Abstract
Background:
Herpes simplex keratitis (HSK) caused by herpes simplex virus 1 (HSV-1), which has high recurrent rate and incidence of severe vision loss, is the leading cause of infectious blindness in the world. The aim was to explore the clinical efficacy of oral ganciclovir (GCV) in the prevention of recurrent HSK.
Methods:
A multicenter, prospective, randomized, single-blind, and controlled clinical trial was conducted from April 2010 to June 2013. One hundred seventy-three patients (173 eyes involved) who were diagnosed as recurrent HSK definitely, including stromal keratitis and corneal endotheliitis, were divided into three groups randomly: negative control (placebo) group was topically administered with 0.15% GCV ophthalmic gel, 4 times per day and 0.1% fluorometholone eye drops, 3 times per day until resolution of HSK; positive control acyclovir (ACV) group was topically adopted the same ophthalmic gel and eye drops and additionally received oral ACV 400 mg 5 times a day for 10 weeks and followed by 400 mg 2 times per day for 6 months; test GCV group was topically adopted the same treatment as negative control group and additionally received oral GCV 1000 mg 3 times per day for 8 weeks. The symptoms and signs were evaluated before and after the therapy 1st week, 2nd week and then followed up every 2 weeks until recovery. Furthermore, we followed up recurrence of HSK for every 3 months after recovery and then assessed the cure time, recurrent rate and adverse reactions.
Results:
One hundred and seventy-three patients were followed up 7–48 months (mean 32.1 ± 12.3 months), but 34 patients were failed to follow-up. The cure time was 12.1 ± 4.3, 11.9 ± 4.0 weeks in negative control (placebo) group and positive control ACV group respectively (P = 0.991), which was longer than that in test GCV group (8.6 ± 2.8 weeks) and there was a significant difference between test GCV group and negative control (placebo) group or positive control ACV group (P = 0.000). Furthermore, the recurrent rate was higher in negative control (placebo) group (47.3%) than that in positive control group ACV (26.7%) and test GCV group (17.2%), and there was a great significant difference among the three groups (P = 0.007), but there was no significant difference between positive control ACV group and test GCV group (P = 0.358). In addition, there was no obvious adverse reaction expect neutropenia (only one patient in test GCV group).
Conclusion:
Short-term oral GCV could cure recurrent HSK and endotheliitis, shorten the course, reduce recurrent rate of HSK and have confirmed safety.
Keywords: Corneal Endotheliitis, Ganciclovir, Herpes Simplex Keratitis, Recurrence, Stromal Keratitis
INTRODUCTION
Herpes simplex keratitis (HSK) caused by herpes simplex virus 1(HSV-1), which has high recurrent rate and incidence of severe vision loss, is the leading cause of infectious blindness in the world.[1,2]
Many studies have indicated that HSV establishes a latent infection in the trigeminal or other sensory ganglia, and recurrent viral shedding can lead to disease of one or both eyes.[3,4,5] Although topical therapy with antiviral drugs inhibits the HSV-1 replication and development of HSK, topical drugs cannot completely clear HSV-1 that resides in eyes or trigeminal ganglia. Once immune function is disordered, HSV-1 will reactivate to cause recurrence of HSK. Thus, the key point of prophylaxis of recurrent HSK is systemic antiviral therapy and regulating the immune resistant to the virus. Multicenter, randomized, double-blind controlled clinical trials suggested[6,7,8,9] that there was no significant difference of efficacy in the treatment of herpes stromal keratitis between oral acyclovir (ACV) therapy and placebo therapy, but further studies[9,10,11,12,13,14,15] found that long-term and low doses of oral ACV played a role in the prevention of recurrent HSK and oral herpes. However, ganciclovir (GCV) exhibited higher antiviral activity, longer half-life, and lower drug resistance rate than ACV.[16,17]
We conducted a prospective, multicenter, randomized, single-blind, controlled study to access the efficacy of oral GCV in the treatment of recurrent HSK, and further observe the efficacy and safety of oral GCV in the prevention of recurrent HSK, aiming at seeking for a potent and specific antiviral agent for patients with recurrent HSK.
METHODS
Subjects
With reference of stromal keratitis (subdivided into necrotizing stromal keratitis, immune stromal keratitis) and endotheliitis in four main categories proposed by Holland et al.,[18] patients who were diagnosed with recurrent HSK in Departments of Ophthalmology, EYE and ENT Hospital of Fudan University, Hangzhou First People's Hospital, and Nanjing First People's Hospital from April 2010 to December 2013 were recruited in this study.
Diagnostic criterion
The HSK was diagnosed by examination of corneal scrapings and determination of HSV DNA in collected tears using real-time polymerase chain reaction (RT-PCR) assay based on classification system–four main categories of HSK proposed by Holland et al.[18]
Groups
Patients were divided into three groups using random number table method. The negative control (placebo) group was topically administered with 0.15% GCV gel (one drop each time, 4 times per day, dripped into conjunctival sac of the eye) and 0.1% fluorometholone eye drops (1 drop each time, 3 times a day, dripped into conjunctival sac of the eye) until complete recovery.[19] The positive control ACV group was topically administered with the therapy identical to the negative control (placebo) group, combined with oral ACV (400 mg each time, 5 times per day, for 10 weeks)[20] followed by oral ACV at a dose of 400 mg given twice a day for lasting 6 months.[13,14] The test GCV group was topically administered with the therapy identical to the negative control (placebo) group, in combination with oral GCV (1000 mg each time, 3 times per day for 8 weeks).[21,22,23]
Inclusion criteria and exclusion criteria
Patients recruited in this study were not adopting any drug or systemic antiviral drugs or had stopped antiviral drugs for at least 1 week, and was strictly prohibited from adopting other antiviral drugs during this trial, they had no other eye problems and had normal kidney functions (creatinine clearance rate ≥70 ml/min). Patients were excluded in the study if they were pregnant or breast-feeding, or had severe heart, lung, liver, kidney dysfunctions or histories of diabetes and malignant tumors. Patients recruited in the study signed an informed consent approved by the Ethics Committee of EYE and ENT Hospital of Fudan University. Patients failed to keep scheduled follow-up or adopted other drugs that might impact the efficacy assessment during the study were not selected for analysis of efficacy. Patients who developed severe adverse reactions were also excluded for statistical analysis of efficacy, but included in the statistical analysis of adverse reactions.
Clinical observation and assessment parameters
Patients were asked about histories of eye conditions and systemic diseases, followed up before treatment and 1 week, 2 week, 4 week, 6 week and 8 week after treatment, and subsequently followed up every 2 weeks until complete recovery. Patients were examined eyesight and eye pressure. The anterior segment was carefully examined using a slit lamp to determine drug's efficacy. During follow-up, all subjects were asked about whether any discomfort occurred during therapy. All patients orally administered with drugs underwent routine blood and urine examinations as well as liver and kidney function examinations to monitor adverse reactions of drugs. For cytopenia, a reduction in leukocytes was defined as the count of leukocytes <3.0 × 109/L (excluding subjects with lower levels before treatment), and the count of leukocytes <1.0 × 109/L was regarded as a severe reduction in leukocytes; a reduction in platelets was defined as the count of platelets <5.0 × 109/L (excluding subjects with lower levels before treatment), and the count of platelets <2.0 × 109/L was regarded as a severe reduction in platelets.
Follow-up and recurrence of herpes simplex keratitis
After recovery, patients had a follow-up[12] every 3 months for 3–5 years to assess the recurrence of HSK. If any recurrence of HSK was reported by the patient or observed during follow-up, the diagnosis was made by re-examination of corneal scraping and determination of HSV DNA in collected tears using RT-PCR.
Statistical analysis
All data were statistically analyzed using SPSS 15.0 for Windows (SPSS Inc., IL, USA). The data for age, follow-up time and course time were shown as mean ± standard deviation (SD). The patients’ ages among three groups were compared using analysis of variance (ANOVA), and a comparison between two groups was performed using least significant difference t-test. The recovery periods among three groups were compared using nonparametric rank-sum test (Kruskal-Wallis), and a comparison between two groups was performed using Student-Newman-Keuls test. The differences in sex, eye, HSK type and the number of cases with recurrent HSK among three groups were compared using Pearson chi-square test. The HSK-free durations among three groups were compared using survival curve analysis (Kaplan-Meier Log Rank). A value of P < 0.05 was considered as statistically significant.
RESULTS
General data
A total of 173 cases (173 eyes involved) were included in this study, with 58 cases in the placebo group, 55 cases in the positive control ACV group and 60 cases in the test GCV group. The mean follow-up was 32.1 ± 12.3 months (range: 7–48 months). No significant differences were observed in age, sex, eye, HSK classification among three groups [Table 1].
Table 1.
General data and follow-up in three groups
| Items | Placebo | ACV | GCV | Sum | P |
|---|---|---|---|---|---|
| n | 55 | 60 | 58 | 173 | 0.810 |
| Age (mean ± SD, years) | 55.1 ± 14.7 | 55.4 ± 12.5 | 56.1 ± 12.5 | 55.5 ± 13.7 | 0.286 |
| Sex (male:female) | 29:26 | 38:22 | 43:15 | 110:63 | 0.061 |
| Eye (right:left) | 32:23 | 25:35 | 27:31 | 84:89 | 0.195 |
| Stromal keratitis: endothelial keratitis (n/n)) | 36:19 | 45:15 | 46:12 | 127:46 | 0.131 |
| Mean follow-up time (mean ± SD, months) | 30.4 ± 11.6 | 31.8 ± 13.1 | 34.1 ± 12.0 | 32.1 ± 12.3 | 0.334 |
ACV: Acyclovir; GCV: Ganciclovir.
Course of herpes simplex keratitis
The course was defined as the time of topical application until no improvement of the symptoms and signs. The mean course of all groups were 10.9 ± 4.1 weeks. The mean course of HSK in the test GCV group was 8.6 ± 2.8 weeks, which were significantly shorter than the placebo group (12.1 ± 4.3 weeks) and the ACV group (11.9 ± 4.0 weeks), and the differences between placebo group or positive control ACV group vs. test GCV group were statistically significant (all P = 0.000). But there was no significant difference between placebo and positive control ACV groups (P = 0.991).
Recurrence of herpes simplex keratitis
There were six cases lost in the placebo group, 15 cases in the ACV group and 13 cases in the GCV group respectively during follow-up. There was no statistical significance in the dropout rate among three groups (χ2 = 4.029, P = 0.133). After recovery, there were 26 (47.3%) patients with HSK recurrence during follow-up in the placebo group, 16 (26.7%) in the ACV group and 10 (17.2%) in the GCV group respectively. The symptoms and signs could be cured after treatment. There was statistically significant difference in recurrence rate among three groups (χ2 = 14.056, P = 0.007) [Table 2]. The recurrence rate in the GCV group was significantly lower than that in the placebo group (χ2 = 12.102, P = 0.002), but no statistically significant difference was observed between the GCV group and the ACV group (χ2 = 2.057, P = 0.358). The recurrence rate in the ACV group was statistically lower compared with the placebo group (χ2 = 6.726, P = 0.035).
Table 2.
Comparison of recurrence in three groups (n)
| Conditions | Placebo group | ACV group | GCV group | Chi- square | P |
|---|---|---|---|---|---|
| No recurrence | 23 | 29 | 35 | 14.056 | 0.007* |
| Recurrence | 26 | 16 | 10 | ||
| Lost to follow-up | 6 | 15 | 13 |
*Placebo versus ACV (χ2 = 6.726, P = 0.035); Placebo versus GCV (χ2 = 12.102, P = 0.002); ACV versus GCV (χ2 = 2.057, P = 0.358). ACV: Acyclovir; GCV: Ganciclovir.
According to the survival curve analysis [Figure 1], there was statistically significant difference in recurrent time of HSK among three groups (χ2 = 11.712, P = 0.003). During the 12-month follow-up, the cumulative proportion surviving at the time was 84.0% in the placebo group, 78.0% in the ACV group and 89.0% in the GCV group, and 71.1%, 71.0% and 79.3%, during the 18-month follow-up respectively. The cumulative proportion surviving at the time during the full follow-up was 43.4%, 62.9% and 76.4% respectively. The GCV group had significantly higher cumulative proportion than the placebo group (χ2 = 11.445, P = 0.001), but there was no statistically significant difference between the GCV group and the ACV group (χ2 = 2.399, P = 0.121). Also, no statistically significant difference was observed between the placebo group and the ACV group (χ2 = 3.209, P = 0.073).
Figure 1.
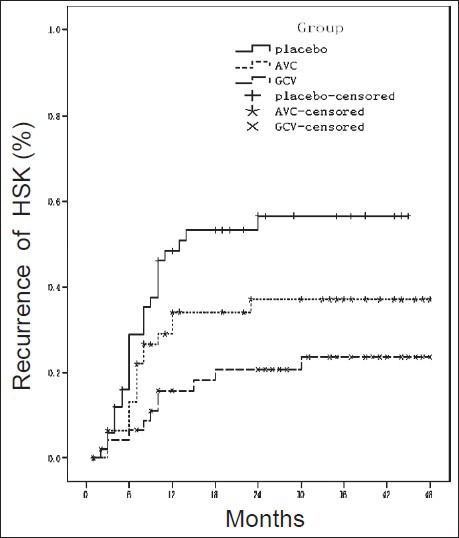
Survival curve analysis in three groups. Data on patients who did not have a recurrence were censored at the time of the last study visit.
Adverse reactions of test drug
No adverse reactions were found in the placebo group. In the ACV group, 1 patient individually discontinued treatment and withdrew from the study 3 months after treatment due to abnormal liver function, and one patient felt painful in the liver area 1 month after treatment although no abnormality was found by routine blood, urine examinations and liver, kidney examinations. However, the patient requested to discontinue the study. In the GCV group, one patient wanted to stop treatment and withdraw from the study due to reduction of granulocytes 1 month after treatment. There was no statistically significant difference among three groups (χ2 = 1.871, P = 0.392).
DISCUSSION
Recently, the incidence of HSK has been on the rise and getting worse.[1] Multicenter studies showed that the incidence of HSK increased up to 31.5/105.[24] Most of them were recurrent patients, with an incidence of 18.3/105. There is fewer statistical data about HSK in developing countries. However, the prevalence rate and incidence in developing countries are higher compared with developed countries. And people in developing countries tend to develop HSK at an earlier age, especially in the young population. Most patients have recurrent and prolonged HSK, which makes it tricky for healthcare providers. In China, besides ocular injury, HSK is the biggest cause for corneal perforation.[1]
Studies have demonstrated that stromal keratitis is an important high-risk factor for recurrence of HSK.[12,20] The recurrent HSV-1 infection is often manifested by stromal keratitis and even ulcerative keratitis. The inflammation induced by keratocyte-mediated immune response results in corneal neovascularization, edema, tissue damage, corneal opacity and ultimately causes blindness.[11,25]
In addition, a study has showed that half of HSK impacts the morphology and function of corneal endothelial cells; after recovery, the reduction of the density of endothelial cells in the affected eye increases faster, and the recurrence of HSK is more likely to cause irreversible damage in the corneal endothelial cells.[26] ACV is an effective measure for HSK. However, evidence has showed that drug resistance of HSV-1 to ACV is an important factor for increased recurrent HSK.[27] Corneal blindness caused by HSK has been the biggest cause for keratoplasty.[28] The recurrence of HSK after keratoplasty substantially increases the risk of immunological rejection, which reduces the success possibility of keratoplasty. Therefore, how to effectively prevent and treat recurrent HSK is of great concern for the healthcare providers in the field of ophthalmology.
GCV selectively inhibits replication of HSV through multiple processes and makes the host immune system prevent the virus from invasion, thereby repairing damaged tissue or mitigating the condition to prevent the development of clinical symptoms. The concentration of active ingredient (GCV triphosphate) in infected cells is 100 times more than that in normal cells, so it can effectively treat HSK. GCV is activated at least 5 times faster than ACV. Moreover, GCV activity in infected cells is at least 60 times stronger than ACV, and maintains at an active concentration for a long time. Few clinical studies reported drug resistance associated with GCV. It is also sensitive to mutant strains induced by other drugs such as foscarnet sodium, ACV and bromethylene deoxyuridine.[16,17] GCV has become one of the drugs that have the broadest spectrum and most powerful antiviral effect for DNA-containing virus.
This study aimed at assessing clinical significance of GCV in the prevention and treatment of recurrent stromal keratitis and endotheliitis. Patients included in the study had recurrent HSK presented by stromal keratitis and endotheliitis. They had substantial visual impairment and had high risk for recurrence, so oral antiviral drugs to reduce the recurrence rate of HSK was of protective significance.[11,12,29,30,31]
In this study, no statistically significant difference was observed in the course between the placebo group and the ACV group, showing that oral ACV had no benefit for the treatment of HSK, which was consistent with previous studies.[6,7,8,9] However, it was noteworthy that the course in the GCV group (oral GCV plus tropical application to the eye) was significantly shorter compared with the placebo group and the ACV group, suggesting that short-term oral GCV was of great significance in the treatment of recurrent stromal keratitis and endotheliitis, and might shorten the course of the disease.
The recurrence rate in the ACV group (26.7%) was significantly lower than that in the placebo group (47.3%), indicated that long-term low-dose oral ACV had effect in the prevention of recurrent HSK. This result was consistent with previous studies.[9,10,11,12,13,14,15,32] The recurrence rate of the GCV group was lower than that of the placebo, but similar to that of the ACV group, indicating that GCV had clinical potential in the prevention of recurrent HSK, almost the same as ACV.
According to the survival curve analysis, the cumulative proportions surviving at time substantially decreased in three groups within 12-month follow-up, and slowly and steadily decreased in the subsequent follow-up, suggesting that the recurrence of HSK peaked at the first 12-month follow-up, which was consistent with previous study.[12] The GCV group had a higher cumulative proportion than the placebo group, but no significant difference from the ACV group, which was consistent with the comparison of recurrence rate. However, the ACV group had a higher cumulative proportion than the placebo group, but with no statistical significance, which might be associated with long follow-up, poor compliance and high dropout rate in the study, due to consideration of time distribution and missing data in the survival curve analysis.
The dropout rate was similar between the GCV group and the ACV group, which was higher than the placebo group, showing that patients with oral systemic drugs should be subjected to better compliance, besides, education and communication to patients was necessary to release their concerns toward therapy. The adverse reactions occurred in individual cases in both GCV group and ACV group, indicating that attention should be attached to the safety during clinical service.
Above all, this study suggested that short-term high-dose oral GCV could shorten the course of recurrent stromal keratitis and endotheliitis. It also substantially reduced the recurrence rate of HSK, which was similar to long-term low doses of ACV.
Footnotes
Edited by: Xin Chen
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: An epidemiologic update. Surv Ophthalmol. 2012;57:448–62. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biser SA, Perry HD. Corneal and Anterior Segment Diseases – Herpes Simplex Keratitis. Ophthal Hyperguide: Corneal and Anterior Segment Diseases. c2006. [Last cited 2007 Jan 10]. Availabe from: http://ophthalmic.hyperguides.com .
- 3.Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46:241–7. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remeijer L, Maertzdorf J, Doornenbal P, Verjans GM, Osterhaus AD. Herpes simplex virus 1 transmission through corneal transplantation. Lancet. 2001;357:442. doi: 10.1016/S0140-6736(00)04011-3. [DOI] [PubMed] [Google Scholar]
- 5.Remeijer L, Maertzdorf J, Buitenwerf J, Osterhaus AD, Verjans GM. Corneal herpes simplex virus type 1 superinfection in patients with recrudescent herpetic keratitis. Invest Ophthalmol Vis Sci. 2002;43:358–63. [PubMed] [Google Scholar]
- 6.Barron BA, Gee L, Hauck WW, Kurinij N, Dawson CR, Jones DB, et al. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology. 1994;101:1871–82. doi: 10.1016/s0161-6420(13)31155-5. [DOI] [PubMed] [Google Scholar]
- 7.Oral acyclovir for herpes simplex virus eye disease: Effect on prevention of epithelial keratitis and stromal keratitis. Herpetic Eye Disease Study Group. Arch Ophthalmol 2000. 118:1030–6. [PubMed] [Google Scholar]
- 8.A controlled trial of oral acyclovir for the prevention of stromal keratitis or iritis in patients with herpes simplex virus epithelial keratitis. The Epithelial Keratitis Trial. The Herpetic Eye Disease Study Group. Arch Ophthalmol. 1997;115:703–12. doi: 10.1001/archopht.1997.01100150705001. [DOI] [PubMed] [Google Scholar]
- 9.Nunes Oda S, Pereira Rde S. Regression of herpes viral infection symptoms using melatonin and SB-73: comparison with Acyclovir. J Pineal Res. 2008;44:373–8. doi: 10.1111/j.1600-079X.2007.00538.x. [DOI] [PubMed] [Google Scholar]
- 10.Guess S, Stone DU, Chodosh J. Evidence-based treatment of herpes simplex virus keratitis: a systematic review. Ocul Surf. 2007;5:240–50. doi: 10.1016/s1542-0124(12)70614-6. [DOI] [PubMed] [Google Scholar]
- 11.Knickelbein JE, Hendricks RL, Charukamnoetkanok P. Management of herpes simplex virus stromal keratitis: an evidence-based review. Surv Ophthalmol. 2009;54:226–34. doi: 10.1016/j.survophthal.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group. N Engl J Med. 1998;339:300–6. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- 13.Jansen AF, Rijneveld WJ, Remeijer L, Völker-Dieben HJ, Eggink CA, Geerards AJ, et al. Five-year follow-up on the effect of oral acyclovir after penetrating keratoplasty for herpetic keratitis. Cornea. 2009;28:843–5. doi: 10.1097/ICO.0b013e318198399a. [DOI] [PubMed] [Google Scholar]
- 14.Goldblum D, Bachmann C, Tappeiner C, Garweg J, Frueh BE. Comparison of oral antiviral therapy with valacyclovir or acyclovir after penetrating keratoplasty for herpetic keratitis. Br J Ophthalmol. 2008;92:1201–5. doi: 10.1136/bjo.2008.138065. [DOI] [PubMed] [Google Scholar]
- 15.van Rooij J, Rijneveld WJ, Remeijer L, Völker-Dieben HJ, Eggink CA, Geerards AJ, et al. Effect of oral acyclovir after penetrating keratoplasty for herpetic keratitis: a placebo-controlled multicenter trial. Ophthalmology. 2003;110:1916–9. doi: 10.1016/S0161-6420(03)00798-X. [DOI] [PubMed] [Google Scholar]
- 16.Wilson SS, Fakioglu E, Herold BC. Novel approaches in fighting herpes simplex virus infections. Expert Rev Anti Infect Ther. 2009;7:559–68. doi: 10.1586/eri.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufman HE, Haw WH. Ganciclovir ophthalmic gel 0.15%: Safety and efficacy of a new treatment for herpes simplex keratitis. Curr Eye Res. 2012;37:654–60. doi: 10.3109/02713683.2012.692846. [DOI] [PubMed] [Google Scholar]
- 18.Holland EJ, Schwartz GS. Classification of herpes simplex virus keratitis. Cornea. 1999;18:144–54. doi: 10.1097/00003226-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelmus KR, Gee L, Hauck WW, Kurinij N, Dawson CR, Jones DB, et al. Herpetic Eye Disease Study. A controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology. 1994;101:1883–95. doi: 10.1016/s0161-6420(94)31087-6. [DOI] [PubMed] [Google Scholar]
- 20.Wilhelmus KR, Dawson CR, Barron BA, Bacchetti P, Gee L, Jones DB, et al. Risk factors for herpes simplex virus epithelial keratitis recurring during treatment of stromal keratitis or iridocyclitis. Herpetic Eye Disease Study Group. Br J Ophthalmol. 1996;80:969–72. doi: 10.1136/bjo.80.11.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadziyannis SJ, Manesis EK, Papakonstantinou A. Oral ganciclovir treatment in chronic hepatitis B virus infection: a pilot study. J Hepatol. 1999;31:210–4. doi: 10.1016/s0168-8278(99)80215-3. [DOI] [PubMed] [Google Scholar]
- 22.Lalezari JP, Friedberg DN, Bissett J, Giordano MF, Hardy WD, Drew WL, et al. High dose oral ganciclovir treatment for cytomegalovirus retinitis. J Clin Virol. 2002;24:67–77. doi: 10.1016/s1386-6532(01)00229-3. [DOI] [PubMed] [Google Scholar]
- 23.Keven K, Basu A, Tan HP, Thai N, Khan A, Marcos A, et al. Cytomegalovirus prophylaxis using oral ganciclovir or valganciclovir in kidney and pancreas-kidney transplantation under antibody preconditioning. Transplant Proc. 2004;36:3107–12. doi: 10.1016/j.transproceed.2004.11.092. [DOI] [PubMed] [Google Scholar]
- 24.Labetoulle M, Auquier P, Conrad H, Crochard A, Daniloski M, Bouée S, et al. Incidence of herpes simplex virus keratitis in France. Ophthalmology. 2005;112:888–95. doi: 10.1016/j.ophtha.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 25.Hillenaar T, van Cleynenbreugel H, Verjans GM, Wubbels RJ, Remeijer L. Monitoring the inflammatory process in herpetic stromal keratitis: The role of in vivo confocal microscopy. Ophthalmology. 2012;119:1102–10. doi: 10.1016/j.ophtha.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Hillenaar T, Weenen C, Wubbels RJ, Remeijer L. Endothelial involvement in herpes simplex virus keratitis: an in vivo confocal microscopy study. Ophthalmology. 2009;116:2077–86.e1. doi: 10.1016/j.ophtha.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 27.Duan R, de Vries RD, van Dun JM, van Loenen FB, Osterhaus AD, Remeijer L, et al. Acyclovir susceptibility and genetic characteristics of sequential herpes simplex virus type 1 corneal isolates from patients with recurrent herpetic keratitis. J Infect Dis. 2009;200:1402–14. doi: 10.1086/606028. [DOI] [PubMed] [Google Scholar]
- 28.Colin J. Ganciclovir ophthalmic gel, 0.15%: A valuable tool for treating ocular herpes. Clin Ophthalmol. 2007;1:441–53. [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Pavan-Langston D, Colby KA. Pediatric herpes simplex of the anterior segment: Characteristics, treatment, and outcomes. Ophthalmology. 2012;119:2003–8. doi: 10.1016/j.ophtha.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Revere K, Davidson SL. Update on management of herpes keratitis in children. Curr Opin Ophthalmol. 2013;24:343–7. doi: 10.1097/ICU.0b013e32836227d8. [DOI] [PubMed] [Google Scholar]
- 31.Sudesh S, Laibson PR. The impact of the herpetic eye disease studies on the management of herpes simplex virus ocular infections. Curr Opin Ophthalmol. 1999;10:230–3. doi: 10.1097/00055735-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh S, Jhanji V, Lamoureux E, Taylor HR, Vajpayee RB. Acyclovir therapy in prevention of recurrent herpetic keratitis following penetrating keratoplasty. Am J Ophthalmol. 2008;145:198–202. doi: 10.1016/j.ajo.2007.10.005. [DOI] [PubMed] [Google Scholar]


