Abstract
Background:
Liposarcomas, which represent 20% of all adult sarcomas, are the most common histological type of malignant soft tissue tumors. The aim of this study was to define the prognostic factors that predict the postoperative survival period for patients with primary retroperitoneal liposarcoma.
Methods:
The clinical data and prognoses of 71 patients with primary retroperitoneal liposarcoma who were treated in the General Hospital of the People's Liberation Army of China between January 1, 2000 and December 31, 2007 were retrospectively reviewed and analyzed.
Results:
The primary tumor from each patient was resected; 54.9% (39/71) were deemed R0 resections, 31.0% (22/71) were R1 resections and 14.1% (10/71) were deemed R2 resections (palliative operations). The median follow up was 68 months (range: 1-160 months). Of the patients who received an R1 or R2 resection of their primary tumor, 96.7% (59/61) had tumor recurrence. The 1-year, 3-year, and 5-year recurrence-free rates were 77.0%, 29.8% and 19.7%, respectively. As of April 2013, 53 of the 71 patients had died from tumor recurrence. The overall 1-year, 3-year, 5-year, and 10-year survival rates were 88.7%, 76.1%, 61.7%, and 30.4%, respectively. The factors that were significantly associated with prognosis in the univariate analysis were age (as a categorical variable) (P = 0.006), modus operandi (P = 0.000), histologic subtype (P = 0.000), tumor grade (P = 0.000), ascites (P = 0.000), postoperative metastasis (P = 0.000) and adjuvant therapy (P = 0.030). However, in the multivariate analysis, the modus operandi (P = 0.000), tumor grade (P = 0.006), ascites (P = 0.027), postoperative metastasis (P = 0.023) and age (as a categorical variable) (P = 0.002) were the only significant predictors of survival.
Conclusions:
Complete resection remains the most effective method for treating liposarcoma. High grade, old age (≥60 years old), postoperative metastasis, and ascites predict poor prognoses.
Keywords: General Surgery, Liposarcoma, Retroperitoneal Neoplasms, Survival, Therapeutics
INTRODUCTION
Liposarcomas, which represent 20% of all adult sarcomas, are the most common histological type of malignant soft-tissue tumors. These tumors originate from mesenchymal tissue,[1] and approximately 10%-36% arise from the retroperitoneum.[2] Primary retroperitoneal liposarcoma is clinically rare, and because the retroperitoneum can be quite large, retroperitoneal tumors can grow silently without inducing any clinical symptoms for an extended period. Because of the characteristic slow development of symptoms, patients tend to report to the hospital only when the tumor is enormous. All of these features make it difficult for surgeons to achieve complete liposarcoma resections, which contribute to the poor prognoses observed. Due to the inefficiency of adjuvant therapy, surgery remains the only effective treatment.[3] In this study, we aim to define the prognostic factors that predict the postoperative survival periods for patients with primary retroperitoneal liposarcoma through retrospective analysis.
METHODS
Patients were identified using the phrase “retroperitoneal liposarcoma” to search our prospective database of all patients with retroperitoneal neoplasms treated in the General Hospital of Chinese People's Liberation Army between January 1, 2000 and December 31, 2007. Patients with recurrent metastatic liposarcoma and those with liposarcomas arising from other tissues were excluded. In total, 73 patients were enrolled in the study, and all received postoperative pathological reaffirmation.
Clinical data included age, gender, modus operandi, histological subtype, tumor size, tumor position (upper abdomen vs. lower abdomen), adjacent organ resection (yes or no), adjuvant therapy (yes or no), ascites (yes or no), and postoperative metastasis (yes or no).
The routine preoperative examinations of all patients consisted of computed tomography and/or magnetic resonance imaging scans to evaluate the general tumor characteristics. The surgical treatment was divided into three strategies. R0 resection was achieved when the tumor was completely resected with clean microscopic margins that were confirmed by the surgeon and pathologist. R1 resection was defined as complete tumor resection with positive microscopic margins. If the tumor's pseudocapsule was cut open during operation, the resection was also defined as R1, irrespective of the margins. If any macroscopic tumor tissue remained, the surgery was considered an R2 resection (palliative operation). The tumor size was determined by gross pathological examinations following surgery or by cross-sectional imaging (for some palliative operations) to determine the maximum diameter. Tumors were classified according to their position relative to the navel (i.e. either upper abdominal or lower abdominal tumors [including the pelvis]). Tumors that were large enough to occupy both the upper and lower abdomen were grouped according to the region that contained >50% of the tumor's maximum diameter.
The same pathologist in the Department of Pathology examined all resected tumor specimens. The histological subtype and degree of malignancy were classified according to the criteria of the World Health Organization's Classification of Tumors of Soft Tissue and Bone.[4] Liposarcomas were divided into the following five subtypes: well-differentiated liposarcoma, dedifferentiated liposarcoma, myxoid liposarcoma, pleomorphic liposarcoma, and mixed liposarcoma. A two-tiered system (high grade vs. low-grade) was utilized to indicate the degree of malignancy, and 29 tumors were categorized as high grade, while 42 were low grade.
All patients were continuously followed up until they died (due to tumor recurrence) or until April of 2013, which was the final date of contact for living patients. The follow-up period for each patient began on the operation date. Two patients from our series were lost during follow-up. The median follow-up time was 68 months (range: 1-160 months). In our univariate analyses, the Kaplan–Meier method was used to describe and evaluate the survival rate, and the log-rank test was used to compare the survival curves. In our multivariate analysis, the Cox proportional hazards regression model was used to define independent factors that predict postoperative survival. All tests were performed using SPSS 13.0 for Windows (SPSS, USA) with a significance level set at P < 0.05.
RESULTS
Patients and clinical data
Of the 71 patients in our study, 47 were men and 24 were women; the male: female ratio was 2.0:1. The mean age of the patients was (49.86 ± 13.20) years (range: 23–85 years). The clinical symptoms were nonspecific; the most common symptom was abdominal distention (35.2% [25/71]), which was followed by abdominal pain (21.1% [15/71]), fever (11.3% [8/71]), emaciation (5.6% [4/71]), backache (4.2% [3/71]), lack of appetite (2.8% [2/71]), fatigue (2.8% [2/71]), scrotal swelling (1.4% [1/71]), acid reflux (1.4% [1/71]), cough (1.4% [1/71]), and belching (1.4% [1/71]). In addition, 11.3% (8/71) of patients were accidently diagnosed without any clinical symptoms during physical examinations for other purposes. Upon systemic examinations, palpable masses were found in 63.4% (45/71) of the patients.
In this cohort, 85.9% (61/71) of the patients had their tumors completely resected, of which 54.9% (39/71) were deemed R0 resections, 31.0% (22/71) were R1 resections, and 14.1% (10/71) were R2 resections (palliative operations). The maximum diameter of the resected tumor was 80.0 cm, the minimum diameter was 4.0 cm and the mean diameter was (21.78 ± 12.07) cm. To achieve complete resection, 22 of the patients underwent concomitant resections of at least one adjacent infiltrated organ [Table 1]. Postoperative complications included incision liquefaction in two cases and venous thrombosis in the lower limbs, urinary fistula, and pancreatic fistula in one patient each. All of these complications were addressed by symptomatic treatment. All patients were discharged uneventfully, and there were no deaths in the perioperative period.
Table 1.
Organ resection
| Resected organs | Number |
|---|---|
| Stomach | 3 |
| Psoas major | 1 |
| Small intestine | 3 |
| Colon | 8 |
| Spleen | 4 |
| Body and tail of the pancreas | 3 |
| Kidney | 5 |
| Appendix | 2 |
| Adrenal gland | 1 |
| Accessory | 1 |
| Ovary | 1 |
| Gall bladder | 1 |
In this series, 35 patients received some type of adjuvant therapy, including intraoperative radiation in two patients, postoperative radiation in seven patients, postoperative chemotherapy in 15 patients, and postoperative radiotherapy plus chemotherapy in 11 patients.
Data analysis
Of the entire cohort, 53 patients died from tumor recurrence by April 2013, including all 10 patients in the R2 resection group and 43 of the patients in the R0 and R1 resection groups. In addition, four patients died for other diseases, including two from heart attacks, one from a stroke, and one from a pulmonary embolism. The overall 1-year, 3-year, 5-year, and 10-year survival rates were 88.7%, 76.1%, 61.7%, and 30.4%, respectively. Of the 61 patients in the R1 and R2 resection groups, 59 patients had a local recurrence by April 2013. The shortest latency until local recurrence following primary surgery was 1 month, and the longest recurrence latency was 120 months. The median recurrence time was 21 months. The likelihood of remaining free from local recurrence was 77.0% at 1 year, 29.8% at 3 years, and 19.7% at 5 years. Five patients developed distant metastases, two of which were in the lung, two in the liver and one in the mesentery.
The factors that were identified in the univariate analysis as important determinants of postoperative survival are shown in Table 2. Age (as a categorical variable) was a prognostic factor for the postoperative survival time (P = 0.006). Individuals younger than 60 years old had an apparent increase in the postoperative survival time compared to those who were older than 60 years of age. The median survival time of the younger group was 84 (95% confidence interval 63.45, 104.55) months and that of the older group was 38 (20.33, 55.67) months. Patients undergoing R0 and R1 resections had median survival times of 114 (84.89, 143.11) and 55 (37.21, 72.79) months, respectively, which were much better (P = 0.000) than for those undergoing R2 resections, who had a median survival time of only 14 (3.15, 24.85) months. The histological subtype was also a significant indicator of the postoperative survival time (P = 0.000). The median survival times observed for well differentiated, dedifferentiated, myxoid, and pleomorphic tumors were 104 (71.43, 136.57), 18 (0.52, 35.48), 65 (4.51, 125.50), and 29 (0.00, 74.09) months, respectively. Analyzing the relationship between the tumor grade and postoperative survival time revealed that patients with low-grade tumors had improved survival times (P = 0.000). The median survival time for patients with low-grade tumors was 105 (80.72, 129.28) months, and it was 34 (25.25, 42.75) months for patients with high-grade tumors. The presence of ascites was a strong predictor of the survival time, and it led to a drastic reduction in the duration of postoperative survival (P = 0.000). For individuals with ascites, the median survival time was 5 (2.85, 7.15) months, and it was 81 (55.78, 106.22) months for patients without ascites. Patients with distant postoperative metastases had a median survival time of 34 (11.21, 56.79) months, which was shorter than the 81 (55.81, 106.19) months observed in patients without distant metastases (P = 0.000). Adjuvant therapy was also related to the postoperative survival time (P = 0.030). Interestingly, the median survival time was lower for individuals who received adjuvant therapy compared to those who did not. Specifically, the median survival time for patients who had undergone adjuvant therapy was 59 (45.48, 72.52) months versus 91 (63.72, 118.28) months for patients who did not undergo adjuvant therapy. This result will be explained in more detail in the discussion section. No significant effects of gender (P = 0.158), tumor size (as a categorical variable) (P = 0.221), tumor position (P = 0.586) or organ resection (P = 0.757) were observed in the univariate analysis.
Table 2.
Factors influencing the postoperative survival for patients with retroperitoneal liposarcoma
| Items | Number (n=71) | Median survival time (months) | P (univariate) | P (multivariate) | RR |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 47 | 63 (47.32, 78.68)† | 0.158 | ||
| Female | 24 | 98 (70.61, 125.40)† | |||
| Age (years) | |||||
| <60 | 56 | 84 (63.45, 104.55)† | 0.006 | 0.002 | 2.93 (1.47,5.86)† |
| ≥60 | 15 | 38 (20.33, 55.67)† | |||
| Modus operandi | |||||
| R0 resection* | 39 | 114 (84.89, 143.11)† | 0.000 | 0.000 | 3.21 (1.90,5.42)† |
| R1 resection* | 22 | 55 (37.21, 72.79)† | |||
| R2 resection* | 10 | 14 (3.15, 24.85)† | |||
| Subtype | |||||
| Well-differentiated | 37 | 104 (71.43, 136.57)† | 0.000 | ||
| Differentiated | 17 | 18 (0.52, 35.48)† | |||
| Myxoid | 12 | 65 (4.51, 125.50)† | |||
| Pleomorphic | 5 | 29 (0.00, 74.09)† | |||
| Mixed | 0 | 0 | |||
| Ascites | |||||
| Yes | 5 | 5 (2.85, 7.15)† | 0.000 | 0.027 | 6.98 (1.24,39.20)† |
| No | 66 | 81 (55.78, 106.22)† | |||
| Postoperative metastasis | |||||
| Yes | 5 | 34 (11.21, 56.79)† | 0.000 | 0.023 | 4.11 (1.22,13.87)† |
| No | 66 | 81 (55.81, 106.19)† | |||
| Tumor grade | |||||
| Low | 42 | 105 (80.72, 129.28)† | 0.000 | 0.006 | 2.70 (1.34,5.45)† |
| High | 29 | 34 (25.25, 42.75)† | |||
| Adjuvant therapy | |||||
| Yes | 35 | 59 (45.48, 72.52)† | 0.030 | ||
| No | 36 | 91 (63.72, 118.28)† | |||
| Tumor position | |||||
| Upper | 39 | 75 (48.37, 101.63)† | 0.586 | ||
| Lower | 32 | 79 (53.17, 104.83)† | |||
| Tumor size | |||||
| <20 cm | 29 | 75 (45.48, 104.52)† | 0.221 | ||
| ≥20 cm | 42 | 66 (41.01, 90.99)† | |||
| Organ resection | |||||
| Yes | 22 | 81 (28.91, 133.09)† | 0.757 | ||
| No | 49 | 75 (53.38, 96.62)† |
*R0 resection: Complete resection + clean microscopic margins; *R1 resection: Complete resection + positive microscopic margins; *R2 resection: Palliative operations; †(xx, xx): 95% confidence interval; RR: Relative risk.
The variables that were identified as significant in the univariate analysis, such as age (as a categorical variable), modus operandi, histological subtype, tumor grade, ascites, postoperative distant metastasis, and adjuvant therapy, were entered into a Cox model to define the independent factors that predict the postoperative survival time. However, the histological subtype failed the proportional hazards assumption test (histological subtype * LN [T_] X2 = 7.208 P = 0.007), and the results observed for adjuvant therapy were inconsistent with this. Consequently, both of these variables were excluded from the Cox model. As a result, a total of five variables, namely age (as a categorical variable), modus operandi, tumor grade, ascites, and postoperative distant metastasis, were included in the Cox model. With multivariate analysis, modus operandi (i.e. R0 resection vs. R1 resection vs. R2 resection, P = 0.000, RR = 3.21 [95% confidence interval 1.90, 5.42], [Figure 1]), tumor grade (i.e. high grade vs. low grade, P = 0.006, RR = 2.70 [1.34, 5.45], [Figure 2]), ascites (i.e. Yes vs. No, P = 0.027, RR = 6.98 [1.24, 39.20], [Figure 3]), postoperative metastasis (i.e. Yes vs. No, P = 0.023, RR = 4.11 [1.22, 13.87], [Figure 4] and age (as a categorical variable) (i.e. ≥60 years old vs. <60 years old, P = 0.002, RR = 2.93 [1.47, 5.86], [Figure 5]) were all independent factors that significantly predicted the postoperative survival time [Table 2].
Figure 1.
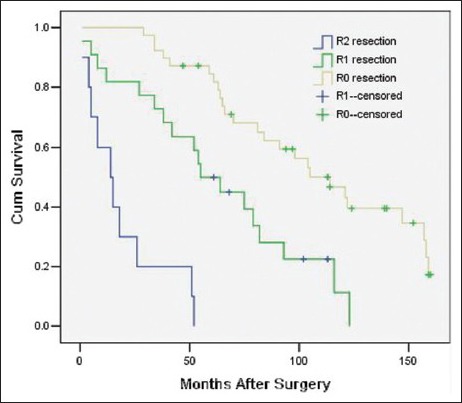
Survival rates as a function of the modus operandi (R0 resection vs. R1 resection vs. R2 resection, P = 0.000 RR = 3.21 [95% confidence interval 1.90, 5.42], 71 patients).
Figure 2.
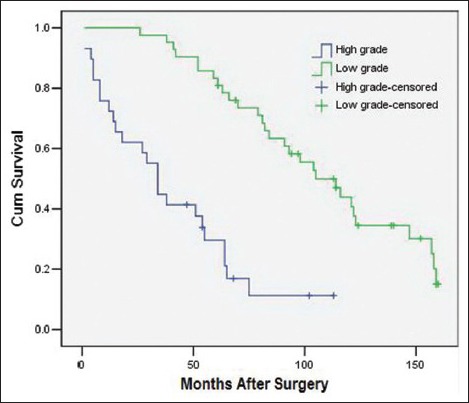
Survival rates as a function of the tumor grade (high grade vs. low grade, P = 0.006, RR = 2.70 [95% confidence interval 1.34, 5.45], 71 patients).
Figure 3.
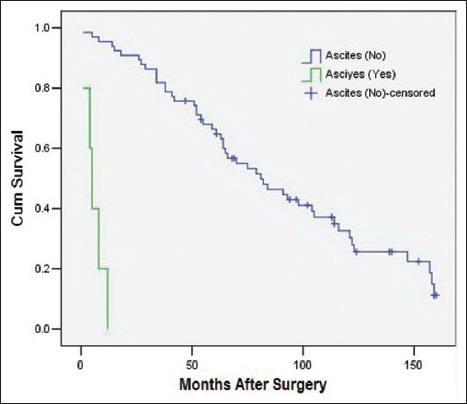
Survival rates as a function of the ascites status (Yes vs. No, P = 0.027 RR = 6.98 [95% confidence interval 1.24, 39.20], 71 patients).
Figure 4.
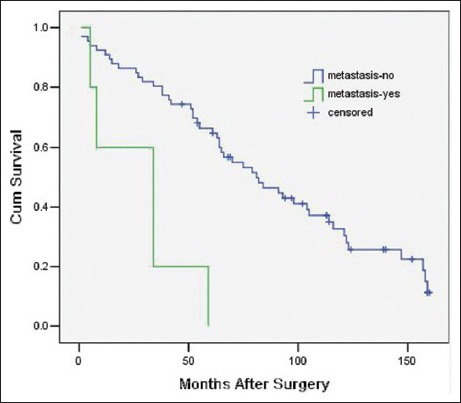
Survival rates as a function of postoperative metastasis (Yes vs. No, P = 0.023 RR = 4.11 [95% confidence interval 1.22, 13.87], 71 patients).
Figure 5.
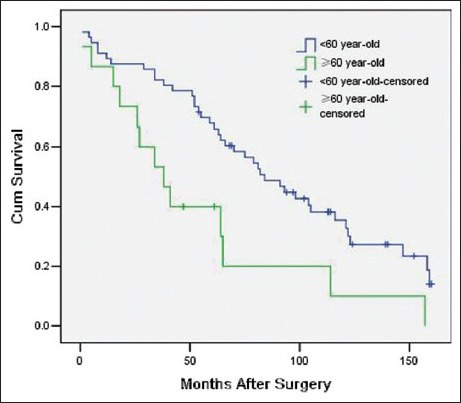
Survival rates as a function of age (≥60 years old vs. <60 years old, P = 0.002 RR = 2.93 [95% confidence interval 1.47, 5.86], 71 patients).
DISCUSSION
Due to the inefficiency of adjuvant therapy, obtaining a complete resection of the tumor remains the only effective treatment of choice[2,5,6,7,8,9,10,11,12,13] and is the most important independent factor predicting the postoperative survival time.[2,5,6] Several authors[2,8] have found resections with a clean microscopic margin can prolong the postoperative survival time compared to resections with a microscopic tumor-positive margin. Our results are in line with previous reports. In our study, regardless of the margin status, patients with complete resection of their tumors had a much better outcome than those who had a palliative operation. In addition, the prognoses of patients undergoing complete resections with a clean microscopic margin are better than those with a positive margin [Figure 1]. Our reports are concordant with previous reports.[2,8] Hence, every effort should be made to achieve a complete resection with a clean microscopic margin.
To achieve a complete resection, it is sometimes inevitable and necessary to excise the infiltrated organs.[14] In the current study, the colon was the most commonly resected organ, followed by the kidney. The role of organ resection in the treatment of retroperitoneal soft tissue tumors remains unclear (P = 0.753). Previous studies[15,16] have demonstrated that organ resections can reduce the local recurrence rates but do not prolong the survival time.[13,15] Additional studies with large numbers of patients will be required to test the efficiency of organ resections in the future.
Several previous studies have reported that tumor grade is an independent predictor for the postoperative survival time in soft tissue sarcoma,[2,17,18] an effect that was also shown in this paper (P = 0.000). Specifically, we observed a much better prognosis for the group with low-grade tumors compared to the group with high-grade tumors [Table 2]. In this cohort, the 3- and 5-year survival rates in the low-grade tumor group were 97.5% and 82.5%, respectively. These values were much higher than those observed in the high-grade group, in which the 3-year and 5-year survival rates were 48.4% and 35.2%, respectively. Based on our data, it is impossible to conclude whether the histological subtype of liposarcoma represents an independent predictor for the postoperative survival time because this variable failed in the proportional hazards assumption test. However, a previous study[2] showed that this variable is, in fact, an important predictor of survival.
Postoperative metastasis is significantly correlated with the postoperative survival time in both univariate and multivariate analyses. Therefore, our data support the conclusion that postoperative metastasis is an independent factor predicting survival. According to our experience, this phenomenon may occur in part because surgery is no longer indicated when a postoperative metastasis is detected and because metastases tend to occur in high-grade tumors even though the metastasis rate is low. Both of these phenomena shorten the life expectancy.
Ascites was identified as a significant predictor of the survival time in both univariate and multivariate analyses. Patients with ascites had much shorter survival times compared to those without ascites (a median survival time of 5 (2.85, 7.15) months with ascites versus 81 (55.78, 106.22) months without ascites). Therefore, we suggest that patients with ascites choose alternative treatment approaches instead of surgery. Our data support age (as a categorical variable) as an independent factor predicting survival. According to our experience, the main reason for this is that young patients are amenable to more operations for resecting local recurrence due to physical conditions, prolonging the life expectancy.
Because of their anatomical position, retroperitoneal soft tissue sarcomas frequently increase in size before clinical symptoms are observed. This phenomenon was observed in the patient cohort from the current study in which 59.2% (42/71) of the patients had tumors with diameters ≥20 cm. Several scholars have suggested that larger tumors have a worse prognosis.[10,19] This has been suggested primarily because large tumors frequently compress and/or infiltrate surrounding structures and tissues, which can significantly complicate complete resections and result in leftover microscopic residual disease. However, several other studies[12,17] have contradicted these findings by showing that there is no significant correlation between the tumor size and survival prognosis. In our report, we did not observe a significant effect of the tumor size on the overall survival of patients (P = 0.221 in univariate analysis). Mäkelä et al.[17] has suggested that the inaccessible, deep location of retroperitoneal liposarcomas, rather than size alone, influences the complete resection rate and, therefore, the postoperative survival time. Singer et al.[2] also reported that when retroperitoneal sarcomas reach a diameter of at least 10 cm, they all exhibit a similarly high risk. Therefore, a large sample of data are required to clarify the relationship between the tumor size and the postoperative survival.
Theoretically, the best option for retroperitoneal sarcoma is complete resection plus adjuvant therapy. However, the efficiency of adjuvant therapy in the treatment of retroperitoneal soft tissue sarcomas remains controversial. To date, no approaches other than surgery have been effective. In our group, patients who received adjuvant therapy had a poorer prognosis than patients who did not (P = 0.030 in univariate analysis). We hypothesize that this effect was likely because we primarily arranged adjuvant therapy for patients with high-grade tumors, resulting in selection bias. A study by Patel et al.[20] showed that adriamycin and ifosfamide might be effective treatments for low-grade liposarcomas. Additionally, Yoon et al.[21] found that neoadjuvant radiotherapy could decrease the tumor volume, making en bloc resections much easier and minimizing the risk of implantation metastases. A recent outcome from a compassionate use program[22] revealed that the trabectedin might promote better prognoses for patients with advanced sarcomas, but future studies are required to evaluate its true efficiency.
In conclusion, complete resection remains the most effective method for treating liposarcoma. High grade, old age (≥60 years old), postoperative metastasis, and ascites predict poor prognoses. However, the generalizability of the results and outcomes observed in the current work are limited by the relatively small sample size and the relatively short follow-up period. Consequently, more patients and a longer follow-up period will be required for future studies.
Footnotes
Edited by: De Wang
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Joshi RM, Gangurde GK, Talathi NP, Telavane PP, Singh R, Hanamshetti SR, et al. Large retroperitoneal liposarcoma – A series of five cases. Indian J Surg. 2013;75(Suppl 1):64–8. doi: 10.1007/s12262-011-0348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer S, Antonescu CR, Riedel E, Brennan MF. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238:358–70. doi: 10.1097/01.sla.0000086542.11899.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng WW, Madewell JE, Wei W, Somaiah N, Lazar AJ, Ghadimi MP, et al. Locoregional disease patterns in well-differentiated and dedifferentiated retroperitoneal liposarcoma: Implications for the extent of resection? Ann Surg Oncol. 2014;21:2136–43. doi: 10.1245/s10434-014-3643-4. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher C, Unni K, Mertens F. In: Pathology and Genetics of Tumors of Soft Tissue and Bone. World Health Organization Classification of Tumors. Kleihues P, Sobin L, editors. Vol. 4. Lyon, France: International Agency for Research on Cancer Press; 2002. p. 427. [Google Scholar]
- 5.Kim ES, Jang SH, Park HC, Jung EH, Moon GB. Dedifferentiated liposarcoma of the retroperitoneum. Cancer Res Treat. 2010;42:57–60. doi: 10.4143/crt.2010.42.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strauss DC, Hayes AJ, Thomas JM. Retroperitoneal tumours: Review of management. Ann R Coll Surg Engl. 2011;93:275–80. doi: 10.1308/003588411X571944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crago AM, Singer S. Clinical and molecular approaches to well differentiated and dedifferentiated liposarcoma. Curr Opin Oncol. 2011;23:373–8. doi: 10.1097/CCO.0b013e32834796e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erzen D, Sencar M, Novak J. Retroperitoneal sarcoma: 25 years of experience with aggressive surgical treatment at the Institute of Oncology, Ljubljana. J Surg Oncol. 2005;91:1–9. doi: 10.1002/jso.20265. [DOI] [PubMed] [Google Scholar]
- 9.Oh SE, Kim HJ, Choi SJ, Oh SY, Roh CR, Kim JH. A case of huge retroperitoneal liposarcoma in pregnancy. Obstet Gynecol Sci. 2014;57:236–9. doi: 10.5468/ogs.2014.57.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An JY, Heo JS, Noh JH, Sohn TS, Nam SJ, Choi SH, et al. Primary malignant retroperitoneal tumors: Analysis of a single institutional experience. Eur J Surg Oncol. 2007;33:376–82. doi: 10.1016/j.ejso.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Hassan I, Park SZ, Donohue JH, Nagorney DM, Kay PA, Nasciemento AG, et al. Operative management of primary retroperitoneal sarcomas: A reappraisal of an institutional experience. Ann Surg. 2004;239:244–50. doi: 10.1097/01.sla.0000108670.31446.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown RE, St Hill CR, Greene QJ, Farmer RW, Reuter NP, Callendar GG, et al. Impact of histology on survival in retroperitoneal sarcomas. Am J Surg. 2011;202:748–52. doi: 10.1016/j.amjsurg.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Linehan DC, Lewis JJ, Leung D, Brennan MF. Influence of biologic factors and anatomic site in completely resected liposarcoma. J Clin Oncol. 2000;18:1637–43. doi: 10.1200/JCO.2000.18.8.1637. [DOI] [PubMed] [Google Scholar]
- 14.Chikatani K, Baba H, Sobajima J, Ishiguro T, Kumamoto K, Kumagai Y, et al. Clinicopathological characteristics and treatment outcome of retroperitoneal liposarcoma. Gan To Kagaku Ryoho. 2012;39:2426–8. [PubMed] [Google Scholar]
- 15.Tseng WW, Wang SC, Eichler CM, Warren RS, Nakakura EK. Complete and safe resection of challenging retroperitoneal tumors: Anticipation of multi-organ and major vascular resection and use of adjunct procedures. World J Surg Oncol. 2011;9:143. doi: 10.1186/1477-7819-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mussi C, Colombo P, Bertuzzi A, Coladonato M, Bagnoli P, Secondino S, et al. Retroperitoneal sarcoma: Is it time to change the surgical policy? Ann Surg Oncol. 2011;18:2136–42. doi: 10.1245/s10434-011-1742-z. [DOI] [PubMed] [Google Scholar]
- 17.Mäkelä J, Kiviniemi H, Laitinen S. Prognostic factors predicting survival in the treatment of retroperitoneal sarcoma. Eur J Surg Oncol. 2000;26:552–5. doi: 10.1053/ejso.2000.0945. [DOI] [PubMed] [Google Scholar]
- 18.Gladdy RA, Qin LX, Moraco N, Agaram NP, Brennan MF, Singer S. Predictors of survival and recurrence in primary leiomyosarcoma. Ann Surg Oncol. 2013;20:1851–7. doi: 10.1245/s10434-013-2876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stojadinovic A, Leung DH, Allen P, Lewis JJ, Jaques DP, Brennan MF. Primary adult soft tissue sarcoma: Time-dependent influence of prognostic variables. J Clin Oncol. 2002;20:4344–52. doi: 10.1200/JCO.2002.07.154. [DOI] [PubMed] [Google Scholar]
- 20.Patel SR, Vadhan-Raj S, Papadopolous N, Plager C, Burgess MA, Hays C, et al. High-dose ifosfamide in bone and soft tissue sarcomas: Results of phase II and pilot studies – Dose-response and schedule dependence. J Clin Oncol. 1997;15:2378–84. doi: 10.1200/JCO.1997.15.6.2378. [DOI] [PubMed] [Google Scholar]
- 21.Yoon SS, Chen YL, Kirsch DG, Maduekwe UN, Rosenberg AE, Nielsen GP, et al. Proton-beam, intensity-modulated, and/or intraoperative electron radiation therapy combined with aggressive anterior surgical resection for retroperitoneal sarcomas. Ann Surg Oncol. 2010;17:1515–29. doi: 10.1245/s10434-010-0935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blay JY, Italiano A, Ray-Coquard I, Le Cesne A, Duffaud F, Rios M, et al. Long-term outcome and effect of maintenance therapy in patients with advanced sarcoma treated with trabectedin: An analysis of 181 patients of the French ATU compassionate use program. BMC Cancer. 2013;13:64. doi: 10.1186/1471-2407-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]


