Abstract
Background:
Antioxidants and the duration of treatment after noise exposure on hearing recovery are important. We investigated the protective effects of an antioxidant substance, edaravone, and its slow-release dosage form, edaravone solid lipid nanoparticles (SLNs), in steady noise-exposed guinea pigs.
Methods:
SLNs loaded with edaravone were produced by an ultrasound technique. Edaravone solution or edaravone SLNs were administered by intratympanic or intravenous injection after the 1st day of noise exposure. Guinea pigs were exposed to 110 dB sound pressure level (SPL) noise, centered at 0.25–4.0 kHz, for 4 days at 2 h/d. After noise exposure, the guinea pigs underwent auditory brainstem response (ABR) threshold measurements, reactive oxygen species (ROS) were detected in their cochleas with electron spin resonance (ESR), and outer hair cells (OHCs) were counted with silvernitrate (AgNO3) staining at 1, 4, and 6 days.
Results:
The ultrasound technique was able to prepare adequate edaravone SLNs with a mean particle size of 93.6 nm and entrapment efficiency of 76.7%. Acoustic stress-induced ROS formation and edaravone exerted a protective effect on the cochlea. Comparisons of hearing thresholds and ROS changes in different animal groups showed that the threshold shift and ROS generation were significantly lower in treated animals than in those without treatment, especially in the edaravone SLN intratympanic injection group.
Conclusions:
Edaravone SLNs show noticeable slow-release effects and have certain protective effects against noise-induced hearing loss (NIHL).
Keywords: Electron Spin Resonance, Intratympanic Injection, Noise-induced Hearing Loss, Reactive Oxygen Species, Solid Lipid Nanoparticles
INTRODUCTION
Noise-induced hearing loss (NIHL) is a major public health problem, and its treatment with traditional therapy strategies is often unsuccessful because of the blood labyrinth barrier (BLB).[1] Due to tight junctions between cells, substances in the systemic circulation with potentially therapeutic effects are prevented from gaining access to inner ear targets. Additionally, the cochlea is a closed space and minor changes in the endolymph and perilymph can affect its function. Therefore, delicate approaches are required to avoid possible damage caused by the delivery method itself. Currently, drugs are commonly administered systemically, but there are some disadvantages of systematic use, such as the inability to get an ideal concentration in the inner ear,[2] the possible occurrence of some deleterious side effects,[3] and lack of clearance of pharmacokinetics from the inner ear.[4] In recent years, many researchers have focused on local delivery for inner ear diseases, and some applications[5] and therapies[6] have been shown to be clinically relevant, so it is necessary to encourage further development of safe and reliable mechanisms for the direct delivery of compounds into the inner ear. Methods for local delivery can be categorized as either intratympanic or intracochlear approaches.
Intratympanic delivery can be accomplished via perfusion of the middle ear with the goal of diffusion through the round window membrane (RWM) into the fluid spaces of the inner ear. This method, introduced more than 50 years ago,[7] remains in common use in the treatment of inner ear diseases. In recent years, the advanced technologies have been used which include hydrogels,[8] nanoparticles,[9] and poloxamers.[10]
Direct intracochlear drug delivery involves the placement of drugs within the cochlear perilymphatic spaces via a cochleostomy in the surrounding bone or the RWM.[11,12] This mode of delivery allows drugs to reach their intended targets more directly than with systemic delivery. Molecules perfused into a perilymphatic compartment have direct access to the cells of the inner ear.[13] Methods of delivery include direct perfusion using micropumps[14,15] and osmotic pumps.
However, with both intratympanic delivery and direct intracochlear drug delivery, there are disadvantages. Because direct intracochlea drug delivery requires surgical implantation, there are patients who cannot accept it and there are also doctors who are not comfortable performing it.
Since the beginning of the 1990s, attention from various research groups has focused on an alternative to polymeric nanoparticles; the solid lipid nanoparticles (SLNs).[16,17] Basically, lipids can be used that are well-tolerated by the body (e.g., glycerides composed of fatty acids, which are present in emulsions for parenteral nutrition). Large scale production can be undertaken in a cost-effective and relatively simple manner, using high-pressure homogenization to create SLNs.[18] An alternative approach is the production of SLNs via microemulsions.[19] Because SLNs can be targeted to specified cell populations, and because they are biodegradable, traceable in vivo, and equipped with controlled drug/gene release, SLNs could overcome the disadvantages of local drug delivery to the inner ear.
Regarding NIHL, research has shown that oxidative stress plays an important role in noise-induced cochlear injury, and these studies have also found that a number of antioxidants and cell death-inhibiting compounds can ameliorate the hearing loss associated with acoustic trauma.[20,21,22] However, these drugs have not been used in clinical settings. Edaravone (1-phenyl-3-methyl-5-pyrazolone) is the first free radical scavenger used in clinical practice in Japan, where it has been used to treat acute cerebral infarction.[23] Edaravone not only inhibits hydroxyl radicals but also ameliorates iron-induced peroxidative injury.[24] There have been some studies showing the effects of edaravone on the inner ear disease.[25,26,27] Takemoto et al.,[28] reported that preexposure by perilymphatic application of edaravone reduced NIHL in guinea pigs.
Thus, in the present study, we administered edaravone to guinea pigs before and after noise exposure, and we investigated the advantages of the slow-release effects of edaravone SLNs for hearing function and hair cell protection. In addition, at various time points after noise exposure we measured levels of ROS in the cochlea to observe the capacity for the free radical scavenging of edaravone solution and edaravone SLNs, as measured by electron spin resonance (ESR) technology.
METHODS
Animals
Ninety-six adult female albino guinea pigs (250–300 g) were obtained from the Central Laboratory of the Naval General Hospital in Beijing, China. This study was performed in accordance with the Public Health Service Policy on the Humane Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee of our institution approved the animal use protocol. All the animals were anesthetized with a mixture of 40 mg/kg ketamine and 1 ml/kg xylazine administered intramuscularly. The animals were placed on the operating table with a heated pad to maintain a rectal temperature of 37 ± 1°C.
The guinea pigs were divided into five groups: Group A, animals exposed to noise and not treated with drugs; Group B, animals exposed to noise and treated with edaravone solution by intravenous injection; Group C, animals exposed to noise and treated with edaravone solution by intratympanic injection; Group D, animals exposed to noise and treated with edaravone SLNs by intravenous injection; and Group E, animals exposed to noise and treated with edaravone SLNs by intratympanic injection.
Noise stimulation
All the exposures were conducted in an inhalation chamber that consisted of a round glass cage (30 cm × 70 cm). For noise exposure, we used the collected stationary noise of a naval vessel, and the main energy of the noise was distributed at 0.25–4 kHz, with the main peak at 500 and 1,000 Hz. The signal was amplified with a power amplifier (Panasonic SA-DV150) and was delivered via stereo speakers (T and T, 8 naval W, Technology of England). The animals were exposed to 110 ± 1 dB sound pressure level (SPL) for 2 h/d for a total of 4 days. Three animals were exposed at the same time, and each of the animals was placed in a separate cage and allowed free access to food and water throughout the exposure. The sound levels were calibrated and measured with a volume level meter at multiple locations within the sound chamber, to ensure uniformity of the stimulus.
Pharmacological protocol
After noise exposure on day 1, the animals were immediately anesthetized. Then, their acoustic vesicles were exposed through a sterile retroauricular incision, and a 0.15 mm × 0.15 mm hole was drilled into each acoustic vesicle, through which was injected 0.1 ml of edaravone solution or SLNs into the middle ear. Both ears of each guinea pig were injected. After drug delivery, the holes were sealed with bone wax, and the animals lay face up for 2 hours. The holes were smeared with erythromycin ointment to prevent infection. Intravenous injection was performed through a unilateral femoral vein, at the same dosage as the intratympanic injections.
Auditory assessment
Click-induced auditory brainstem response (ABR) was measured for both ears of all of the guinea pigs 1 day before and 1, 4, and 6 days after noise exposure and drug delivery. The animals were lightly anesthetized with a mixture of 40 mg/kg ketamine and 1 ml/kg xylazine, administered intramuscularly prior to ABR measurement, and they were lightly restrained in a wooden tube during the recording procedure. Differential needle electrodes were placed subcutaneously below the test ear (reference) and at the vertex (active). A ground electrode was positioned below the contralateral ear. The sound stimulus consisted of a 15 ms tone burst. The sound intensity varied in 5 dB intervals near the threshold. One thousand and twenty-four tone presentations given at a rate of 12.5/s, were averaged using a microcomputer and custom software to obtain a waveform. The hearing threshold was defined as the lowest stimulus intensity that produced a reliable peak III or IV in ABR waveforms.
ESR measurement
After ABR measurement, the guinea pigs were immediately decapitated, and the bilateral temporal bones, open acoustic capsules, and cochleas were extracted. Then, the blood was quickly washed away with ice water and 10 mmol/L phosphate buffered saline (PBS). The crust of the cochlea was opened under an anatomical microscope and placed in a test tube and deep frozen in liquid nitrogen (LN). Finally, the test tube was placed into a resonant cavity for testing. The test conditions were as follows: Microwave frequency X-wave band (9.45 GHz), microwave power 20 mW, modulation frequency 100 kHz, modulation argument 5 Gauss, scanning time 60 seconds, and scanning duration 500 Gauss. Testing below 77 K, scanning one at a time, the acquired signal was amplified by 1.0 × 104 with a computer. The relative value of ROS was defined as the relative altitude of peak II.
Outer hair cell counting
The guinea pigs were decapitated after hearing function measurement, and the temporal bone was obtained from a hole drilled in the apex of the cochlea, opening the round window and oval window and exposing the crista ampullaris at the same time. A solution of 0.5% AgNO3 was perfused into the hole of the cochlea apex three times, and a 4% methanol solution was then infused using the same method. The temporal bone was fixed in the solution for 3 hours. The basal membrane was segregated and exposed to natural light for 1 hour.
Subsequently, the basal membrane was taken under a dissecting microscope, isolated from the whole cochlea, and sealed with glycerin, under an optical microscope to observe the morphology of OHCs and any injury. The light microscope field was ×200, the lossin OHCs were counted from the apex to the base each with a 0.24 mm reticule. The average OHC loss of each 0.24 mm segment was plotted and calculated along the entire length of the cochlea as a cytocochleogram for each group. Statistical differences were evaluated for significance based on the mean and variance data for the OHCs from the apex to base in the cochlea.
Statistical analysis
All data were analyzed statistically by two-way analysis of variance (ANOVA) using the Statistical Package for Social Sciences (SPSS 11.5) software. Data are expressed as mean ± standard deviation (SD) and differences were considered statistically significant when P < 0.05.
RESULTS
Hearing function
The auditory thresholds before noise exposure were essentially equivalent in all of the ears, and there were no significant differences among the groups. Immediately after noise exposure, the average threshold shift was approximately 44 dB SPL. The greatest threshold shift was approximately 50 dB SPL on the 4th day; and at our last observation time point on the 6th day, the ABR threshold still had not recovered to normal and was approximately 50 dB SPL. Noise-induced threshold shifts (TTS), measured 1 day post-noise exposure, were not significantly reduced by treatment with the drug, neither in the local drug delivery groups, in the systematic drug delivery groups, nor in the solution groups or the SLNs groups. NIHL, measured 6 days post-noise, was substantially reduced by treatment with edaravone SLNs administered by intratympanic injection [Figure 1]. Only the edaravone SLNs by local delivery group demonstrated a significant attenuation of the noise-induced threshold shift on the 4th day following exposure.
Figure 1.
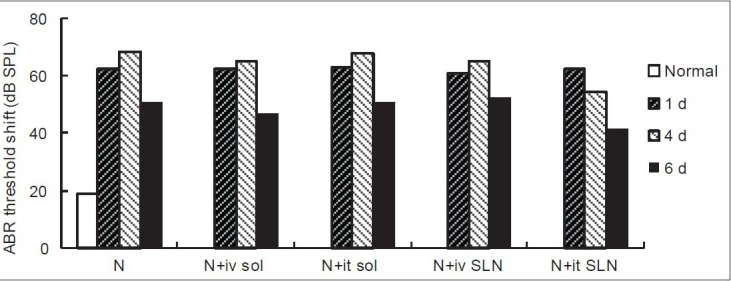
Changes in hearing threshold, measured using auditory brainstem responses in guinea pigs. The animals were divided into five groups: Noise alone (N, control group, N = 6); noise + iv EDA solution (N + iv sol, N = 18); noise + it EDA solution (N + it sol, N = 18); noise + iv EDA SLNs (N + iv SLNs, N = 18); and noise + it EDA SLNs (N + it SLNs, N = 18). Steady state noise was used for 2 h/d for 4 consecutive days. Data are presented as the mean ± SD, and differences were analyzed with ANOVA for repeated measures (two-way), followed by the Student–Newman–Keuls (SNK) test. SPL was statistically different among the time groups after noise exposure to normal animals and statistically different from the drug administered groups compared to normal animals. The sound pressure level of the intravenous injection of edaravone solution, intratympanic injection of edaravone solution, and intravenous injection of edaravone SLNs group remained elevated, while the intratympanic injection of edaravone SLNs group showed some recovery on day 4 and 6. *P < 0.05, P < 0.01. SLN: solid lipid nanoparticle, EDA: edaravone.
Compared to normal animals, the thresholds of all groups at all time points after noise exposure were significant (P < 0.05). Between Group A and C there was no statistically significant difference (P = 0.146); while between Group A and B and GroupD and E, there were statistically significant differences (P < 0.01). Between Group E and the other four groups, there werestatistically significant differences (P < 0.01). Compared to Group B, the hearing threshold of Group C was significantly lower (P < 0.01) and that of Group D was higher (P = 0.039).
ROS in cochleas
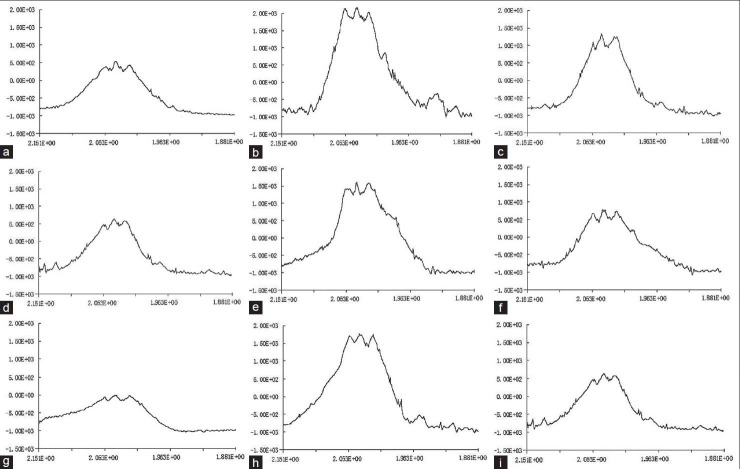
The ESR spectrum of guinea pig cochleas had three main peaks, peak I (e ES II((e E and III (d E [Figure 2]. Peak I was a background peak of resonance, which appeared in all of the specimens for reasons that are unclear, not only in the cochleas,[29] but also in all of the other tissues[30] of animals without peaks found in this site. Peak II was called peak O∥, peak III was called peak O⊥. We could not define the ROS value from peak III precisely because peak coenzyme Q was mixed with this peak, so we choose peak II as the measurement peak to calculate the value of free radicals. We adopted h (cm) to represent the absolute value of ROS, which was calculated by the distance from the crest of the peak to the basal line, and we adopted w (g) to represent the weight of the cochlea, so we represented the relative value of ROS as ΔROS. Thus, ΔROS = h/w (cm/g). The changes in the ΔROS of the cochleas of the five groups are shown in Figure 3.
Figure 2.
ROS spectra of the five animal groups shown in (a) ROS spectra of normal animals’ cochleas. (b-d) ROS spectra of animals at 1, 4, and 6 days after noise exposure. (e) ROS spectra of animals at 1 day after EDA solution (iv). (f) ROS spectra of animals at 1 day after EDA solution (it). (g) ROS spectra of animals at 6 days after EDA SLNs (iv). (h, i) ROS spectra of animals at 1 and 6 days after EDA SLNs (it). ROS = Reactive oxygen species.
Figure 3.
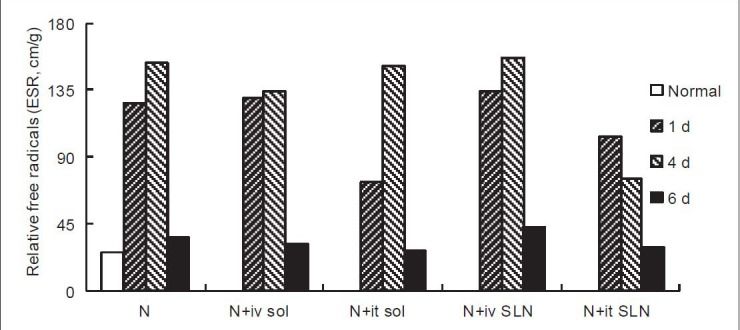
Changes in ROS generation by ESR technology in guinea pigs. Animal grouping, noise exposure methods, and statistical treatment are consistent with Figure 1. ΔROS = Relative value of ROS, ΔROS = h/w (cm/g). ΔROS was statistically different by time after noise exposure compared to normal animals, and statistically different by drug administered group from the noise exposure group. The ROS level of the intravenous injected edaravone solution and intravenous injected edaravone SLNs groups remained elevated on the 1st day, and the intratympanic injected edaravone solution and intravenous injected edaravone SLNs groups remained elevated on the 4th day, while the edaravone SLNs groups showed some recovery on the 6th day. */†P < 0.05, P < 0.01.
Before noise exposure, the ΔROS in the cochlea was approximately 26.52 cm/g, and it immediately increased after noise exposure to 126.39 cm/g. On the 4th day after noise exposure it achieved a maximum of 152.59 cm/g, but on the 6th day, the relative value of ROS was decreased to near the normal level. Compared to group A, ΔROS was significantly lower on the 1st day in Groups C and E, on the 4th day in Groups B and E, and on the 6th day in Groups C and E.
Hair cell loss
Missing hair cells were observed and counted with AgNO3 staining [Figure 4], and the percentages of OHC loss were evaluated as the mean loss for each treatment group [Figure 5]. From the pictures, we can see the inner hair cells (IHC, ed group from the noise exposure group. The ROS level of the intravenous injected edaravone solu↗). But the percentages of OHC loss showed no difference among the groups.
Figure 4.
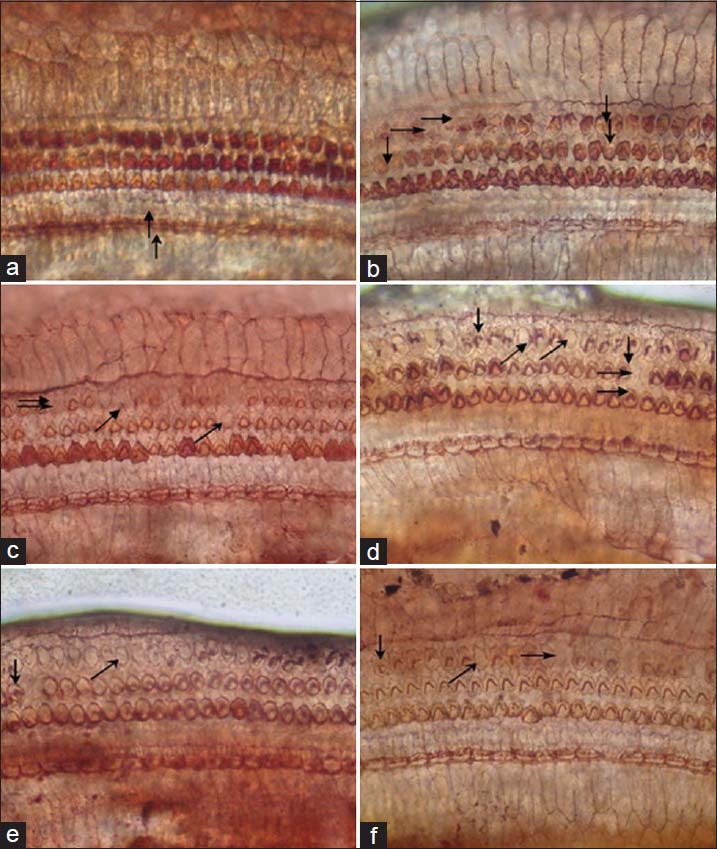
AgNO3 staining in guinea pigs. (a) Hair cells of normal animals. (b-d) Hair cell loss of animals at 1, 4, and 6 days after noise exposure. (e) Hair cell loss of animals on the 6th day after EDA solution (iv). (f) Hair cell loss of animals on the 6th day after EDA SLNs.
Figure 5.

Changes in hair cell loss by AgNO3 staining in guinea pigs. By counting hair cells, we found that the percentages of outer hair cell (OHC) loss showed no difference among the groups.
DISCUSSION
ROS induced inner ear injury primarily occurs via three pathways:[31,32] (1) Oxidizing of lipid molecules and protein molecules by kinds of cellular membranes (CMs), thereby influencing the stability of CMs; (2) Blocking of ion transmission of CMs, leading to disequilibrium of calcium and other ions, further interfering with signal conduction inside and outside the cells; (3) Oxidization of organelles, especially mitochondria, resulting in energy metabolism disturbance, generating more free radicals and resulting in a vicious cycle. All molecular injuries appear morphologically as hair cell changes and finally decrease hearing function.
In comparison to a previous study of edaravone by Japanese researchers, the auditory functional results from this study with edaravone solution did not demonstrate similar protective effects of the drug against exposure to noise. However, edaravone SLNs by local administration showed protective effects on the cochlea from noise exposure. These results demonstrate that edaravone has protective effects on the cochlea against noise exposure, but because intravenous injection cannot achieve a sufficiently high concentration, and edaravone solution cannot be sustained long enough in the inner ear, and these groups could not achieve ideal protective effects.
This study directly detected the changes in ROS in the cochlea after noise exposure, generating evidence of relationships among noise exposure, ROS generation, hearing loss, and hair cell injury. In this study, we did not use free radical capture agents, such as 5,5-dimethyl-1-pyrroline N-oxide (DMPO)[33] or 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide (DEPMPO).[34] We considered that one agent could capture only one corresponding free radical, while noise can induce many kinds of free radicals, so we chose ESR technology without capture agents to assess all of the changes in ROS in the cochlea after noise exposure. In this study, we detected little ROS formation in the cochleas of normal guinea pigs, and the signals of ROS were very weak. After noise exposure, the signal of ROS increased quickly, reached a peak on the 4th day, and decreased to near normal levels by the 6th day. Via drug administration, ROS decreased immediately and significantly, especially in animals given SLNs. Intratympanic administration is better than systematic use and results in slower release of the dosage than from a solution.
Compared to auditory function, the ROS generation in animals on the 1st day following an intratympanic edaravone solution injection was significantly inhibited, similar to the results for the intratympanic edaravone SLN injection. This finding demonstrates that the drug is useful for inhibiting noise-induced reactive oxygen species (ROS) generation in the cochlea; also, the ABR threshold changes were affected not only by ROS injury, but also by some other mechanisms. In this study, the ROS generation in the animals on the 4th and 6th days by intratympanic edaravone solution injection was not inhibited effectively, similar to the results for the animals without drug treatment. This finding demonstrates that SLNs have a detectable slow release effect in intratympanic topical use, and the effects have advantages for inhibiting ROS generation in the cochlea.
In the morphology study, we did not find differences among the noise exposure groups and the drug delivery groups, and we also did not find differences among the two dosages and two delivery methods. We believe that we did not observe protective effects of edaravone on morphology by AgNO3 staining, perhaps because AgNO3 staining was not suitable for calculating the hair cell loss ratio or, because this method could not draw a clear distinction between hair cell loss and cilium disorders. However, it might also be possible that edaravone did not have protective effects against noise-induced hair cell loss. Therefore, our next study will use a well received and efficient morphology method to investigate this surprising result.
CONCLUSIONS
There are relationships among the drug delivery methods, the drug dosage forms, and the protective effects on the cochlea against noise exposure. Our edaravone SLNs demonstrate sustained release of edaravone to the inner ear over a 6-day period by acoustic vesicle injection to the RWM. The edaravone SLNs can inhibit ROS generation in cochleas after noise exposure and decrease hearing thresholds as measured by ABR. These encouraging results support further investigation of SLNs as a novel delivery method for local drug administration to the inner ear.
Footnotes
Edited by: De Wang
Source of Support: This study was supported by a grant from the Capital Health Research and Development of Special Fund (No. 2009-1027).
Conflict of Interest: None declared.
REFERENCES
- 1.Juhn SK. Barrier systems in the inner ear. Acta Otolaryngol Suppl. 1988;458:79–83. doi: 10.3109/00016488809125107. [DOI] [PubMed] [Google Scholar]
- 2.Plontke SK, Siedow N, Wegener R, Zenner HP, Salt AN. Cochlear pharmacokinetics with local inner ear drug delivery using a three-dimensional finite-element computer model. Audiol Neurootol. 2007;12:37–48. doi: 10.1159/000097246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekborn A, Laurell G, Ehrsson H, Miller J. Intracochlear administration of thiourea protects against cisplatin-induced outer hair cell loss in the guinea pig. Hear Res. 2003;181:109–15. doi: 10.1016/s0378-5955(03)00181-3. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Duan M, Lee H, Ruan R, Ulfendahl M. Pharmacokinetics of caroverine in the inner ear and its effects on cochlear function after systemic and local administrations in Guinea pigs. Audiol Neurootol. 2003;8:49–56. doi: 10.1159/000067893. [DOI] [PubMed] [Google Scholar]
- 5.Richardson RT, Wise AK, Andrew JK, O’Leary SJ. Novel drug delivery systems for inner ear protection and regeneration after hearing loss. Expert Opin Drug Deliv. 2008;5:1059–76. doi: 10.1517/17425247.5.10.1059. [DOI] [PubMed] [Google Scholar]
- 6.Salt AN, Plontke SK. Local inner-ear drug delivery and pharmacokinetics. Drug Discov Today. 2005;10:1299–306. doi: 10.1016/S1359-6446(05)03574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuknecht HF. Ablation therapy for the relief of Meniere's disease. Laryngoscope. 1956;66:859–70. doi: 10.1288/00005537-195607000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Endo T, Nakagawa T, Kita T, Iguchi F, Kim TS, Tamura T, et al. Novel strategy for treatment of inner ears using a biodegradable gel. Laryngoscope. 2005;115:2016–20. doi: 10.1097/01.mlg.0000183020.32435.59. [DOI] [PubMed] [Google Scholar]
- 9.Zou J, Saulnier P, Perrier T, Zhang Y, Manninen T, Toppila E, et al. Distribution of lipid nanocapsules in different cochlear cell populations after round window membrane permeation. J Biomed Mater Res B Appl Biomater. 2008;87:10–8. doi: 10.1002/jbm.b.31058. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Lee JE, Baek WY, Lim JO. Regional delivery of vancomycin using pluronic F-127 to inhibit methicillin resistant Staphylococcus aureus (MRSA) growth in chronic otitis media in vitro and in vivo. J Control Release. 2004;96:1–7. doi: 10.1016/j.jconrel.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Salt AN, Stopp PE. The effect of cerebrospinal fluid pressure on perilymphatic flow in the opened cochlea. Acta Otolaryngol. 1979;88:198–202. doi: 10.3109/00016487909137160. [DOI] [PubMed] [Google Scholar]
- 12.Nuttall AL, LaRouere MJ, Lawrence M. Acute perilymphatic perfusion of the guinea pig cochlea. Hear Res. 1982;6:207–21. doi: 10.1016/0378-5955(82)90055-7. [DOI] [PubMed] [Google Scholar]
- 13.Tonndorf J, Duvall AJ, 3rd, Reneau JP. Permeability of intracochlear membranes to various vital stains. Ann Otol Rhinol Laryngol. 1962;71:801–41. doi: 10.1177/000348946207100317. [DOI] [PubMed] [Google Scholar]
- 14.Brown JN, Miller JM, Altschuler RA, Nuttall AL. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear Res. 1993;70:167–72. doi: 10.1016/0378-5955(93)90155-t. [DOI] [PubMed] [Google Scholar]
- 15.Hoffer ME, Kopke RD, Weisskopf P, Gottshall K, Allen K, Wester D. Microdose gentamicin administration via the round window microcatheter: Results in patients with Meniere's disease. Ann N Y Acad Sci. 2001;942:46–51. doi: 10.1111/j.1749-6632.2001.tb03734.x. [DOI] [PubMed] [Google Scholar]
- 16.Heiati H, Phillips NC, Tawashi R. Evidence for phospholipid bilayer formation in solid lipid nanoparticles formulated with phospholipid and triglyceride. Pharm Res. 1996;13:1406–10. doi: 10.1023/a:1016090420759. [DOI] [PubMed] [Google Scholar]
- 17.Mehnert W1, Mäder K. Solid lipid nanoparticles: Production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–96. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 18.Jenning V, Lippacher A, Gohla SH. Medium scale production of solid lipid nanoparticles (SLN) by high pressure homogenization. J Microencapsul. 2002;19:1–10. doi: 10.1080/713817583. [DOI] [PubMed] [Google Scholar]
- 19.Krauel K, Davies NM, Hook S, Rades T. Using different structure types of microemulsions for the preparation of poly (alkylcyanoacrylate) nanoparticles by interfacial polymerization. J Control Release. 2005;106:76–87. doi: 10.1016/j.jconrel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 20.McFadden SL, Ohlemiller KK, Ding D, Shero M, Salvi RJ. The influence of superoxide dismutase and glutathione peroxidase deficiencies on noise-induced hearing loss in mice. Noise Health. 2001;3:49–64. [PubMed] [Google Scholar]
- 21.Harris KC, Bielefeld E, Hu BH, Henderson D. Increased resistance to free radical damage induced by low-level sound conditioning. Hear Res. 2006;213:118–29. doi: 10.1016/j.heares.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Yuki S, Watanabe T, Mitsuka M, Saito KI, Kogure K. Delayed neuronal death prevented by inhibition of increased hydroxyl radical formation in a transient cerebral ischemia. Brain Res. 1997;762:240–2. doi: 10.1016/s0006-8993(97)00490-3. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe T, Yuki S, Egawa M, Nishi H. Protective effects of MCI-186 on cerebral ischemia: Possible involvement of free radical scavenging and antioxidant actions. J Pharmacol Exp Ther. 1994;268:1597–604. [PubMed] [Google Scholar]
- 25.Horiike O, Shimogori H, Ikeda T, Yamashita H. Protective effect of edaravone against streptomycin- induced vestibulotoxicity in the guinea pig. Eur J Pharmacol. 2003;464:75–8. doi: 10.1016/s0014-2999(03)01367-0. [DOI] [PubMed] [Google Scholar]
- 26.Horiike O, Shimogori H, Yamashita H. Effect of edaravone on streptomycin induced vestibul -otoxicity in the Guinea pig. Laryngoscope. 2004;114:1630–2. doi: 10.1097/00005537-200409000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Masuda Y, Tanabe T, Murata Y, Kitahara S. Protective effect of edaravone in inner-ear barotrauma in guinea pigs. J Laryngol Otol. 2006;120:524–7. doi: 10.1017/S0022215106000855. [DOI] [PubMed] [Google Scholar]
- 28.Takemoto T, Sugahara K, Okuda T, Shimogori H, Yamashita H. The clinical free radical scavenger, edaravone, protects cochlear hair cells from acoustic trauma. Eur J Pharmacol. 2004;487:113–6. doi: 10.1016/j.ejphar.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 29.Hirose Y, Sugahara K, Mikuriya T, Hashimoto M, Shimogori H, Yamashita H. Effect of water-soluble coenzyme Q10 on noise-induced hearing loss in guinea pigs. Acta Otolaryngol. 2008;128:1071–6. doi: 10.1080/00016480801891694. [DOI] [PubMed] [Google Scholar]
- 30.Kunz R, Brune HA, Ziegler U, Marzinzig M, Beger HG. Ischemia/reperfusion damage of the liver caused by free radicals-direct radical detection using electron spin resonance (ESR) Langenbecks Arch Chir. 1991;376:139–42. doi: 10.1007/BF00250337. [DOI] [PubMed] [Google Scholar]
- 31.Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol. 1999;4:229–36. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- 32.Lynch ED, Gu R, Pierce C, Kil J. Reduction of acute cisplatin ototoxicity and nephrotoxicity in rats by oral administration of allopurinol and ebselen. Hear Res. 2005;201:81–9. doi: 10.1016/j.heares.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Clerici WJ, Hensley K, DiMartino DL, Butterfield DA. Direct detection of ototoxicant induced reactive oxygen species generation in cochlear explants. Hear Res. 1996;98:116–24. doi: 10.1016/0378-5955(96)00075-5. [DOI] [PubMed] [Google Scholar]
- 34.Bacić G, Spasojević I, Sećerov B, Mojović M. Spin-trapping of oxygen free radicals in chemical and biological systems: New traps, radicals and possibilities. Spectrochim Acta A Mol Biomol Spectrosc. 2008;69:1354–66. doi: 10.1016/j.saa.2007.09.047. [DOI] [PubMed] [Google Scholar]