Abstract
Background:
Hydrothorax, as one of the common complications of malignant tumors, still cannot be sensitively detected in clinical practice, thus requiring a sensitive, specific method for diagnosis. The aim of this study was to analyze the correlation between levels of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) in patients with benign and malignant hydrothorax.
Methods:
The contents of VEGF in the pleural effusion and serum of the patients with malignant pleural effusion (n = 35) and benign pleural effusion (n = 30) were detected by double antibody sandwich enzyme linked immunosorbent assay. The gene copy number level of EGFR in pleural effusion was detected by fluorescence in situ hybridization (FISH). The points with the highest sensitivity and specificity were selected as the critical values to calculate the diagnostic value of the VEGF in pleural effusion and serum, and EGFR gene copy number in pleural effusion.
Results:
The contents of VEGF in pleural effusion and serum of patients with malignant hydrothorax were (384.91 ± 120.18), and (129.62 ± 46.35) ng/L, respectively, which were significantly higher than those of the patients with benign hydrothorax (207.97 ± 64.04), (63.49 ± 24.58) ng/L (P < 0.01). The sensitivity and specificity of detecting VEGF in pleural effusion were 80.0% and 96.7% (the boundary value was 297.06 ng/L), respectively for diagnosing benign and malignant hydrothorax. The sensitivity and specificity of serum were 74.3% and 96.7%, respectively (the boundary value was 99.21 ng/L) for diagnosing benign and malignant hydrothorax. The diagnostic efficiencies of EGFR and VEGF in hydrothorax were similar. There was a significant correlation between EGFR and VEGF in hydrothorax (P < 0.01).
Conclusions:
VEGF and EGFR play important roles in the formation of pleural effusion. VEGF differed significantly in benign and malignant pleural effusions, which contributed to differential diagnosis results of benign and malignant pleural effusions. It is feasible to detect the gene copy number of the pleural effusion cell mass EGFR by FISH technique. Joint detection can improve the diagnostic sensitivity.
Keywords: Enzyme-linked Immunosorbent Assay, Epidermal Growth Factor Receptor, Fluorescence In Situ Hybridization, Hydrothorax, Vascular Endothelial Growth Factor
INTRODUCTION
Hydrothorax is one of the most common complications of malignant tumors, particularly the thoracic tumors, and also the primary cause of initial diagnosis of many patients.[1] Meanwhile, hydrothorax is also common in other non-neoplastic diseases, such as pleural inflammations, tuberculosis, and systemic connective tissue diseases.[2] It is still difficult to identify benign and malignant hydrothorax with current clinically available routine examinations. Cytologic examination of hydrothorax is highly specific, but the sensitivity is far from satisfactory. An experimental technique with both high sensitivity and specificity is urgently required to assist the diagnosis of hydrothorax.[3,4] Currently, fluorescence in situ hybridization (FISH) detection of the epidermal growth factor receptor (EGFR) gene has been widely carried out in China, but it is frequently used for biopsy tissues or surgical specimens. Detection of small specimens such as sputum and pleural effusion remains challenging. Pleural effusion detection will be considered for patients with unavailable pathological tissues.[5,6] Therefore, the aim of this study was to discuss the role of VEGF and EGFR in hydrothorax and their correlation.
METHODS
Subjects
Among the patients with hydrothorax hospitalized in Department of Respiratory Medicine, Affiliated Hospital of Inner Mongolia Medical University from November 2011 to October 2013 and receiving initial diagnosis and treatment, 35 patients of malignant pleural effusions, who were diagnosed with malignant tumors, were subjected to examination of cast-off cells in pleural effusion, biopsy sampling with a fiber bronchoscope, lymph node puncture, percutaneous lung biopsy, and thoracoscope. Thirty patients with benign hydrothorax who were clinically diagnosed as tuberculous pleuritis or parapneumonic effusion received anti-inflammatory or antituberculous treatments. The hydrothorax was well-controlled based on the follow-up results.
Collection of samples
Fasting venous blood (4 ml) was drawn from each of the patients with benign and malignant hydrothorax in the morning, and cryopreserved in a cryogenic refrigerator (-80°C) for detection after the serum was separated. Fresh pleural effusion was obtained by thoracocentesis, centrifuged, and placed in an automatic hydroextractor for dehydration into paraffin cell mass. Cellular morphology and distribution were observed by hematoxylin-eosin (HE) staining after slicing. The paraffin specimens all showed complete morphologies without any obvious overlap.
Detection of VEGF with ELISA
Antibody sandwich enzyme-linked immunosorbent assay (ELISA) was performed in accordance with the instructions of the kit (Mabtech AB, Sweden). An automatic microplate reader (Mabtech AB, Sweden) was used to measure the absorbance at 450 nm. The concentration (ng/L) of VEGF in the specimen was calculated based on the standard curve plotted by the computer.[7]
Detection of gene copy number of EGFR
The cells in pleural effusion were subject to FISH detection after being centrifuged by using a FISH test kit (Beijing GP Medical Technologies, Ltd., Beijing, China). The human No. 7 chromosome centriole probe exhibits green fluorescence due to fluorescein isothiocyanate labeling, and the EGFR probe exhibits red fluorescence due to rhodamine labeling.
Statistical treatment
SPSS 19.0 (IBM, USA) was used for data processing. The measurement data were mostly expressed as mean ± standard deviation (SD). An independent-sample t test was conducted and then a q test was conducted. The difference was considered as statistically significance when P < 0.05. A paired Chi-square test was conducted for the enumeration data to calculate the difference between the two detection methods. The optimal sensitivity and specificity points were selected as the critical values through the area under the receiver operating characteristic (ROC) to calculate the sensitivity, specificity, positive predictive value, and negative predictive value in diagnosing benign and malignant tumors with VEGF and EGFR.
RESULTS
General clinical data
There were 20 males and 15 females in patients with malignant pleural effusion, the mean age was 45.00 ± 8.00 years old (range from 25 to 62 years old); and there were 17 males and 13 females in patients with benign pleural effusion, the mean age was 66.00 ± 7.00 years old (range from 41 to 76 years old). The gender and age between two groups were not significantly different (P > 0.05). In 35 patients with malignant pleural effusion, 16 had adenocarcinoma, 14 had squamous carcinoma, two had mesothelium, two had lymphoma, and one had malignant pleural effusion from carcinoma of unknown primary site.
Results of detecting pleural effusion and serum VEGF with ELISA
VEGF could be detected in the pleural effusions and serum of all patients, but the levels were different. The content and ratio of VEGF in the malignant pleural effusion and serum were higher than those in the benign group (P < 0.05). The concentration was 384.91 ± 120.18 ng/L (range 114.64–617.01 ng/L) in the pleural effusion and 129.62 ± 46.35 ng/L (range 61.52–279.24) in the serum of patients with malignant hydrothorax, while the concentration was 207.97 ± 64.04 ng/L (range 115.35–391.47.6 ng/L) in the pleural effusion and 63.49 ± 24.58 ng/L (range 19.58–101.25 ng/L) in the serum of patients with benign hydrothorax. An independent sample t test was conducted to compare the levels of VEGF in the pleural effusion and serum, and the determination results of VEGF in the pleural effusions and serum of two groups showed significant differences (t = 7.549 and 7.324, respectively, all P < 0.01).
An exact probability test was used to discuss the relationship between the level of VEGF in the serum of the patients with malignant hydrothorax, demographical, and clinical pathological characteristics. There was no significant relationship between the positive rate and gender, age, or smoking (P > 0.05). However, the pathological pattern was significant related to the positive test result. The positive rate of adenocarcinoma was higher than that of squamous carcinoma (P < 0.05) [Table 1].
Table 1.
Relationship between the vascular endothelial growth factor and demographical and pathological characteristics in the serum of the patients with malignant hydrothorax
| Items | Number of cases (n) | Positive numbers (n (%)) | χ2 | P |
|---|---|---|---|---|
| Gender | - | 0.45 | ||
| Male | 20 | 16 (80.0) | ||
| Female | 15 | 10 (66.7) | ||
| Age (years) | - | 0.12 | ||
| <60 | 17 | 15 (88.2) | ||
| ≥60 | 18 | 11 (61.1) | ||
| Smoking | - | 1.00 | ||
| Yes | 19 | 14 (73.7) | ||
| No | 16 | 12 (75.0) | ||
| Histopathology | 10.88 | 0.03 | ||
| Adenocarcinoma | 16 | 14 (87.5) | ||
| Squamous carcinoma | 14 | 10 (71.4) |
An exact probability test was used to discuss the relationship between the level of VEGF in the pleural effusion of the patients with malignant hydrothorax, demographical, and clinical pathological characteristics. There was no significant relationship between the positive rate and gender, age, smoking, or pathological pattern (P > 0.05). The positive rate of adenocarcinoma exceeded that of squamous carcinoma, but there was no significant different [P > 0.05, Table 2].
Table 2.
Relationship between the vascular endothelial growth factor and demographical and pathological characteristics in the pleural effusion of the patients with malignant hydrothorax
| Item | Number of cases (n) | Positive numbers (n (%)) | χ2 | P |
|---|---|---|---|---|
| Gender | - | 0.430 | ||
| Male | 20 | 17 (85.0) | ||
| Female | 15 | 11 (73.3) | ||
| Age (years) | - | 0.090 | ||
| <60 | 17 | 16 (94.1) | ||
| ≥60 | 18 | 12 (66.7) | ||
| Smoking | - | 1.000 | ||
| Yes | 19 | 15 (78.9) | ||
| No | 16 | 13 (81.3) | ||
| Histopathology | 9.330 | 0.053 | ||
| Adenocarcinoma | 16 | 14 (87.5) | ||
| Squamous carcinoma | 14 | 11 (78.6) |
Results of FISH detection of the gene copy number of EGFR in the cell mass of pleural effusion
FISH was used to detect the gene copy number of EGFR in the 30 benign pleural effusion specimens. One of the benign pleural effusion specimens was positive and the remaining specimens were negative. In the malignant pleural effusion, 15 results were negative [Figure 1a] and 20 results were positive [Figure 1b and 1c]. The positive detection rate of malignant hydrothorax was 57.14% (20/35). Among the positive specimens, seven exhibited EGFR gene amplification and 13 exhibited high polysomy of EGFR gene. Among the 16 cases of lung adenocarcinoma, four cases (25.0%) exhibited cluster amplification, 10 cases exhibited punctiform amplification (62.5%), and two cases exhibited no amplification. The adenocarcinoma amplification rate was 87.5%. Among the 14 cases of squamous cell lung carcinoma, three cases exhibited EGFR gene cluster amplification (21.4%) [Figure 1d and 1e], three cases exhibited punctiform amplification (21.4%), and eight cases exhibited no amplification (57.1%). The amplification rate of squamous carcinoma was 42.8%. An exact probability test was used to discuss the relationship between the gene copy number of EGFR in malignant pleural effusion and demographic and clinicopathological characteristics. There was no significant relationship between the detection result and gender, age, or smoking (P > 0.05). However, there was a significant difference between pathological pattern and the detection result, and the positive rate of adenocarcinoma (87.5%) by FISH was higher than that of the squamous carcinoma (42.8%) [P < 0.01, Table 3].
Figure 1.
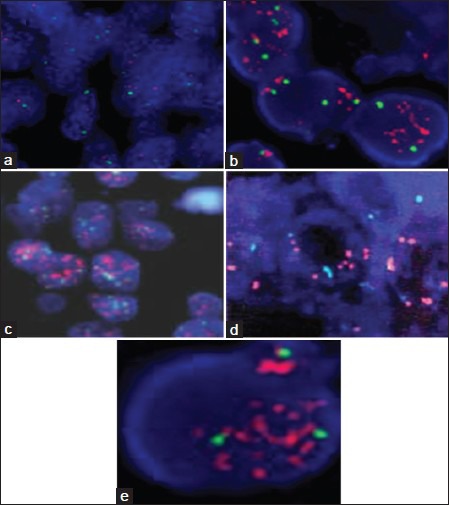
Fluorescence in situ hybridization (FISH) was used to detect the gene copy number of epidermal growth factor receptor (EGFR) in malignant pleural effusion (the red signal was EGFR gene amplification and cluster formation; the green signal was the No. 7 chromosome centromere) (original magnification ×200). (a) Negative result showed no EGFR gene amplification; (b and c) FISH positive detection showed EGFR gene cluster amplification; (d) EGFR FISH positive (high polysomy); (e) EGFR FISH positive (gene amplification).
Table 3.
Relationship between the detection result of epidermal growth factor receptor in the pleural effusion of the patients with malignant hydrothorax and clinical pathological characteristics
| Item | Number of cases (n) | Positive numbers (n (%)) | χ2 | P |
|---|---|---|---|---|
| Gender | - | 0.17 | ||
| Male | 20 | 9 (45.0) | ||
| Female | 15 | 11 (73.3) | ||
| Age (years) | - | >0.05 | ||
| <60 | 17 | 10 (58.8) | ||
| ≥60 | 18 | 10 (55.5) | ||
| Smoking | - | 0.51 | ||
| Yes | 19 | 12 (63.2) | ||
| No | 16 | 8 (50.0) | ||
| Histopathology | 13.85 | 0.01 | ||
| Adenocarcinoma | 16 | 14 (87.5) | ||
| Squamous carcinoma | 14 | 6 (42.9) |
Difference in diagnosis of benign and malignant hydrothorax between VEGF and EFGR in pleural effusion
As shown in Table 4, there was a difference in the diagnosis of benign and malignant hydrothorax between VEGF and EFGR in pleural effusion (χ2 = 3.50, P = 0.06), indicating that there was no difference between both methods for diagnosing benign and malignant hydrothorax. A Spearman's correlation analysis was conducted to calculate the correlation between EGFR and VEGF. There was a significant correlation between EGFR and VEGF in hydrothorax (r = 0.56, P = 0.00). The points with the optimal sensitivity and specificity were selected as the critical values in accordance with the ROC curve to calculate the efficiency of detection of pleural effusion, serum VEGF, and EGFR gene copy number in diagnosing benign and malignant hydrothorax. The sensitivity and specificity of detecting VEGF in pleural effusion were 80.0% and 96.7% (the boundary value was 297.06 ng/L), respectively for diagnosing benign and malignant hydrothorax. The sensitivity and specificity of serum were 74.3% and 96.7%, respectively (the boundary value was 99.21 ng/L) for diagnosing benign and malignant hydrothorax [Table 5].
Table 4.
Difference between vascular endothelial growth factor and epidermal growth factor receptor of pleural effusion for diagnosis of benign and malignant hydrothorax
| VEGF | EGFR | χ2 | P | |
|---|---|---|---|---|
| Positive | Negative | |||
| Positive | 18 | 11 | 3.50 | 0.06 |
| Negative | 3 | 33 | ||
Chi-square test compared two positive results. VEGF: Vascular endothelial growth factor, EGFR: Epidermal growth factor receptor.
Table 5.
Efficiency evaluation for diagnosing benign and malignant hydrothorax with pleural effusion and serum vascular endothelial growth factor, and pleural effusion epidermal growth factor receptor
| Detection indexes | Sensitivity (%) | Specificity (%) | Positive predicted value | Negative predicted value | Negative likelihood ratio |
|---|---|---|---|---|---|
| Pleural effusion VEGF | 80.0 | 96.7 | 0.966 | 0.806 | 24.24 |
| Serum VEGF | 74.3 | 96.7 | 0.963 | 0.763 | 22.52 |
| EGFR gene copy number | 57.1 | 96.7 | 0.952 | 0.659 | 17.30 |
| Pleural effusion VEGF + serum VEGF | 77.1 | 96.7 | 0.964 | 0.784 | 23.36 |
| Pleural effusion VEGF + pleural effusion EGFR | 85.7 | 99.3 | 0.968 | 0.853 | 12.79 |
| Serum VEGF + pleural effusion EGFR | 82.9 | 100 | 1 | 0.833 | - |
| Serum + pleural effusion VEFG + pleural effusion EGFR | 82.9 | 100 | 1 | 0.833 | - |
The joint detection used the multiple process, i. e., it was considered malignant when any of the detection indexes was >critical value; critical value: Pleural effusion VEGF >297.06 ng/L, serum VEGF >96.67 ng/L. VEGF: Vascular endothelial growth factor; EGFR: Epidermal growth factor receptor.
DISCUSSION
Currently, a number of detection approaches are available for identifying the nature of the pleural effusion, such as computed tomography (CT)/positron emission tomography (PET), biochemical criteria (light standard, pH ofpleural effusion), cytologic examinations, tumor markers: Carcino-embryonic antigen (CEA), cancer antigen (CA) 54-9, thyroid transcription factor-1 (TTF-1), neuron-specific enolase (NSE)/telomerase, serum cytokeratin 19 fragment (CYFRA21-1); cytokines: Adenosine deaminase (ADA), tumor necrosis factor alpha (TNF-α), interferon-γ (IFN-γ), interleukin (IL) and their receptors. However, only 7%–12% of the cases with cytologically negative pleural effusion can be diagnosed accurately.[8,9,10] Identification of benign and malignant hydrothorax remains very difficult. Hence, a detection approach with superior performance is required for the identification of pleural effusions.
In this study, we collected the pleural effusions and serum of the patients with benign and malignant hydrothorax to detect the VEGF levels with ELISA. The levels of VEGF in pleural effusion and serum of patients with malignant hydrothorax were significantly higher than those of the benign group (P < 0.01), indicating its overexpression in malignant pleural effusion. This can provide a certain reference for the differential diagnosis of benign hydrothorax. The pleural effusions were made into paraffin samples after being centrifuged. The EGFR gene amplification was detected by FISH. The fluorescence signals were intense under a microscope, showing significant red and green contrast. The cell nucleus had a clear outline after being re-stained with 4’, 6-diamidino-2-phenylindole (DAPI). All these facilitated the microscopic analyses. A relatively noninvasive specimen collection platform was provided for patients who received no definite cytological diagnosis or whose tissue specimens were unavailable by employing surgery or biopsy. The results were of evident clinical significance.[11,12,13,14] Since pleural effusion herein can be obtained easily, it is feasible to detect EGFR gene of the cell mass in the pleural effusion. Its amplification rate was 57.14% in malignant pleural effusion and the diagnosis sensitivity was 57.1%. An elevated tumor marker level usually indicates a malignant effusion. The sensitivity was moderate possibly because, similar to EGFR gene mutation, not all patients with malignant pulmonary tumors underwent EGFR gene amplification.[15,16,17,18,19]
Moreover, the accuracy of diagnostic tests was calculated by the area under the ROC curve. EGFR and VEGF of the pleural effusion had equivalent significance for the identification of benign and malignant hydrothorax (P > 0.05). The sensitivity and specificity of separate detection of serum, pleural effusion VEGF, and pleural effusion EGFR were lower than those of the VEGF and EGFR pairwise detection in pleural effusion. The efficiency of triple joint detection was also lower than that of this pairwise joint detection, suggesting that joint detection of VEGF and EGFR in pleural effusion can elevate the efficiency of diagnosis.[20,21,22] The sensitivity of pairwise joint detection in pleural effusion was higher than that of triple joint detection, which may be associated with the number of samples. The Spearman's correlation analysis showed that there was a significant positive correlation between pleural effusion EGFR and VEGF (P < 0.01), indicating that there may be a negative correlation between serum VEGF and the remaining two indices, thus affecting the sensitivity of the triple joint detection. Further studies need to be conducted to evaluate the molecular biological relationship between EGFR and VEGF in oncogenesis.
In this study, one patient with benign hydrothorax showed FISH positive, which has never been reported hitherto. The possible reasons may included: (1) there were excessive purulent pleural effusion cells. The cells significantly overlapped after slicing of paraffin cell mass, which affected the counting of red signals in the cell nucleus and led to a false negative result; and (2) the patients may have malignant tumors that were not detected. The detection of pleural effusion and lung CT should be observed continuously in follow-up period for this patient.
In conclusion, VEGF and EGFR play crucial roles in the formation of pleural effusion, of which VEGF differs significantly in benign and malignant pleural effusions, contributing to differential diagnosis for malignant or benign diseases. It is feasible to detect the gene copy number of EGFR in pleural effusion cell mass by FISH technique that is superior to amplification refractory mutation system (ARMS) method. VEGF and EGFR in pleural effusion are correlated, so joint detection can improve the diagnostic sensitivity. This method can be applied to the detection of small-scale samples such as sputum and pleural effusion, which provides a possible solution for the patients whose pathological tissues cannot be collected.
Footnotes
Edited by: Xin Chen
Source of Support: This work was supported by a grant from the Scientific Research Project of Institutions of Higher Education (No. NJZY12146).
Conflict of Interest: None declared.
REFERENCES
- 1.Ruiz E, Aleman C, Alegre J, Monasterio J, Segura RM, Armadans L, et al. Angiogenic factors and angiogenesis inhibitors in exudative pleural effusions. Lung. 2005;183:185–95. doi: 10.1007/s00408-004-2533-0. [DOI] [PubMed] [Google Scholar]
- 2.Pathak AK, Bhutani M, Kumar S, Mohan A, Guleria R. Circulating cell-free DNA in plasma/serum of lung cancer patients as a potential screening and prognostic tool. Clin Chem. 2006;52:1833–42. doi: 10.1373/clinchem.2005.062893. [DOI] [PubMed] [Google Scholar]
- 3.Yoon KA, Park S, Lee SH, Kim JH, Lee JS. Comparison of circulating plasma DNA levels between lung cancer patients and healthy controls. J Mol Diagn. 2009;11:182–5. doi: 10.2353/jmoldx.2009.080098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–77. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–90. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Ou W, Yang H, Liu QW, Zhang SL, Wang BX, et al. A randomized phase 2 trial of erlotinib versus pemetrexed as second-line therapy in the treatment of patients with advanced EGFR wild-type and EGFR FISH-positive lung adenocarcinoma. Cancer. 2014;120:1379–86. doi: 10.1002/cncr.28591. [DOI] [PubMed] [Google Scholar]
- 7.Hsu MY, Yang CY, Hsu WH, Lin KH, Wang CY, Shen YC, et al. Monitoring the VEGF level in aqueous humor of patients with ophthalmologically relevant diseases via ultrahigh sensitive paper-based ELISA. Biomaterials. 2014;35:3729–35. doi: 10.1016/j.biomaterials.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 8.American Thoracic Society. Management of malignant pleural effusion. Am J Respir Crit Care Med. 2000;162:1987–2001. doi: 10.1164/ajrccm.162.5.ats8-00. [DOI] [PubMed] [Google Scholar]
- 9.Xia H, Yu CH, Zhang YM, Zhang W, Li YJ, Guo NN. Wedge resection and thermal cautery for malignant pleural effusion caused by lung cancer under vats. Nat Med J China. 2011;91:775–7. [PubMed] [Google Scholar]
- 10.Wu GP, Ba J, Zhao YJ, Wang EH. Diagnostic value of CEA, CYFRA 21-1, NSE and CA 125 assay in serum and pleural effusion of patients with lung cancer. Acta Cytol. 2007;51:679–80. [PubMed] [Google Scholar]
- 11.Guber A, Greif J, Rona R, Fireman E, Madi L, Kaplan T, et al. Computerized analysis of cytology and fluorescence in situ hybridization (FISH) in induced sputum for lung cancer detection. Cancer Cytopathol. 2010;118:269–77. doi: 10.1002/cncy.20094. [DOI] [PubMed] [Google Scholar]
- 12.Voss JS, Kipp BR, Halling KC. Detection of lung cancer in bronchial brushing specimens by FISH. Expert Rev Mol Diagn. 2012;12:679–81. doi: 10.1586/erm.12.69. [DOI] [PubMed] [Google Scholar]
- 13.Liu YZ, Wang Z, Fang LL, Li L, Cao J, Xu X, et al. A potential probe set of fluorescence in situ hybridization for detection of lung cancer in bronchial brushing specimens. J Cancer Res Clin Oncol. 2012;138:1541–9. doi: 10.1007/s00432-012-1232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Shi J, Wang Q, Li H, Cai K, Hou X, et al. Assessment of the pre-clinical immunogenicity of a new VEGF receptor Fc-fusion protein FP3 with ELISA and BIACORE. Cancer Immunol Immunother. 2010;59:239–46. doi: 10.1007/s00262-009-0744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jankowska R, Porebska I, Dyla T. Evaluation of vascular endothelial Growth factor (VEGF) in neoplastic and tuberculosis effusions--preliminary results. Pneumonol Alergol Pol. 2002;70:258–64. [PubMed] [Google Scholar]
- 16.Yatabe Y, Takahashi T, Mitsudomi T. Epidermal growth factor receptor gene amplification is acquired in association with tumor progression of EGFR-mutated lung cancer. Cancer Res. 2008;68:2106–11. doi: 10.1158/0008-5472.CAN-07-5211. [DOI] [PubMed] [Google Scholar]
- 17.Cappuzzo F. EGFR FISH versus mutation: different tests, different end-points. Lung Cancer. 2008;60:160–5. doi: 10.1016/j.lungcan.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Eberhard DA, Giaccone G, Johnson BE. Non-Small-Cell Lung Cancer Working Group. Biomarkers of response to epidermal growth factor receptor inhibitors in Non-small-cell Lung Cancer Working Group: standardization for use in the clinical trial setting. J Clin Oncol. 2008;26:983–94. doi: 10.1200/JCO.2007.12.9858. [DOI] [PubMed] [Google Scholar]
- 19.Chang JW, Liu HP, Hsieh MH, Fang YF, Hsieh MS, Hsieh JJ, et al. Increased epidermal growth factor receptor (EGFR) gene copy number is strongly associated with EGFR mutations and adenocarcinoma in non-small cell lungcancers: a chromogenic in situ hybridization study of 182 patients. Lung Cancer. 2008;61:328–39. doi: 10.1016/j.lungcan.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Gupta R, Dastane AM, McKenna R, Jr, Marchevsky AM. The predictive value of epidermal growth factor receptor tests in patients with pulmonary adenocarcinoma: review of current “best evidence” with meta-analysis. Hum Pathol. 2009;40:356–65. doi: 10.1016/j.humpath.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Sibilia M, Kroismayr R, Lichtenberger BM, Natarajan A, Hecking M, Holcmann M. The epidermal growth factor receptor: from development to Tumorigenesis. Differentiation. 2007;75:770–87. doi: 10.1111/j.1432-0436.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 22.Huang SF, Liu HP, Li LH, Ku YC, Fu YN, Tsai HY, et al. High frequency of epidermal growth factor receptor mutations with complex patterns in non-small cell lung cancers related to gefitinib responsiveness in Taiwan. Clin Cancer Res. 2004;10:8195–203. doi: 10.1158/1078-0432.CCR-04-1245. [DOI] [PubMed] [Google Scholar]


