Abstract
Background:
Hepatocellular carcinoma (HCC) is a common cancer in China, an area of high hepatitis B virus (HBV) infection. Although several staging systems are available, there is no consensus on the best classification to use because multiple factors, such as etiology, clinical treatment and populations could affect the survival of HCC patients.
Methods:
This study analyzed 743 HBV-related Chinese HCC patients who received surgery first and evaluated the predictive values of eight different commonly used staging systems in the clinic.
Results:
The overall 1-, 3-, 5-year survival rates and a median survival were 91.5%, 70.3%, 55.3% and 72 months respectively. Barcelona Clinic Liver Cancer (BCLC) staging systems had the best stratification ability and showed the lowest Akaike information criterion (AIC) values (2896.577), followed by tumor-node-metastasis 7th (TNM 7th) (AIC = 2899.980), TNM 6th (AIC = 2902.17), Japan integrated staging score (AIC = 2918.085), Tokyo (AIC = 2938.822), Cancer of the Liver Italian Program score (AIC = 2941.950), Chinese University Prognostic Index grade (AIC = 2962.027), and Okuda (AIC = 2979.389).
Conclusions:
BCLC staging system is a better staging model for HBV infection patients with HCC in Chinese population among the eight currently used staging systems. These identifications afford a large group of Chinese HCC patients with HBV infection and could be helpful to design a new staging system for a certain population.
Keywords: Chinese Population, Clinical Staging Ssystem, Hepatocellular Carcinoma, Survival
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide with increasing annual incidence worldwide.[1,2,3] Although the incidence of HCC increases in recent years due to hepatitis C virus (HCV) infection, alcohol-related cirrhosis and possibly nonalcoholic fatty liver disease in western countries, more than half of the new cases still occur in China which is an area of high hepatitis B virus (HBV) infection.[4] In spite of many other patterns of treatment, such as radiofrequency, transarterial therapy, chemotherapy or radiotherapy, and so on, are frequently recommended and have been used to treat the HCC patients, surgery is still the main first-treatment to HCC patients in clinic. Clinical staging systems are important which are not only able to provide evidence for disease assessment, but also help to make therapeutic decisions. Staging systems for most solid cancers are available and consistently used in clinic, but the system for HCC is more difficult and remains unknown[5] because there are much biological variability related to different etiologies and incomplete understanding of its natural history.
Currently, eight HCC staging systems used in clinic mainly include two tumor-node-metastasis classification 6th and 7th (TNM),[6,7] the Okuda staging,[8] Cancer of the Liver Italian Program score (CLIP),[9] the Barcelona Clinic Liver Cancer staging system (BCLC),[10] the model for the Chinese University Prognostic Index grade (CUPI),[11] the Japan integrated staging score (JIS)[12] and Tokyo score.[13] Nevertheless, clinical trials and comparative studies revealed that no appropriate staging system could be applied in all the population due to the different etiologies of HCC, clinical performance, and treatments.[14,15,16] Among these staging systems, BCLC and CLIP staging systems had been proved more effective than others in western countries where the main etiologies are HCV infection and alcohol abuse, and for Asian-Pacific region, TNM and JIS staging systems seemed to be more applicable. Recently, CUPI system, the biggest clinical research program focusing Chinese populations, was mainly applied for the HCC patients in late stage and had the complicated calculation method, which might limit its clinical application. Considering the clinic treatment, hepatic resection is the primary treatment method for patients with resectable HCC, who have adequate liver function.[17] In this study, we included 743 HBV-related Chinese HCC patients who received surgery first and evaluated the predictive values of eight different staging systems, which were commonly used in the clinic.
METHODS
Patients
In this study, 743 patients with HCC underwent surgery were recruited from January 1999 to December 2010 at the Hepatobiliary Surgery Center, Cancer Hospital, Chinese Academy of Medical Sciences, Beijing, China. All the patients were confirmed histopathologically by at least two pathologists in our hospital. Characteristics and medical information, including age, sex, etiology of chronic liver disease, pathology results, serum biochemistries, and treatment were obtained from patients’ medical records. The distributions of selected characteristics among patients are shown in Table 1. Overall survival time of patients was measured from the date of surgery to the date of last follow-up or death. Whether and when a patient had died was obtained from inpatient and outpatient records, patients’ families or local Public Security Census Register Office through follow-up telephone calls. The last date of follow-up was December 31, 2011, and no patients were lost to follow-up. Patients alive on the last follow-up date were considered censored. The median follow-up time was 62 months. Written informed consent was obtained from all patients and this study was approved by the Institutional Review Board of the Chinese Academy of Medical Sciences Cancer Institute.
Table 1.
Distributions of Selected Characteristics of 743 Patients with HCC in this Study
| Number (%) | |
|---|---|
| Status | |
| Death | 253 (34.1) |
| Survival | 490 (65.9) |
| Gender | |
| Male | 643 (86.5) |
| Female | 100 (13.5) |
| Age | |
| ≤60 median age | 531 (71.5) |
| >60 | 212 (28.5) |
| Alcohol drinking | |
| Drinker | 153 (20.6) |
| Nondrinker | 590 (79.4) |
| Family history | |
| Yes | 148 (19.9) |
| No | 595 (80.1) |
| Tumor size (cm) (median, range) | 4.1 (0.50-18.0) |
| Numbers of tumor sites | |
| Single | 614 (82.6) |
| Multiple | 129 (17.4) |
| Tumor position | |
| Left | 169 (22.7) |
| Right | 548 (73.8) |
| Caudate lobe | 4 (0.5) |
| Both | 22 (3.0) |
| Serum biochemistry (median, range) | |
| Albumin (g/dl) | 4.18 (3.87-4.48) |
| Bilirubin (μmol/L) | 14.00 (10.50-18.80) |
| AFP (μg/ml) | 30.83 (5.07-505.75) |
| Child-Pugh stage | |
| A | 724 (97.4) |
| B | 19 (2.6) |
| Edmandson stages | |
| I-II | 147 (19.8) |
| III-IV | 596 (80.2) |
| Vascular involvement by pathology | 70 (9.4) |
| Major vascular invasion | 19 (2.6) |
| Regional lymph node metastasis | 7 (0.9) |
| Direct invasion of adjacent organs other than gallbladder or with perforation of visceral peritoneum | 27 (3.6) |
| Surgery methods | |
| Curative hepatectomy | 700 (94.2) |
| Palliative hepatectomy | 9 (1.2) |
| PEI | 15 (2.0) |
| RFA | 19 (2.6) |
| Hepatic inflow occlusion | 110 (14.8) |
| Adjuvant treatment | |
| Yes | 348 (46.8) |
| No | 395 (53.2) |
HCC: Hepatocellular carcinoma; AFP: Alpha-fetoprotein; PEI: Percutaneous ethanol injection; RFA: Radiofrequency ablation.
Treatment
All the 743 patients underwent surgery first in our center. The etiology of HCC of all the patients was HBV infection, whose serological detection of hepatitis B surface antigen was positive. During the surgery process, four types of surgery methods, curative hepatectomy, radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), palliative hepatectomy were given to the patients. Curative hepatectomy and RFA were used to remove or ablate all lesions with a tumor-free margin; palliative hepatectomy and PEI were applied to stanch the blood by tumor rupture or reduce tumor burden. Other adjuvant therapies including transarterial therapy, chemotherapy/target therapy/placebo therapy, or radiotherapy were given to selected patients with preserved liver functions and distant tumor metastasis after surgery.
Classification of staging systems
All the patients were retrospectively assigned to different stages according to the criteria of classification of eight different staging systems, respectively as followed: (1) TNM classification system, (2) the Okuda staging, (3) CLIP, (4) the BCLC, (5) the model for the CUPI, (6) the JIS (7) and Tokyo score.[13] All the classifications were stringently based on the patients’ clinical information.
Statistical methods
For each of the selected staging system, we performed Cox proportional hazards regression adjusted by age and sex to evaluate the association between patients in various stages and their overall survival in different staging systems, respectively. Hazard ratios and their 95% confidence intervals were calculated. Kaplan–Meier survival estimates were plotted, and P values were assessed using log-rank tests. In order to neutralize the potential bias in comparing prognostic scores with different numbers of stages, Akaike information criterion (AIC) was calculated by the results of Cox's regression. The AIC analysis represents an overall assessment of a certain staging system and is the most important statistic method for the comparison across different staging systems. The lower AIC values, the more accurate and informative explained by a certain stage. All the P < 0.05 were considered as statistically significance. All statistical analyses were performed using the packages in R.
RESULTS
Patient characteristics
The characteristics of 743 patients are shown in Table 1. The median age of all the patients was 55 years old (range = 19–85) and the majority of them were male (n = 643, 86.5%). All the patients were receiving surgery as their first clinical treatment: 700 patients (94.2%) received curative hepatectomy, 19 patients (2.6%) received RFA, 15 patients (2.0%) received PEI and 9 patients (1.2%) received palliative hepatectomy, respectively. Considering the treatment after the surgery, 348 patients (46.8%) received adjuvant therapies including transarterial therapy (n = 331), chemotherapy/target therapy/placebo (n = 62) or radiotherapy (n = 17). The median size of the tumor is 4.0 cm, and 614 patients (82.6%) have a single tumor. Vascular involvement was identified in 70 patients (9.4%) by pathology while major vascular invasion was detected in 19 patients (2.6%) by imaging studies. The median AFP value of serum biochemistry was 30.83 μg/ml.
By the last date of follow-up, 253 (34.1%) patients were dead and 490 (65.9%) patients still survived. The overall 1-, 3-, 5-year survival rates and median survival were 91.5%, 70.3%, 55.3% and 72 months.
Comparison of different staging systems
Eight staging systems were used to stratify the HCC patients into different stages, respectively. The comparisons of survival distributions according to different stages are illustrated in Figure 1a (BCLC), Figure 1b (TNM 7th), Figure 1c (TNM 6th), Figure 1d (JIS), Figure 1e (Tokyo), Figure 1f (CLIP), Figure 1g (CUPI), Figure 1h (Okuda), respectively. Significant survival difference was found across all groups of these staging systems (P < 0.05) except for the comparison between TNM 7th Stage IV versus III (P = 0.1574), TNM 7th Stage IV versus III (P = 0.5366), Tokyo score 1 versus 0 (P = 0.3930), Tokyo score 4 versus 3 (P = 0.5691), Tokyo score 5 versus 4 (P = 0.0823), CLIP score 3 + 4 versus 2 (P = 0.9283), Okuda Stage I versus Stage 0 (P = 0.1107), Okuda Stage II versus Stage I (P = 0.7077) [Table 2].
Figure 1.
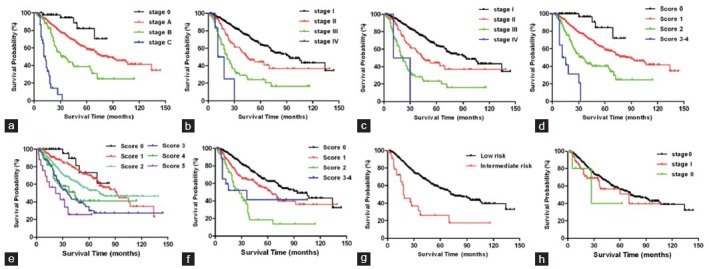
Kaplan–Meier curves by different stages in eight staging systems. (a) Barcelona Clinic Liver Cancer, (b) Tumor-node-metastasis (TNM) 7th, (c) TNM 6th, (d) Japan integrated staging, (e) Tokyo, (f) Cancer of the Liver Italian Program, (g) Chinese University Prognostic Index, (h) Okuda.
Table 2.
Associations of Different Staging Systems with Overall Survival of HCC Patients
| Number (%) | MST | HR (95% CI) | P | AIC value | |
|---|---|---|---|---|---|
| BCLC | 2896.58 | ||||
| 0 | 48 (6.5) | 94 | 1.00 (reference) | ||
| A | 574 (77.3) | 84 | 2.89 (1.19-7.03) | 0.0195 | |
| B | 89 (12.0) | 36 | 2.17 (1.55-3.04) | 6.36×10−6 | |
| C | 32 (4.3) | 13 | 5.49 (3.11-9.68) | 4.19×10−9 | |
| Ptrend | 1.50×10−24 | ||||
| TNM 7th | 2899.98 | ||||
| Stage I | 535 (72.0) | 94 | 1.00 (reference) | ||
| Stage II | 109 (14.7) | 45 | 2.00 (1.43-2.78) | 4.27×10−5 | |
| Stage III | 92 (12.4) | 22 | 2.09 (1.41-3.09) | 0.0002 | |
| Stage IV | 7 (0.9) | 14.5 | 1.95 (0.77-4.94) | 0.1574 | |
| Ptrend | 3.10×10−23 | ||||
| TNM 6th | 2902.17 | ||||
| Stage I | 535 (72.0) | 94 | 1.00 (reference) | ||
| Stage II | 109 (14.7) | 45 | 2.00 (1.43-2.78) | 4.27×10−5 | |
| Stage III | 97 (13.1) | 22 | 2.15 (1.46-3.17) | 0.0001 | |
| Stage IV | 2 (0.3) | 20.5 | 1.56 (0.38-6.47) | 0.5366 | |
| Ptrend | 1.63×10−22 | ||||
| JIS score | 2918.085 | ||||
| 0 | 47 (6.3) | 94 | 1.00 (reference) | ||
| 1 | 539 (72.5) | 90 | 3.56 (1.32-9.61) | 0.0122 | |
| 2 | 142 (19.1) | 35 | 2.28 (1.72-3.03) | 1.36×10−8 | |
| 3+4 | 15 (2.0) | 13 | 2.99 (1.54-5.80) | 0.0012 | |
| Ptrend | 3.97×10−18 | ||||
| Tokyo score | 2938.82 | ||||
| 0 | 32 (4.3) | 94 | 1.00 (reference) | ||
| 1 | 277 (37.3) | 92 | 1.49 (0.60-3.70) | 0.3930 | |
| 2 | 265 (35.7) | 72 | 1.46 (1.07-2.00) | 0.0173 | |
| 3 | 111 (14.9) | 36 | 1.77 (1.26-2.48) | 0.0009 | |
| 4 | 41 (5.5) | 44 | 0.86 (0.51-1.45) | 0.5691 | |
| 5 | 17 (2.3) | 22 | 1.96 (0.92-4.18) | 0.0823 | |
| Ptrend | 4.70×10−12 | ||||
| CLIP score | 2941.95 | ||||
| 0 | 429 (57.7) | 94 | 1.00 (reference) | ||
| 1 | 239 (32.2) | 64 | 1.64 (1.25-2.15) | 0.0004 | |
| 2 | 56 (7.5) | 27 | 2.21 (1.49-3.30) | 9.30×10−5 | |
| 3+4 | 19 (2.6) | 36 | 0.97 (0.45-2.07) | 0.9283 | |
| Ptrend | 6.94×10−12 | ||||
| CUPI | 2962.03 | ||||
| Low risk | 708 (95.3) | 74 | 1.00 (reference) | ||
| Intermediate risk | 35 (4.7) | 18 | 3.36 (2.14-5.28) | 1.34×10−7 | |
| Okuda | 2979.39 | ||||
| Stage 0 | 673 (90.6) | 72 | 1.00 (reference) | ||
| Stage I | 64 (8.6) | 70 | 1.41 (0.92-2.16) | 0.1107 | |
| Stage II | 6 (0.8) | 27 | 1.33 (0.30-5.81) | 0.7077 | |
| Ptrend | 0.0685 |
MST: Median survival time. HR (95% CI) and P were calculated using Cox proportional hazards regression adjusted by age and sex and the lower stage or score was as the reference when the risk progressed from lower stage or score to the higher one. AIC: Akaike information criterion was calculated using the additive model of each staging system; HCC: Hepatocellular carcinoma; HR: Hazard ratio; CI: Confidence interval; BCLC: Barcelona clinic liver cancer; TNM: Tumor-node-metastasis; JIS: Japan integrated staging; CLIP: Cancer of the Liver Italian Program; CUPI: Chinese University Prognostic Index.
Using additive model, all the staging systems except Okuda staging system stratified overall survival rates of HCC patients and furthermore, we measured the AIC values from Cox's regression analysis to compare the major HCC staging systems [Table 2]. BLCL staging systems showed the lowest AIC values (2896.577), followed by TNM 7th (AIC = 2899.980), TNM 6th (AIC = 2902.17), JIS (AIC = 2918.085), Tokyo (AIC = 2938.822), CLIP (AIC = 2941.950), CUPI (AIC = 2962.027), and Okuda (AIC = 2979.389).
DISCUSSION
A superior liver cancer staging system was still needed to divide patients clearly into different groups based on their risk factors, objective, quantitative data, and simple calculation methods. Therefore, clinical doctors can easily make a decision on further treatment or recruitment of clinical research based on these factors and predict patients’ prognosis. The discrepancies of HCC staging systems among studies might be attributed to several factors that potentially influenced results, including tumor related variables, etiologies, first-line treatment, different populations, samples sizes, and so on. It has been demonstrated that HBV-related HCC and HCV-related HCC have different mechanisms in hepatocarcinogenesis and clinical manifestations and the relation between the etiologies of HCC and its prognosis remains controversial. The aim of this study is to find a superior staging system for predicting survival of patients with HCC after surgery for HBV-related Chinese populations. Two strengths of our study are that all the patients were HBV-infected and received the same treatment model of surgery as their first-line treatment. We compared eight different staging systems for 743 Chinese HCC patients who are all HBV infection. All staging systems except Okuda staging system stratified overall survival rates of HCC patients and BCLC staging system showed the best prognostic ability for cancer staging in terms of long-term survival outcome.
The BCLC staging classification has been widely accepted for stratification of HCC patients in clinical practice guidelines, as it not only incorporates Child-Pugh stages, presence of portal hypertension, tumor morphology, and performance status as variables but has also been validated by renowned scientific societies as a useful tool able to differentiate the prognosis of HCC patients.[18,19,20] The prominent character of BCLC staging is that it includes evidence-based clinical treatment, and its surgical indications have also been recognized as a guideline for surgical treatment of HCC in a number of countries.[21] BCLC has been externally validated more effective than others in western countries where main etiologies are HCV infection and alcohol abuse[22] and for Asia area, some studies also proved it be a superior staging system. Recently, a study of 1717 HCC candidates (72.1% for HBV related-HCC) showed BCLC was the best prognostic model in a large-scale Korean cohort.[23] In our study, BCLC proved to be the best staging system for HBV-related HCC (AIC = 2896.58) as well. Although some studies showed that BCLC staging system performed poorly in predicting the survival of patients with early HCC after liver resection,[24] our study demonstrated that BCLC staging has a superior discriminable ability for early stage HCC between Stage 0 and Stage A (P = 0.0195).
Six other staging systems all have the discriminatory ability for death in long-term survival prediction after surgery. TNM staging system (American Joint Committee on Cancer staging [AJCC]) for HCC has been modified several times to the recent seventh edition in 2009.[7] The major change between the 6th and the 7th AJCC staging system is that the new system imposes heavier prognostic weight on major vascular invasion as a potential predictive factor for poor prognosis.[25] Our results showed that the prognostic performance of TNM 7th and TNM 6th staging system are the second and third best staging system associated with overall survival of HCC patients among all staging systems, respectively. This is consistent with the traditional view that TNM staging is suitable for Asian-Pacific population who received resection.[26] However, the limitation of TNM staging was without the consideration of the category of liver function to classify the patients, which is well accepted as a prominent factor for HCC overall survival and more and more scholars highlighted the importance of liver function factor in HCC patients staging categories. However, all the patients included in our study had relatively well-preserved liver function and could receive surgery as their first clinical treatments, more than 90% of patients under the same curative hepatectomy, so the impact of cirrhosis factor on survival could be minimized. Therefore, it is reasonable two TNM staging systems have better prognostic performances over others. The JIS score is consisted of the Japanese TNM staging and the Child-Pugh Sore and appears to be one of the most promising classification systems in the Asia-Pacific region.[27] It has been noted that the JIS system may be limited in its ability to stratify patients with advanced scores because it uniformly assigns tumor stage and live function. Although JIS score did not show superiority in prediction performance over BCLC staging system in our study, it remains the forth suitable staging system and all the four stages could be clearly stratified (all P < 0.05). Tokyo score was established from the study of consecutive patients with HCC treated by percutaneous ablation in 2005.[13] The advantage of this staging is its simplicity only including albumin, bilirubin, tumor size and tumor number as the variants. The original aim developing this staging system was to evaluate the prognosis of patients at early-stage who receive radical treatment. Our study also showed that Tokyo staging had poor stratification ability for late-stage (sore 3 versus 4; sore 4 versus 5), which was also consistent with its disadvantage in late-stage candidates. CLIP system was established from the study of 435 patients with HCC from 16 Italian institutions.[9] It was widely proven to be a better prognostic model for late-stage HCC population,[28] and a good staging system in some cohort studies from Europe populations,[29] Japanese,[30] populations in middle-east regions,[31] and the region of Taiwan of China,[1] and so on. However, there are still some limitations of CLIP staging: Firstly, the definition of tumor morphology in CLIP staging category is relatively too broad to distinguish staging subgroups correctly due to the development of imaging technology in recent years; secondly, all studies to date showed a high proportion of patients were categorized as CLIP score 0–2,[20] which means its low discriminatory ability for late-stage candidates with CLIP scores of 4–6.[29,32] The results of our study were consistent with previous literature results, only 19 (2.6%) HCC patients were classified into score 3–4 group and did not show discriminatory ability for late-stage candidates with the P value of score 3 + 4 versus score 2 being 0.9283. The CUPI score was developed by the Chinese University in Hong Kong in 2002[11] and the only system used widely for Chinese HCC patients with HBV infection. However, some studies in Chinese populations showed that CUPI had limited efficacy in predicting survival of HCC patients undergoing surgical resection.[33] CUPI score was based on the fifth edition of the TNM, but the TNM 6th and TNM 7th was broadly reported superior in discriminating survival among patients in different stages, so it needs to be further modified. The CUPI staging calculation seems relatively complicated, and it contains only three risk subgroups, and both of them limit its clinical use. In our study, only two CUPI stages, low risk and intermediate risk, were included and patients in late-stage using TNM or BCLC staging systems could not be precisely classified to high-risk group in CUPI system and this relatively decreased the analysis power of our study. Furthermore, only a small proportion (10.4%) of early-stage HCC patients received surgery original enrollment of CUPI study,[11] so its prognostic ability for early HCC was restricted.
The Okuda classification was the first staging system combining tumor size and liver function by Japanese study in 1999.[8] However, as it contains fewer tumor factors and high threshold of bilirubin level, this staging system has been gradually losing favor with the emergency of newer staging systems.[34] Our study has proved that Okuda staging was the only staging system which had no significant prognostic ability in determining overall survival, which was in accordance with previous studies.[26,35]
Our study has several strengths. First, all the HCC patients included in this study were Chinese Han populations with HBV infection that exclude the effect of other etiologies or population heterogeneities on the survival of patients. A homogeneous group of patients may enhance our ability to find a suitable staging system. Second, all the patients received surgical resection as their first clinical treatment which not only provided a correct pathology diagnosis for all the patients but also could decrease the effect of different treatments to the overall survival of HCC patients. Third, we collected more than 20 variables which might associate with overall survival of HCC patients, which help us comprehensively compared eight different staging systems based on the same populations. Fourth, reviewing previous literature, sample volume of 743 cases for homogenous HBV-related HCC ethnic Chinese population is relatively large.
There are also some limitations in our study. Firstly, the proportion of patients in late-stage was relative small which could decrease the analysis power. However, all the patients had the same etiology, and first clinical treatment could help us to identify a suitable staging system in a homogeneous patients. Secondly, this is a single-center experience, and the study design is retrospective, it remains to be warranted to validate the prognostic value of this staging system in other well-designed cohorts.
In all, we identify the BCLC staging system is a better staging model for HBV infection patients with HCC in Chinese population among the eight currently used staging systems. These identifications afford a large group of Chinese HCC patients with HBV infection and could be helpful to design a new staging system for a certain population.
Footnotes
Edited by: Xiu-Yuan Hao
Source of Support: This study was supported by a grant from National Science and Technology Major Project Grant (No. 2008ZX10002-025).
Conflict of Interest: None declared.
REFERENCES
- 1.Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Lee PC, et al. Selecting an optimal staging system for hepatocellular carcinoma: comparison of 5 currently used prognostic models. Cancer. 2010;116:3006–14. doi: 10.1002/cncr.25044. [DOI] [PubMed] [Google Scholar]
- 2.Moore SW, Millar AJ, Hadley GP, Ionescu G, Kruger M, Poole J, et al. Hepatocellular carcinoma and liver tumors in South African children: A case for increased prevalence. Cancer. 2004;101:642–9. doi: 10.1002/cncr.20398. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–23. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Chen TW, Chu CM, Yu JC, Chen CJ, Chan DC, Liu YC, et al. Comparison of clinical staging systems in predicting survival of hepatocellular carcinoma patients receiving major or minor hepatectomy. Eur J Surg Oncol. 2007;33:480–7. doi: 10.1016/j.ejso.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Green F. Liver (including intrahepatic bile ducts) In: Green F, Fleming I, editors. AJCC Cancer Staging Handbook. 6th ed. New York: Springer; 2002. pp. 131–44. [Google Scholar]
- 7.Edge SB, Compton . 7th ed. Chicago: Springer; 2009. AJCC Cancer Staging Manual; p. 237. [Google Scholar]
- 8.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–28. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: The Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–5. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 11.Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–9. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 12.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–15. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 13.Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419–25. doi: 10.1136/gut.2003.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan SL, Mo FK, Johnson PJ, Liem GS, Chan TC, Poon MC, et al. Prospective validation of the Chinese University Prognostic Index and comparison with other staging systems for hepatocellular carcinoma in an Asian population. J Gastroenterol Hepatol. 2011;26:340–7. doi: 10.1111/j.1440-1746.2010.06329.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen CH, Huang GT, Yang PM, Chen PJ, Lai MY, Chen DS, et al. Hepatitis B-and C-related hepatocellular carcinomas yield different clinical features and prognosis. Eur J Cancer. 2006;42:2524–9. doi: 10.1016/j.ejca.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Shiratori Y, Shiina S, Imamura M, Kato N, Kanai F, Okudaira T, et al. Characteristic difference of hepatocellular carcinoma between hepatitis B-and C-viral infection in Japan. Hepatology. 1995;22:1027–33. doi: 10.1016/0270-9139(95)90605-3. [DOI] [PubMed] [Google Scholar]
- 17.Kaibori M, Ishizaki M, Saito T, Matsui K, Kwon AH, Kamiyama Y. Risk factors and outcome of early recurrence after resection of small hepatocellular carcinomas. Am J Surg. 2009;198:39–45. doi: 10.1016/j.amjsurg.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 18.Vitale A, Morales RR, Zanus G, Farinati F, Burra P, Angeli P, et al. Barcelona Clinic Liver Cancer staging and transplant survival benefit for patients with hepatocellular carcinoma: a multicentre, cohort study. Lancet Oncol. 2011;12:654–62. doi: 10.1016/S1470-2045(11)70144-9. [DOI] [PubMed] [Google Scholar]
- 19.Meier V, Ramadori G. Clinical staging of hepatocellular carcinoma. Dig Dis. 2009;27:131–41. doi: 10.1159/000218345. [DOI] [PubMed] [Google Scholar]
- 20.Marrero JA, Kudo M, Bronowicki JP. The challenge of prognosis and staging for hepatocellular carcinoma. Oncologist. 2010;15(Suppl 4):23–33. doi: 10.1634/theoncologist.2010-S4-23. [DOI] [PubMed] [Google Scholar]
- 21.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 22.Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–16. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 23.Kim BK, Kim SU, Park JY, Kim do Y, Ahn SH, Park MS, et al. Applicability of BCLC stage for prognostic stratification in comparison with other staging systems: single centre experience from long-term clinical outcomes of 1717 treatment-naïve patients with hepatocellular carcinoma. Liver Int. 2012;32:1120–7. doi: 10.1111/j.1478-3231.2012.02811.x. [DOI] [PubMed] [Google Scholar]
- 24.Nathan H, Mentha G, Marques HP, Capussotti L, Majno P, Aldrighetti L, et al. Comparative performances of staging systems for early hepatocellular carcinoma. HPB (Oxford) 2009;11:382–90. doi: 10.1111/j.1477-2574.2009.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chun YH, Kim SU, Park JY, Kim do Y, Han KH, Chon CY, et al. Prognostic value of the 7th edition of the AJCC staging system as a clinical staging system in patients with hepatocellular carcinoma. Eur J Cancer. 2011;47:2568–75. doi: 10.1016/j.ejca.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Sirivatanauksorn Y, Tovikkai C. Comparison of staging systems of hepatocellular carcinoma. HPB Surg 2011. 2011:818217. doi: 10.1155/2011/818217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, et al. Validation of a new prognostic staging system for hepatocellular carcinoma: The JIS score compared with the CLIP score. Hepatology. 2004;40:1396–405. doi: 10.1002/hep.20486. [DOI] [PubMed] [Google Scholar]
- 28.Collette S, Bonnetain F, Paoletti X, Doffoel M, Bouché O, Raoul JL, et al. Prognosis of advanced hepatocellular carcinoma: comparison of three staging systems in two French clinical trials. Ann Oncol. 2008;19:1117–26. doi: 10.1093/annonc/mdn030. [DOI] [PubMed] [Google Scholar]
- 29.Llovet JM, Bruix J. Prospective validation of the Cancer of the Liver Italian Program (CLIP) score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;32:679–80. doi: 10.1053/jhep.2000.16475. [DOI] [PubMed] [Google Scholar]
- 30.Ueno S, Tanabe G, Sako K, Hiwaki T, Hokotate H, Fukukura Y, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology. 2001;34:529–34. doi: 10.1053/jhep.2001.27219. [DOI] [PubMed] [Google Scholar]
- 31.Siddique I, El-Naga HA, Memon A, Thalib L, Hasan F, Al-Nakib B. CLIP score as a prognostic indicator for hepatocellular carcinoma: Experience with patients in the Middle East. Eur J Gastroenterol Hepatol. 2004;16:675–80. doi: 10.1097/01.meg.0000108338.41221.ec. [DOI] [PubMed] [Google Scholar]
- 32.Yen YH, Changchien CS, Wang JH, Kee KM, Hung CH, Hu TH, et al. A modified TNM-based Japan Integrated Score combined with AFP level may serve as a better staging system for early-stage predominant hepatocellular carcinoma patients. Dig Liver Dis. 2009;41:431–41. doi: 10.1016/j.dld.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Yang T, Zhang J, Lu JH, Yang LQ, Yang GS, Wu MC, et al. A new staging system for resectable hepatocellular carcinoma: Comparison with six existing staging systems in a large Chinese cohort. J Cancer Res Clin Oncol. 2011;137:739–50. doi: 10.1007/s00432-010-0935-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol. 1999;31:133–41. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 35.Giannini E, Risso D, Botta F, Romagnoli P, Malfatti F, Fumagalli A, et al. Prognosis of hepatocellular carcinoma in anti-HCV positive cirrhotic patients: a single-centre comparison amongst four different staging systems. J Intern Med. 2004;255:399–408. doi: 10.1046/j.1365-2796.2003.01284.x. [DOI] [PubMed] [Google Scholar]


