Abstract
Background:
Green tea has been shown to improve cholesterol metabolism in animal studies, but the molecular mechanisms underlying this function have not been fully understood. Long non-coding RNAs (lncRNAs) have recently emerged as a major class of regulatory molecules involved in a broad range of biological processes and complex diseases. Our aim was to identify important lncRNAs that might play an important role in contributing to the benefits of epigallocatechin-3-gallate (EGCG) on cholesterol metabolism.
Methods:
Microarrays was used to reveal the lncRNA and mRNA profiles in green tea polyphenol(-)-epigallocatechin gallate in cultured human liver (HepG2) hepatocytes treated with EGCG and bioinformatic analyses of the predicted target genes were performed to identify lncRNA-mRNA targeting relationships. RNA interference was used to investigate the role of lncRNAs in cholesterol metabolism.
Results:
The expression levels of 15 genes related to cholesterol metabolism and 285 lncRNAs were changed by EGCG treatment. Bioinformatic analysis found five matched lncRNA-mRNA pairs for five differentially expressed lncRNAs and four differentially expressed mRNA. In particular, the lncRNA AT102202 and its potential targets mRNA-3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) were identified. Using a real-time polymerase chain reaction technique, we confirmed that EGCG down-regulated mRNA expression level of the HMGCR and up-regulated expression of AT102202. After AT102202 knockdown in HepG2, we observed that the level of HMGCR expression was significantly increased relative to the scrambled small interfering RNA control (P < 0.05).
Conclusions:
Our results indicated that EGCG improved cholesterol metabolism and meanwhile changed the lncRNAs expression profile in HepG2 cells. LncRNAs may play an important role in the cholesterol metabolism.
Keywords: Epigallocatechin-3-gallate, Tea Catechins, Cholesterol, Green Tea Polyphenol(-)-epigallocatechin Gallate in Cultured Human Liver, Long Non-coding RNA
INTRODUCTION
Atherosclerosis is a leading cause of morbidity and mortality worldwide.[1] Hyperlipidemia, which results from abnormalities in lipid metabolism, especially low-density lipoprotein (LDL) cholesterol, has a central role in the development of atherosclerotic plaques and is one of the key risk factors of atherosclerosis-related diseases. With the increasing incidence of hyperlipidemia, therapeutic and dietary approaches to their treatment and prevention are highly relevant.[2,3]
The beneficial health effects of green tea have been attributed mainly to the high levels of catechins, among which, epigallocatechin-3-gallate (EGCG) has been extensively investigated as it is the dominant catechin, accounting for up to 65% of the total catechin content in green tea.[4] In clinical studies, green tea has been shown to decrease serum total cholesterol and LDL-cholesterol and be associated with a reduction in the risk of cardiovascular disease.[5,6] Animal studies have shown that tea catechins inhibited cholesterol synthesis and up-regulated the LDL receptor and lowered plasma cholesterol.[7,8] Furthermore, tea catechins have been reported to directly influence cholesterol metabolism in hepatocytes.[9,10] However, the molecular mechanisms underlying the plasma cholesterol-lowering effect of tea catechins are not fully understood.
Increasing studies have revealed that long non-coding RNAs (lncRNA), defined as a class of non-protein-coding transcripts over 200 nucleotides long, have a variety of important physiological processes, including X-chromosome inactivation, genomic imprinting, and embryonic stem cell differentiation.[11] Recently, lncRNAs have been recognized to play an important role in cardiac development[12,13] and be associated with susceptibility to coronary artery disease.[14,15] Thus, we hypothesize that lncRNAs may also be involved in the regulation of cholesterol metabolism. In the present study, we performed microarray analysis using green tea polyphenol(-)-epigallocatechin gallate in cultured human liver (HepG2) cells treated with EGCG to clarify the effects of EGCG on hepatic cholesterol metabolism and explore the role of lncRNAs in the regulation of cholesterol metabolism.
METHODS
Cell culture
The human hepatoblastoma cell line, HepG2 (from ATCC, Manassas, VA, USA), were incubated in Dulbecco's modified Eagle's medium (GIBCO, Grand Island, NY, USA) containing 10% charcoal/dextran-treated fetal bovine serum, 100 units/ml penicillin, 100 mg/ml streptomycin (Invitrogen, Carlsbad, CA, USA), with and without EGCG (Sigma, St Louis, MO, USA). After incubation for 24 hours, the cells were harvested for RNA preparation.
Long non-coding RNA and mRNA microarray
Total RNA was extracted using TRIZOL Reagent (Life Technologies) following the manufacturer's instructions and quality assessment was conducted using Bioanalyzer 2100 (Agilent technologies, Santa Clara, CA, US) and Total RNA integrity number values were between 8.9 and 9.8 (average: 9.3). Total RNA were amplified, labeled and purified using Affymetrix Amplication and labeling kits followed the manufacturer's instructions to obtain the biotin labeled complementary DNA. One hundred ng of total RNA were processed in parallel with an external microarray quality control A RNA to control robustness of data. Labeled DNA mean yield was 7.19 μg (min: 6.27 μg; max: 7.57 μg). Affymetrix GeneChip® Human Transcriptome 2.0 ST microarrays were hybridized with 4.7 μg of labeled DNA. Arrays were hybridized in the Gene Chip Hybridization Oven 640 (Affymetrix) for 16 hours at 45°C rotating at 60 rpm. Washing, staining, and scanning of the arrays were done with the GeneChip Expression Wash, Stain, and Scan Kit (Affymetrix) and the Gene Chip Fluidics Station 450 (Affymetrix). Quantile normalization and subsequent data processing were performed using the Command Console Software 3.1 (Affymetrix) with default settings. Differentially expressed mRNA and lncRNAs were considered significantly differentially expressed when fold-change was >1.5 or < −1.5, and P < 0.05.
Long non-coding RNAs targets prediction
Differentially expressed lncRNAs were selected for target prediction. Two independent algorithms were used. The first algorithm searches for target genes acting in cis. With the help of gene annotations at University of California, Santa Cruz (UCSC) (http://genome.ucsc.edu/), lncRNAs and potential target genes were paired and visualized using UCSC genome browser. The genes transcribed within a 10 kbp window upstream or downstream of lncRNAs were considered as cis target genes.[16] The second algorithm is based on mRNA sequence complementarity and RNA duplex energy prediction, assessing the impact of lncRNA binding on complete mRNA molecules. It uses the BLAST software for first round screening. Finally, RNAplex in contrast to similar programs, can recover short, highly stable interactions between two RNAs, by introducing a per nucleotide penalty and thus was used to choose trans-acting target genes.[17] RNAplex parameters were set as –e −20. Then we integrated the predicted potential lncRNA targets above with the differently expressed mRNAs in the profile.
Quantitative real-time polymerase chain reaction validation
Quantitative real-time polymerase chain reaction (qRT-PCR) was carried out for verification of the microarray data. The qRT-PCR was performed on a real-time detection instrument ABI PRISM7900 system (Applied Biosystems, Foster City, CA, USA) using 2 × PCR master mix (SuperArray Bioscience, Frederick, MD, USA) at the following conditions: 3 minutes at 95°C, 40 cycles: 30 seconds at 95°C and 40 seconds at 60°C. They were used to quantitate relative amounts of product using glyceraldehyde 3-phosphate dehydrogenase as an endogenous control. Expression ratios were subjected to a log 2 transform to produce fold change data. Student's t-test was used to test for significant differences between control and EGCG-treated groups. (Statistical Package for the Social Sciences (SPSS) version 17, SPSS Inc., Chicago, IL, USA). The primers used are listed in Table 1.
Table 1.
Primer sequences used for qRT-PCR
| Genes | Primer sequences (5’-3’) | Product length (bp) |
|---|---|---|
| 3-hydroxy-3-methylglutaryl-CoA reductase | GCAGCAAACATTGTCACCG (F) | 166 |
| CACCACCCACCGTTCCTAT (R) | ||
| LDL receptor | GGTCTTTACGTGTTCCAAGG (F) | 143 |
| CGCAGTTTTCCTCGTCAGAT (R) | ||
| AT102202 | AAAGTTTGCCCTCAGTTCCA (F) | 170 |
| GCAGCCAAAGCAGCACATAA (R) | ||
| GADPH | CATGAGAAGTATGACAACAGCCT (F) | 113 |
| AGTCCTTCCACGATACCAAAGT (R) |
PCR: Polymerase chain reaction.
Small interfering RNA to knockdown long non-coding RNA AT102202
Three different Small is interfering RNAs (siRNAs) that targeted AT102202 RNA, and a scrambled siRNA control were purchased from Life Technologies. The siRNA molecules are 21 base-pair double-stranded RNA oligonucleotides with proprietary chemical modifications. The BLOCK-iT RNA interference (RNAi) designer was used to find gene-specific 21 nucleotide siRNA molecules. It uses gene specific targets for RNAi analysis and reports up to 10 top scoring siRNA targets. The freeze-dried siRNAs were dissolved in RNase free-water and stored as aliquots at 20°C. These siRNAs were respectively transfected into HepG2 cells with lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction. Knockdown efficiency was tested 24 hours after transfection. The siRNA with the sequence cucuuguugaaugucuugutt (siRNA 124) with the optimal concentration of 18 nmol/L yielded the highest degree of AT102202 knockdown and thus was selected for subsequent functional studies. Briefly, a total of 250,000 cells were cultured in serum free medium (without antibiotics) for 24 hours. siRNA 124 was transfected at 18 nmol/L concentration into HepG2 cells with lipofectamine 2000 (Invitrogen). Cells were incubated for 24 hours at 37°C in a CO2 incubator, and the transfected HepG2 cells were treated for 24 hours with EGCG (10, 25 μM). The level of expression of predicted target gene was assessed by qRT-PCR.
RESULTS
Effects of epigallocatechin-3-gallate on hepatic cholesterol metabolism and long non-coding RNA expression
To comprehensively investigate the effects of EGCG on hepatic cholesterol metabolism and lncRNA expression, we performed microarray analysis using HepG2 cells treated with 25 μmol/L EGCG. The microarrays contain probes to target nearly 40,000 ncRNAs and 240,000 mRNAs. In total, we identified 2737 differentially expressed transcripts with a ±1.5-fold. As shown in Table 2, the expression levels of 15 genes categorised in sterol metabolic process were changed by EGCG treatment, among which, the highest expression level was LDL receptor (2.6-fold) and the lowest expression level was 3-hydroxy-3-methyl glutaryl coenzyme A reductase (HMGCR) (−3.5-fold). The results suggest that EGCG directly affects cholesterol metabolism in hepatocytes. In addition, a total of 285 lncRNAs were differentially expressed after EGCG treatment, among which 29 were changed with a ±2-fold [Table 3].
Table 2.
Changes in gene expression of cholesterol metabolic process in HepG2 cells treated with 25 μmol/L epigallocatechin gallate relative to the vehicle control
| Genes | Fold (EGCG/control) | Genebank accession no. |
|---|---|---|
| 3-hydroxy-3-methylglutaryl-CoA reductase | −3.50 | NM_000859 |
| 3-Hydroxy-3-methylglutaryl-coenzyme A synthase 1 | 2.58 | NM_001098272 |
| 7-Dehydrocholesterol reductase | 2.34 | NM_001163817 |
| Mevalonate (diphospho) decarboxylase | 2.98 | NM_002461 |
| LDL receptor | 3.25 | NM_000527 |
| VLDL receptor | 2.56 | NM_001018056 |
| PPARγ | 2.25 | NM_005037 |
| Acetyl-CoA acetyltransferase 2 | −2.76 | NM_005891 |
| Cytochrome P450, family 51, subfamily A, polypeptide 1 | −2.40 | NM_000786 |
| 24-Dehydrocholesterol reductase | 2.21 | NM_014762 |
| Acyl-CoA synthetase short-chain family member 2 | −2.7 | NM_001076552 |
| Lanosterol synthase | 2.97 | NM_002340 |
| Sterol-C5-desaturase-like | 1.97 | NM_001024956 |
| Niemann-Pick disease, type C1 | −2.84 | NM_000271 |
| Retinoid X receptor, beta | 1.65 | NM_021976 |
EGCG: Epigallocatechin gallate; LDL: Low-density lipoprotein; VLDL: Very low-density lipoprotein.
Table 3.
Changes in long non-coding RNAs with a fold> ± 2-fold in HepG2 cells treated with 25 μmol/L epigallocatechin gallate relative to the vehicle control
| lncRNAs | Change | Source of database |
|---|---|---|
| N342928 | ↓ | NONCODE |
| NONHSAT015959 | ↓ | NONCODE |
| N333444 | ↓ | NONCODE |
| AT088005 | ↓ | NONCODE |
| TM4SF4-2 | ↓ | Rinn lincRNAs |
| AT009251 | ↓ | NONCODE |
| AT008445 | ↓ | NONCODE |
| AT068602 | ↓ | NONCODE |
| AT020294 | ↓ | NONCODE |
| AT122449 | ↓ | NONCODE |
| AT068591 | ↓ | NONCODE |
| PHF17-1 | ↓ | TUCP |
| AT098264 | ↓ | NONCODE |
| n382512 | ↓ | NONCODE |
| n409611 | ↓ | NONCODE |
| DCLK3-3 | ↓ | TUCP |
| AT101123 | ↓ | NONCODE |
| ISLR2-3 | ↓ | Rinn lincRNAs |
| AT102202 | ↑ | NONCODE |
| AT115872 | ↑ | NONCODE |
| AT017383 | ↑ | NONCODE |
| AT004532 | ↑ | NONCODE |
| AT027943 | ↑ | NONCODE |
| ITGB2-3 | ↑ | Rinn lincRNAs |
| AT078273 | ↑ | NONCODE |
| AT016514 | ↑ | NONCODE |
| AT097214 | ↑ | NONCODE |
| FAM75A3-1 | ↑ | TUCP |
| FAM75A3-2 | ↑ | TUCP |
lncRNAs: Long non-coding RNAs; EGCG: Epigallocatechin gallate.
Potential targets of the differentially expressed long non-coding RNAs
Since lncRNAs regulate the expression of its target genes; the next step is to construct a relationship between the expression profile of the mRNA involved in the cholesterol metabolism and the differentially expressed lncRNAs via target prediction programs. As a result, we found five matched lncRNA-mRNA pairs for five differentially expressed lncRNAs and four differentially expressed mRNA [Table 4]. In particular, the lncRNA AT102202 and its potential targets mRNA-HMGCR were identified. AT102202 is a length of 303 nucleotides lncRNA, containing four exons which three exons highly overlap with the HMGCR gene exons 4–6 (from UCSC genome database), indicating that HMGCR is potential cis-regulated by AT102202.
Table 4.
Long non-coding RNAs and predicted target genes
| lncRNA | Length (bp) | Chromosomal localization | Predicted gene | Chromosomal localization | Regulation |
|---|---|---|---|---|---|
| AT102202 | 303 | Chr5 | 3-hydroxy-3-methylglutaryl-CoA reductase | Chr5 | cis |
| N342928 | 17,002 | Chr6 | acetyl-CoA acetyltransferase 2 | Chr6 | cis |
| N333444 | 6900 | Chr7 | Cytochrome P450, family 51, subfamily A, polypeptide 1 | Chr7 | cis |
| AT088005 | 499 | Chr3 | PPARγ | Chr3 | cis |
| AT115872 | 1718 | Chr6 | acetyl-CoA acetyltransferase 2 | Chr6 | trans |
lncRNAs: Long non-coding RNAs.
Quantitative real-time polymerase chain reaction validation
In the present study, we focused on investigating the effect of EGCG on HMGCR, AT102202 and LDL receptor expression, and qRT-PCR was carried out to confirm the effect of EGCG on expression levels of these genes. As expected, the addition of 10 and 25 μM of EGCG significantly increased the level of expression of AT102202 and LDL receptor, meanwhile decreased HMGCR expression [Figure 1]. Furthermore, we confirmed that the expression of AT102202 and its predicted target gene-HMGCR was linked.
Figure 1.

Effects of the addition of epigallocatechin gallate (EGCG) on the level of AT102202 (a), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) (b), and LDL receptor (LDLR) mRNA expression (c). Total RNA were harvested from HepG2 cells treated with vehicle control, 10 or 25 μmol/L-EGCG for 24 hours and the level mRNA expression was measured using quantitative real-time PCR and normalised to the mRNA expression level of the GADPH gene. The levels of the vehicle-control-treated group are set at 100%, and the levels are presented as fold inductions relative to the vehicle-control-treated group. Values are means, with their standard error (n=6). *P <0.05 vs. control group.
Knockdown of long non-coding RNA AT102202 in green tea polyphenol(-)-epigallocatechin gallate in cultured human liver cells
To investigate the functional role of AT102202, we used siRNA to downregulate AT102202 expression in HepG2 cells. Three different siRNA molecules were tested for their knockdown efficiency, the most efficient of which (siRNA 124) was selected for subsequent functional studies [Figure 2]. To determine the optimal concentration for knockdown, several different concentrations of siRNA were examined. When these cells were transfected with 18 nmol/L of siRNA, at least 60% AT102202 silencing was observed. Therefore, subsequent functional studies were performed with a maximum of 18 nmol/L siRNA.
Figure 2.
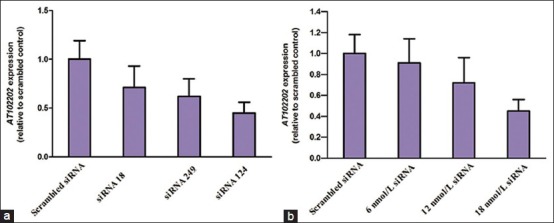
Knockdown of AT102202 in HepG2 cells. The expression of AT102202 following knockdown by three different siRNA (a). And knockdown efficiency was tested following different concentrations (6, 12, and 18 nmol/L) of siRNA 124 transfection for 24 hours (b). The level of knockdown efficiency was determined by quantitative real-time PCR. Error bars indicate the standard error of the mean for 6 technical replicates and expression values are normalized to scramble siRNA controls.
Given the correlated expression of AT102202 and HMGCR, we next aimed to determine the effect of AT102202 knockdown on HMGCR expression in HepG2 cells treated with or without EGCG. Using qRT-PCR, we determined the expression of HMGCR following siRNA-mediated knockdown of AT102202. As a result, we found that the level of HMGCR expression was significantly increased following AT102202 knockdown relative to the scrambled siRNA control [Figure 3]. These results suggest that AT102202 regulates HMGCR expression, and EGCG inhibits the HMGCR expression partially through by AT102202.
Figure 3.

The expression of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) mRNA expression following AT102202 knockdown (siRNA124 at 18 nmol/L) in HepG2 cells with EGCG (10 or 25 μmol/L) treatment for 24 hours. The level of HMGCR expression was measured using quantitative real-time PCR and error bars indicate the standard error of the mean for 6 technical replicates and expression values are normalized to scramble siRNA controls.
DISCUSSION
In this study, the microarray analysis reveals that EGCG improves cholesterol metabolism directly through up-or down-regulate multiple genes involved in cholesterol biosynthesis and uptake. In addition, many lncRNAs were differentially expressed after EGCG treatment, and we identified one such transcript, AT102202, which mapped within the HMGCR gene. Knockdown of AT102202 resulted in a markedly increase of HMGCR expression. These findings suggest that lncRNAs play an important role in the regulation of cholesterol metabolism.
In the present study, EGCG has been shown to greatly decrease HMGCR expression and increase LDL receptor expression. HMGCR is the rate-regulating enzyme in the cholesterol biosynthetic pathway and the primary site of cholesterol feedback regulation.[18] LDL receptor is important for mediating cellular LDL uptake and mainly regulated by cholesterol feedback.[19] When the levels of hepatocellular sterols drop, the key transcription factors-sterol regulatory element-binding proteins enters the nucleus, where it activates the expression of LDL receptor.[20] Thus, the decrease in HMGCR expression, leading to LDL receptor up-regulation and subsequently cholesterol uptake by hepatic cell, is an important contributor to the efficacy of EGCG on improvement of LDL-cholesterol. Due to the central role in cholesterol synthesis, HMGCR is the target of several hypocholesterolemic drugs, of which statins are the most extensively studied and among the most widely prescribed drugs worldwide.[21,22] Recently, in efforts to identify nonconventional treatments for hypercholesterolemia, tea catechins have been tested successfully both in vitro and vivo as cholesterol-lowering agents.[7,8,9,10] EGCG, the most pharmacologically active molecule of green tea catechins, was found to potently inhibit the in vitro activity of HMGCR by competitively binding to the nicotinamide adenine dinucleotide phosphate binding site of the enzyme[23] and decrease hepatic HMGCR expression at transcriptional levels.[24] However, the mechanism by which EGCG regulates this rate-limiting enzyme in cholesterol synthesis remains unclear.
Increasing evidence has confirmed lncRNAs to be one of the most important factors controlling gene expression.[25] Therefore, we evaluated the lncRNA expression profile in HepG2 to reveal the potential role of lncRNAs in cholesterol metabolism. Microarray techniques revealed a set of differentially expressed lncRNAs in HepG2 cells, indicating that EGCG may potentially regulate gene expression through by lncRNAs other than microRNAs.[26]
Recent studies demonstrated that lncRNAs can guide changes in gene expression either in cis (on neighboring genes) or in trans (distantly located genes) manner that is not easily predicted based on lncRNA sequence.[27,28] By target prediction programs, we constructed the relationship between the lncRNA and mRNA involved in cholesterol metabolism and found five matched lncRNA-mRNA pairs for five differentially expressed lncRNAs and four differentially expressed mRNA. Noteworthily, we identified one lncRNA-AT102202 and its potential targets mRNA-HMGCR. AT102202 is mapped within the HMGCR gene locus, which has prompted the hypothesis that lncRNAs AT102202 may have cis-acting effects within HMGCR gene locus. Following siRNA-mediated knockdown of AT102202 in HepG2 cells, a significant increase in HMGCR expression level was observed, confirming that HMGCR was cis-regulated by AT102202.
Since lncRNAs regulate gene expression by a variety of mechanisms, including chromatin modification, transcription, post-transcriptional processing,[11] the mechanism by which AT102202 regulate HMGCR expression remain unclear. Recently, the long intergenic RNA HOTAIR was shown to regulate metastatic progression in human breast cancer. This RNA recruits Polycomb Repressive Complex 2 to specific target genes in the genome that lead to histone H3 lysine 27 trimethylation and epigenetic silencing of metastatic suppressor genes.[29] In addition, a number of studied lncRNAs, at transcriptional level, influenced the expression (either positively or negatively) of the local protein-coding gene by RNAi, recruiting and modulating the activities of RNA-binding protein, or recruitment of activator and repressor proteins (transcription factors).[30,31,32] Thus, whether AT102202 regulate HMGCR expression through by epigenetic effects or transcriptional regulation needed to be further explored in future.
In contrast to the group of cis-regulatory lncRNAs, there are a couple of examples of lncRNAs that exert their transcriptional effects across chromosomes in trans. Here we have introduced the program RNAplex algorithms which reduces the time needed to localize putative hybridization sites, mainly by neglecting intramolecular interactions and by using a slightly simplified energy model.[17] As a consequence, we found that acetyl-CoA acetyltransferase 2, which is involved in absorbing dietary cholesterol and in storing cholesteryl esters as lipid droplets,[33] may also be regulated by lncRNA (N342928) in trans, but confirmation and elucidation of this relationship requires further study.
The present study indicates that EGCG improves cholesterol metabolism through decrease in HMGCR expression and up-regulation of LDL receptor. In addition, we found that lncRNA AT102202 may play an important role in the regulation of HMGCR expression. Furthermore, the roles of other differentially expressed lncRNAs in cholesterol metabolism from the array data need further verification and analysis.
Footnotes
Edited by: Yi Cui
Source of Support: The present study was supported by a grant from the National Natural Science Foundation of China (No. 81241007).
Conflict of Interest: None declared.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics-2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson TA. The lower the better in hypercholesterolemia therapy: A reliable clinical guideline? Ann Intern Med. 2000;133:549–54. doi: 10.7326/0003-4819-133-7-200010030-00015. [DOI] [PubMed] [Google Scholar]
- 3.Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365:2078–87. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 4.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–21. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng XX, Xu YL, Li SH, Liu XX, Hui R, Huang XH. Green tea intake lowers fasting serum total and LDL cholesterol in adults: A meta-analysis of 14 randomized controlled trials. Am J Clin Nutr. 2011;94:601–10. doi: 10.3945/ajcn.110.010926. [DOI] [PubMed] [Google Scholar]
- 6.Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 2006;296:1255–65. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 7.Bursill CA, Abbey M, Roach PD. A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and upregulating the LDL receptor in the cholesterol-fed rabbit. Atherosclerosis. 2007;193:86–93. doi: 10.1016/j.atherosclerosis.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Lee SM, Kim CW, Kim JK, Shin HJ, Baik JH. GCG-rich tea catechins are effective in lowering cholesterol and triglyceride concentrations in hyperlipidemic rats. Lipids. 2008;43:419–29. doi: 10.1007/s11745-008-3167-4. [DOI] [PubMed] [Google Scholar]
- 9.Bursill CA, Roach PD. Modulation of cholesterol metabolism by the green tea polyphenol (2)-epigallocatechin gallate in cultured human liver (HepG2) cells. J Agric Food Chem. 2006;54:1621–6. doi: 10.1021/jf051736o. [DOI] [PubMed] [Google Scholar]
- 10.Lee MS, Park JY, Freake H, Kwun IS, Kim Y. Green tea catechin enhances cholesterol 7alpha-hydroxylase gene expression in HepG2 cells. Br J Nutr. 2008;99:1182–5. doi: 10.1017/s0007114507864816. [DOI] [PubMed] [Google Scholar]
- 11.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–41. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152:570–83. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grote P, Wittler L, Hendrix D, Koch F, Währisch S, Beisaw A, et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell. 2013;24:206–14. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holdt LM, Beutner F, Scholz M, Gielen S, Gäbel G, Bergert H, et al. ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler Thromb Vasc Biol. 2010;30:620–7. doi: 10.1161/ATVBAHA.109.196832. [DOI] [PubMed] [Google Scholar]
- 15.Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z, Sharpless NE. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010;6:e1001233. doi: 10.1371/journal.pgen.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–7. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tafer H, Hofacker IL. RNAplex: A fast tool for RNA-RNA interaction search. Bioinformatics. 2008;24:2657–63. doi: 10.1093/bioinformatics/btn193. [DOI] [PubMed] [Google Scholar]
- 18.Bloch K. The biological synthesis of cholesterol. Science. 1965;150:19–28. doi: 10.1126/science.150.3692.19. [DOI] [PubMed] [Google Scholar]
- 19.Jeon H, Blacklow SC. Structure and physiologic function of the low-density lipoprotein receptor. Annu Rev Biochem. 2005;74:535–62. doi: 10.1146/annurev.biochem.74.082803.133354. [DOI] [PubMed] [Google Scholar]
- 20.Brown MS, Goldstein JL. Cholesterol feedback: From Schoenheimer's bottle to Scap's MELADL. J Lipid Res. 2009;50(Suppl):S15–S27. doi: 10.1194/jlr.R800054-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carbonell T, Freire E. Binding thermodynamics of statins to HMG-CoA reductase. Biochemistry. 2005;44:11741–8. doi: 10.1021/bi050905v. [DOI] [PubMed] [Google Scholar]
- 22.Sarver RW, Bills E, Bolton G, Bratton LD, Caspers NL, Dunbar JB, et al. Thermodynamic and structure guided design of statin based inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Med Chem. 2008;51:3804–13. doi: 10.1021/jm7015057. [DOI] [PubMed] [Google Scholar]
- 23.Cuccioloni M, Mozzicafreddo M, Spina M, Tran CN, Falconi M, Eleuteri AM, et al. Epigallocatechin-3-gallate potently inhibits the in vitro activity of hydroxy-3-methyl-glutaryl-CoA reductase. J Lipid Res. 2011;52:897–907. doi: 10.1194/jlr.M011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cocci P, Mosconi G, Palermo FA. Partial cloning, tissue distribution and effects of epigallocatechin gallate on hepatic 3-hydroxy-3-methylglutaryl-CoA reductase mRNA transcripts in goldfish (Carassius auratus) Gene. 2014;545:220–5. doi: 10.1016/j.gene.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 25.Khachane AN, Harrison PM. Mining mammalian transcript data for functional long non-coding RNAs. PLoS One. 2010;5:e10316. doi: 10.1371/journal.pone.0010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milenkovic D, Jude B, Morand C. MiRNA as molecular target of polyphenols underlying their biological effects. Free Radic Biol Med. 2013;64:40–51. doi: 10.1016/j.freeradbiomed.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 27.Hung T, Chang HY. Long noncoding RNA in genome regulation: Prospects and mechanisms. RNA Biol. 2010;7:582–5. doi: 10.4161/rna.7.5.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagano T, Fraser P. No-nonsense functions for long noncoding RNAs. Cell. 2011;145:178–81. doi: 10.1016/j.cell.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martianov I, Ramadass A, Serra Barros A, Chow N, Akoulitchev A. Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature. 2007;445:666–70. doi: 10.1038/nature05519. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Arai S, Song X, Reichart D, Du K, Pascual G, et al. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454:126–30. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berghoff EG, Clark MF, Chen S, Cajigas I, Leib DE, Kohtz JD. Evf2 (Dlx6as) lncRNA regulates ultraconserved enhancer methylation and the differential transcriptional control of adjacent genes. Development. 2013;140:4407–16. doi: 10.1242/dev.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang TY, Chang CC, Lin S, Yu C, Li BL, Miyazaki A. Roles of acyl-coenzyme A: Cholesterol acyltransferase-1 and -2. Curr Opin Lipidol. 2001;12:289–96. doi: 10.1097/00041433-200106000-00008. [DOI] [PubMed] [Google Scholar]


