Abstract
Background:
Contrast-enhanced ultrasound is a dynamic and continuous modality providing real-time view of vascularization and flow distribution patterns of different organs and tumors. In order to evaluate the diagnostic significance of intraoperative contrast-enhanced ultrasound in assessing the resection degree of brain glioma by transmission electron microscopic (TEM) examination, it is important to have specific knowledge about contrast-enhanced ultrasound.
Methods:
Ultrasound contrast was applied in operations of 120 cases of brain glioma, to evaluate the degree of tumor resection. Biopsy tissues were obtained the suspicious residual tumors surrounding the tumor cavity. The sensitivity and specificity of the residual tumors were determined by the intraoperative ultrasound contrast according to TEM examination results.
Results:
There were 44 cases of low-grade gliomas and 76 cases of high-grade gliomas. Three hundred and sixty biopsy tissues were obtained. The sensitivity of intraoperative ultrasound contrast in diagnosing the residual tumor was 62.2%, while the specificity degree of it was 92.8%. The consistency coefficient of the ultrasound contrast diagnosis and TEM examination results was 0.584 (Kappa = 0.584), which was between 0.4 and 0.6, therefore it was of medium consistency.
Conclusions:
Intraoperative ultrasound contrast was of a high sensitivity and specificity in evaluating the excision degree of tumor. The consistency of the residual tumor rate detected, respectively, by ultrasound contrast and TEM examination was of medium consistency. The application of intraoperative ultrasound contrast can improve the resection rate of brain glioma.
Keywords: Brain Glioma, Intra-surgery, Transmissionelectron Microscopic, Resection Degree, Ultrasound Contrast
INTRODUCTION
Malignant gliomas are the most commonly seen brain tumors among adults, accounting for 70% of primary malignant brain tumors and 40% of all primary brain tumors. Nowadays, the treatment method of best therapeutic efficacy in treating brain glioma is microscopic surgical resection. In recent years, the clinical application of intraoperative ultrasound imaging techniques can significantly improve tumor removal rate, for it can show the tumor more clearly. Ultrasound imaging technique was used in the operation to extract the specimens of tumor cavity tissues after resection. Referring to the postoperative transmission electron microscopic (TEM) examination results, the sensitivity and specificity of intraoperative ultrasound contrast in diagnosing the residual tumors was evaluated.
METHODS
Patients
One hundred and twenty patients, 67 males and 53 females, aged from 24 to 72 years, with a mean age of 39.6 ± 6.8, admitted by our neurosurgery department from January 2012 to June 2014, were imaging diagnosed as brain glioma by preoperative magnetic resonance imaging (MRI) and enhanced examinations. They were all diagnosed as supratentorial gliomas, among which there were 47 cases of frontal glioma, 41 cases of temporal glioma, 24 cases of top glioma, and 8 cases of occipital glioma.
Instruments
The time–intensity curve analysis software was assigned randomly to work with the α-10 Colour Ultrasonic Scanner, produced by Aloka. The UST-9133dedicated small convex array probe, whose probe frequency was 3–6 MHz, was of good mobility and small size. It could directly contact with the surface of the brain. The imaging technique used in this experiment was coded phase-inversion (CPI) harmonic ultrasound, randomly configured by Alokaα-10, whose ultrasonic output power was of a low mechanical index (MI: 0.10 – 0.12).
Ultrasound contrast agent was SonoVue (Bracco), which were microbubbles of the phospholipids microencapsulated sulfur hexafluoride (SF6). The average diameter of the microbubble was 2.5 μm and its pH value ranged from 4.5 to 7.5. Each agent contained 59 mg of sulfur hexafluoride gas and 25 mg of lyophilized powder. SF6 microbubbles suspension was prepared before the contrast by injecting 5 ml of sodium chloride into 59 mg of SonoVue.
Scanning methods
The craniotomy was located by referring to the preoperative MRI and/or computed tomography (CT) imaging data. The ultrasound was used to locate the lesions after opening the bone flap and cutting the endocranium of the patient. The probe was applied with the coupling agents on its surface and protected by sterile plastic sleeve cover. It moved on the cerebral cortex to observe the tumor location, borders, shape, internal echo, its relationship with the surrounding edematous brain tissue and normal brain tissue, and to measure the lesion size and its depth to the surface of brain. The color Doppler flow imaging (CDFI) was used to observe the signal features of the peripheral and internal blood flow of lesions. Then the ultrasound contrast screen was cut into after the best lesion facet was selected. Put the observation target on the screen center, adjusting the depth and focus, starting the coding ultrasound contrast harmonic function, and setting the MI to 0.10 – 0.12. Five milliliter of microbubble sulfur hexafluoride suspension was bolus injected via the femoral vein and then 10 ml of saline was injected for washing, followed by injection of the contrast agent while starting the built-in timer of the ultrasound scanner to make real-time dynamic observation of tumor blood flow perfusion and its enhanced features and to record all the image data for storage. The ultrasound scanning should be operated by neurosurgeons, which had accepted certain ultrasound technology training and had intraoperative ultrasound experience for more than 1 year. The identification of ultrasonic images should be done by ultrasound physicians with extensive experience in intraoperative ultrasound. The intraoperative ultrasound contrast operation was completed by the same doctor and the injection of the ultrasound contrast agent was also carried out by the same nurse.
Sampling methods
After the neurosurgeon judged that the tumor was totally removed, a complete hemostasis was carried out and then the cotton pieces and hemostatic materials were removed from the residue cavity. After washing repeatedly, the residual cavity was filled with saline and another ultrasound contrast was carried out on the patient. Following the procedure mentioned above, determining whether there was any residual tumor after the surgery by scanning the residual tumor cavity and its adjacentbrain surface. Biopsy was taken on the three randomly-selected points inside three tumor cavity walls, respectively, and then marked on their corresponding ultrasonic images; when there was any tumor residue detected by the ultrasound contrast, threebiopsy points would be taken on the surface of tumor residue and labeling would be done on their corresponding ultrasound images.
Examination results
The examination results were positive if there was any abnormally high-enhanced region of contrast agent inside the cavity wall, in the form of pellet or ribbon, with the thickness ≥5 mm, detected by ultrasound contrast examination and it would be suspected as tumor residue; if there was no abnormally high-enhanced region which was in the form of pellet or ribbon (thickness < 5 mm), then the results were negative. All the intraoperative biopsies were sent to the electron microscopy room to receive transmission electron microscopy examination.
The specimens for TEM sampling, fixed with 2.5% glutaraldehyde under the temperature of 4°C for 2 hours, were dehydrated in a graded ethanol and routinely embedded by epoxy resin 812. Ultrathin section was carried out after the semi-thin slice positioning. The TEM observation was taken after the uranyl acetate-lead citrate double staining.
Evaluation on the sensitivity and specificity of ultrasound contrast in detecting the tumor residues
Cases diagnosed simultaneously by sensitivity = gold standard (TEM examination) and the new diagnosis method (intraoperative ultrasound contrast) as positive/cases diagnosed by gold standard as positive. Cases diagnosed simultaneously by sensitivity = gold standard (TEM examination) and the new diagnosis method (intraoperative ultrasound contrast) as negative/cases diagnosed by gold standard as negative.
RESULTS
TEM observation results
The common features of the astrocytoma tumor cells were as followed: chromatin inside the nucleus was evenly distributed and there were glial filaments of varying amounts inside the cytoplasm and cell protrusion, including hairy cell type and endomorphytype tumor cells [Figure 1]; the nucleus of glioblastoma tumor cells were large but irregular. Its nuclear membrane was in the shape of invaginated serrate and multicores, megakaryocytes, weird nucleus tumor cells, and intranuclear pseudoinclusion could be commonly seen inside the membrane. There were many unevenly distributed heterochromatin and the inter chromatin granules with reticular or rod-shaped prominent nucleoli or multiple nucleoli could also be seen [Figure 2].
Figure 1.
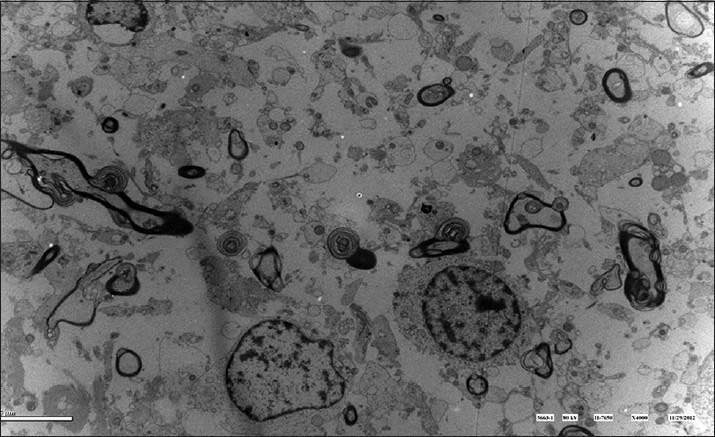
Tumor cells distributed diffusedly while some of them aggregated. Tumor cells were ill-defined with little cytoplasm, nucleus of varied size, and myelin with diffusively degenerated mesenchyme. It was consistent with the features of star-oligodendroglioma.
Figure 2.
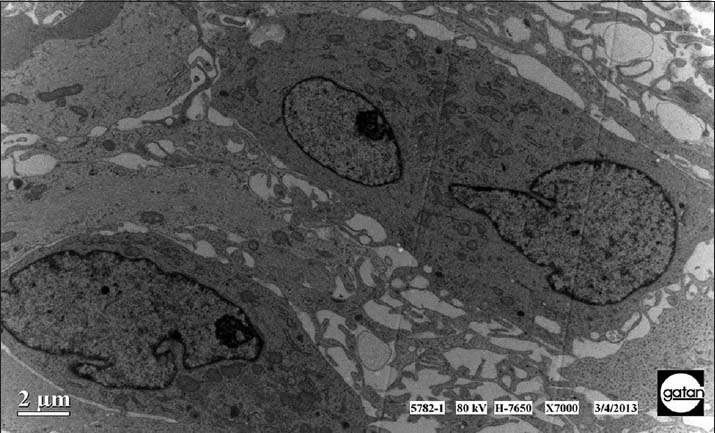
The tumor cells distributed densely along the blood vessels. They were of different size and shapes with nucleus of irregular shapes and nucleolus. Sometimes it could be seen the double-core tumor cells. All the features mentioned above were in line with those of glioblastoma.
There were altogether 360 samples in this operation and 87 tumor residues detected by the intraoperative ultrasound imaging [Figure 3], 69 residues of which were verified by the TEM examination. The sensitivity of ultrasound contrast was 69/111 = 62.2%. There were 273 samples without tumor residues detected by intraoperative ultrasound contrast [Figure 4], 231 of which were verified by the TEM examination. The specificity of ultrasound contrast was 231/249 = 92.8%. The comparison and contrast of the results obtained, respectively, by ultrasound contrast and TEM examination are shown in [Table 1].
Figure 3.
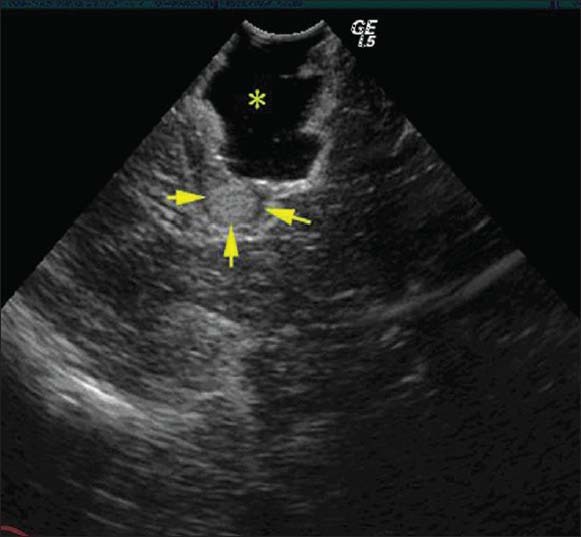
The tumor residues diagnosed by intraoperative ultrasound contrast which had been verified by TEM examination. Arrows in the Figure marked the location of tumor residues. The star showed the residual cavity after surgery which was filled with saline.
Figure 4.
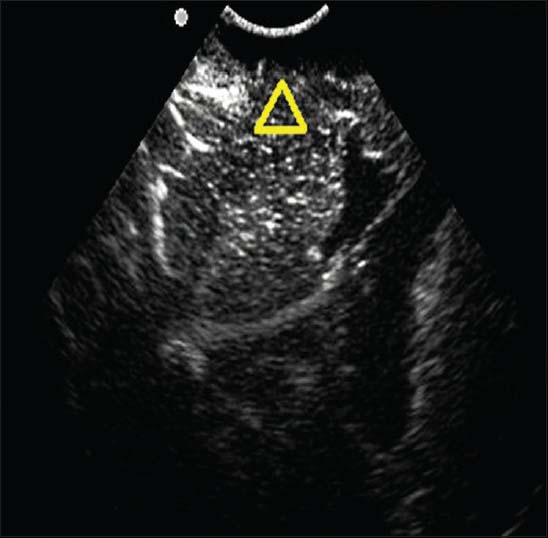
The banded medium-strong echo area and its surrounding normal brain tissue examined by ultrasound contrast showed homogeneous enhancement (marked by the triangle in the Figure). It was not considered as tumor residue by ultrasound contrast, while was proved to be gliocyteproliferation by postoperative TEM examination.
Table 1.
Comparison and contrast of results obtained, respectively, by ultrasound contrast and TEM examination on patients with brain glioma
| TEM examination | Total | ||
|---|---|---|---|
| With residue | Without residue | ||
| Intraoperative ultrasound with residue | 69 | 18 | 87 |
| Contrast without residue | 42 | 231 | 273 |
| Total | 111 | 249 | 360 |
TEM: Transmission electron microscopy.
The consistency coefficient of ultrasound contrast diagnosis and TEM examination results was 0.584 (Kappa = 0.584, 0.4–0.6), and was of medium consistency.
DISCUSSION
Brain gliomais the most commonly seen malignant brain tumor, whose priority of treatment is surgery. The main purpose of surgical treatment is to maximize the removal of the tumor and to control tumor growth and recurrence, while preserving normal nerve function in order to improve patients’ quality of life.[1,2] The most important determinant of the postoperative reoccurrence and clinical prognosis is the extent of glioma resection.[3] However, brain glioma grows invasively in the brain, with no obvious boundaries from normal brain tissues; therefore, it was difficult to completely remove it by surgical excision. In this aspect, real-time intraoperative judgment of tumor resection degree is a major problem bothering neurosurgery.[4]
Intraoperative color ultrasound scanning, whose utilization rate is of growing popularity, is simple and convenient, real-time, and accurate. It is easy to operate and can be applied repeatedly.[5] However, there are some errors for general ultrasound contrast to identify tumor boundaries and to distinguish the edematous brain tissue surrounding the tumor from the normal brain tissue.[6] In recent years, the application of ultrasound contrast agent makes up for the flaws of general ultrasound contrast by showing the tumor location and boundaries more clearly after contrast enhancement.[7]
He et al.,[8] found that the application of ultrasound contrast in brain tumor could accurately and immediately locate the tumor and display the relationship between the internal blood vessels and peripheral blood vessels. Because the blood supply pattern of tumor is different from the one of normal brain tissue surrounding it, the ultrasound contrast has huge advantage in displaying the brain tumor boundaries over the ordinary intraoperative ultrasound, while displaying the tumor blood vessels.[9] It is of strong specificity and sensitivity and can determine whether there are tumor residues or not, according to the changes of local enhancement.[10]
Electron microscopy technology is of significant value in terms of diagnosis and differentiation of brain glioma, by observing the ultrastructure of tissue in the micron level.[11,12] Electron microscopy, light microscopy, and immunohistochemistry can complement each other's function in diagnosis, treatment, basic research, and prognosis prediction of brain glioma.[13] The available data would be limited, if solely relying on light microscopy and immunohistochemistry, therefore the assist of electron microscopy is necessary.[14]
There are some reports on the ultrastructure of primary intracranial tumors; domestic and overseas.[15] It played an important role in promoting the diagnosis and differential diagnosis of brain tumors.[16] Transmission electron microscopy can be used as an auxiliary tool of the pathological diagnosis and differential diagnosis of brain glioma.[17]
To study on the specificity and sensitivity of intraoperative ultrasound contrast, 120 patients were selected and imaging diagnosed as brain glioma by preoperative MRI scanning and enhanced examinations. Three places of tissue surrounding the residual tumor cavity after resection were taken for biopsy and 360 samples (120 × 3) were detected by biopsy. In this study, the intraoperative ultrasound contrast is of high sensitivity and specific in detecting the tumor residues. The application of it can improve the removal rate of brain glioma. The consistency coefficient is 0.584 (kappa = 0.584), which is less than 0.6. In this aspect, it is of medium instead of high consistency (0.6 – 0.8), which indicates that nowadays ultrasound contrast cannot improve the brain glioma removal rate significantly.
No abnormally high-enhanced region or banded-enhanced region were clearly displayed by ultrasound contrast examinations; or there were some display of the regions but with the thickness <5 mm in 42 cases, results of which could be regarded as negative. But these specimens were proved to be tumor residues by TEM examination. In this aspect, the result obtained by the ultrasound contrast was false negative. The misdiagnosis rate was 42/231 = 16%. The edema regions surrounding the tumor showed a strong echo under the ultrasound contrast, which was easily to be regarded as false negative. The preoperative MRI could be combined to test the tumor peripheral edema regions.
There were 18 cases of false-positive biopsy verified by TEM examination as gliosis proliferation. In high malignant gliomas, gliosis proliferation regions could be seen between the tumor tissues and edema regions, which were caused by gliosis proliferation of tumor peripheraltissues. The intraoperative ultrasound contrast of the gliosis proliferation region showed medium-high echo and the one of partial thrombosis also showed strong echo. It would be mistaken as residual tumor, if the physician did not take fully consideration.[18,19] However, it can be found that there were boundaries between the gliosis proliferation regions and tumor tissue and the echo was homogeneous by careful observation. In addition, in patients with reoccurring glioma after radiation therapy, the recurrent tumor focus, radioactive necrotic tissues, and peripheral edema tissues all showed medium-strong echo and there were no obvious boundaries between them, both of which made it difficult to identify the tumor removal degree.[20,21] In this study, there were 12 in 18 false-positive cases of recurrent glioma which were treated with radiotherapy. Therefore, it is believed that the application of intraoperative ultrasound contrast will be limited in evaluating the tumor removal degree of patients with recurrent gliomas or patients with gliomas after radiotherapy.
In conclusion, intraoperative ultrasound contrast is simple and convenient, immediate and accurate, noninvasive and safe, and economic and affordable. It is of a high sensitivity and specificity in detecting the intraoperative tumor residues. The diagnosing results of intraoperative ultrasound contrast and postoperative TEM diagnosis are of medium consistency. The application of intraoperative ultrasound contrast can improve brain glioma removal rate. It is believed in the near future that with the continuous improvement of intraoperative ultrasound imaging techniques, the intraoperative ultrasound contrast will be applied more widely in neurosurgery.
Footnotes
Edited by: Li-shao Guo
Source of Support: This study was supported by a grant from Capital Medical University Cooperation Fund of Basic Medical- clinical Key Scientific Research Projects (No. 12JL08).
Conflict of Interest: None declared.
REFERENCES
- 1.Solheim O, Selbekk T, Jakola AS, Unsgard G. Ultrasound-guided operations in unselected high-gradegliomas--overall results, impact of image quality and patient selection. Acta Neurochir (Wien) 2010;152:1873–86. doi: 10.1007/s00701-010-0731-5. [DOI] [PubMed] [Google Scholar]
- 2.Vyberg M, Ulhøi BP, Teglbjaerg PS. Neuronal features of oligodendrogliomas--an ultrastructural and immunohistochemical study. Histopathology. 2007;50:887–96. doi: 10.1111/j.1365-2559.2007.02686.x. [DOI] [PubMed] [Google Scholar]
- 3.Moiyadi AV, Shetty PM, Mahajan A, Udare A, Sridhar E. Usefulness of three-dimensional navigable intraoperative ultrasound in resection of brain tumors with a special emphasis on malignant gliomas. Acta Neurochir (Wien) 2013;155:2217–25. doi: 10.1007/s00701-013-1881-z. [DOI] [PubMed] [Google Scholar]
- 4.Wang LS, He W, Liu HZ. Controlstudy on pathological evaluation on brain glioma removal degree by intraoperative ultrasound contrast. J Med Ultrasound. 2009;6:5002–6. [Google Scholar]
- 5.Kanno H, Ozawa Y, Sakata K, Sato H, Tanabe Y, Shimizu N, et al. Intraoperative power Doppler ultrasonography with a contrast-enhancing agent for intracranial tumors. J Neurosurg. 2005;102:295–301. doi: 10.3171/jns.2005.102.2.0295. [DOI] [PubMed] [Google Scholar]
- 6.Wang YQ, Yu SQ, Wang JS, Ji N, Ren T, Li DL, et al. Application of intraoperative contrast-enhanced ultrasound in different pathological grades of glioma. Zhonghua Yi Xue Za Zhi. 2012;92:1495–7. [PubMed] [Google Scholar]
- 7.Xu HX. Contrast-enhanced ultrasound: The evolving applications. World J Radiol. 2009;1:15–24. doi: 10.4329/wjr.v1.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He W, Jiang XQ, Wang S, Zhang MZ, Zhao JZ, Liu HZ, et al. Intraoperative contrast-enhanced ultrasound for brain tumors. Clin Imaging. 2008;32:419–24. doi: 10.1016/j.clinimag.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Prada F, Perin A, Martegani A, Aiani L, Solbiati L, Lamperti M, et al. Intraoperative contrast-enhanced ultrasound for brain tumor surgery. Neurosurgery. 2014;74:542–52. doi: 10.1227/NEU.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 10.Kopp R, Zürn W, Weidenhagen R, Meimarakis G, Clevert DA. First experience usingin traoperative contrast-enhanced ultrasound during endovascular aneurysm repair for infrarenal aortic aneurysms. J Vasc Surg. 2010;51:1103–10. doi: 10.1016/j.jvs.2009.12.050. [DOI] [PubMed] [Google Scholar]
- 11.Wierzba-Bobrowicz T, Lewandowska E, Matyja E, Dziduszko J, Koszewski W, Stepień T, et al. Granular cell astrocytoma. A case report with immunohistochemical and ultrastructural characterization. Folia Neurpathol. 2008;46:286–93. [PubMed] [Google Scholar]
- 12.Neumann M, Kunz U, Lehmann H, Gabel D. Determination of the subcellular distribution of mercaptoundecahydro-closo-dodecaborate (BSH) in human glioblastoma multiforme by electron microscopy. J Neurooncol. 2002;57:97–104. doi: 10.1023/a:1015737010621. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, He YS. Application of electron microscopy in study of brain glioma. Int Neurol Neurosurg. 2010;37:175–8. [Google Scholar]
- 14.Zhang QQ, Ma L, Wang FH, Mi ZK. Application of electron microscopy technique in clinical pathology diagnosis. Chin Med Front. 2011;6:65–7. [Google Scholar]
- 15.Wang J, Liu X, Hou WH, Dong G, Wei Z, Zhou H, et al. The relationship between Intraoperative ultrasonography and Pathological Grade in Cerebral Glioma. J Int Med Res. 2008;36:1426–34. doi: 10.1177/147323000803600632. [DOI] [PubMed] [Google Scholar]
- 16.Frontczak-Baniewicz M, Czajkowska D, Andrychowski J, Walski M. The immature endothelial cell in humanglioma. Ultrastructural features of blood capillary vessels. Folia Neuropathol. 2008;46:49–56. [PubMed] [Google Scholar]
- 17.Bian XW, Bai JQ, Liu FX, Wang XR, Feng H, Zhang KC. Observation of 68 cases of brain glioma by transmission electron microscopy and scanning electron microscopy and its diagnosing and differentiation significance. Electron Microsc Society. 2002;21:907–10. [Google Scholar]
- 18.Serra C, Stauffer A, Actor B, Burkhardt JK, Ulrich NH, Bernays RL, et al. Intraoperative high frequency ultrasound in intracerebral high-grade tumors. Ultraschall Med. 2012;33:E306–12. doi: 10.1055/s-0032-1325369. [DOI] [PubMed] [Google Scholar]
- 19.Gulati S, Berntsen EM, Solheim O, Kvistad KA, Håberg A, Selbekk T, et al. Surgical resection of high-grade gliomas in eloquent regions guided by blood oxygenation level dependent functional magnetic resonance imaging, diffusion tensor tractography, and intraoperative navigated 3D ultrasound. Minim Invasive Neurosurg. 2009;52:17–24. doi: 10.1055/s-0028-1104566. [DOI] [PubMed] [Google Scholar]
- 20.D’Agostino DP, Olson JE, Dean JB. Acute hyperoxia increases lipid peroxidation and induces plasma membrane blebbing in human U87 glioblastoma cells. Neuroscience. 2009;159:1011–22. doi: 10.1016/j.neuroscience.2009.01.062. [DOI] [PubMed] [Google Scholar]
- 21.Leong-Poi H. Molecular imaging using contrast-enhanced ultrasound: Evaluation of angiogenesis and cell therapy. Cardiovasc Res. 2009;84:190–200. doi: 10.1093/cvr/cvp248. [DOI] [PubMed] [Google Scholar]


