased the survival rates
The OS rate in the G-BM group and the G-PBSC group were (76.3 ± 6.6)% and (70.3 ± 4.6)%, respectively. Both were significantly higher than the SS-BM group ([45.1 ± 6.6]%, P < 0.05) [Figure 3a]. The incidences of LFS in the G-BM and G-PBSC were (68.7 ± 7.2)% and (68.9 ± 4.7)%, both were significantly higher than that in the SS-BM group ([45.3 ± 6.6]%, P < 0.05) [Figure 3b]. The cumulative incidence of TRM in the SS-BM group was (41.2 ± 6.7)%, which was significantly higher than that in the G-PBSC and the G-BM groups ([19.0 ± 3.9]%, P < 0.05; [12.0 ± 5.0]%, P < 0.05) [Figure 3c]. However, there were no significant differences in leukemia relapse rates between any two groups (G-BM, [23.9 ± 7.0]%; G-PBSC, [20.3 ± 4.4]%; SS-BM, [24.0 ± 7.0]%) (P > 0.1) [Figure 3d].
Figure 3.
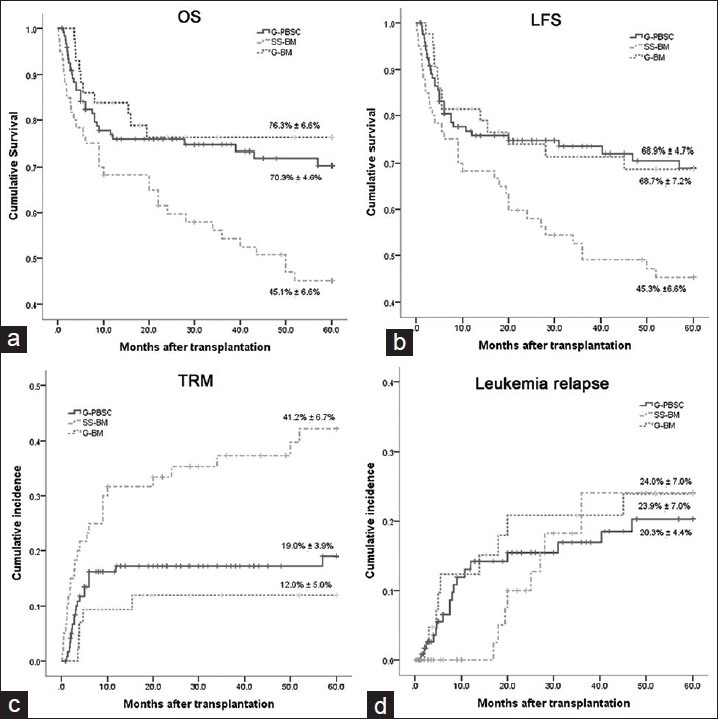
Survival of the entire cohort by therapy. (a) Overall survival. (b) Leukemia-free survival. (c) Transplant-related mortality. (d) Leukemia relapse.
Table 2 shows the causes of death in each group. A total of 33 of 60 patients (55%) died due to a variety of factors (leukemia relapse, GVHD, infections, interstitial pneumonia/idiopathic pneumonia syndrome, graft rejection, etc.) in the SS-BM group. In contrast, only 25.6% (11/43) of the G-BM group and 26.0% (32/123) of the G-PBSC group died due to the same reasons.
Table 2.
Causes of death
| Items | G-BM (n = 43) | SS-BM (n = 60) | G-PBSC (n = 123) |
|---|---|---|---|
| Leukemia relapse | 6 | 8 | 11 |
| aGVHD (or with infections) | 1 | 6 | 7 |
| cGVHD | 1 | 5 | 4 |
| Infections | 1 | 5 | 5 |
| Interstitial pneumonia/idiopathic pneumonia syndrome | 0 | 2 | 3 |
| Graft rejection | 1 | 2 | 0 |
| HVOD | 0 | 1 | 0 |
| Cerebral hemorrhage | 0 | 1 | 0 |
| Organ dysfunction | 1 | 0 | 1 |
| Gastric cancer | 0 | 1 | 0 |
| Hemocytolysis | 0 | 1 | 0 |
| Asthma | 0 | 0 | 1 |
| Hepatitis B | 0 | 1 | 0 |
| Total (n (%)) | 11 (25.6) | 33 (55.0) | 32 (26.0) |
aGVHD: Acute graft-versus-host disease; cGVHD: Chronic graft-versus-host disease; HVOD: Hepatic veno-occlusive disease; G-PBSC: G-peripheral blood stem cell; G-BM: G-CSF-primed bone marrow.
Meanwhile, there was no significant difference between AML and CML on OS ([59.8 ± 5.0]% versus [67.7 ± 4.7]%, P > 0.05), LFS ([57.3 ± 5.1]% vs. [66.0 ± 4.8]%, P > 0.05), and TRM ([23.7 ± 4.4]% versus [24.1 ± 4.4]%, P > 0.05). However, AML is associated with more leukemia relapse compared to CML ([27.7 ± 5.1]% versus [16.4 ± 4.2]%, P < 0.05).
DISCUSSION
Steady-state bone marrow, G-PBSC, and G-BM are the main stem-cell sources in allogeneic HSCT. A number of groups have reported on the clinical outcomes of patients who received G-BM, G-PBSC, or SS-BM during HLA-identical sibling HSCT.[7,8,9,10,11,12,13,14,15,16] These studies show that G-BM and G-PBSC resulted in significantly faster neutrophil and platelet recovery than SS-BM, which is consistent with the results presented here. However, previous studies indicated that G-BM appeared to have no advantage in survivals when compared to SS-BM.[7,8,9] In this study, compared with patients treated with SS-BM, those patients who were treated with G-BM and G-PBSC had significantly higher OS [Figure 3a] and LFS [Figure 3b]. As to TRM and III-IV grade aGVHD, the incidences of patients treated with G-BM were significantly lower chance to develop aGVHD and greater chance of survival than those treated with SS-BM [Figure 2a and b]. Interestingly, G-BM could significantly decrease the incidences of cGVHD and extensive cGVHD, compared with G-PBSC [Figures 2c and d], which was consistent with a previous study.[13] However, G-BM did not increase the incidences of leukemia relapse [Figure 2d]. These results clearly indicated that G-BM shared the advantages of both G-PBSC and SS-BM, which was characterized by fast engraftment, low incidences of very severe aGVHD/cGVHD/TRM, and high incidences of LFS/OS. This report was the first study that included all three main stem-cell sources (G-BM, G-PBSC, SS-BM), and we concluded that G-BM was an excellent stem-cell source for HLA-identical sibling HSCT.
Many groups have compared the different clinical outcomes of G-BM with SS-BM or G-PBSC in HLA-identical sibling HSCT,[7,8,9,10,11,12,13,15,19] however, the results have been very controversial, especially regarding the OS. Our study indicated that G-BM was superior to SS-BM and G-PBSC, and that a significant improvement in OS was observed in the G-BM group. The variances might be caused by several factors. First was the number of the enrolled patient and the follow-up durations. Previous reports studied fewer patients (22, 48, and 50 patients) with shorter follow-up duration (the median time <12 months).[7,8,9] We have conducted the current study with more patients (226) and longer follow-up time (median time, G-BM: 60 months; G-PBSC: 30 months; SS-BM: 40 months), which could reach a sufficient statistical power. The second was the G-CSF priming schedules. Previous studies have shown that G-CSF stimulation caused a 1.4–1.7-fold increase in CD34 + cell count, a 3-fold increase in colony-forming cells, and 50–90-fold increase in short-term repopulating cells in G-BM, when compared to SS-BM.[20] However, different priming schedules (doses and duration of G-CSF administration) might cause controversial results regarding the engraftment capability of G-BM,[21,22] which would in turn cause different treatment outcomes. The priming schedules in previous studies were very diverse.[10,11,12,13,15,19] The dose of G-CSF for BM priming ranged from 5 μg∙kg-1∙d-1 to 12.1 μg∙kg-1∙d-1 and the length of G-CSF treatment ranged from 2 to 5 days. In the present study, all donors received a uniformed G-CSF priming schedule, which was 5.0 μg∙kg-1∙d-1 for 3 consecutive days. In addition, the disease status before the transplantation would have an impact on the outcome. To rule out the interference of disease status, we only included patients with de novo AML (CR1) and CML (CP1). We found that AML patients had higher leukemia relapse rates than CML, which suggested that different underlying disease before transplantation might play a role in the treatment effect of HSCT. Finally, the preparative regimens, the GVHD prophylaxis, and the supportive care might play a role in the outcome. We used the classic Cy routine combined with fractionated TBI as preparative regimens and standard CSA plus short-term MTX as prophylactic measures to prevent GVHD in all patients. The uniform management could reduce the interferences of these factors and give more credible results. Further investigation is needed to address how these factors influenced the treatment effects in HSCT.
Despite the availability of many new immune suppressive drugs and antibodies, GVHD still remained the most serious complications in HSCT. To obtain the optimal results, we need to decrease the GVHD while enhance the graft-versus-leukemia (GVL) effect. To effect, a lot of approaches have been developed to minimize the GVHD, such as the depletion of T-cells in vitro and in vivo, and the introduction of suicide gene into T-cells.[23,24] These approaches could decrease the incidence of GVHD, but the lower GVHD rates might be due to using of anti-thymocyte globulin, depletion of T-cells, or other factors. In this study, we found that the GVL effect in G-BM was superior to G-PBSC and SS-BM. As presented in this study, G-BM could remarkably lower the risk of GVHD (III-IV grade GVHD, cGVHD, extensive cGVHD), and accordingly improve the survival. However, the incidence of leukemia relapse did not increase, suggesting that G-BM might be an alternative way to enhance GVL effect and lower the risk of GVHD. Meanwhile, the use of G-BM in HSCT might be a simple and “low prices” approach to separate GVHD and GVL effects which did not require additional immunosuppression drugs or special measurements.
This was the first retrospective report on HLA-identical sibling HSCT that was conducted in a single center with all three main grafts (G-BM, G-PBSC, SS-BM). Compared with previous studies, the overall clinical outcomes of G-BM, G-PBSC, and SS-BM in this study might be more credible because the number of patients was higher and the treatments (preparative regimens, GVHD prophylaxis, and supportive care) were uniform. Recently, a phase 3, randomized trial comparing G-BM and SS-BM is ongoing,[15] and another randomized multicenter study comparing G-PBSC and G-BM in patients receiving HLA-identical sibling HSCT is also being conducted in Canada.[25] Although the corresponding results are not released yet, we believe that these studies will further illuminate the advantages of G-BM in HSCT.
This study has several limitations including the retrospective nature, the heterogeneous patient cohorts, and lacking of molecular typing. However, the results are remarkable because of a comparatively large number of patients with a long follow-up as well as uniform data collection.
In summary, our study has provided sufficient data to conclude that G-BM is an excellent stem-cell source in HLA-identical sibling HSCT. G-BM transplant is characterized by fast engraftment, low incidence of severe aGVHD/cGVHD, and improved survival. We recommend G-BM rather than G-PBSC or SS-BM for patients receiving HLA-identical sibling HSCT. Meanwhile, G-BM is potentially a good stem-cell source in unrelated and haploidentical HSCT, which need to be further investigated in the future.
Footnotes
Edited by: Limin Chen
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Arcese W, Aversa F, Bandini G, De Vincentiis A, Falda M, Lanata L, et al. Clinical use of allogeneic hematopoietic stem cells from sources other than bone marrow. Haematologica. 1998;83:159–82. [PubMed] [Google Scholar]
- 2.Schmitz N, Beksac M, Hasenclever D, Bacigalupo A, Ruutu T, Nagler A, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002;100:761–7. doi: 10.1182/blood-2001-12-0304. [DOI] [PubMed] [Google Scholar]
- 3.Storek J, Gooley T, Siadak M, Bensinger WI, Maloney DG, Chauncey TR, et al. Allogeneic peripheral blood stem cell transplantation may be associated with a high risk of chronic graft-versus-host disease. Blood. 1997;90:4705–9. [PubMed] [Google Scholar]
- 4.Waller EK, Logan BR, Harris WA, Devine SM, Porter DL, Mineishi S, et al. Improved survival after transplantation of more donor plasmacytoid dendritic or naïve T cells from unrelated-donor marrow grafts: Results from BMTCTN 0201. J Clin Oncol. 2014;32:2365–72. doi: 10.1200/JCO.2013.54.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mo XD, Xu LP, Zhang XH, Liu DH, Wang Y, Chen H, et al. Haploidentical hematopoietic stem cell transplantation in adults with Philadelphia-negative acute lymphoblastic leukemia: No difference in the high- and low-risk groups. Int J Cancer. 2014 doi: 10.1002/ijc.29146. [DOI] [PubMed] [Google Scholar]
- 6.De Angelis G, Santarone S, Cerretti R, Picardi A, Bavaro P, Olioso P, et al. Non T-cell depleted, G-CSF primed bone marrow transplantation from haploidentical donors for patients with high-risk acute myeloid leukaemia. Bone Marrow Transplant. 2011;46:S39. [Google Scholar]
- 7.Ji SQ, Chen HR, Wang HX, Yan HM, Pan SP, Xun CQ. Comparison of outcome of allogeneic bone marrow transplantation with and without granulocyte colony-stimulating factor (lenograstim) donor-marrow priming in patients with chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8:261–7. doi: 10.1053/bbmt.2002.v8.pm12064363. [DOI] [PubMed] [Google Scholar]
- 8.Chiang KY, Haight A, Horan J, Olson E, Gartner A, Hartman D, et al. Clinical outcomes and graft characteristics in pediatric matched sibling donor transplants using granulocyte colony-stimulating factor-primed bone marrow and steady-state bone marrow. Pediatr Transplant. 2007;11:279–85. doi: 10.1111/j.1399-3046.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 9.Ji SQ, Chen HR, Xun CQ, Wang HX, Pan SP, Xiao MH. The effect of G-CSF-stimulated donor marrow on engraftment and incidence of graft-versus-host disease in allogeneic bone marrow transplantation. Clin Transplant. 2001;15:317–23. doi: 10.1034/j.1399-0012.2001.150503.x. [DOI] [PubMed] [Google Scholar]
- 10.Isola L, Scigliano E, Fruchtman S. Long-term follow-up after allogeneic granulocyte colony-stimulating factor-primed bone marrow transplantation. Biol Blood Marrow Transplant. 2000;6:428–33. doi: 10.1016/s1083-8791(00)70034-6. [DOI] [PubMed] [Google Scholar]
- 11.Couban S, Messner HA, Andreou P, Egan B, Price S, Tinker L, et al. Bone marrow mobilized with granulocyte colony-stimulating factor in related allogeneic transplant recipients: A study of 29 patients. Biol Blood Marrow Transplant. 2000;6:422–7. doi: 10.1016/s1083-8791(00)70033-4. [DOI] [PubMed] [Google Scholar]
- 12.Serody JS, Sparks SD, Lin Y, Capel EJ, Bigelow SH, Kirby SL, et al. Comparison of granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood progenitor cells and G-CSF – stimulated bone marrow as a source of stem cells in HLA-matched sibling transplantation. Biol Blood Marrow Transplant. 2000;6:434–40. doi: 10.1016/s1083-8791(00)70035-8. [DOI] [PubMed] [Google Scholar]
- 13.Morton J, Hutchins C, Durrant S. Granulocyte-colony-stimulating factor (G-CSF)-primed allogeneic bone marrow: Significantly less graft-versus-host disease and comparable engraftment to G-CSF-mobilized peripheral blood stem cells. Blood. 2001;98:3186–91. doi: 10.1182/blood.v98.12.3186. [DOI] [PubMed] [Google Scholar]
- 14.Ostronoff F, Ostronoff M, Souto-Maior AP, Domingues M, Sucupira A, Manso DA, et al. Prospective trial of mycophenolate mofetil-cyclosporine A prophylaxis for acute GVHD after G-CSF stimulated allogeneic bone marrow transplantation with HLA-identical sibling donors in patients with severe aplastic anemia and hematological malignancies. Clin Transplant. 2009;23:33–8. doi: 10.1111/j.1399-0012.2008.00894.x. [DOI] [PubMed] [Google Scholar]
- 15.Frangoul H, Nemecek ER, Billheimer D, Pulsipher MA, Khan S, Woolfrey A, et al. A prospective study of G-CSF primed bone marrow as a stem-cell source for allogeneic bone marrow transplantation in children: A Pediatric Blood and Marrow Transplant Consortium (PBMTC) study. Blood. 2007;110:4584–7. doi: 10.1182/blood-2007-07-101071. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Min WS, Cho BS, Eom KS, Kim SY, Kim YJ, et al. Overcoming various comorbidities by G-CSF-primed unmanipulated BM SCT in adult patients with AML. Bone Marrow Transplant. 2009;44:345–51. doi: 10.1038/bmt.2009.42. [DOI] [PubMed] [Google Scholar]
- 17.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 19.Isola LM, Scigliano E, Skerrett D, Shank B, Ross V, Najfeld V, et al. A pilot study of allogeneic bone marrow transplantation using related donors stimulated with G-CSF. Bone Marrow Transplant. 1997;20:1033–7. doi: 10.1038/sj.bmt.1701029. [DOI] [PubMed] [Google Scholar]
- 20.Shier LR, Schultz KR, Imren S, Regan J, Issekutz A, Sadek I, et al. Differential effects of granulocyte colony-stimulating factor on marrow and blood derived hematopoietic and immune cell populations in healthy human donors. Biol Blood Marrow Transplant. 2004;10:624–34. doi: 10.1016/j.bbmt.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Damiani D, Fanin R, Silvestri F, Grimaz S, Infanti L, Geromin A, et al. Randomized trial of autologous filgrastim-primed bone marrow transplantation versus filgrastim-mobilized peripheral blood stem cell transplantation in lymphoma patients. Blood. 1997;90:36–42. [PubMed] [Google Scholar]
- 22.Martínez C, Urbano-Ispizua A, Rozman M, Rovira M, Marín P, Montfort N, et al. Effects of short-term administration of G-CSF (filgrastim) on bone marrow progenitor cells: Analysis of serial marrow samples from normal donors. Bone Marrow Transplant. 1999;23:15–9. doi: 10.1038/sj.bmt.1701526. [DOI] [PubMed] [Google Scholar]
- 23.Alyea EP, Soiffer RJ, Canning C, Neuberg D, Schlossman R, Pickett C, et al. Toxicity and efficacy of defined doses of CD4(+) donor lymphocytes for treatment of relapse after allogeneic bone marrow transplant. Blood. 1998;91:3671–80. [PubMed] [Google Scholar]
- 24.Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–24. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 25.Chang YJ, Huang XJ. Use of G-CSF-stimulated marrow in allogeneic hematopoietic stem cell transplantation settings: A comprehensive review. Clin Transplant. 2011;25:13–23. doi: 10.1111/j.1399-0012.2010.01298.x. [DOI] [PubMed] [Google Scholar]


