Abstract
Background:
Calreticulin (CRT) is major Ca2+-binding chaperone mainly resident in the endoplasmic reticulum (ER) lumen. Recently, it has been shown that non-ER CRT regulates a wide array of cellular responses. We previously found that CRT was up-regulated during hypoxia/reoxygenation (H/R) and this study was aimed to investigate whether CRT nuclear translocation aggravates ER stress (ERS)-associated apoptosis during H/R injury in neonatal rat cardiomyocytes.
Methods:
Apoptosis rate and lactate dehydrogenase (LDH) leakage in culture medium were measured as indices of cell injury. Immunofluorescence staining showed the morphological changes of ER and intracellular translocation of CRT. Western blotting or reverse transcription polymerase chain reaction was used to detect the expression of target molecules.
Results:
Compared with control, H/R increased apoptosis rate and LDH activity. The ER became condensed and bubbled, and CRT translocated to the nucleus. Western blotting showed up-regulation of CRT, Nrf2, activating transcription factor 4 (ATF4), CHOP and caspase-12 expression after H/R. Exogenous CRT overexpression induced by plasmid transfection before H/R increased cell apoptosis, LDH leakage, ER disorder, CRT nuclear translocation and the expression of ERS-associated molecules. However, administration of the ERS inhibitor, taurine, or CRT siRNA alleviated cell injury, ER disorder, and inhibited ERS-associated apoptosis.
Conclusions:
Our results indicated that during H/R stress, CRT translocation increases cell apoptosis and LDH leakage, aggravates ER disorder, up-regulates expression of nuclear transcription factors, Nrf2 and ATF4, and activates ERS-associated apoptosis.
Keywords: Apoptosis, Calreticulin, CCAAT/Enhancer-binding Protein Homologous Protein, Endoplasmic Reticulum, Hypoxia/Reoxygenation
INTRODUCTION
Calreticulin (CRT) is a major calcium-binding chaperone that is highly concentrated in the lumen of the endoplasmic reticulum (ER) and is vital to the regulation of protein folding and Ca2+ homeostasis in the ER.[1] Recent studies have shown that non-ER CRT located in the cytosol, cell membrane, and extracellular matrix, regulates a wide array of cellular responses, such as adhesion, migration, apoptosis, phagocytosis, and immunoregulation.[2]
Calreticulin is essential for embryonic cardiac development with the absence being embryonically lethal.[3] After birth, CRT expression in the myocardium decreases; however, during cardiac hypertrophy and heart failure, CRT expression is increased.[4] This profile suggests that CRT may play an important role in the generation of postnatal/adult cardiac pathology.
Our previous work demonstrated that the endogenous protective phenomena, hypoxic preconditioning (HPC) and hypoxia/reoxygenation (H/R) injury, both upregulate CRT expression, but to different magnitudes. Moderate expression of CRT induced by HPC can protect cardiomyocytes from oxidative damage, whereas CRT overexpression induced by H/R induces myocardial damage. Notably, there is a positive correlation between the amount of CRT and the cytoplasm calcium ion concentration.[5] The signaling cascades responsible for this CRT dichotomy have not yet been elucidated. It has been shown that either oxidative damage or a posttranslational modification of CRT can affect both its function and subcellular localization.[2,6] As an inefficient substrate for the proteasome, and thus avoiding the usual ubiquitylation and degradation following translocation from the ER to the cytosol, CRT is the only known mammalian protein that is translocated from the ER without being degraded.[6] Over the past decade, the effects of non-ER CRT located in other parts of the cell, plasma membrane, and extracellular matrix in many physiological processes have been well described, such as cell adhesion, phagocytosis, apoptosis and tumor recognition.[2,6] The exact mechanism underlying the pro-apoptotic effect of non-ER CRT has not been clarified.
We hypothesized that during H/R, CRT is translocated to the nucleus, promoting expression of ER stress (ERS)-associated transcription factors such as Nrf2 and activating transcription factor 4 (ATF4), causing ER damage and subsequent apoptosis. To test this hypothesis, CRT was overexpressed by either plasmid transfection or knockdown by siRNA interference in cardiomyocytes. Results showed that CRT overexpression aggravated its’ nuclear translocation and ERS-associated apoptosis induced by H/R. ERS inhibitor, taurine, and CRT siRNA alleviated its’ translocation and cell injury.
METHODS
Cell culture and experimental protocol
All procedures were performed in accordance with the guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (Publication No. 85-23, revised 1996), and approved by the animal care and use committee of Chinese People's Liberation Army General Hospital. Primary cultures of cardiac myocytes from neonatal Sprague-Dawley rats (born within 24 hours) were prepared as previously described.[7] Briefly, ventricular tissue was enzymatically dissociated, and the resulting cell suspension was enriched. The dispersed cells were preplated for 1.5 hours to minimize fibroblast contamination. Cells were plated at 2.5–3.0 × 105 cells/ml onto poly-D-lysine-coated coverslips, well plates or dishes and cultured in Dulbecco's modified Eagle's medium (Gibco-Invitrogen, Carlsbad, CA, USA) supplemented with 10% neonatal bovine serum (PAA, Linz, Austria), 3.7 g/ml sodium bicarbonate and 100 mg/ml ampicillin. Cardiomyocytes were randomly divided into the following groups for treatment: (1) normal control (C); (2) hypoxia (95% N2-5% CO2) for 8 hours and reoxygenation (95% air-5% CO2) for 16 hours (H/R); (3) overexpression by transfection with pcDNA3.1-CRT for 24 hours prior to H/R (CRT); (4) vector control transfection with pcDNA3.1 for 24 hours prior to H/R (pcDB); (5) stealth RNAi transfection for 24 hours prior to H/R (siCRT); (6) negative control siRNA transfection for 24 prior to H/R (si-neg). 40 mmol/L taurine was administrated just before hypoxia to inhibit ERS. The treatments of the experiment were shown in Table 1.
Table 1.
Experiment Treatments
| Experiment groups | Plasmid/siRNA transfection | Taurine | Hypoxia/reoxygenation |
|---|---|---|---|
| C | - | - | - |
| H/R | - | - | √ |
| CRT | √ | - | √ |
| pcDB | √ | - | √ |
| siCRT | √ | - | √ |
| si-neg | √ | - | √ |
| CRT+tau | √ | √ | √ |
| pcDB+tau | √ | √ | √ |
C: Normal control; H/R: Hypoxia/reoxygenation; CRT: CRT overexpression+H/R; pcDB: pcDB plasmid transfection+H/R; siCRT: CRT siRNA interference+H/R; si-neg: Negative control siRNA+H/R; CRT+tau: CRT overexpression+40 mM taurine+H/R; pcDB+tau: pcDB plasmid transfection+40 mM taurine+H/R. √: The treatment was taken; CRT: Calreticulin.
Plasmid construction
The open reading frame of rattus norvegicus CRT (rCRT) gene deposited in GenBank database (accession number NM_022399) was cloned from a cDNA library of rat spleen by polymerase chain reaction (PCR) with the primers 5’-CCTCGGCCCACCATGCTCC-3’ and 5’-GT GGCCTCTACAGCT CATCC-3’. rCRT full-length gene was linked using pGEM-T Easy (Invitrogen, Carlsbad, CA, USA) and subcloned into pcDNA3.1/Myc-His(-) B (Invitrogen).
Stealth RNAi™ siRNA construction
Calreticulin Stealth RNAi™ siRNA duplexes were designed and synthesized by Invitrogen Co., CA, USA. The sequences were designed to target the rattus norvegicus CRT (rCRT) gene (NM_022399). Sense primer: 5’-UUGGCUUGUCUGCAACCCUUUAUCC-3’. Antisense primer: 5’-GGAUAAAGGGUUGCAGACAAGCCA A-3’).
Cardiomyocyte viability, lactate dehydrogenase leakage, and apoptosis
Cell viability was measured using a Trypan Blue Exclusion test as previously described.[8] Lactate dehydrogenase (LDH) activity in the culture medium was measured by the LDH assay kit (Sigma) according to the kit protocol. The cell apoptosis rate was analyzed by the annexin V-propidium iodide (PI) apoptosis detection kit (KeyGen) according to the manufacturer's instructions, using quantitative fluorescence activated cell sorter (BD FACSCalibur, America).
Immunocytofluorescence staining
Cardiomyocytes were grown on coverslips at a density of 104/cm2 and immunofluorescence was performed as previously described.[9] Briefly, the cells were fixed in prechilled methanol at −20°C for 5 minutes and 4% paraformaldehyde at room temperature for another 15 minutes, the slides were then blocked by the addition of 10% donkey serum in phosphate-buffered saline containing 0.1% triton X-100 for 30 minutes. We identified cells by indirect immunofluorescent staining with an anti-CRT rabbit polyclonal antibody (1:50, Stressgen), and anticoncanavalin A[10] goat polyclonal antibody (1:100, vector laboratories) overnight at 4°C. Then they were stained with TexasRed-conjugated donkey antirabbit (Santa Cruz Biotechnology) and fluorescein isothiocyanate-conjugated donkey antigoat IgG (Santa Cruz Biotechnology). The coverslips were mounted on glass slides with mounting medium and DAPI (Vector Laboratories, Burlingame, CA, USA). Images were obtained under a confocal scanning microscope (Zeiss LSM-510 Meta, Jena, Germany). A ×60 oil immersion objective with a numerical aperture of 1.4 was used.
Isolation of total RNA and reverse transcription polymerase chain reaction (PCR)
The total RNA isolation and reverse transcription PCR involved the use of the EasyScript First-strand cDNA Synthesis Super-Mix Kit (TransGen, Beijing, China) according to the manufacturer's instructions. Primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and CRT were in Table 2. The PCR products were separated on 1.5% agarose gel and photographed for analysis.
Table 2.
Primer Sequences for RT-PCR
| Sequences (forward and reverse) | Product length (bp) |
|---|---|
| CRT | |
| P1:5’-CAAGGATATCCG GTG TAA GGA-3’ | 445 |
| P2: 5’-CAT AGA TAT TCG CAT CGG GG-3’ | |
| GAPDH | |
| P1:5’-TGC TGA GTA TGT CGT GGA G-3’ | 288 |
| P2: 5’-GTC TTC TGA GTG GCA GTG AT-3 |
CRT: Calreticulin; GAPDH: Glyceraldehydes-3-phosphate dehydrogenase; RT-PCR: Real time-polymerase chain reaction.
Separation of cytoplasmic and nuclear extracts
The NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific. Cat No. 78833) were used to separate cytoplasmic and nuclear extracts from cardiomyocytes, according to the manufacturer's instructions.[11] The antibody for Histone H3 (a nucleus control protein, Cell Signaling, Cat No. 9715) and the antibody for Rho guanine nucleotide dissociation inhibitor-α (GDI-α) (H-43) (a cytoplasmic control protein, Santa Cruz, Cat No. sc-292544) were used to verify the effectiveness of separation. The relative level of analyzed nuclear proteins was normalized to that of Histone H3, and the relative level of cytoplasmic proteins was normalized to that of GDI.
Western blotting analyses
Cardiomyocytes were lysed, and protein extraction was performed as previously described.[12] The soluble supernatant of the extracts was determined by the Bradford method, and 16 samples of 50 mg protein were prepared and separated on 10% acrylamide gels. The separated proteins were electrophoretically transferred to nitrocellulose membranes, blocked with 5% bovine serum albumin in tris-buffered saline Tween 20, containing 20 mmol/l tris-HCl (pH 7.6), 137 mmol/l NaCl and 0.1% Tween 20. The membranes were incubated with the antibodies against CRT (1:1000, Stressgen), Nrf2 (1:1000, Santa Cruz), ATF4 (1:1000, Santa Cruz), glucose-regulated protein 78 (GRP78) (1:1000, Santa Cruz), CHOP (1:200, Santa Cruz), caspase-12 (1:1000, Santa Cruz), caspase-3 (1:1000, Santa Cruz), Bax (1:200, Santa Cruz), Bcl-2 (1:200, Santa Cruz) and GAPDH (1:500, Santa Cruz), respectively overnight at 4°C. After incubation for 2 hours with horseradish peroxidase-conjugated secondary antibodies, the reaction was visualized using an enhanced chemiluminescence kit (Santa Cruz Biotechnology). The integrated optical density (mean intensity × area) of proteins was quantified using Image-Pro Plus. The relative level of analyzed protein expression was normalized to that of GAPDH.
Statistical analysis
Each experiment was performed at least in triplicate and cardiomyocytes were pooled from three to four different rat litters. Our data from three to four experiments was pooled and analyzed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA); data are presented as mean ± standard deviation. Differences observed between the two groups were analyzed by two-sample t-tests for independent samples and among groups by one-way analysis of variance with Newman–Keuls posttest analysis. A P < 0.05 was considered statistically significant.
RESULTS
Cardiomyocyte injury represented by cell viability, lactate dehydrogenase leakage, and apoptosis rate
We examined H/R-induced cell injury and the effects of either CRT overexpression or RNA interference. Cell viability was measured using the Trypan Blue Exclusion test, and LDH activity in the culture medium was used to reflect cellular membrane permeability. The cellular apoptosis rate was identified using annexin V-PI double staining and quantified using a BD Biosciences FACSCalibur flow cytometer [Figure 1a]. Compared with control, the cell apoptosis rate and LDH activity in a medium increased in the H/R group [Figure 1c] (P < 0.05), while cell survival rate decreased [Figure 1d]. The CRT over-expression exaggerated H/R injury, and compared with H/R group, apoptosis rate and LDH activity increased [Figure 1b and 1c] (P < 0.05), while cell survival rate decreased [Figure 1d] (P < 0.05). Either siRNA interference or taurine protected H/R injury. Compared with H/R group, apoptosis rate and LDH activity both decreased [Figure 1b and 1c] (P < 0.05), while cell survival rate increased [Figure 1d] (P < 0.05). Either the pcDNA3.1 plasmid (vector) or negative control siRNA transfection showed no significant differences compared with H/R (P > 0.05). To conclude, H/R induced cell apoptosis and LDH leakage, CRT overexpression exaggerated H/R injury, while CRT siRNA and taurine protected H/R injury.
Figure 1.
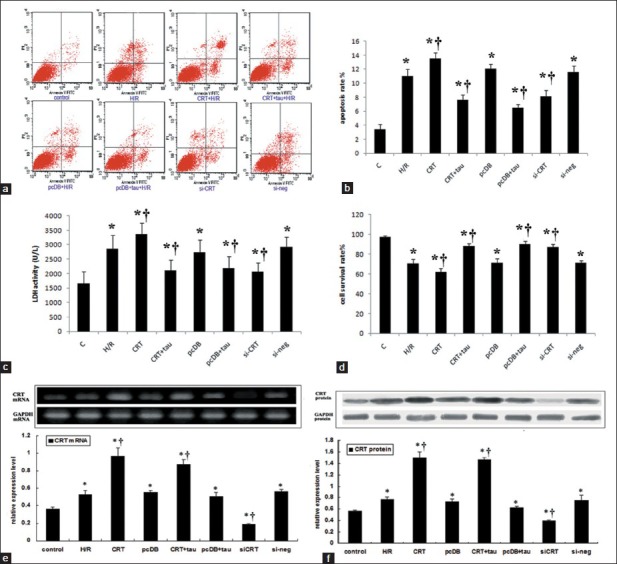
The cell injuries induced by hypoxia/reoxygenation (H/R) and the effects of calreticulin (CRT) over-expression and siRNA interference and taurine administration. Cardiomyocytes were transfected by either pcDNA3.1-CRT or pcDNA3.1 or either stealth siRNA or negative control siRNA for 24 hours, then hypoxia for 8 hours and reoxygenation for 16 hours. 40 mmol/L taurine was administrated prior to hypoxia to inhibit endoplasmic reticulum stress. (a) Images of flow cytometry. (b) The statistical analysis graph of apoptosis rate analyzed by flow cytometry. (c) Lactate dehydrogenase activity of culture medium. (d) cardiomyocyte survival rate. (e) The mRNA expression and the statistical analysis graph of CRT and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (f) The protein expression and the statistical analysis graph of CRT and GAPDH (P < 0.05 vs. control, †P <0.05 vs. H/R). Each experiment had been repeated for 3 times.
mRNA and protein expression of CRT
Reverse transcription polymerase chain reaction and Western blot were used to detect the mRNA and protein expression of CRT [Figure 1e and 1f]. H/R upregulated CRT mRNA and protein expression compared with control (P < 0.05). There was successful overexpression of CRT induced by plasmid transfection compared with vector transfection (P < 0.05). Stealth siRNA transfection both down-regulated CRT expression at mRNA and protein level compared with negative control siRNA transfection (P < 0.05). It indicated that CRT overexpression and knockdown were successful.
Immunocytofluorescence staining
Concanavalin A is a nonspecific ER stain, which could stain the nuclear envelope, the rough ER, and the matrix of some multivesicular bodies. However, it has been used as an accepted marker to visualize the structure of ER.[13] Figure 2 showed the results of co-immunocytoflurescene of concanavalin A (green) and CRT (red). During the resting state, the ER displayed as homogeneous faint green fluorescence, distributed around the nuclei. The red fluorescence representing CRT was mainly co-localized with concanavalin A, showing weak intensity in the nuclear area. After H/R, the green fluorescence of ER became condensed, grainy and bubbled. The fluorescence intensity of CRT increased, no longer overlapping with concanavalin A, and the low fluorescence area of the nuclei disappeared, suggesting that CRT translocated from ER to nuclei. CRT overexpression aggravated the structural disorder of ER, and the CRT nuclear translocation became obvious. RNA interference knockdown the total expression of CRT and the phenomena of ER bubbling and CRT nuclear translocation eventually decreased. While the control plasmid (pCDB) or negative control siRNA (si-neg) transfection had no effect on ER disorder and CRT re-distribution induced by H/R.
Figure 2.
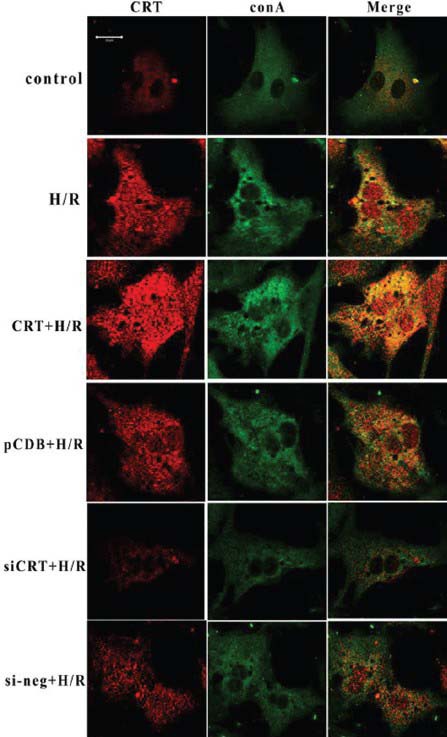
Co-immunocytoflurescene of concanavalin A (green) and calreticulin (CRT) (Red). During the resting state, the endoplasmic reticulum displayed a homogeneous faint green fluorescence, distributed around the nuclei. The red fluorescence representing CRT was mainly co-localized with concanavalin A, showing weak intensity in the nuclear area. After hypoxia/reoxygenation (H/R), the green fluorescence of the endoplasmic reticulum (ER) became condensed, grainy and bubbled. The fluorescence intensity of CRT increased, no longer overlapped with concanavalin A, and the low fluorescence area of the nuclei disappeared, suggesting that CRT translocated from ER to the nuclei. CRT overexpression aggravated the structural disorder of ER, and the CRT nuclear translocation became obvious. RNA interference knocked-down the total expression of CRT and the phenomena of ER bubbling and CRT nuclear translocation eventually decreased. The control plasmid (pCDB) or negative control siRNA (si-neg) transfection had no effect on ER disorder and CRT re-distribution induced by H/R. CRT + H/R represented transfection with CRT plasmid 24 hours before H/R. pcDB + H/R represented transfection with control plasmid 24 hours before H/R. siCRT + H/R represented transfection with CRT siRNA 24 hours before H/R. si-neg + H/R represented transfection with siRNA control 24 hours before H/R. The cell density for immunocytofluorescence analysis was 1 × 104 cell/cm2 and the transfection efficiency of our experiment was about 65%. Each experiment was repeated in triplicate.
Protein expression of transcription factors and markers of endoplasmic reticulum -stress and apoptosis
Cytoplasmic and nuclear extracts were separated by NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific. Cat No. 78833). Following H/R, the protein expression of CRT in the nuclear extracts increased compared with the control and hypoxic preconditioning (HPC) groups (P < 0.05). Hypoxic preconditioning also induced CRT nuclear translocation, but the extent was much lower than the H/R group (P < 0.05) [Figure 3a and 3b]. Along with the translocation of CRT from ER to nucleus, the expression of transcription factors such as Nrf2 and ATF4 in nuclear extracts increased [Figure 3a and 3b compared with control] (P < 0.05). H/R notably up-regulated the specific ERS markers, such as GRP78, CHOP and caspase-12 [Figure 4] (P < 0.05). It suggested that H/R induces ERS. Among these markers, GRP78 is a protective molecule and CRT overexpression, siRNA interference and taurine had no obvious effects on the up-regulation of GRP78 induced by H/R [Figure 4b] (P > 0.05). With regard to the pro-apoptotic molecules such as CHOP and caspase-12, CRT over-expression increased their expressions to a higher degree [Figure 4b] (P < 0.05); however, taurine administration and CRT siRNA interference decreased the expression of CHOP and caspase-12 [Figure 4b] (P < 0.05). CRT overexpression aggravated the apoptosis-promoting effect of H/R associated with ERS, while taurine and CRT siRNA alleviated ERS-associated apoptosis. In regard to general apoptosis-associated markers [Figure 5a], H/R up-regulated the pro-apoptotic molecules caspase-3 and Bax, while down-regulated the antiapoptotic molecule Bcl-2 [Figure 5b] (P < 0.05). CRT over-expression exacerbated the H/R effect, and the expression of caspase-3 and Bax increased while Bcl-2 expression decreased [Figure 5b] (P < 0.05). Taurine and CRT siRNA inhibited the up-regulation of caspase-3 and Bax, and the down-regulation of Bcl-2 [Figure 5b] (P < 0.05), indicating that these treatments relieved apoptosis induced by H/R.
Figure 3.
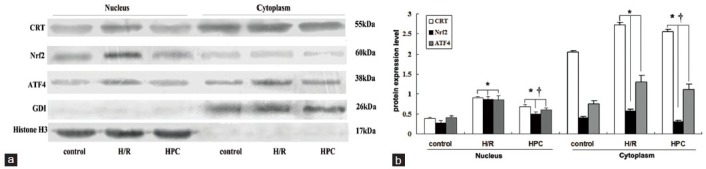
Cytoplasmic and nuclear extracts were separated using NE-PER nuclear and cytoplasmic extraction reagents. The relative amounts of protein expression levels of calreticulin (CRT), Nrf2, activating transcription factor 4 (ATF4). (a) Results of Western blotting of CRT, Nrf2, ATF4, Rho guanine nucleotide dissociation inhibitor-α (GDI-α) and Histone H3. Rho GDI was used as the endogenous loading control for cytoplasmic protein, and Histone H3 was used as the endogenous loading control of nuclear protein. (b) Statistical analysis of relative expression levels of nuclear and cytoplasmic proteins. The relative level of analyzed nucleus proteins was normalized to that of histone H3, and the relative level of cytoplasmic proteins was normalized to that of GDI. After hypoxia/reoxygenation (H/R), the protein expression of CRT in nuclear extracts increased compared with the control group and hypoxic preconditioning (HPC) group (P < 0.05). Hypoxic preconditioning also induced CRT nuclear translocation, but the extent was much lower than H/R group (P < 0.05) [Figure 4a and 4b]. Each experiment was repeated in triplicate (*P < 0.05 vs. control, †P <0.05 vs. H/R).
Figure 4.
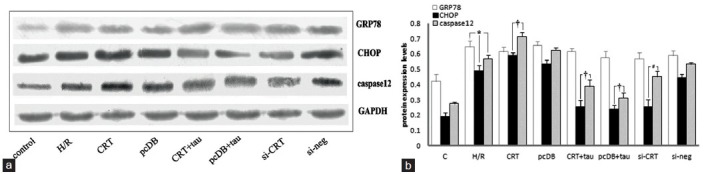
The relative amounts of protein expression levels of molecular markers associated with ER stress, such as glucose-regulated protein 78 (GRP78), CHOP and caspase-12. (a) Results from Western blotting of GRP78, CHOP, caspase-12 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (b) The statistical analysis graph of relative expression levels of these markers normalized to that of GAPDH. Hypoxia for 8 hours and reoxygenation for 16 hours (hypoxia/reoxygenation [H/R]) up-regulated the expressions of GRP78, CHOP and caspase-12. Among them, GRP78 was a protective molecule and calreticulin (CRT) over-expression, siRNA interference and taurine administration had no obvious effects on up-regulation of GRP78 induced by H/R. With regard to the pro-apoptotic molecules such as CHOP and caspase-12, CRT over-expression increased, while siRNA interference and taurine administration decreased their expression. Each experiment was repeated in triplicate. *P < 0.05 vs. control (†P <0.05 vs. H/R).
Figure 5.
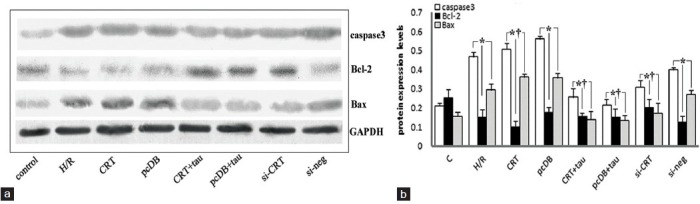
The relative amounts of protein expression levels of molecular markers associated with apoptosis, such as caspase-3, Bcl-2 and Bax. (a) Results of Western blotting of caspase-3, Bcl-2, Bax and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (b) The statistical analysis graph of relative expression levels of these markers normalized to that of GAPDH. Hypoxia for 8 hours and reoxygenation for 16 hours (hypoxia/reoxygenation [H/R]) up-regulated the expressions of caspase-3 and Bax, and down-regulated Bcl-2 expression. calreticulin overexpression induced by plasmid transfection 24 h before H/R further aggravated the changes induced by H/R, while siRNA interference and taurine administration had the opposite effects. Each experiment was repeated in triplicate (*P < 0.05 vs. control. †P <0.05 vs. H/R).
DISCUSSION
Myocardial ischemia-reperfusion injury is a serious concern to human health. Thrombolytic therapy is widely used to rescue ischemic myocardium, but its beneficial effect is often limited by cardiomyocyte oxidative stress induced by either I/R or H/R. Such injury threatens cardiomyocyte integrity and survival, and it is associated with ER dysfunction.[14] The ER is an important sub-cellular organelle for protein folding, lipid synthesis, and calcium storage. Disturbance of ER homeostasis results in ERS, acting as a mediator between survival and apoptosis. This study demonstrates that CRT, a major ER Ca2+ binding chaperone with multiple functions, translocates from the ER to the other parts of the cell during cardiomyocyte H/R, promotes ER dysfunction, and induces ERS-associated apoptosis.
Calreticulin has long been known as an ER luminal protein, and previous reports mainly focused on its function as a calcium binding chaperone, regulating intracellular Ca2+ homeostasis and protein folding. However, it is difficult to explain the controversial effects of CRT during apoptosis induced by ischemia, hypoxia or oxidative stress in different cell types. In recent years, it has been found that CRT has multiple functions in the other intracellular compartments, cell surface, and extracellular matrix.[2,6] Agents that directly lower ER Ca2+ levels can induce the rapid translocation of presynthesized CRT from the ER.[6] Once CRT translocates to the cytosol, it can be posttranslationally modified by arginyl transferase, which attaches an arginine residue to the N-terminal aspartic acid residue of the protein and localizes CRT to cytoplasmic stress granules.[15] It has also been reported that CRT can bind to the KxGFFKR sequence of many nuclear transcription factors, such as glucocorticoid receptor, regulating gene transcription.[16]
Cardiomyocytes over-expressing CRT exhibit increased intracellular calcium as well as either an expression or activity of pro-apoptotic factors such as Bax, p53, caspase-8 and caspase-3, together with reduced antiapoptotic factor Bcl-2.[17] The direct inhibition of CRT overexpression induced by either I/R or H/R injury through the endogenous protective phenomena such as IPC or hypoxic preconditioning (HPC), alleviates cardiomyocyte apoptosis,[5] inhibits caspase-12 activation in vitro, limits myocardial infarct size, improves heart function in vivo, and is consistent with its effect in rat skeletal muscle cells.[18] Recently, we found that CRT inhibition by antisense oligonucleotides weakened hypoxic postconditioning protection, and at the same time, attenuated H/R injury.[5] Based on previous studies, this work focused on the changes of CRT subcellular localization induced by H/R and its effect on ERS-associated apoptosis. We further hypothesized that H/R injury may up-regulate CRT expression and affect its subcellular localization. Other than its functions of re-establishing ER Ca2+ homeostasis and restoring proper protein folding in the ER lumen, CRT may have other effects on ERS -associated apoptosis. Either brief ischemic or hypoxic stress lead to the induction of CRT as an ER chaperone, and further succeeds in re-establishing ER homeostasis, while prolonged ischemia or hypoxia may induce CRT overexpression and translocation from the ER to the cytosol and disturb the anti- and pro-apoptotic balance of unfolded protein response, leading to an apoptotic fate.
This study showed that hypoxia re-oxygenation increased cell apoptosis and the permeability of the cell membrane, the appearance of ER condensation, and the bubbling phenomena. Along with the nuclear translocation of CRT, the expression of transcription factors such as Nrf2[19] and ATF4 in nuclear extracts also increased. The expression of ER-associated apoptosis markers, such as CHOP and caspase-12 increased, and the expression ratio of Bcl-2 to Bax decreased. Exogenous CRT overexpression aggravated cardiomyocyte H/R injury, causing cell apoptosis, and an increase in necrosis rate and cell membrane permeability. Additionally, the activity of LDH in the culture medium was increased, and ER morphology injury was aggravated. The nuclear translocation of CRT and the activation of ERS-associated apoptosis pathway further increased. There was a positive correlation between the over-expression of CRT induced by H/R and the up-regulation of pro-apoptotic molecules such as CHOP and caspase-12 associated with ERS. Taurine is a conditionally essential amino acid, which is high in the myocardium (10–40 mmol/L), playing cytoprotective role, including calcium modulation, membrane stabilization, osmoregulation, antioxidation, protein phosphorylation, and ion flux. Our previous work has confirmed that taurine inhibits ERS in the myocardium,[20] and in our in vitro experiment we used 40 mmol/L of taurine as an ERS inhibitor according to the dose reported in the literature.[21] This work showed that taurine and CRT siRNA interference both attenuated injuries induced by H/R, showing reversed damages to ER structure, decreased CRT distribution in ER and nucleus, and the expression of pro-apoptotic molecules. When ERS is overactive, as a multifunctional protein, CRT may retro-translocate to the other parts of the cell and no longer play a protective role, promoting ER dysfunction and participating in the activation of the ERS-associated apoptosis cascade. H/R induced CRT over-expression and translocation, aggravating ER dysfunction and activating the ERS-associated apoptosis pathway to enhance cardiomyocyte damage.
It has been reported that during ERS-associated apoptosis, the expression of CRT, Nrf2 and ATF4 were up-regulated,[22] but the exact relationship between these factors has not been clarified. We found that along with CRT nuclear translocation, the expression of Nrf2 and ATF4 in nuclear extraction increased, suggesting that CRT nuclear translocation induced by H/R might participate in regulating nuclear transcription factor expression and gene transcription. CHOP, also named growth arrest and DNA damage inducible gene 153 (GADD153), can directly regulate nuclear target genes to increase the tendency of cell apoptosis. CHOP up-regulation increased cell sensitivity to apoptosis induced by ERS.[23] CHOP over-expressing cells displayed cell cycle arrest, Bcl-2 down-regulation and Bax translocation from the cytoplasm to the mitochondria and eventually to apoptosis. Furthermore, CHOP-induced apoptosis may be blocked by either Bcl-2 overexpression or the knockout of Bax. In our study, CRT over-expression up-regulated CHOP and Bax, and down-regulated Bcl-2, while taurine administration and the siRNA interference had opposite effects, indicating that CRT overexpression may be involved in the CHOP-related apoptotic pathway during cardiomyocyte H/R injury.
Casapse-12 is localized to the ER and activated by ERS, including the disruption of ER calcium homeostasis and the accumulation of excess proteins in the ER, but not by either membrane- or mitochondrial-targeted apoptotic signals. Mice that are deficient in casapse-12 are resistant to ERS-induced apoptosis, but their cells undergo apoptosis in response to other death stimuli. Thus, casapse-12 mediates an ER specific apoptosis pathway.[24] In our study, CRT overexpression up-regulated expression of caspase-12 and caspase-3, while taurine administration and siRNA interference had opposite effects, indicating that CRT overexpression may be involved in the ER-specific apoptosis pathway during cardiomyocyte H/R injury.
Collectively, CRT is a major calcium-binding chaperone that is primarily located within the ER. Over the past decade, CRT has also been implicated into a variety of processes that occur outside of the ER lumen. From the results of the study, we speculated that CRT nuclear translocation might regulate the transcriptional activities of nuclear transcription factors such as Nrf2 and ATF4, affecting the transcription and translation of ERS-associated proteins and causing ER damage and subsequent apoptosis.
Footnotes
Edited by: Xiu-Yuan Hao
Source of Support: This study was supported by the National Natural Science Foundation of China (No. 81170140 and No. 31471094).
Conflict of Interest: None declared.
REFERENCES
- 1.Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417:651–66. doi: 10.1042/BJ20081847. [DOI] [PubMed] [Google Scholar]
- 2.Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM, et al. Calreticulin: Non-endoplasmic reticulum functions in physiology and disease. FASEB J. 2010;24:665–83. doi: 10.1096/fj.09-145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesaeli N, Nakamura K, Zvaritch E, Dickie P, Dziak E, Krause KH, et al. Calreticulin is essential for cardiac development. J Cell Biol. 1999;144:857–68. doi: 10.1083/jcb.144.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zwadlo C, Borlak J. Disease-associated changes in the expression of ion channels, ion receptors, ion exchangers and Ca (2+)-handling proteins in heart hypertrophy. Toxicol Appl Pharmacol. 2005;207:244–56. doi: 10.1016/j.taap.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Xu F, Fu Y, Liu F, Sun S, Wu X. Calreticulin induces delayed cardioprotection through mitogen-activated protein kinases. Proteomics. 2006;6:3792–800. doi: 10.1002/pmic.200500906. [DOI] [PubMed] [Google Scholar]
- 6.Afshar N, Black BE, Paschal BM. Retrotranslocation of the chaperone calreticulin from the endoplasmic reticulum lumen to the cytosol. Mol Cell Biol. 2005;25:8844–53. doi: 10.1128/MCB.25.20.8844-8853.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Wu X, Cai L, Tang C, Su J. Hypoxic preconditioning of cardiomyocytes and cardioprotection: Phophorylation of HIF-1alpha induced by p42/p44 mitogen-activated protein kinases is involved. Pathophysiology. 2003;9:201–5. doi: 10.1016/s0928-4680(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 8.Thornton JD, Liu GS, Downey JM. Pretreatment with pertussis toxin blocks the protective effects of preconditioning: Evidence for a G-protein mechanism. J Mol Cell Cardiol. 1993;25:311–20. doi: 10.1006/jmcc.1993.1037. [DOI] [PubMed] [Google Scholar]
- 9.John LM, Lechleiter JD, Camacho P. Differential modulation of SERCA2 isoforms by calreticulin. J Cell Biol. 1998;142:963–73. doi: 10.1083/jcb.142.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeffery E, Peters LR, Raghavan M. The polypeptide binding conformation of calreticulin facilitates its cell-surface expression under conditions of endoplasmic reticulum stress. J Biol Chem. 2011;286:2402–15. doi: 10.1074/jbc.M110.180877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai NP, Lin YL, Tsui YC, Wei LN. Dual action of epidermal growth factor: Extracellular signal-stimulated nuclear-cytoplasmic export and coordinated translation of selected messenger RNA. J Cell Biol. 2010;188:325–33. doi: 10.1083/jcb.200910083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Wu X, Cai L, Sun S. Calreticulin downregulation is associated with FGF-2-induced angiogenesis through calcineurin pathway in ischemic myocardium. Shock. 2008;29:140–8. doi: 10.1097/shk.0b013e318123e822. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda Y, Gohra H, Hamano K, Zempo N, Ueyama T, Ohkusa T, et al. Effects of cardioplegic arrest and reperfusion on rabbit cardiac ryanodine receptors. Jpn Circ J. 2001;65:330–4. doi: 10.1253/jcj.65.330. [DOI] [PubMed] [Google Scholar]
- 14.Chen YH, Wu XD, Yao ST, Sun S, Liu XH. Calcineurin is involved in cardioprotection induced by ischemic postconditioning through attenuating endoplasmic reticulum stress. Chin Med J. 2011;124:3334–40. [PubMed] [Google Scholar]
- 15.Decca MB, Carpio MA, Bosc C, Galiano MR, Job D, Andrieux A, et al. Post-translational arginylation of calreticulin: a new isospecies of calreticulin component of stress granules. J Biol Chem. 2007;282:8237–45. doi: 10.1074/jbc.M608559200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olkku A, Mahonen A. Calreticulin mediated glucocorticoid receptor export is involved in beta-catenin translocation and Wnt signalling inhibition in human osteoblastic cells. Bone. 2009;44:555–65. doi: 10.1016/j.bone.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Lim S, Chang W, Lee BK, Song H, Hong JH, Lee S, et al. Enhanced calreticulin expression promotes calcium-dependent apoptosis in postnatal cardiomyocytes. Mol Cells. 2008;25:390–6. [PubMed] [Google Scholar]
- 18.Zhang ZY, Liu XH, Guo XS, Liu FY. Calreticulin is involved in ischemic postconditioning-induced attenuation of ischemia/reperfusion injury in rat skeletal muscle. Acta Physiologica Sinica. 2007;59:643–50. [PubMed] [Google Scholar]
- 19.Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, et al. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29:1843–50. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M, Wang XR, Wang C, Song DD, Liu XH, Shi DZ. Panax quinquefolium saponin attenuates ventricular remodeling after acute myocardial infarction by inhibiting chop-mediated apoptosis. Shock. 2013;40:339–44. doi: 10.1097/SHK.0b013e3182a3f9e5. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Yang L, Yang YJ, Liu XY, Jia JG, Qian JY, et al. Low-dose taurine upregulates taurine transporter expression in acute myocardial ischemia. Int J Mol Med. 2013;31:817–24. doi: 10.3892/ijmm.2013.1264. [DOI] [PubMed] [Google Scholar]
- 22.Kelsen SG, Duan X, Ji R, Perez O, Liu C, Merali S. Cigarette smoke induces an unfolded protein response in the human lung: a proteomic approach. Am J Respir Cell Mol Biol. 2008;38:541–50. doi: 10.1165/rcmb.2007-0221OC. [DOI] [PubMed] [Google Scholar]
- 23.Zhang ZY, Liu XH, Ye YJ, Sun S, Rong F, Guo XS, et al. C/EBP homologous protein-mediated endoplasmic reticulum stress-related apoptosis pathway is involved in abdominal aortic constriction-induced myocardium hypertrophy in rats. Acta Physiologica Sinica. 2009;61:161–8. [PubMed] [Google Scholar]
- 24.Yang YY, Shang J, Liu HG. Role of endoplasmic reticular stress in aortic endothelial apoptosis induced by intermittent/persistent hypoxia. Chin Med J. 2013;126:4517–23. [PubMed] [Google Scholar]


