Abstract
Background:
Food and Drug Administration announcements have highlighted the standard rate of mesh-related complications. We aimed to report the short-term results and complications of tension-free polypropylene mesh (PROSIMA™) surgical repair of pelvic organ prolapse (POP) using the standard category (C), timing (T), and site (S) classification system.
Methods:
A prospective cohort study of 48 patients who underwent PROSIMA™ mesh kit-related surgical repairs were followed for two years at Peking Union Medical College Hospital. Recurrence was defined as symptomatic POP quantification (POP-Q) Stage II or higher (leading edge ≥ −1 cm). The Patient Global Impression of Change Questionnaire, the Chinese version of the Pelvic Floor Impact Questionnaire short-form-7 and POP/Urinary Incontinence Sexual Questionnaire short-form-12 were used to evaluate the self-perception and sexual function of each patient. Mesh-related complications conformed to the International Urogynecological Association/International Continence Society joint terminology. The paired-sample t-test, one-way analysis of variance, Fisher's exact test, Kaplan-Meier survival analysis and log-rank test were used to analyze data.
Results:
All patients were followed up for ≥12 months; 30 (62.5%) patients completed the 24 months study. We observed a 93.8% (45/48) positive anatomical outcome rate at 12 months and 90.0% (27/30) at 24 months. Recurrence most frequently involved the anterior compartment (P < 0.05). Pelvic symptoms improved significantly from baseline (P < 0.05), although the patients’ impressions of change and sexual function were not satisfying. Vaginal complication was the main complication observed (35.4%, 17/48). The survival analysis did not identify any relationship between vaginal complication and anatomical recurrent prolapse (POP-Q ≥ Stage II) (P = 0.653).
Conclusions:
Tension-free polypropylene mesh (PROSIMA™)-related surgical repair of POP has better short-term anatomical outcomes at the apical and posterior compartments, but a low patient satisfaction rate. The mesh complications were not the definitive cause of recurrence.
Keywords: Pelvic Organ Prolapse, Postoperative Complication, PROSIMA™ Mesh Kit, Quality of Life
INTRODUCTION
Pelvic organ prolapse (POP) adversely affects the quality of life (QoL) of parous women.[1,2] Women having high recurrence rates using traditional techniques or native tissue repairs are increasingly likely to choose mesh-related surgical treatments.[3] In the past several decades, various mesh kits or materials have resulted in varying reported success rates. However, mesh safety also gained much attention as Food and Drug Administration announcements have reported mesh-related complications.[4] In 2011, the International Urogynecological Association (IUGA)/International Continence Society (ICS) issued a joint report on those complications arising directly from the insertion of synthetic materials (prostheses) in female pelvic floor surgeries, which standardized the measurement of complications.[5]
The PROSIMA™ pelvic floor repair system is a mesh kit with a nonanchored, tension-free polypropylene mesh and vaginal support device (VSD) that is inserted into the lumen of the vagina at the completion of surgery.[6,7] This kit was reported to advantageously reduce recurrence and complication rates.[8] However, limited results are available regarding the clinical outcomes and complications in Chinese women. Although this mesh kit has been withdrawn from the market by the manufacturer and is not widely used today. The patients’ QoL with this surgery should be considered the most important part of treatment. In addition, there are limited results about the measurement of complications of these surgeries using the standard CTS classification system. The aim of this study was to evaluate the clinical outcomes, the QoL and especially mesh-related complications using the standard CTS classification system in a cohort of women who received total pelvic reconstructive surgical repair with the mesh kit. We also aimed to identify the relationships between the mesh complications and recurrence.
METHODS
Study participants
From July 2010 to July 2012 at Peking Union Medical College Hospital (PUMCH), 48 consecutive patients were enrolled in this study and underwent vaginal repair with mesh kits (PROSIMA™ pelvic floor repair system). All participants provided signed informed consent. This study was approved by the Institutional Ethics Committee at PUMCH. All the surgeries were performed by the same senior urogynecologist. The following inclusion criteria were applied: first-time patients who were predominantly diagnosed with symptomatic Stage III or IV POP using the POP quantification (POP-Q) system.[9] We excluded the patients with genital malignancies or other severe physiological diseases and those with mental illness (Gynecare PROSIMA™ Pelvic Floor Repair System [J and J]).
Study design
The baseline evaluation included standard medical and (uro) gynecological history, urodynamics, a 1-hour pad test when the patient complained of urine leakage, and a 1-hour pad test when the prolapse was reduced. For sexually active women, the Chinese version of the POP/Urinary Incontinence Sexual Questionnaire short-form-12 (PISQ-12) was administered to evaluate sexual function,[10] and the Chinese version of the Pelvic Floor Impact Questionnaire short-form-7 (PFIQ-7), which evaluates three domains (i.e. prolapse, urinary and colorectal symptoms), was used to measure the impact of prolapse on QoL before surgery, as well as the degree of postoperative symptom improvement.[11] The patients underwent the total vaginal mesh procedure with or without concomitant vaginal hysterectomy; the surgical procedure was clearly described in a previous study.[6] The VSD was removed 3–4 weeks after the surgery, and Visual Analog Scales (VAS) were used to evaluate the degree of pain.[12] The indwelling urinary catheter was removed 24 or 48 hours after the operation, and postvoid residual urine was evaluated by scan after the third void. Preoperative data, the operation time, pre- and post-operative complications, blood loss, and postoperative short-term voiding difficulties, were also abstracted.
Patients were consulted regarding postoperative symptoms, and they underwent physical examinations in the clinic at 1 month, 3 months and every 12 months after surgery. Anatomical outcomes were evaluated using the POP-Q system. Prolapse recurrence was defined as symptomatic (a bulge or something falling out that could be seen or felt in the vaginal area) Stage II or higher POP (leading edge ≥ −1 cm). The patients were also telephoned at 24 months to complete three questionnaires; the patient global impression of change (PGI-C) inventory assessed the women's perception of improvement of their prolapse condition using a five-point Likert scale ranging from “much worse” to “much better”,[13] and PISQ-12 and PFIQ-7 were used to measure the subjective perception of symptom improvement. We conformed to the IUGA/ICS joint terminology for mesh-related complications.
Statistical analyses
A paired-sample t-test was used to compare the continuous variables between the groups. Fisher's exact test was used to evaluate nominal variables. One-way analysis of variance (ANOVA) was used to compare the POP measurements between the three groups (i.e. prolapse, urinary and colorectal symptoms) at the pre- and post-operative time points. P ≤ 0.05 was considered as statistical significant. The Kaplan-Meier survival analysis estimated the relapse-free survival rates. The log-rank test compared the complication-free survival rate. SPSS version 16.0 (SPSS Inc., USA) was used to perform the statistical analyses.
RESULTS
All patients were followed for 12 months or much longer (median, 21 months; range, 12–29 months). Thirty (62.5%) patients completed the 24 months follow-up. All patients completed the telephone interview questionnaire at 24 months. The average age was 65.0 ± 5.8 years, the mean course of POP was 5.6 ± 8.8 years, and all patients were POP-Q Stage III or higher. Of the 48 patients, 10 (20.8%) showed stress urinary incontinence (1-hour pad test, 4.30 ± 1.2 g) before surgery. Three (6%) patients had concomitant anti-incontinence surgeries (tension-free vaginal tape obturator [TVT-O]). Eight (16.7%) patients had undergone previous hysterectomy. The patients had a median operation time of 50 minutes with concomitant vaginal hysterectomy and 30 minutes without it. The median blood loss was 50 ml (20–150 ml), and the median hospital stay was 5 days (3–10 days). The postoperative VAS score was 4.0 ± 2.9 at 24 hours and 1.0 ± 1.4 at three or four weeks after surgery [Table 1].
Table 1.
Baseline characteristics of the 48 patients (n = 48)
| Baseline characteristics | Values |
|---|---|
| Age, mean (SD), year | 65.0 (5.8) |
| BMI, mean (SD) | 24.9 (2.6) |
| Pregnancies, median (range) | 3 (2–4) |
| Deliveries, median (range) | 2 (1–3) |
| Course of the disease, mean (SD), year | 5.6 (8.8) |
| Pelvic surgical history, n (%) | 8 (16.7) |
| Average urine flow rate, mean (SD), ml | 14.3 (5.8) |
| Bladder residue urine, n (%) | 14 (29.2) |
| Operation time, median (range), min | |
| Without concomitant vaginal hysterectomy | 30 (20–30) |
| With concomitant vaginal hysterectomy | 50 (30–70) |
| Bleeding, median (range), ml | 50 (20–150) |
| Hospital stay, median (range), day | 5 (3–10) |
| Morbidity, n (%) | 11 (22.90) |
| VSD retention time, median (range), day | 28 (19–55) |
| VAS, mean (SD) | |
| Pain at 24 hours after surgery | 4.0 (2.9) |
| Pain at 3–4 weeks after surgery | 1.0 (1.4) |
SD: Standard deviation; BMI: Body mass index; VSD: Vaginal support device; VAS: Visual analogue scale.
Clinical outcomes
Anatomical outcomes
As shown in Table 2, the POP-Q stages were significantly improved from baseline. The two years follow-up assessment showed a 93.8% (45/48) positive anatomic outcome rate (POP-Q Stage 0, I or II without symptoms) at 12 months and 90.0% (27/30) at 24 months. Surgical repair showed better treatment for the apical and posterior compartments than the anterior at the 12- and 24-month follow-ups (P < 0.05). Three patients had symptomatic recurrences at 12 months after surgery; all recurrences were at the anterior compartment. None of them chose a second surgical intervention.
Table 2.
Pelvic organ prolapse quantification stages by compartment at baseline and at different follow-UPS, (n (%))
| Variables | Preoperative (n = 48) | Postoperative | ||
|---|---|---|---|---|
| 3 months (n = 48) | 12 months (n = 48) | 24 months (n = 30) | ||
| Anterior stage | ||||
| 0 | 41 (85.4) | 23 (47.9) | 10 (33.3) | |
| I | 4 (8.3) | 16 (33.3) | 12 (40.0) | |
| II | 2 (4.2) | 3 (6.3) | 9 (18.8) | 8 (26.7) |
| III | 43 (89.6) | |||
| IV | 3 (6.3) | |||
| Apical stage | ||||
| 0 | 48 (100) | 45 (93.8) | 27 (90.0) | |
| I | 4 (8.3) | 2 (4.2) | 2 (6.7) | |
| II | 4 (8.3) | 1 (2.1) | 1 (3.3) | |
| III | 38 (79.2) | |||
| IV | 2 (4.2) | |||
| Posterior stage | ||||
| 0 | 37 (77.1) | 21 (73.3) | ||
| I | 1 (2.1) | 48 (100) | 10 (20.8) | 8 (26.7) |
| II | 17 (35.4) | 1 (2.1) | 1 (3.3) | |
| III | 25 (52.1) | |||
| IV | 4 (8.3) | |||
| Overall success rate | 48 (100) | 45 (93.8) | 27 (90.0) | |
| Recurrence rate | 3 (6.3)* | 3 (10.0)* | ||
| P | 0.003† | 0.006† | ||
*Patients felt a vaginal bulge and had POP-Q stage ≥ II (leading edge ≥ −1 cm), †Fisher's exact test of the POP-Q stages among the anterior, apical and posterior compartments (POP-Q stage ≥ II vs. POP-Q stage < II). The apical and posterior compartments showed better anatomic treatment outcomes compared to the anterior compartment at the 12- and 24-month follow-ups (P = 0.003, P = 0.006). POP-Q: Pelvic organ prolapse quantification.
Functional outcomes
Twenty-eight (58.3%) patients were satisfied with the postoperative change (PGI-C score 4 or 5), whereas seven (14.6%) patients felt disappointed (PGI-C score 1 or 2). Statistically significant improvements were observed in the symptom scores compared with those at baseline as recorded by the PFIQ-7 (P < 0.05). Univariate one-way ANOVA demonstrated that the greatest symptom improvement occurred in the prolapse domain while the least improvement was noted in the urinary domain. Only a third (7/21) of the patients were sexually active after the surgery, and the PISQ-12 questionnaire did not show significant improvement (P = 0.082) [Table 3].
Table 3.
Symptom scores before and after prosima™ reconstructive pelvic floor surgery (n = 48)
| Variables | Preoperative | Postoperative | P |
|---|---|---|---|
| PGI-C, (n (%)) | |||
| 1 | − | 2 (4.2) | |
| 2 | - | 5 (10.4) | |
| 3 | - | 13 (27.1) | |
| 4 | - | 21 (43.8) | |
| 5 | - | 7 (14.6) | |
| PISQ-12 | |||
| Mean ± SD | 28.6 ± 6.2 | 32.3 ± 6.2 | 0.082* |
| n (%) | 21 (43.8) | 7 (14.6) | |
| PFIQ-7, mean (SD) | |||
| POPIQ-7 | 33.50 (26.6) | 8.40 (17.5) | <0.001* |
| UIQ-7 | 28.80 (28.5) | 10.40 (18.9) | <0.001* |
| CRAIQ-7 | 12.40 (21.2) | 2.00 (7.4) | 0.004* |
| P | 0.002† |
*Paired-sample t-test of the symptom scores before and after surgery; †Univariate one-way ANOVA of the difference between the improvements of POPIQ-7, UIQ-7 and CRAIQ-7. SD: Standard deviation; PGI-C: Patient global impression of change; PISQ: Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire; PFIQ: Pelvic Floor Impact Questionnaire; POPIQ: Pelvic organ Prolapse Impact Questionnaire; UIQ: Urinary Impact Questionnaire; CRAIQ: Colorectal-anal Impact Questionnaire; ANOVA: Analysis of variance.
Postoperative short-term voiding difficulty was observed in 25% (12/48) of the patients (postvoid residual urine measured by bladder scan ≥100 ml or >1/3 of the voiding volume). Catheterization was prolonged from 72 hours to six days. Half of these patients (6/12) reported residual urine ≥100 ml before surgery. None of the patients had concomitant anti-incontinence surgeries (TVT-O). All patients with postoperative voiding difficulty recovered in seven days. Frequency and nocturia were the most frequent complaints. Four cases of de novo urinary incontinence were reported at three (3/4) and 12 months (1/4) after surgery.
Mesh-related complication
Table 4 shows all mesh-related complications that occurred during the follow-up; all shown complications conform to IUGA/ICS joint terminology. Retropubic hematoma (7A) was found in 2/48 (4.2%) patients (3.5 cm × 2.3 cm, 5.4 cm × 2.6 cm) at 48 hours after surgery, but it became asymptomatic and disappeared in seven days. Vaginal complication (C1–C3, mesh contraction or exposure) was the main complication (35.4%, 17/48), but 88.2% (15/17) of these patients were asymptomatic. Of the patients with vaginal complications, 29.4%(5/17) were clinically diagnosed over 12 months (T4) after surgery. The anterior vaginal wall was frequently involved (64.7%, 11/17). One patient complained of dyspareunia whereas the other had spontaneous pain. Patients with vaginal complications and repeated mesh exposure resolved with an exposed mesh excision in the clinic and topical estrogen treatment.
Table 4.
The IUGA/ICS complications classification during the follow-up time (n = 48)
| Patients | Code | Explanation of complications |
|---|---|---|
| 1 | 7A/T1/S3 | Retropubic hematoma (3.5 cm×2.3 cm, first 48 hours) |
| 2 | 7A/T1/S3 | Retropubic hematoma (5.4 cm×2.6 cm, first 48 hours) |
| 3 | 2Aa/T2/S1 | A midline vaginal exposure of mesh (1 cm in anterior wall) at 1-month, asymptomatic |
| 4 | 2Aa/T2/S1 | A midline vaginal exposure of mesh (2 cm×1 cm in anterior wall, 1 cm×1 cm in the posterior wall) at 1-month and 1 cm mesh exposure in the right vaginal wall at 24 months, asymptomatic |
| 2Aa/T4/S2 | ||
| 3Aa/T2/S1 | ||
| 5 | 3Aa/T2/S1 | A midline vaginal exposure of mesh (1.5 cm in posterior wall) at 2 months, asymptomatic |
| 6 | 3Be/T2/S2 | Lateral vaginal exposure of mesh (2 cm×1 cm in vaginal fornix) at 1-month, with spontaneous pain |
| 7 | 1Aa/T3/S2 | Lateral vaginal exposure of mesh (mesh fiber in vaginal fornix) at 12 months, asymptomatic |
| 8 | 2Aa/T3/S2 | Lateral vaginal exposure of mesh (0.8 cm in vaginal fornix) at 11 months, asymptomatic |
| 9 | 2Aa/T3/S1 | A midline vaginal exposure of mesh (0.3 cm in anterior wall) at 3 months, asymptomatic |
| 10 | 2Aa/T3/S1 | A midline vaginal exposure of mesh (0.5 cm in anterior wall, mesh fiber in posterior wall) at 3 months, asymptomatic |
| 1Aa/T3/S1 | ||
| 11 | 2Aa/T3/S1 | A midline vaginal exposure of mesh (1 cm in upper anterior wall) at 6 months, asymptomatic |
| 12 | 2Aa/T3/S1 | A midline vaginal exposure of mesh (0.3 cm in posterior wall) at 12 months, asymptomatic |
| 13 | 3Aa/T3/S2 | Lateral vaginal exposure of mesh (3 cm×0.5 cm in anterior wall) at 6 months, asymptomatic |
| 14 | 3Aa/T3/S1 | A midline vaginal exposure of mesh (2 cm×1.5 cm in anterior wall) at 4 months, asymptomatic |
| 15 | 3Ac/T3/S1 | A midline vaginal exposure of mesh (3 cm in anterior wall and 1/3 mesh in the posterior wall) at 11 months, with dyspareunia and recurrence |
| 16 | 1Aa/T4/S2 2Aa/T4/S1 | Mesh contraction in right anterior wall and 1.5 cm mesh exposure in the middle line of the posterior wall at 13 months, asymptomatic |
| 17 | 1Aa/T4/S1 | Mesh fiber in the posterior wall at 13 months, asymptomatic |
| 18 | 2Aa/T4/S2 2Aa/T4/S1 | Mesh exposure (0.3 cm in the right anterior wall and 1 cm in the middle line of posterior wall) at 14 months, asymptomatic |
| 19 | 1Aa/T4/S1 | Mesh fiber in the anterior wall at 13 months, asymptomatic |
IUGA: International Urogynecological Association; ICS: International Continence Society.
We aimed to identify the relationship between vaginal complication (mesh exposure) and the degeneration of POP-Q stage. We found that the anterior compartment was the compartment most often involved with recurrence and vaginal complications. However, the Kaplan-Meier and log-rank survival analyses did not find any significant relationship between anatomical recurrent prolapse (POP-Q Stage ≥ II) and vaginal complications (P = 0.653) [Figure 1].
Figure 1.
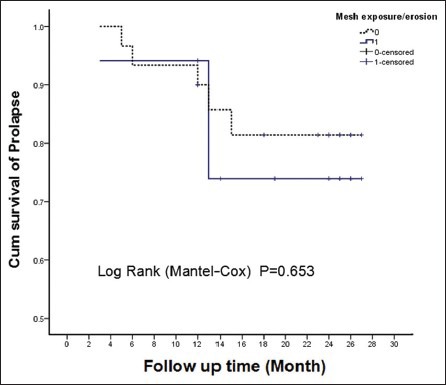
The Kaplan-Meier survival curve of prolapse-free survival rate and a log-rank test of the complication-free survival rate during the longest follow-up time.
DISCUSSION
The PROSIMA™ mesh kit was designed by Carey et al.[6] to reinforce the vaginal repair procedure with mesh and to support the vagina with the VSD for four weeks to reduce the intra-abdominal pressure that may adversely affect healing after the procedure, which could lead to surgical failure and recurrent prolapse.[14] Although this mesh kit has been frequently used in pelvic reconstructive surgeries over the past decade, there have been few reports on the efficacy and safety of the kit in China. This mesh kit is not widely used today. Patients’ QoL should not be overestimated or overlooked after this surgery. In this study, this tension-free mesh-related surgical repair appeared to have better short-term anatomic outcomes for advanced POP in the apical and posterior compartments than in anterior compartment, but with a high rate of complications. The low VAS scores of the patients can be interpreted as an advantage of the tension-free mesh. The overall cure rate appeared to be better than the one year results reported by Withagen et al. (86.9%), Carey et al. (85.0%), and Sayer et al. (84.5%)[6,14,15] but aligned with those of Fan et al.[16] PROSIMA™ mesh-related surgical repair is clearly a better treatment for the apical and posterior compartments. Recurrence frequently involved the anterior compartment, which is consistent with transvaginal prolapse repair studies that have established this compartment as the most vulnerable to recurrent prolapse.[17,18] We might also explain this phenomenon by the inclusion of patients with predominantly advanced anterior compartment prolapse before the surgery. The free tension of this mesh kit may be another defect that provides better and stronger long-term support for the anterior compartment. However, this report had a short duration with unknown long-term results. Compared with the improvement of anatomic outcomes, we did not identify equal improvements in self-perception of the patients based on the PGI-C questionnaire, which should be more important than objective improvement. Only half of the patients were satisfied with the change after surgery. Bothersome symptoms included repeated mesh exposure (7/48), recurrence (3/48), de novo urinary incontinence (4/48), voiding dysfunction (3/48) and urinary frequency or urgency (2/48). However, none of these needed surgical intervention. We recommended these patients are given more care, longer follow-up time, shorter follow-up span and regular topical estrogen application.
The complications of mesh-related surgical repair are important issues that have recently gained much attention. Many reports of mesh exposure have been released, but few have conformed to the IUGA/ICS joint terminology, which clearly describes the category, time and site of complications. The cumulative mesh complication rate in our study was 39.6% (19/48) during our follow-up period. Two postoperative hematoma (7A/T1/S3) complaints disappeared one week after surgery. Seventeen patients had vaginal complications (C1–C3) with no urinary or intestinal tract involvement. The mesh exposure resolved with excision of the exposed mesh and topical estrogen treatment. To date, no infection has been reported. Compared with many other mesh-augmented surgeries, we found that mesh exposure appeared earlier at higher rates in PROSIMA™-related surgical repairs than reported by other systematic reviews (0–7.1% or 10.3%).[19,20] Compared with the posterior and apical compartments, mesh exposure requiring excision was more commonly seen after anterior compartment transvaginal mesh repairs, which was in line with Nguyen et al.[21] As there were no standard measurements in prior studies, direct comparison with our results is inappropriate and hence the difference in mesh exposure between these studies should be interpreted with caution. However, in our unpublished data that evaluated complications after PROLIFT™ mesh kit-related surgical repair of POP, we also identified a much lower vaginal complication rate (10.4%). Patients with low PGI-C scores also complained that repeated mesh excision was painful and annoying. We observed that the anterior compartment was prone to both recurrence and vaginal complications. As patients with vaginal mesh exposure underwent repeated mesh excision, whether these two factors were associated with each other is unknown. However, during our follow-up time, we did not identify any relationship between vaginal complication and the survival rate of anatomical recurrent prolapse nor were there any other potential risk factors. This finding may be attributed to the short follow-up time and limited patient number; therefore, long-term follow-up is still recommended. The high risk of mesh complication may be directly attributable to the unique characteristics of the PROSIMA™ mesh kit. We hypothesize that the long retention time of VSD in the vagina puts pressure on the vagina and results in tissue ischemia, which impedes tissue regeneration and favors mesh exposure. The earlier appearance of mesh exposure supports this hypothesis. A pathomorphology study is needed to confirm this hypothesis.
Few studies have discussed the postoperative short-term voiding difficulty, which directly prolonged hospital stays and increased medical costs. We found postoperative voiding difficulty (postvoid residual urine measured by a bladder scan ≥100 ml or >1/3 of the voiding volume) in 25% (12/48) of the patients. However, the true cut-off value of postoperative short-term voiding difficulty has not been defined by the ICS. Many patients with residual urine ≥100 ml were asymptomatic and recovered rapidly. The definition of postoperative voiding difficulty in this study may be arbitrary, and more study is needed to evaluate this topic. Limited studies have identified potential risk factors for this complication. We recommend that patients with low preoperative average urine flow rates be given sufficient preoperative explanations about this problem. Several studies have found that these patients are prone to postoperative short-term urinary retention.[22,23]
The strengths of this study include the use of a new standard IUGA/ICS classification system. All surgeries were performed by the same surgeon, which controlled for the influence of surgical experience level and technique. The limitations of our study include the short follow-up period, lack of a control group, limited number of patients and the fact that this mesh kit has been withdrawn from the market and is not widely used today.
In conclusion, tension-free polypropylene mesh (PROSIMA™)-related surgical repair of POP has better short-term anatomic outcomes at the apical and posterior compartments. However, this method is related to a low postoperative patient satisfaction rate and a high risk of mesh complication. We did not identify a relationship between the vaginal complication and degeneration of the anatomic outcome in this limited patient cohort. Long-term studies of larger patient cohorts are still needed. Postoperative short-term voiding difficulty should be given more attention because it resulted in prolonged hospital stays and increased medical costs; both factors affect the doctor-patient relationship. Patients’ QoL is often overestimated or overlooked after surgery. Therefore, more attention and care should be given to these patients.
ACKNOWLEDGMENTS
We would like to thank Dr. Xin-Wen Shi for her professional data input.
Footnotes
Edited by: Huan Liu
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Pelvic Floor Impact Questionnaire Short Form: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160–6. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- 2.Hui SY, Chan SC, Lam SY, Lau TK, Chung KH. A prospective study on the prevalence of hydronephrosis in women with pelvic organ prolapse and their outcomes after treatment. Int Urogynecol J. 2011;22:1529–34. doi: 10.1007/s00192-011-1504-2. [DOI] [PubMed] [Google Scholar]
- 3.Chan SS, Cheung RY, Yiu KW, Lee LL, Pang AW, Chung TK. Symptoms, quality of life, and factors affecting women's treatment decisions regarding pelvic organ prolapse. Int Urogynecol J. 2012;23:1027–33. doi: 10.1007/s00192-012-1698-y. [DOI] [PubMed] [Google Scholar]
- 4.Murphy M, Holzberg A, van Raalte H, Kohli N, Goldman HB, Lucente V, et al. Time to rethink: An evidence-based response from pelvic surgeons to the FDA Safety Communication: “UPDATE on Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse”. Int Urogynecol J. 2012;23:5–9. doi: 10.1007/s00192-011-1581-2. [DOI] [PubMed] [Google Scholar]
- 5.Haylen BT, Freeman RM, Swift SE, Cosson M, Davila GW, Deprest J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) and grafts in female pelvic floor surgery. Int Urogynecol J. 2011;22:3–15. doi: 10.1007/s00192-010-1324-9. [DOI] [PubMed] [Google Scholar]
- 6.Carey M, Slack M, Higgs P, Wynn-Williams M, Cornish A. Vaginal surgery for pelvic organ prolapse using mesh and a vaginal support device. BJOG. 2008;115:391–7. doi: 10.1111/j.1471-0528.2007.01606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reisenauer C, Shiozawa T, Huebner M, Slack M, Carey MP. Anatomic study of prolapse surgery with nonanchored mesh and a vaginal support device. Am J Obstet Gynecol. 2010;203:590.e1–7. doi: 10.1016/j.ajog.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Zyczynski HM, Carey MP, Smith AR, Gauld JM, Robinson D, Sikirica V, et al. One-year clinical outcomes after prolapse surgery with nonanchored mesh and vaginal support device. Am J Obstet Gynecol. 2010;203:587.e1–8. doi: 10.1016/j.ajog.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–7. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhu L, Yu S, Xu T, Yang X, Lu Y, Lang J. Validation of the Chinese version of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire short form (PISQ-12) Int J Gynaecol Obstet. 2012;116:117–9. doi: 10.1016/j.ijgo.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Zhu L, Yu S, Xu T, Yang X, Lu Y, Li B, et al. Chinese validation of the Pelvic Floor Impact Questionnaire Short Form. Menopause. 2011;18:1030–3. doi: 10.1097/gme.0b013e31820fbcbe. [DOI] [PubMed] [Google Scholar]
- 12.Aitken RC. Measurement of feelings using visual analogue scales. Proc R Soc Med. 1969;62:989–93. [PMC free article] [PubMed] [Google Scholar]
- 13.Wren PA, Janz NK, Brubaker L, Fitzgerald MP, Weber AM, LaPorte FB, et al. Reliability of health-related quality-of-life measures 1 year after surgical procedures for pelvic floor disorders. Am J Obstet Gynecol. 2005;192:780–8. doi: 10.1016/j.ajog.2004.10.603. [DOI] [PubMed] [Google Scholar]
- 14.Withagen MI, Milani AL, den Boon J, Vervest HA, Vierhout ME. Trocar-guided mesh compared with conventional vaginal repair in recurrent prolapse: a randomized controlled trial. Obstet Gynecol. 2011;117:242–50. doi: 10.1097/AOG.0b013e318203e6a5. [DOI] [PubMed] [Google Scholar]
- 15.Sayer T, Lim J, Gauld JM, Hinoul P, Jones P, Franco N, et al. Medium-term clinical outcomes following surgical repair for vaginal prolapse with tension-free mesh and vaginal support device. Int Urogynecol J. 2012;23:487–93. doi: 10.1007/s00192-011-1600-3. [DOI] [PubMed] [Google Scholar]
- 16.Fan HL, Chan SS, Cheung RY, Chung TK. Tension-free vaginal mesh for the treatment of pelvic organ prolapse in Chinese women. Hong Kong Med J. 2013;19:511–7. doi: 10.12809/hkmj133948. [DOI] [PubMed] [Google Scholar]
- 17.Whiteside JL, Weber AM, Meyn LA, Walters MD. Risk factors for prolapse recurrence after vaginal repair. Am J Obstet Gynecol. 2004;191:1533–8. doi: 10.1016/j.ajog.2004.06.109. [DOI] [PubMed] [Google Scholar]
- 18.Shull BL. Pelvic organ prolapse: Anterior, superior, and posterior vaginal segment defects. Am J Obstet Gynecol. 1999;181:6–11. doi: 10.1016/s0002-9378(99)70427-8. [DOI] [PubMed] [Google Scholar]
- 19.Shah HN, Badlani GH. Mesh complications in female pelvic floor reconstructive surgery and their management: a systematic review. Indian J Urol. 2012;28:129–53. doi: 10.4103/0970-1591.98453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abed H, Rahn DD, Lowenstein L, Balk EM, Clemons JL, Rogers RG, et al. Incidence and management of graft erosion, wound granulation, and dyspareunia following vaginal prolapse repair with graft materials: a systematic review. Int Urogynecol J. 2011;22:789–98. doi: 10.1007/s00192-011-1384-5. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen JN, Jakus-Waldman SM, Walter AJ, White T, Menefee SA. Perioperative complications and reoperations after incontinence and prolapse surgeries using prosthetic implants. Obstet Gynecol. 2012;119:539–46. doi: 10.1097/AOG.0b013e3182479283. [DOI] [PubMed] [Google Scholar]
- 22.Dawson T, Lawton V, Adams E, Richmond D. Factors predictive of post-TVT voiding dysfunction. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1297–302. doi: 10.1007/s00192-007-0324-x. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Shin SH, Oh MM, Park JY, Lee JG, Bae JH. Factors affecting transient urinary retention after transobturator tape mid-urethral sling surgery for female patients with stress urinary incontinence: a single center experience. Eur J Obstet Gynecol Reprod Biol. 2013;168:107–11. doi: 10.1016/j.ejogrb.2012.12.013. [DOI] [PubMed] [Google Scholar]


